Abstract
Background:
Persons with MS commonly report word-finding difficulty clinically, yet this language deficit remains underexplored.
Objective:
To investigate the prevalence and nature of word-finding difficulty in persons with early MS on three levels: patient report, cognitive substrates, neuroimaging.
Methods:
Two samples of early MS patients (n=185 and n=55; ≤5 years diagnosed) and healthy controls (n=50) reported frequency/severity of cognitive deficits and underwent objective assessment with tasks of rapid automatized naming (RAN, measuring lexical access speed), memory, word generation, and cognitive efficiency. High-resolution brain MRIs derived measurements of regional cortical thickness, global and deep gray matter volume, and T2 lesion volume. Relationships among patient-reported word-finding difficulty, cognitive performance, and neural correlates were examined.
Results:
Word-finding difficulty was the most common cognitive complaint of MS patients and the only complaint reported more by patients than healthy controls. Only RAN performance discriminated MS patients with subjective word-finding deficits from those without subjective complaints and from healthy controls. Thinner left parietal cortical gray matter independently predicted impaired RAN performance, driven primarily by the left precuneus.
Conclusions:
Three levels of evidence (patient-report, objective behavior, regional gray matter) support word-finding difficulty as a prevalent, measurable, disease-related deficit in early MS linked to left parietal cortical thinning.
Keywords: multiple sclerosis, language, cognition, parietal lobe, memory, demyelinating diseases
INTRODUCTION
Cognitive decline is a prevalent and debilitating clinical feature of multiple sclerosis (MS), with most research focused on processing speed and memory.1–3 Anecdotally, MS patients often report word-finding difficulty, but there is little research on this deficit. Patients describe embarrassment and frustration in social and occupational settings due to word-finding difficulty. Some studies have reported deficits in rapid word generation (verbal fluency) and picture naming (especially in long-standing progressive disease),4–9 but the prevalence, cognitive substrates, and neural basis of word-finding difficulty remain poorly understood, particularly early in disease.
MS has historically been understood as a diffuse demyelinating disease leading to a subcortical pattern of cognitive decline: slowed processing speed with secondary deficits in other functions (i.e., memory).10 This model may have dissuaded investigation of independent language deficits. MS is now recognized as a neuroinflammatory and neurodegenerative disease with cerebral gray matter atrophy11 occurring early in disease12–14 and linked to cognitive deficits.15–17 Recent cross-sectional and longitudinal MRI studies in MS highlight prominent left parietal atrophy14,18 as one of the earliest cortical regions to atrophy.14 This may have clinical implications for word-finding.
Here we investigate word-finding difficulty in a research cohort of 185 patients with early MS, with replication in an independent clinical MS sample. We (a) compare prevalence of subjective word-finding difficulty versus other cognitive complaints across patients and healthy controls, and (b) examine links between patient-reported word-finding and objective cognitive performance including lexical access speed assessed with rapid automatized naming (RAN). Research on RAN originated in the dyslexia literature to assess lexical access speed,19 which is linked to left parietal function.20 RAN may be more sensitive to subtle disease-related language changes underlying word-finding than traditional word generation tasks and processing speed. Finally, we (c) identify patterns of regional cerebral gray matter associated with word-finding.
METHODS
Participants
RADIEMS Sample:
The Reserve Against Disability in Early MS (RADIEMS) is a study of risk and protective factors for cognitive decline in early MS (aged 20 to 50 years, diagnosed with relapsing-remitting MS or clinically isolated syndrome ≤5 years).21 Key exclusions: other neurologic or neurodevelopmental conditions, major psychiatric illness (e.g., bipolar disorder, schizophrenia, current major depressive episode), dyslexia, pregnancy, and clinical relapse within six weeks. All patients were proficient in English as a requirement of enrollment.
Healthy Controls:
Healthy controls meeting the same inclusion criteria were enrolled. There were no demographic differences between RADIEMS patients and healthy controls (Table 1).
Table 1.
Sample Characteristics
| RADIEMS MS | Healthy | Clinical MS | ||
|---|---|---|---|---|
| Sample Size1 | 185 | 50 | 55 | |
| Age (mean ± sd) | 34.4±7.5a | 32.9±7.5a | 37.5±7.2b | b>a, p<.01 |
| Sex (% Female) | 66.5 | 64.0 | 65.5 | |
| Handedness (% Right) | 87.6 | 82.0 | 85.5 | |
| Maternal Education (% ≥16 years)2 | 52.4 | 60.0 | 41.8 | |
| Verbal IQ (mean ± sd) | 108.2±9.2a | 111.0±9.3b | 104.8±9.4c | a>c, p<.05; b>c, p<.001 |
| Disease Course (RRMS / CIS)19 | 165/20 | 52/3 | ||
| Years since Diagnosis (mean ± sd) | 2.2 ± 1.4 | 2.1 ± 1.7 | ||
| Timed 25-Foot-Walk (median, IQR) | 4.1, 3.7-4.4a | 4.4, 4.0-4.9b | b>a, p<.001 | |
| EDSS (median, IQR)3 | 1.0, 0.0-1.5 | |||
| EDSS score (n): 0.0 (63), 1.0 (52), 1.5 (34), 2.0 (21), ≥2.5(15) | ||||
Two enrolled patients were not permitted to undergo research MRIs due to metal in their bodies. These patients had not reported this contraindication prior to enrollment because the metal had not precluded previous clinical MR imaging. The sample size for imaging analyses was therefore 183.
Maternal education is a better marker of socioeconomic status than a person’s own education because (a) it represents socioeconomic status during childhood and adolescence and (b) the current sample was relatively young, with 15% of the RADIEMS sample younger than 25 years of age.
EDSS scores were not collected for the clinical MS group.
Clinical MS Sample:
Patients at Mount Sinai are referred for baseline cognitive evaluations. We performed retrospective chart reviews. Data from all patients meeting aforementioned inclusion criteria and evaluated before 04/30/2019 comprised an independent replication sample. This clinical sample was slightly older with lower albeit average verbal IQ versus RADIEMS and healthy control groups (Table 1). Data collection was approved by Institutional Review Boards at Mount Sinai Hospital and Columbia University Medical Center.
Subjective Cognitive Deficits
All participants completed the Perceived Deficits Questionnaire (PDQ)22 in which they rated the frequency/severity of 20 cognitive deficits (Table 2), to which we added one additional item to assess word-finding difficulty: “Having a word on the tip of your tongue but having difficulty getting it out.” Severity of each cognitive complaint was endorsed as “never,” “rarely,” “sometimes,” “fairly often,” or “very often.” Established subscale scores for attention, executive function, retrospective memory, and prospective memory were derived.
Table 2. Subjective Cognitive Complaints by MS Patients and Healthy Controls.
Percentages of subjects reporting each level of severity for each cognitive complaint are reported separately for healthy controls, RADIEMS MS patients, and clinical MS patients. For comparisons of RADIEMS MS patients versus healthy controls, p-values are reported for unadjusted responses (unadj) and responses adjusted for mood (mood). The same comparisons are made for clinical MS patients versus healthy controls, plus an additional comparison controlling for both mood and demographics (mood, demo). Word-finding difficulty is the cognitive complaint reported more by both patient samples versus healthy controls.
| Healthy Controls (HC) | RADIEMS MS Sample | RADIEMS MS vs HC | Clinical MS Sample | Clinical MS Sample vs HC | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Fairly Often | Very Often | Never | Rarely | Sometimes | Fairly Often | Very Often | P-Value (unadj) | P-Value (mood) | Never | Rarely | Sometimes | Fairly Often | Very Often | P-Value (unadj) | P-Value (mood) | P-Value (mood, demo) | |
| ATTENTION | 0.916 | 0.406 | 0.829 | 0.667 | 0.649 | |||||||||||||||
| losing your train of thought | 6.0% | 22.0% | 56.0% | 12.0% | 4.0% | 5.9% | 24.9% | 44.3% | 15.7% | 9.2% | 0.588 | 0.925 | 5.5% | 21.8% | 38.2% | 18.2% | 16.4% | 0.151 | 0.161 | 0.287 |
| trouble concentrating on what people are saying | 24.0% | 40.0% | 30.0% | 6.0% | 0.0% | 25.9% | 30.8% | 33.0% | 8.1% | 2.2% | 0.533 | 0.921 | 21.8% | 30.9% | 27.3% | 14.5% | 5.5% | 0.178 | 0.156 | 0.063 |
| difficulty doing more than one thing at a time | 20.0% | 50.0% | 20.0% | 10.0% | 0.0% | 29.2% | 35.7% | 25.4% | 6.5% | 3.2% | 0.768 | 0.397 | 130.9% | 25.5% | 21.8% | 10.9% | 10.9% | 0.547 | 0.463 | 0.320 |
| taking a long time to finish things | 22.0% | 36.0% | 24.0% | 18.0% | 0.0% | 23.2% | 35.1% | 30.3% | 9.2% | 2.2% | 0.705 | 0.143 | 27.3% | 25.5% | 27.3% | 10.9% | 9.1% | 0.814 | 0.997 | 0.542 |
| trouble holding a string of numbers in your head | 24.0% | 44.0% | 22.0% | 6.0% | 4.0% | 32.4% | 27.6% | 27.0% | 8.1% | 4.9% | 0.958 | 0.845 | 41.8% | 21.8% | 16.4% | 14.5% | 5.5% | 0.563 | 0.604 | 0.333 |
| EXECUTIVE FUNCTION | 0.589 | 0.614 | 0.719 | 0.632 | 0.496 | |||||||||||||||
| trouble getting things organized | 22.0% | 50.0% | 22.0% | 2.0% | 4.0% | 24.3% | 37.8% | 28.6% | 5.4% | 3.8% | 0.481 | 0.964 | 18.2% | 36.4% | 18.2% | 16.4% | 10.9% | 0.053 | 0.057 | 0.209 |
| difficulty planning what to do in the day | 50.0% | 30.0% | 16.0% | 4.0% | 0.0% | 37.3% | 37.8% | 16.8% | 4.9% | 3.2% | 0.124 | 0.439 | 40.0% | 27.3% | 20.0% | 9.1% | 3.6% | 0.139 | 0.143 | 0.140 |
| trouble getting started even if you had lots to do | 24.0% | 30.0% | 34.0% | 6.0% | 6.0% | 29.2% | 30.3% | 25.4% | 9.2% | 5.9% | 0.570 | 0.090 | 27.3% | 25.5% | 27.3% | 10.9% | 9.1% | 0.811 | 0.927 | 0.472 |
| finding your mind drifting or your mind going blank | 24.0% | 32.0% | 36.0% | 6.0% | 2.0% | 20.5% | 30.8% | 34.6% | 7.0% | 7.0% | 0.363 | 0.876 | 25.5% | 32.7% | 20.0% | 14.5% | 7.3% | 0.719 | 0.665 | 0.644 |
| trouble making decisions | 22.0% | 44.0% | 26.0% | 4.0% | 4.0% | 28.1% | 34.6% | 23.8% | 10.8% | 2.7% | 0.984 | 0.467 | 41.8% | 23.6% | 21.8% | 9.1% | 3.6% | 0.297 | 0.304 | 0.534 |
| RETROSPECTIVE MEMORY | 0.030 | 0.223 | 0.245 | 0.174 | 0.273 | |||||||||||||||
| difficulty learning names of new people or places | 12.0% | 40.0% | 34.0% | 12.0% | 2.0% | 11.9% | 33.0% | 33.0% | 14.1% | 8.1% | 0.260 | 0.573 | 21.8% | 23.6% | 27.3% | 14.5% | 10.9% | 0.607 | 0.692 | 0.908 |
| forgetting if you had already done something | 28.0% | 38.0% | 28.0% | 6.0% | 0.0% | 16.8% | 34.6% | 35.1% | 9.7% | 3.8% | 0.024 | 0.100 | 23.6% | 21.8% | 30.9% | 14.5% | 9.1% | 0.036 | 0.029 | 0.079 |
| trouble remembering where you put something | 28.0% | 42.0% | 26.0% | 4.0% | 0.0% | 18.4% | 39.5% | 24.9% | 10.3% | 7.0% | 0.029 | 0.113 | 20.0% | 32.7% | 27.3% | 10.9% | 9.1% | 0.038 | 0.031 | 0.164 |
| forgetting details of a recent conversation | 24.0% | 38.0% | 28.0% | 8.0% | 2.0% | 24.3% | 26.5% | 36.8% | 7.0% | 5.4% | 0.342 | 0.832 | 29.1% | 21.8% | 29.1% | 10.9% | 9.1% | 0.439 | 0.410 | 0.842 |
| trouble recalling what happened during the last week | 30.0% | 42.0% | 24.0% | 4.0% | 0.0% | 24.9% | 36.2% | 26.5% | 7.6% | 4.9% | 0.126 | 0.303 | 45.5% | 10.9% | 32.7% | 5.5% | 5.5% | 0.901 | 0.984 | 0.739 |
| PROSPECTIVE MEMORY | 0.187 | 0.881 | 0.152 | 0.091 | 0.209 | |||||||||||||||
| forgetting what you came into the room for | 8.0% | 44.0% | 44.0% | 4.0% | 0.0% | 12.4% | 30.3% | 40.5% | 8.6% | 8.1% | 0.157 | 0.295 | 10.9% | 27.3% | 32.7% | 16.4% | 12.7% | 0.031 | 0.015 | 0.113 |
| forgetting appointments or meetings you had planned | 54.0% | 32.0% | 8.0% | 6.0% | 0.0% | 42.2% | 37.8% | 13.0% | 4.9% | 2.2% | 0.133 | 0.709 | 32.7% | 30.9% | 23.6% | 7.3% | 5.5% | 0.008 | 0.006 | 0.059 |
| forgetting what day of the week it is | 54.0% | 26.0% | 14.0% | 4.0% | 2.0% | 54.1% | 31.9% | 8.6% | 2.2% | 3.2% | 0.768 | 0.116 | 54.5% | 25.5% | 9.1% | 7.3% | 3.6% | 0.958 | 0.780 | 0.969 |
| forgetting to do things like turning on your alarm | 62.0% | 32.0% | 4.0% | 0.0% | 2.0% | 50.8% | 35.7% | 8.1% | 3.8% | 1.6% | 0.107 | 0.364 | 61.8% | 16.4% | 10.9% | 7.3% | 3.6% | 0.524 | 0.600 | 1.000 |
| forgetting to do routine things without reminders | 58.0% | 22.0% | 12.0% | 6.0% | 2.0% | 43.8% | 28.6% | 18.9% | 4.3% | 4.3% | 0.098 | 0.801 | 40.0% | 34.5% | 10.9% | 12.7% | 1.8% | 0.097 | 0.097 | 0.320 |
| WORD FINDING | ||||||||||||||||||||
| word on tip of your tongue but difficulty getting it out | 16.0% | 50.0% | 24.0% | 8.0% | 2.0% | 11.4% | 26.5% | 37.8% | 12.4% | 11.9% | <0.001 | 0.008 | 14.5% | 25.5% | 25.5% | 23.6% | 10.9% | 0.008 | 0.006 | 0.044 |
Objective Cognitive Evaluation
RADIEMS patients and healthy controls completed a comprehensive neuropsychological battery of tasks assessing cognitive efficiency, memory, rapid word generation, and rapid automatized naming (RAN). Multiple measures of each construct were included to calculate separate composite scores (see below). Performance on each task was regression-adjusted for age, sex, and estimated premorbid verbal ability (Wechsler Test of Adult Reading). Cognitive Efficiency: Symbol Digit Modalities Test (SDMT): 90-second task wherein participants orally provide digits that match visual symbols based on nine digit-symbol pairings in a key as quickly as possible.23 NIH Toolbox Pattern Comparison (PC): 90-second tablet-based task wherein participants rapidly indicate via button press whether two pictures are the same or different.24 Decision Speed: 100-second task wherein participants are presented with sixty rows each containing pictures of four common objects (e.g., dress, car, book, paper clip); participants quickly circle the object in each row that is largest in real life (e.g., car). Stroop Color and Word Test (SCWT25): Participants quickly name the ink color (red, green, blue) of non-matching printed words (e.g., “red” written in green ink). Memory: Selective Reminding Test (SRT) and Brief Visuospatial Memory Test, Revised (BVMT-R) are the verbal (word list learning) and nonverbal (geometric shapes/locations) memory tests of the Brief Repeatable Battery for MS.2 We also assessed verbal and nonverbal paired-associate learning with Verbal Paired Associate Learning (V-PAL) requiring participants to learn 12 unrelated word pairs across four trials and CANTAB Paired Associate Learning (PAL; Cambridge Cognition, Cambridge, UK) requiring participants to recall the spatial locations where visual stimuli were previously presented. Rapid Word Generation (Verbal Fluency): Phonemic Fluency (FAS) and Semantic Fluency (Animals) word generation tasks ask participants to quickly retrieve as many words as possible starting with target letters across three 60-second trials (FAS) or belonging to a semantic category in one 60-second trial (Animals).26 RAN: Rapid Object Naming (RAN-Object) and Rapid Color Naming (RAN-Color) are classic RAN procedures to assess speed of lexical access.27 During RAN-Objects participants were shown pictures of twenty common objects (e.g., leaf, apple, turtle) and were told the name of each object (to ensure that single words were used, i.e., not “maple leaf’). Participants were then shown four rows of ten pictures (each of the twenty objects was used twice). Participants named objects as quickly as possible and time to completion was recorded. RAN-Color was assessed with the color naming condition of the aforementioned SCWT in which persons name the color of X’s printed in red, blue, or green as quickly as possible in 45 seconds. Composite Scores: Residualized scores for each of these tasks (adjusted for age, sex, WTAR) were converted to z-scores based on means and standard deviations of the healthy control group (signs adjusted so positive z-scores reflect better performance). Composite measures of cognitive efficiency, memory, rapid word generation, and RAN were computed as the mean z-score of tasks within each domain.
Clinical MS Sample:
The same or similar tasks were used in the clinical replication sample: Cognitive Efficiency assessed with SDMT and Decision Speed; Memory assessed with CANTAB PAL and the Hopkins Verbal Learning Test, Revised (HVLT-R); Rapid Word Generation (Verbal Fluency) assessed with Phonemic Fluency (Letter F) and Semantic Fluency (Animals). RAN assessed with same RAN-Object as above and a variant RAN-Color in which patients rapidly named the color of boxes printed in red, blue, green, or yellow ink. Visual Confrontation Naming: Patients completed 15-item Boston Naming Test (BNT) requiring them to name of lower-frequency objects presented as line drawings (not administered in RADIEMS).
Neuroimaging (RADIEMS Only)
Patients (n=183) underwent 3D T1-weighted (TR=2400ms, TE=2.0ms, FOV=256mm, 176 slices with 1.0 mm thickness: voxel size=1.0mm3) and 3D T2-weighted (TR=3200ms, TE=566.0ms, FOV=230mm, 224 slices with 0.9 mm thickness: voxel size=0.9mm3) 3.0-Tesla brain MRIs (Siemens Skyra; 16 channel head coil) at Mount Sinai Hospital. T2 lesion volumes (T2LV) were quantified using a local thresholding segmentation technique (Jim 6.0, Xinapse System, www.xinapse.com) by trained postdoctoral fellows, with adjudication by a neurologist. SIENAX and FIRST were performed on lesion-filled T1-weighted images to derive normalized volumes of total brain (nBV), total gray matter (nGM), and total deep gray matter (nDGM), and applying the volume-scaling factor (SIENAX) to each. Mean cortical thickness and cortical matter thickness for the 68 Desikan-Killiany regions of interest (ROIs) were derived using FreeSurfer 5.0 on the same T1-weighted images. Automated segmentations were visually-inspected and manually-corrected as needed. FreeSurfer templates were used to calculate mean cortical thickness for left and right frontal, temporal, parietal, occipital and cingulate cortices, (https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation), which were regression-adjusted for age, sex, and mean overall cortical thickness. A subsample of healthy controls (n=35) also underwent identical neuroimaging procedures and analyses (except for T2LV).
Statistical Analysis
Subjective Cognitive Deficits.
Mann-Whitney tests assessed differences in cognitive complaints (20 PDQ items, 4 PDQ subscales, word-finding) across RADIEMS patients and healthy controls, with correction for multiple comparisons (Bonnferoni p=.002). We predicted that patients would report word-finding deficits more than healthy controls and that word-finding would be the largest disease-related deficit. We then investigated for this expected finding within the independent clinical sample versus healthy controls. Given that this second analysis was a replication in a smaller sample, we used an alpha of .01 to correct for multiple comparisons. Adjusted alphas in both samples are rigorous and conservative given our a priori hypothesis. We repeated analyses controlling for mood and demographic variables differing across groups.
Objective Cognitive Performance:
Spearman correlations investigated relationships between patient-reported word-finding and performance on all objective cognitive tests and composite scores within the MS RADIEMS sample, with correction for multiple comparisons (Bonnferoni p=.0031). All scores were already regression-adjusted for age, sex, and premorbid verbal ability. Analyses were repeated for the clinical MS replication sample. To further characterize objective performance across levels of patient-reported word-finding difficulty, MANOVA investigated differences in performance composites across three levels of patients reporting word-finding difficulty (a) never/rarely, (b) sometimes, or (c) fairly or very often (to ensure adequate sample sizes per group). For any composites linked to word-finding, we also performed independent t-tests to assess performance differences between levels of patient-reported word-finding and healthy controls. These latter analyses were not repeated for the clinical MS sample due to the smaller sample size and different tests used for composites.
Neuroimaging Correlates (RADIEMS only):
Spearman correlations investigated relationships between patient-reported word-finding and neuroimaging outcomes. Next, separate stepwise linear regressions identified neuroimaging metrics independently related to objective performance on cognitive outcomes linked to patient-reported word-finding in previous analyses, with correction for multiple comparisons (Bonnferoni entry p=.0033). If region(s) of cortical thickness (e.g., left parietal) were retained, follow-up analyses examined correlations with components of that region (e.g., left supramarginal gyrus). To be thorough, we repeated the stepwise regression including aforementioned neuroimaging variables and all 68 regions of cortical thickness (Bonnferoni entry p=.0006).
RESULTS
Subjective Cognitive Deficits
RADIEMS:
Word-finding difficulty was the only cognitive complaint reported more by patients than healthy controls (p<.001, Table 2). Nearly a quarter of RADIEMS patients reported “fairly often” (12.4%) or “very often” (11.9%) experiencing word-finding problems, compared with only 8.0% and 2.0% of healthy controls, respectively. No other cognitive complaints significantly differed between groups when employing conservative (p=0.002) or even liberal (p=0.01) corrections for multiple comparions. These findings highlight word-finding difficulty as the most prevalent patient-reported cognitive deficit in early MS.
We repeated analyses after regression-adjusting all cognitive complaints for mood assessed by the Mental Health Inventory (MHI-5) and Beck Depression Inventory-Fast Screen. Analysis of group differences on adjusted responses again identified word-finding difficulty as the only complaint reported more by patients than healthy controls (Table 2).
Clinical MS Sample:
Word-finding difficulty (p=.008) and forgetting appointments / meetings (p=.008) were the only complaints that differed between clinical patients and healthy controls (Table 2). A third of clinical patients reported “fairly often” (23.6%) or “very often” (10.9%).
Our clinic sample completed the Hospital Anxiety and Depression Scale (HADS), not MHI-5 or BDI-FS. To control for mood, clinic patients and controls were classified as having no (HADS-D<8 or BDI-FS<4) or ≥ mild depression symptoms (HADS-D ≥8 or BDI-FS ≥4; 18.2% and 18.0% respectively). When PDQ items were regression-adjusted for depression symptoms (none versus ≥mild), word-finding difficulty (p=.006) and forgetting appointments/meetings (p=.006) were still more by patients than controls (Table 2). We also adjusted PDQ responses for age and verbal ability given group differences (Table 1), and maternal education (trend difference). Word-finding was the only complaint robust against these controls (Table 2).
Results from both RADIEMS and clinical MS patients show that word-finding difficulty was the only cognitive complaint reported more by early MS patients than healthy persons (Figure 1).
FIGURE 1. Subjective Cognitive Complaints across Patients and Healthy Controls.
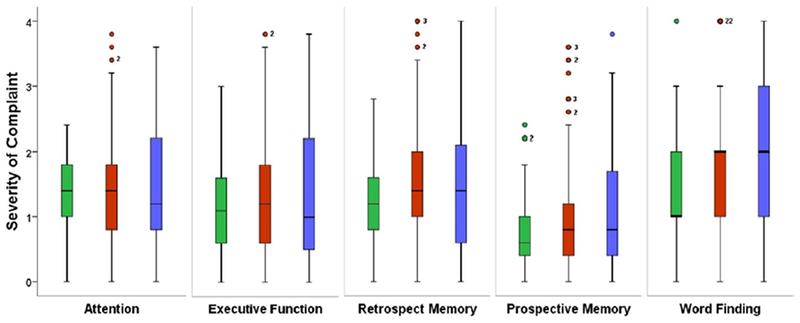
Boxplots depict medians and interquartile ranges for the four PDQ subscales and word-finding are compared across healthy controls (green), RADIEMS MS patients (red), and clinical MS patients (blue). Frequency / severity of subjective difficulty on the y-axis: never (0), rarely (1), sometimes (2), fairly often (3), very often (4). Numbers adjacent to circles indicate the frequency of participants with that same value (if >1). Kruskal Wallis tests assessing median differences for each subjective complaint across groups was significant for Word-Finding (p=.003) but not for any other cognitive domains (Ps>.10). As shown in Table 2, both MS groups reported worse word-finding difficulty than healthy controls.
Objective Performance
RADIEMS:
Patient-reported word-finding difficulty was specifically linked to slower speed of lexical access on RAN-Composite and RAN-Object but not other cognitive domains or tasks (Table 3). Moreover RAN-Composite and RAN-Object were independently linked to patient-reported word-finding even when adjusted for other cognitive composites (Table 3).
Table 3. Correlations between Cognitive Performance and Patient-Reported Word-Finding.
Spearman correlations are reported between patient-reported word-finding and objective cognitive performance for both the RADIEMS MS and clinical MS samples. Correlations withstanding correction for multiple comparisons are in bold: RAN-Composite and RAN-Object. Both RAN-Composite and RAN-Object remained robustly related to patient-reported word-finding even when scores were regression-adjusted for composites of cognitive efficiency, memory, or word list generation in the RADIEMS (Ps≤.01) and clinical MS (Ps≤.05) cohorts. [We did not include healthy controls in the table because so few controls reported word-finding difficulty fairly often (n=4) or very often (n=1). There was only one significant correlation: a difficult to interpret (likely random) link between worse word-finding and better PAL (p=.032).]
| PRO Word-Finding | |||
|---|---|---|---|
| RADIEMS | Clinical MS | ||
| COGNITIVE EFFICIENCY | rho | −0.148 | −0.087 |
| p | 0.045 | 0.526 | |
| SDMT | rho | −0.108 | 0.034 |
| p | 0.145 | 0.804 | |
| Decision Speed | rho | −0.062 | −0.136 |
| p | 0.398 | 0.322 | |
| Pattern Comparison | rho | −0.033 | |
| p | 0.659 | ||
| SCWT (Stroop) | rho | −0.169 | |
| p | 0.022 | ||
| MEMORY | rho | −0.101 | −0.169 |
| p | 0.170 | 0.218 | |
| SRT / HVLT-R | rho | −0.130 | −0.049 |
| p | 0.078 | 0.722 | |
| PAL | rho | −0.046 | −0.182 |
| p | 0.532 | 0.183 | |
| BVMT-R | rho | −0.045 | |
| p | 0.546 | ||
| VPAL | rho | −0.077 | |
| p | 0.297 | ||
| WORD LIST GENERATION | rho | −0.095 | −0.082 |
| p | 0.198 | 0.550 | |
| FAS / F | rho | −0.034 | 0.027 |
| p | 0.646 | 0.845 | |
| Animals | rho | −0.124 | −0.238 |
| p | 0.093 | 0.081 | |
| RAPID AUTOMATIZED NAMING | rho | −0.227 | −0.321 |
| p | 0.002 | 0.017 | |
| RAN-Object | rho | −0.229 | −0.374 |
| p | 0.002 | 0.005 | |
| RAN-Color | rho | −0.181 | −0.219 |
| p | 0.014 | 0.108 | |
MANOVA investigated differences in performance composites across patients never/rarely (n=70), sometimes (n=70), and often (n=55) reporting word-finding difficulty. (All composites were normally-distrubuted, K-S Ps>.05). There was an effect for RAN (F[2]=6.38, p=.002, ηp2=.066) whereby patients often reporting word-finding difficulty (mean z [95% CI]: −0.612 [−0.954, −0.270]) performed worse than patients rarely/never (0.181 [−0.093, 0.455], p<001) or sometimes (−0.099 [−0.373, 0.176], p=.022) reporting difficulty. The latter two groups did not differ (p=.823). There were no effects for cognitive efficiency (F[2]=2.43, p=.091, ηp2=.026), memory (F[2]=0.645, p=.526, ηp2=.007) or rapid word generation (F[2]=0.806, p=.448, ηp2=.009). Also, patients often reporting word-finding difficulty performed worse on RAN than healthy controls (t[93]=2.51, p=.016, d=0.51), but there were no differences on RAN between healthy controls and patients never/rarely or sometimes reporting difficulty (Ps>.35).
Clinical MS Sample:
The same pattern of correlations was replicated among clinical MS patients, with patient-reported word-finding difficulty linked to poorer RAN-Composite and RAN-Object (Table 3), but not other cognitive domains or tests. Clinical MS patients also completed the BNT, which was unrelated to patient-reported word-finding (rho=.082, p=.553).
Healthy Controls:
There were no relationships between subjective report of word-finding and RAN-Composite or RAN-Object among healthy controls (Ps>.50).
Neuroimaging Correlates
Patient-Reported Word-Finding.
Worse patient-reported word-finding was only related to thinner left parietal cortex (rho=−.175, p=.018), although this did not withstand correction for multiple comparisons.
Objective Performance.
We investigated neuroimaging metrics related to RAN-Composite and RAN-Object, as these were specifically linked to patient-reported word-finding in previous analyses. RAN-Composite and RAN-Object were both independently predicted by only left parietal cortex thickness and nDGM within stepwise regressions (Table 4, Figure 2). Within left parietal cortex, RAN-Composite and RAN-Object were both most strongly linked to precuneus and inferior parietal thickness (Table 5). When regressions considered all 68 regions of interest, RAN-Composite was only independently predicted by left parietal cortex thickness and nDGM, whereas RAN-Object was only independently predicted by left precuneus thickness (Table 4).
Table 4. Results of Stepwise Regressions predicting RAN in RADIEMS Sample.
Results for stepwise regressions predicting RAN-Composite (A) and RAN-Object (B) that considered only core neuroimaging variables, and results for stepwise regressions predicting RAN-Composite (C) and RAN-Object (D) with core neuroimaging variables plus all 68 ROIs.
|
A. Stepwise Regression Predicting RAN-Composite with Core Neuroimaging Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||||
| Predictor | β | t | p | rp | β | t | p | rp |
| Left Parietal CT | 0.292 | 4.095 | .000064 | 0.292 | 0.301 | 4.305 | .000023 | 0.309 |
| nDGM | 0.243 | 3.513 | .000561 | 0.254 | ||||
| R2 Δ | .085 | .059 | ||||||
| F for R2 Δ | 16.77 | 12.34 | ||||||
| p for R2 Δ | .000064 | .000561 | ||||||
| F for Model | 16.77 | 15.08 | ||||||
| R2 for Model | .085 | .144 | ||||||
| P for Model | .000064 | <.000001 | ||||||
|
B. Stepwise Regression Predicting RAN-Object with Core Neuroimaging Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||||
| Predictor | β | t | p | rp | β | t | p | rp |
| Left Parietal CT | 0.306 | 4.305 | .000027 | 0.306 | 0.314 | 4.539 | .000010 | 0.321 |
| nDGM | 0.227 | 3.275 | .001268 | 0.238 | ||||
| R2 Δ | .088 | .051 | ||||||
| F for R2 Δ | 18.53 | 10.73 | ||||||
| p for R2 Δ | .000027 | .001268 | ||||||
| F for Model | 18.53 | 15.13 | ||||||
| R2 for Model | .088 | .145 | ||||||
| P for Model | .000027 | <.000001 | ||||||
|
C. Stepwise Regression Predicting RAN-Composite with Core Neuroimaging + 68 ROIs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||||
| Predictor | β | t | p | rp | β | t | p | rp |
| Left Parietal CT | 0.292 | 4.095 | .000064 | 0.292 | 0.301 | 4.305 | .000023 | 0.309 |
| nDGM | 0.243 | 3.513 | .000561 | 0.254 | ||||
| R2 Δ | .085 | .059 | ||||||
| F for R2 Δ | 16.77 | 12.34 | ||||||
| p for R2 Δ | .000064 | .000561 | ||||||
| F for Model | 16.77 | 15.08 | ||||||
| R2 for Model | .085 | .144 | ||||||
| P for Model | .000064 | <.000001 | ||||||
|
D. Stepwise Regression Predicting RAN-Object with Core Neuroimaging + 68 ROIs | ||||
|---|---|---|---|---|
| Model 1 |
||||
| Predictor | β | t | p | rp |
| Left Precuneus CT | 0.334 | 4.753 | <.000001 | 0.334 |
| F for Model | 22.59 | |||
| R2 for Model | .112 | |||
| P for Model | <.000001 | |||
Note: Bonferroni-corrected entry p=.0033 for Models A & B, and p=.0006 for Models C & D.
RAN: Rapid Automatized Naming; CT: cortical thickness; nDGM: normalized deep grey matter volume
FIGURE 2. Neuroimaging Predictors of Rapid Automatized Naming.
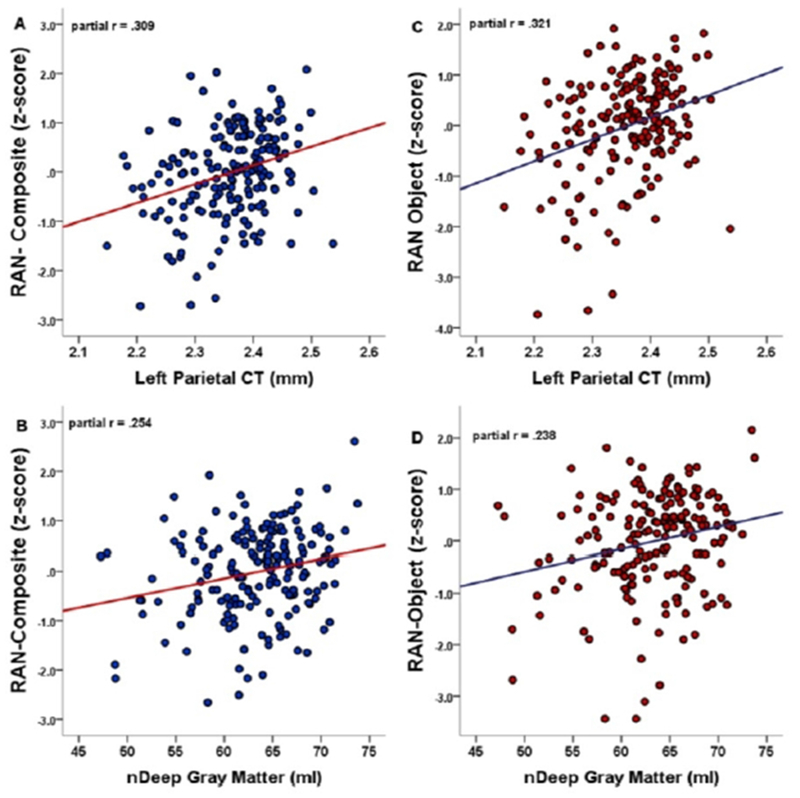
Results of stepwise regressions identified two independent predictors of RAN performance (Table 4: A & B): thicker left parietal cortex predicted better RAN-Composite (A) and RAN-Object (B); higher nDGM predicted better RAN-Composite (C) and RAN-Object (D).
Table 5. Correlations between RAN and Left Parietal ROIs.
Correlations (r) and partial correlations (rp ; controlling for nDGM) are presented between RAN performance (RAN-Composite, RAN-Object) and cortical thickness of the five subcomponents of the left parietal region.
| RAN-Composite (r) | RAN-Composite (rp) | RAN-Object (r) | RAN-Object (rp) | ||
|---|---|---|---|---|---|
| left inferior parietal | r / rp | 0.25391 | 0.27937 | 0.27058 | 0.29579 |
| p | 0.00052 | 0.00013 | 0.00021 | 0.00005 | |
| left postcentral | r / rp | 0.22897 | 0.22286 | 0.19725 | 0.19049 |
| p | 0.00182 | 0.00250 | 0.00744 | 0.01000 | |
| left precuneus | r / rp | 0.26347 | 0.26239 | 0.33383 | 0.33411 |
| p | 0.00031 | 0.00035 | <0.00001 | <0.00001 | |
| left superior parietal | r / rp | 0.13732 | 0.16425 | 0.16016 | 0.18705 |
| p | 0.06379 | 0.02671 | 0.03033 | 0.01146 | |
| left supramarginal | r / rp | 0.20884 | 0.23047 | 0.17730 | 0.19763 |
| p | 0.00455 | 0.00175 | 0.01635 | 0.00749 | |
Disease-Specificity.
Neither RAN-Composite nor RAN-Object correlated with left parietal or left precuneus thicknesses in healthy controls (Ps>.50). Patients with impaired performance (z≤−1.5) on both RAN-Composite and RAN-Object had thinner left precuneus (t[52]=2.03, p=.048, d=0.54) and trending thinner left parietal cortex (t[52]=1.86, p=.068, d=0.51) than healthy controls. Patients with intact RAN (z>−1.5) did not differ from healthy controls (Ps>.10).
Supplemental Analyses
Eleven RADIEMS patients learned English as a second language, but estimated premorbid English verbal ability was the same as patients with English as a first language (WTAR Verbal IQ: 107.9±8.7 vs. 108.2±9.3, p=.914). All results were maintained when re-analyzed without these 11 patients. Given possible links between handedness and eloquent cortex, we also re-analzyed all neuroimaging results in right-handers only (n=161). Results were nearly identical to the total sample. Patients reported worse word-finding relative to healthy controls with and without controlling for mood (Table 2). Results are also maintained for both RADIEMS and clinical MS groups when additionally controlling for fatigue (Fatigue Severity Scale, Ps<.05).
DISCUSSION
Word-finding difficulty was the most common cognitive complaint in both cohorts of early MS patients, and the only complaint reported more by patients than healthy controls. This subjective complaint corresponded with an objective deficit in speed of lexical access during retrieval of target words on RAN tasks. Patient-reported word-finding was unrelated to other cognitive domains. In RADIEMS (with neuroimaging), slower lexical access during RAN was specifically associated with thinner left parietal cortex.
Successful word-finding relies on a network of left temporal, parietal, and frontal areas that are each necessary but none sufficient for the function.28,29 Neural correlates of word-finding difficulty for any disease population should depend on aspects of the network most impacted by that disease (e.g., temporal lobe in temporal lobe epilepsy,30 parietal cortex in logopenic progressive aphasia31). Our findings linking word-finding difficulty to thinner left parietal cortex align with recent neuroimaging work establishing parietal gray matter thinning as a prominent non-random disease-related pattern of cortical atrophy in persons with MS,14,18 which occurs early in disease.14 In one investigation, significant regions of atrophy included bilateral posterior cingulate, bilateral temporal pole, and bilateral parietal cortex.18 Of these, bilateral posterior cingulate and temporal pole thickness were related to a composite measure of processing speed and memory; however, word-finding was not assessed.18 Research employing a data-driven event-based model to determine the temporal sequence of regional gray matter atrophy in MS showed very early atrophy of the precuneus.14 Our results identify thinner left parietal cortex (especially precuneus) as the best predictor of poor RAN performance, and therefore reveal a potential behavioral consequence of this early disease-related parietal atrophy. Moreover, the assertion of a disease-related observation is supported by significantly thinner left precuneus gray matter among MS patients with impaired RAN versus healthy controls.
Cognitive evaluations in MS have relied heavily on consensus test batteries focused on cognitive speed and memory.1–3 Some early studies reported no naming deficit among MS patients on the Boston Naming Test (BNT),2,15 likely diverting attention away from word-finding in MS. Importantly, confrontation naming tasks (e.g., BNT) are more sensitive to severe rather than milder naming deficits characterized by failure to name a picture within 20-seconds, often seen in aphasia or dementia.32 Patient-reported word-finding was unrelated to BNT in our clinical MS sample. In contrast, RAN tasks challenge the automaticity/ease of naming.27 RAN tasks involve pathways from perception and recognition of the stimuli, integration with orthographic and phonological representations, and access/retrieval of phonological labels through to motor articulation.33 This need for speedy word retrieval may explain why RAN reflects patient-reported deficits of word-finding, such as tip-of-the tongue phenomena. In contrast, verbal fluency tasks require patients to generate any words that fit a broad cue (e.g., target letter), which may not reflect naturalistic demands to quickly and efficiently retrieve the specific word one needs within the context of discourse. RAN tasks are also dissimilar from real-life situations in which persons need to spontaneously retrieve a word without strong external cues. Instead, RAN assessed maximal efficiency of one’s lexical access capabilities. It is possible that lexical access speed during RAN is sensitive to word-finding deficits early in disease but that confrontation naming or word generation tasks may emerge as sensitive to word-finding later in disease.
Our cross-sectional results provide the groundwork for longitudinal work on the evolution of word-finding deficits over time in MS. We presented data from patients with early relapsing MS, but renewed exploration in older patients and those with progressive disease is needed. Recent evidence suggested decline in expressive language over two years in a prospective study of secondary progressive MS patients (i.e., orally defining words, articulating similarities in verbal constructs, p=0.058),34 which is notable given that such language abilities are not expected to decline until around the eighth decade of life35 (baseline age of aforementioned sample was 51.3±6.9 years). Together with our results, changes in language combined with recent observations of prominent disease-related parietal atrophy14,18 suggest that decline in word-finding and language ability is more common and significant in MS than previously thought.
Our study is limited by the small healthy control and clinical MS samples, and evaluation of patient-reported word-finding with only one question. More thorough assessment of subjective and objective expressive language function is still needed. Deficits on speed of lexical access during RAN provide initial insights into word-finding as a language-related deficit independent of general processing speed, but work is needed to identify underlying cognitive mechanisms (e.g., phonological processing, multisensory semantic cuing). Likewise, links to left parietal thinning in early MS highlight a region of interest, but they do not elucidate precise disease-related neuroanatomical and neurophysiological bases of word-finding deficits due to MS, which may evolve with disease progression. Comprehensive behavioral and multimodality neuroimaging studies are needed to characterize the nature, extent, evolution, etiology, and treatment of language dysfunction in persons with MS, which likely extends beyond word-finding to other language-related functions.
ACKNOWLEDGEMENTS
We thank Angeliki Tsapanou, Korhan Buyukturkoglu, Peipei Li, Alessio Pepe, and Amgad Droby for neuroimaging analysis support. We also thank other collaborators on the grant, including Yaakov Stern and Christian Habeck. Finally, sincere thanks to patients of the RADIEMS cohort from the Corinne Goldsmith Dickinson Center for MS at Mount Sinai Hospital and the MS Center at Columbia University Medical Center.
FUNDING
This study was funded by the National Institutes for Health (R01 HD082176 to JFS).
Footnotes
DISCLOSURES
The authors have no disclosures relevant to this publication.
REFERENCES
- 1.Benedict RH, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). Journal of the International Neuropsychological Society : JINS. July 2006;12(4):549–558. [DOI] [PubMed] [Google Scholar]
- 2.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. May 1991;41(5):685–691. [DOI] [PubMed] [Google Scholar]
- 3.Sumowski JF, Benedict R, Enzinger C, et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology. February 6 2018;90(6):278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty WW, Goodkin DE, Beatty PA, Monson N. Frontal lobe dysfunction and memory impairment in patients with chronic progressive multiple sclerosis. Brain and cognition. September 1989;11(1):73–86. [DOI] [PubMed] [Google Scholar]
- 5.Beatty WW, Goodkin DE, Monson N, Beatty PA. Cognitive disturbances in patients with relapsing remitting multiple sclerosis. Archives of Neurology. 1989;46(10):1113–1119. [DOI] [PubMed] [Google Scholar]
- 6.Joly H, Cohen M, Lebrun C. Demonstration of a lexical access deficit in relapsing-remitting and secondary progressive forms of multiple sclerosis. Revue neurologique. Aug-Sep 2014;170(8-9):527–530. [DOI] [PubMed] [Google Scholar]
- 7.Sepulcre J, Peraita H, Goni J, et al. Lexical access changes in patients with multiple sclerosis: a two-year follow-up study. Journal of clinical and experimental neuropsychology. February 2011;33(2):169–175. [DOI] [PubMed] [Google Scholar]
- 8.Kujala P, Portin R, Ruutiainen J. Language functions in incipient cognitive decline in multiple sclerosis. Journal of the neurological sciences. September 15 1996;141(1-2):79–86. [DOI] [PubMed] [Google Scholar]
- 9.Friend KB, Rabin BM, Groninger L, Deluty RH, Bever C, Grattan L. Language functions in patients with multiple sclerosis. The Clinical neuropsychologist. February 1999;13(1):78–94. [DOI] [PubMed] [Google Scholar]
- 10.Rao SM. Neuropsychology of multiple sclerosis: a critical review. Journal of clinical and experimental neuropsychology. October 1986;8(5):503–542. [DOI] [PubMed] [Google Scholar]
- 11.Geurts JJ, Calabrese M, Fisher E, Rudick RA. Measurement and clinical effect of grey matter pathology in multiple sclerosis. The Lancet. Neurology. December 2012;11(12):1082–1092. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese M, Atzori M, Bernardi V, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol. September 2007;254(9):1212–1220. [DOI] [PubMed] [Google Scholar]
- 13.Bergsland N, Horakova D, Dwyer MG, et al. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol September 2012;33(8):1573–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshaghi A, Marinescu RV, Young AL, et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain : a journal of neurology. June 1 2018;141(6):1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedict RB, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R. Prediction of neuropsychological impairment in multiple sclerosis: Comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Archives of Neurology. 2004;61(2):226–230. [DOI] [PubMed] [Google Scholar]
- 16.Sailer M, Fischl B, Salat D, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. August 2003;126(Pt 8):1734–1744. [DOI] [PubMed] [Google Scholar]
- 17.Rocca MA, Amato MP, De Stefano N, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. The Lancet. Neurology. March 2015;14(3):302–317. [DOI] [PubMed] [Google Scholar]
- 18.Steenwijk MD, Geurts JJ, Daams M, et al. Cortical atrophy patterns in multiple sclerosis are nonrandom and clinically relevant. Brain : a journal of neurology. January 2016;139(Pt 1):115–126. [DOI] [PubMed] [Google Scholar]
- 19.Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. J Educ Psychol September 1999;91(3):415–438. [Google Scholar]
- 20.Wiig EH, Nielsen NP, Minthon L, Garrett GE. Parietal lobe: Activation in rapid, automatized naming by adults. Percept Motor Skill. June 2002;94(3):1230–1244. [DOI] [PubMed] [Google Scholar]
- 21.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet. Neurology. February 2018;17(2):162–173. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan MJL, Edgley K, Dehoux E. A survey of multiple sclerosis. Part 1: Perceived cognitive problems and compensatory strategy use. Canadian Journal of Rehabilitation. 1990;4(2):99–105. [Google Scholar]
- 23.Smith A. Symbol digit modalities test: Manual. Los Angeles: Western Psychological Services. Manual; 1982. [Google Scholar]
- 24.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. March 12 2013;80(11 Suppl 3):S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden C, Freshwater S. Stroop Color and Word Test, Revised 2002 Adult Manual for Clinical and Experimental Uses. Wood Dale, IL: Stoelting; 2002. [Google Scholar]
- 26.Tombaugh TN, Kozak J, Rees L. Normative Data Stratified by Age and Education for Two Measures of Verbal Fluency: FAS and Animal Naming. Archives of Clinical Neuropsychology. 1999/February/01/ 1999;14(2):167–177. [PubMed] [Google Scholar]
- 27.Denckla MB, Rudel R. Rapid “Automatized” Naming of Pictured Objects, Colors, Letters and Numbers by Normal Children. Cortex. 1974/June/01/ 1974;10(2):186–202. [DOI] [PubMed] [Google Scholar]
- 28.Wright P, Stamatakis EA, Tyler LK. Differentiating hemispheric contributions to syntax and semantics in patients with left-hemisphere lesions. The Journal of neuroscience : the official journal of the Society for Neuroscience. June 13 2012;32(24):8149–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. May-Jun 2004;92(1-2):101–144. [DOI] [PubMed] [Google Scholar]
- 30.Trebuchon-Da Fonseca A, Guedj E, Alario FX, et al. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain : a journal of neurology. October 2009;132(Pt 10):2772–2784. [DOI] [PubMed] [Google Scholar]
- 31.Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. May 24 2011;76(21):1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domoto-Reilly K, Sapolsky D, Brickhouse M, Dickerson BC, Alzheimer’s Disease Neuroimaging I. Naming impairment in Alzheimer’s disease is associated with left anterior temporal lobe atrophy. NeuroImage. October 15 2012;63(1):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: implications for understanding and treatment of reading disabilities. Annual review of psychology. 2012;63:427–452. [DOI] [PubMed] [Google Scholar]
- 34.Chan D, Binks S, Nicholas JM, et al. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: secondary analyses from the MS-STAT randomised, placebo-controlled trial. The Lancet Neurology. 2017;16(8):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan JJ, Sattler JM, Lopez SJ. Age effects on Wechsler Adult Intelligence Scale-III subtests. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. May 2000;15(4):311–317. [PubMed] [Google Scholar]


