Abstract
Background/Objectives:
Most older adults have multimorbidity that impairs physical functioning but it is difficult to quantify using claims data. We previously developed and validated a multimorbidity-weighted index (MWI) that embeds physical functioning through disease weightings. Here, we mapped these conditions to ICD-9-CM codes and compared them with existing indices.
Design:
Population-based prospective cohort.
Setting:
Respondents to the 2006–2016 waves of the Health and Retirement Study (HRS) with linked Medicare claims data and continuous enrollment in 2006.
Participants:
Community-dwelling Medicare-eligible HRS participants (N=9,923, mean age 75.5 ± 8.5).
Measurements:
Individuals were followed for future physical functioning (2006–2014) and mortality (2006–2016). MWI conditions were mapped to ICD-9-CM codes to produce an ICD-coded MWI (MWI-ICD). We compared MWI-ICD, simple disease count, Charlson, Elixhauser, and the health-related quality of life comorbidity index (HRQOL-CI) through distributions, hazard ratios for mortality, and relationships with future physical functioning.
Results:
MWI-ICD exhibited the broadest distribution and most unique values (5891). Left-censoring was most pronounced for Charlson (34.3% score=0) and Elixhauser (13.1% score=0) versus MWI (5.0% score=0). Hazard ratios and C-statistics for mortality across extreme quartiles were similar for MWI-ICD, Elixhauser, and Charlson but lower for disease count and the HRQOL-CI. For physical functioning, MWI-ICD yielded the greatest contrast across extreme quartiles and overall R2.
Conclusion:
MWI-ICD was significantly associated with mortality and future physical functioning and comparable to established metrics for mortality prediction though not weighted to mortality. MWI-ICD successfully captures diseases accumulation and functioning in claims data.
Keywords: multimorbidity, comorbidity, physical functioning, administrative claims data, Medicare
INTRODUCTION
Among adults with chronic disease, most have multimorbidity, the coexistence of multiple chronic conditions.1 Despite the clinical and epidemiologic importance of accurately quantifying this population with multimorbidity, traditional methods have largely relied upon a simple disease count or comorbidity indices such as the Charlson comorbidity index2 and Elixhauser comorbidity score3 and recalibrated versions of these indices4, 5 that were specifically designed to predict inpatient mortality. In absence of more specific tools for risk-adjustment, these indices have frequently been generalized beyond their intended utility despite being poorly suited for this.6 For example, the Charlson index poorly predicts future physical impairment in community-dwelling adults.7 For adults surviving with chronic disease, health-related quality of life (HRQOL) and physical functioning are universally valued but neither consistently collected nor available in clinical and administrative data.
We developed and validated a multimorbidity-weighted index (MWI) that weights 81 chronic diseases and conditions by their impact on physical HRQOL in three large cohorts of community-dwelling adults.8 To assess its generalizability, we externally validated it in the nationally-representative Health and Retirement Study (HRS).9 Importantly, MWI captured both the low and high extremes of multimorbidity better than other measures of multimorbidity and provided a better model fit and generally stronger associations with mortality and future physical functioning.7 MWI is also associated with physical and cognitive performance, basic and instrumental activities of daily living (ADL, IADL) limitations, HRQOL, and future physical functioning and mortality using a joint analysis.9–12
Although the MWI has been extensively validated, its use is currently limited to datasets using self-reported conditions. To make MWI available for claims-based analyses, we developed and validated an ICD-coded MWI (MWI-ICD) based on mapping self-reported conditions to ICD, Ninth Revision, Clinical Modification (ICD-9-CM) codes. We compared the distribution and performance of MWI-ICD on future physical functioning and mortality so that MWI-ICD could be compared with the original MWI based on self-reported conditions and with existing comorbidity measures.2–5, 13 The overall goal was to make MWI readily available for use in administrative and claims data.
METHODS
Study sample
We linked data from the HRS and Centers for Medicare and Medicaid Services (CMS) claims. The HRS is a population-based, longitudinal cohort of more than 38,000 U.S. adults aged 51 years and older followed since 1992.14 Participants are interviewed biennially in several domains, including health and health services utilization, psychosocial and lifestyle factors, functioning, and demographics. A subset of participants permitted their individual-level data to be linked to CMS claims of ICD-coded diagnoses associated with healthcare encounters and other utilization.
At baseline in 2006, 10,581 participants had HRS-CMS linked data. We excluded adults aged <50 years (N=93, 0.8%) and those not continuously enrolled in Medicare parts A and B for the entire year (N=565, 5.3%). The final sample included 9,923 Medicare-eligible adults with at least one ICD-9-CM claim from the outpatient, inpatient, and carrier files in 2006. For the mortality outcome, participants were followed through March 31, 2016. For the physical functioning outcome, participants were followed 8 years after baseline to 2014.
Multimorbidity measurement
The MWI includes chronic conditions generally considered to be permanent conditions that require lifelong treatment or lifestyle modification for maintenance and prevention (e.g. hypertension).8 MWI is computed by identifying these chronic diseases and summing their weights derived from their effects on physical functioning (Supplementary Appendix S1).8
Simple disease count includes the same conditions as MWI but conditions are equally weighted. The Charlson index includes 19 chronic conditions weighted 1, 2, 4, or 6 based on their associations with 1-year mortality prediction in hospitalized patients.2 The Elixhauser score includes 30 conditions associated with inpatient mortality, cost and length of stay, and are each weighted 1 point.3 Schneeweiss reweighted Charlson index conditions by applying New Jersey Medicare weights (0–4 or 6 points),4 and van Walraven reweighted Elixhauser score conditions with −7 to 12 points using data from hospitalizations at the Ottawa Hospital, Canada.5 Last, the HRQOL-comorbidity index (HRQOL-CI) weights 20 clinical classification codes by 1–3 points based on their association with the Short Form-12 physical component summary.13
Mapping MWI to ICD codes
Starting with the original MWI based on self-reported physician-diagnosed conditions, we created a corresponding index mapped to ICD-9-CM codes for each condition (Supplementary Table S1). ICD codes were identified based on a comprehensive literature review of published studies that mapped chronic conditions to ICD codes and reported diagnostic tests such as sensitivity and specificity for validation. We also examined mapped ICD codes from commonly used indices, such as the CMS Chronic Conditions Data Warehouse,15 an adaptation of the Charlson index,16 and the Elixhauser comorbidity score.3 Finally, we checked ICD code mappings with CMS fiscal year 2015 (October 1, 2014 to September 30, 2015) ICD-9-CM, a comprehensive list of ICD-9 codes and corresponding conditions.17 We verified the accuracy of codes from the literature search and existing indices by locating codes in the CMS 2015 ICD-9-CM list. Codes were identified and checked independently for agreement by four individuals (including MYW, DR).
We further compared the prevalence of 18 chronic conditions self-reported by participants in HRS versus ICD codes from Medicare claims mapped to these conditions. (Supplementary Table S2).
Mortality measurement
We assessed mortality between January 1, 2007 and March 31, 2016. We used the “date of death” outcome from the CMS Master Beneficiary Summary File. Most death information (98%) was obtained from the Social Security Administration. Other sources included death status updates submitted by family members, Medicare claims data from the Medicare Common Working File, and the Railroad Retirement Board.18 All deaths (100%) were validated.19
Physical functioning measurement
We used a modified Short Form (SF)-36 10-item physical functioning scale20 described previously.9, 12 Ten physical functioning items included vigorous activities such as running, moderate activities such as pushing large objects, lifting or carrying over 10 pounds, climbing several flights of stairs, climbing one flight of stairs, kneeling or stooping, walking more than a mile, walking several blocks, walking one block, and bathing or dressing oneself. Nine physical functioning items were equivalent to the SF-36 physical functioning scale while the tenth item “walking more than a mile” was intermediate between two questions, “walking several blocks” and “running/jogging about a mile” and imputed. The physical functioning items were weighted, rescaled and standardized according to the SF-36 protocol.20 The HRS physical functioning scale ranged from 0 (difficulty with all items) to 100 (no difficulty performing any physical functioning item).
At the start of 8-year follow-up in 2014, 6,424 participants were alive. Of these, 4,950 reported physical functioning. We excluded 1,467 (23%) who did not complete the SF-36 physical functioning and 7 (0.14%) who responded to less than half the items. For participants missing less than half of the 10-item physical functioning scale (N=57 of 4,950 participants with physical functioning at 8-year follow-up, 1.2%), physical functioning was imputed using the average value of the answered items per SF-36 protocol.20
Statistical analysis
We examined the distribution of comorbidity metrics to describe how well multimorbidity is described at both low and high ends. To illustrate distributions, we examined histograms, left-censoring based on the prevalence of participants with no computable value (i.e., score of 0), and number of unique values to demonstrate the potential spread. Given the asymmetric distribution of multimorbidity, we examined the robust scale estimator Sn,21 in which larger values indicate greater variability or granularity in a given variable, a desired trait for describing the breadth and depth of multimorbidity among participants.
Finally, we computed the Gini index,22 a measure of statistical dispersion used to measure (in)equality in the distribution of multimorbidity values among participants. A score of 0 indicates perfect equality, whereby every participant has the same value of multimorbidity and no detectable difference exists among participants. A score of 1.0 indicates maximum inequality where every participant except one has no morbidity, and a single individual has all possible morbidities. Because both extreme values reflect metrics incapable of stratifying the full population, an ideal multimorbidity metric has a Gini index of ~0.5. To illustrate the Gini index, we plotted the Lorenz curve of distribution.22
To compare the performance of the multimorbidity measures, we examined 10-year mortality and future 8-year physical functioning prediction. We used Cox proportional hazards models23 to determine the hazard ratios and 95% confidence intervals for each measure with mortality. MWI-ICD, simple disease count, Charlson, Elixhauser, and HRQOL-CI associations with mortality were examined using quadratic and cubic transformations (Supplementary Table S3) and categorically in quartiles due to the non-normal distributions. We compared model fit using the Akaike Information Criterion (AIC), where the lowest AIC indicated the best performance. To compare how accurately the models discriminated between survival outcomes, we computed the concordance (C) statistic, or area under the receiver operating characteristic (ROC) curve, for survival.24
Given the expected correlation and collinearity between MWI-ICD and disease count (which use the same underlying diseases but weigh them differently) but not with other metrics, we directly compared MWI-ICD with disease count as a sensitivity analysis. First, we included them concurrently in the same model with mortality to determine which index had the greatest magnitude association with mortality, even in the presence of collinearity. By effectively adjusting for disease count in the model, we could examine the effect of disease weights alone on mortality (i.e., the effect of weighted diseases beyond counting diseases). As a second comparison between MWI-ICD and disease count, we examined the effect of MWI-ICD adjusted for disease count on mortality using the residual method.25
Our final association of interest was between multimorbidity with physical functioning from baseline to 8 years (2006–2014). We used general linear models to determine the associations of each measure in quartiles and non-linear transformations (Supplementary Table S4) with future physical functioning. We compared the magnitude of regression coefficients, P and T values to quantify the strength of the association between each measure with future physical functioning. We also quantified the extent each measure explained and predicted future physical functioning by computing the coefficient of determination (R2), the proportion of total variation of physical functioning explained by each measure. Lastly, we adjusted MWI-ICD by sex (Supplementary Tables S3, S4). All analyses were conducted using SAS 9.4 (Cary, North Carolina, 2013). This study was approved by the University of Michigan Institutional Review Board.
RESULTS
In 2006, 9,923 participants had a mean (SD) age of 75.5 (8.5) years and mean values for MWI-ICD of 9.1 (7.7), disease count of 5.1 (3.3), Charlson index of 1.9 (2.2) and Elixhauser score of 3.2 (2.7). During follow-up, 4,465 (45%) deaths occurred.
Self-reported versus ICD-coded conditions
A comparison of the prevalence of conditions based on self-report versus ICD codes from Medicare claims is shown in Supplementary Table S2. The top 3 conditions were the same using self-report and ICD-codes but their prevalences differed. Based on the self-reported MWI, the most prevalent conditions were arthritis (70.8%), hypertension (66.4%), and diabetes (23.8%). The corresponding prevalences based on ICD-coded Medicare claims were hypertension (71.3%), diabetes (29.7%), and arthritis (29.0%). Self-reported and ICD-coded conditions were strongly correlated (Spearman’s rho=0.88, p<0.001).
Multimorbidity distribution
There was left-censoring of multimorbidity across all measures that was most pronounced in Charlson and Elixhauser (Figure 1). More participants had a multimorbidity of 0 with Charlson (34.3%) and Elixhauser (13.1%), compared with MWI-ICD or disease count (5.0%). In contrast, MWI-ICD had the highest value of Sn, indicating the greatest variability (Table 1).
Figure 1.
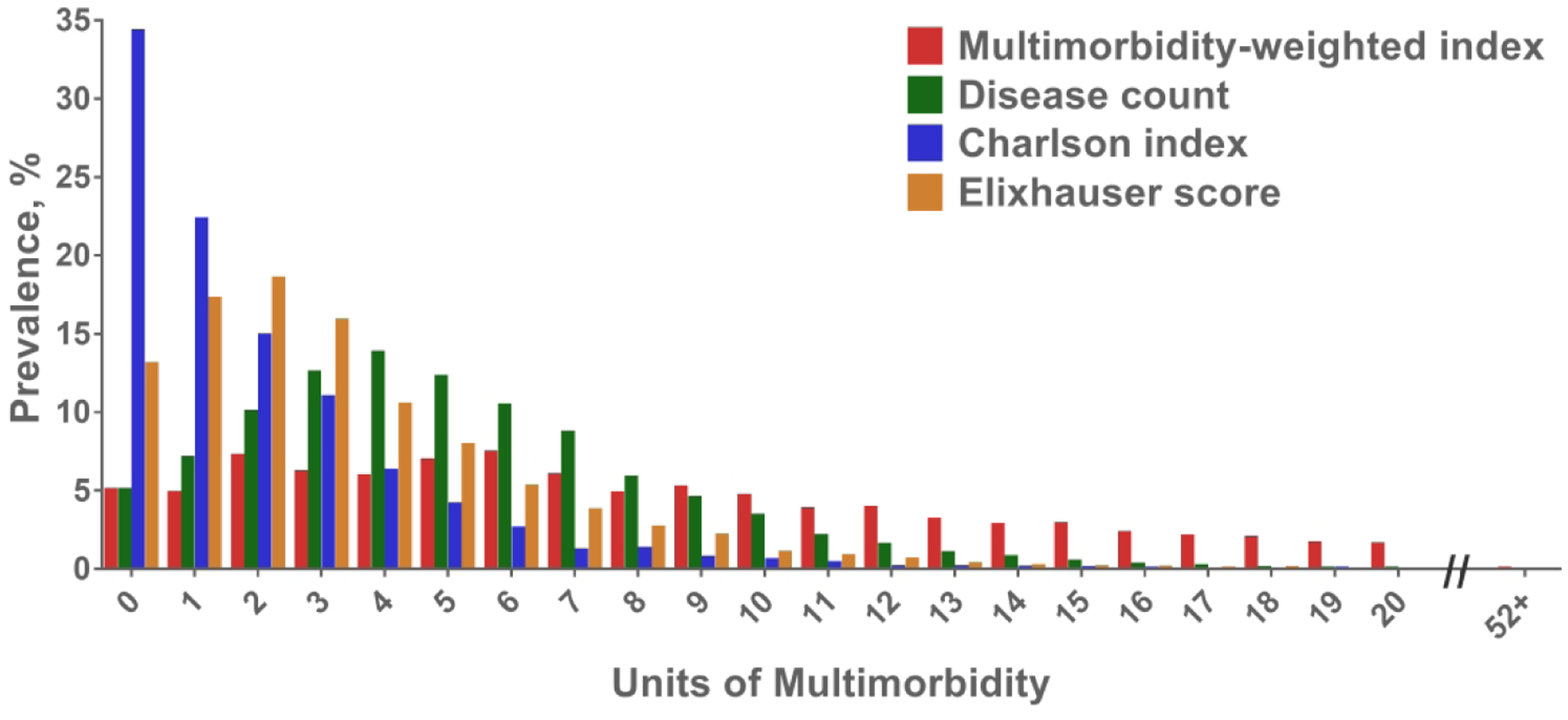
Distribution of multimorbidity based on the ICD-coded multimorbidity-weighted index, simple disease count, Charlson, and Elixhauser measures.
Table 1.
Distribution of multimorbidity measures in Health and Retirement Study-Medicare participants at baseline, 2006.
| Multimorbidity Weighted Index (MWI)-ICD | Disease Count | Charlson index | Elixhauser score | |
|---|---|---|---|---|
| Mean (SD) | 9.1 (7.7) | 5.1 (3.3) | 1.9 (2.2) | 3.2 (2.7) |
| Median (IQR) | 7.1 (3.3–13.0) | 5.0 (3.0–7.0) | 1.0 (0.0–3.0) | 3.0 (1.0–4.0) |
| Range | 0–52.4 | 0–25 | 0–19 | 0–18 |
| Number of unique values | 5891 | 24 | 19 | 19 |
| % with value of 0 | 5.04 | 5.04 | 34.31 | 13.07 |
| Robust scale estimator Sn (SE) | 6.5 (6.5) | 3.6 (3.6) | 1.2 (1.2) | 2.4 (2.4) |
| Gini index (0.5 = balance) | 0.43 | 0.35 | 0.60 | 0.45 |
Abbreviations: IQR, interquartile range; MWI-ICD, International Classification of Diseases, Ninth Revision coded multimorbidity-weighted index; SD, standard deviation; SE, standard error
MWI-ICD had the most unique values, with 5,891 values of multimorbidity at baseline in 2006. There were only 24 observed values of multimorbidity when using disease count, and 19 values for both Charlson and Elixhauser.
The Gini index for MWI-ICD and Elixhauser were 0.43 and 0.45, respectively, indicating near-balance in the breadth of multimorbidity values (Table 1). The Gini index was 0.35 for disease count, indicating more homogeneity in the multimorbidity values. The Charlson index had a Gini index of 0.60, which also indicates greater departure from balance in the spread of multimorbidity values (Supplementary Figure S1).
In HRS-Medicare, MWI-ICD most closely followed an exponential distribution (Supplementary Figure S2).
Multimorbidity and mortality
There were monotonic increasing associations between mortality risk and increasing quartiles of all multimorbidity measures (Table 2). Participants with the highest quartile MWI-ICD had more than triple the hazard ratio of mortality than those in the lowest quintile (HR=3.28, 95%CI: 3.01–3.57, C-statistic=0.63; Figure 2).
Table 2.
Hazard ratios for mortality by standardized metrics after 10-year follow-up, 2006–2016
| N=9,923 | ||||
|---|---|---|---|---|
| Deaths | N=4465 | |||
| Model | HR (95% CI) | P Value | AIC | C-statistic (SE) |
| Multimorbidity-Weighted Index-ICD coded conditions, continuous | 1.60 (1.56–1.64) | <0.001 | 78651.71 | 0.642 (0.004) |
| Multimorbidity-Weighted Index-ICD coded conditions, quartiles | 3.28 (3.01–3.57) | <0.001 | 78763.31 | 0.632 (0.004) |
| Q4 | 1.80 (1.64–1.97) | <0.001 | ||
| Q3 | 1.10 (1.00–1.22) | 0.05 | ||
| Q2 | 1.0 | |||
| Q1 (reference) | P trend <0.001 | |||
| Simple Disease Count, continuous | 1.44 (1.40–1.48) | <0.001 | 79152.34 | 0.602 (0.004) |
| Simple Disease Count, quartiles | 2.22 (2.04–2.42) | <0.001 | 79255.97 | 0.592 (0.004) |
| Q4 | 1.33 (1.21–1.46) | <0.001 | ||
| Q3 | 1.02 (0.93–1.12) | 0.30 | ||
| Q2 | 1.0 | |||
| Q1 (reference) | P trend <0.001 | |||
| Charlson Index, continuous | 1.54 (1.50–1.57) | <0.001 | 78743.11 | 0.643 (0.004) |
| Charlson Index, quartiles | 78789.17 | 0.634 (0.004) | ||
| Q4 | 3.23 (2.99–3.49) | <0.001 | ||
| Q3 | 2.13 (1.94–2.34) | <0.001 | ||
| Q2 | 1.47 (1.34–1.61) | <0.001 | ||
| Q1 (reference) | 1.0 | |||
| P trend <0.001 | ||||
| Elixhauser Score, continuous | 1.62 (1.58–1.66) | <0.001 | 78597.46 | 0.642 (0.004) |
| Elixhauser Score, quartiles | 78849.09 | 0.624 (0.004) | ||
| Q4 | 2.90 (2.61–3.23) | <0.001 | ||
| Q3 | 1.48 (1.30–1.67) | <0.001 | ||
| Q2 | 1.11 (0.99–1.24) | 0.09 | ||
| Q1 (reference) | 1.0 | |||
| P trend <0.001 | ||||
| HRQOL-Comorbidity Index, continuous | 1.48 (1.45, 1.53) | <0.001 | 79035.36 | 0.614 (0.004) |
| HRQOL-Comorbidity Index, quartiles | ||||
| Q4 | 2.49 (2.28–2.72) | <0.001 | 79169.68 | 0.602 (0.004) |
| Q3 | 1.49 (1.35–1.64) | <0.001 | ||
| Q2 | 1.11 (1.00–1.22) | 0.04 | ||
| Q1 (reference) | 1.0 | |||
Abbreviations: AIC, Akaike Information Criterion; C, concordance; HR, hazard ratio; HRQOL, health-related quality of life; Q, quartile; ICD, International Classification of Diseases; SE, standard error
Figure 2. Kaplan-Meier survival curve for the ICD-coded multimorbidity-weighted index quartiles, January 1, 2006 to March 31, 2016.
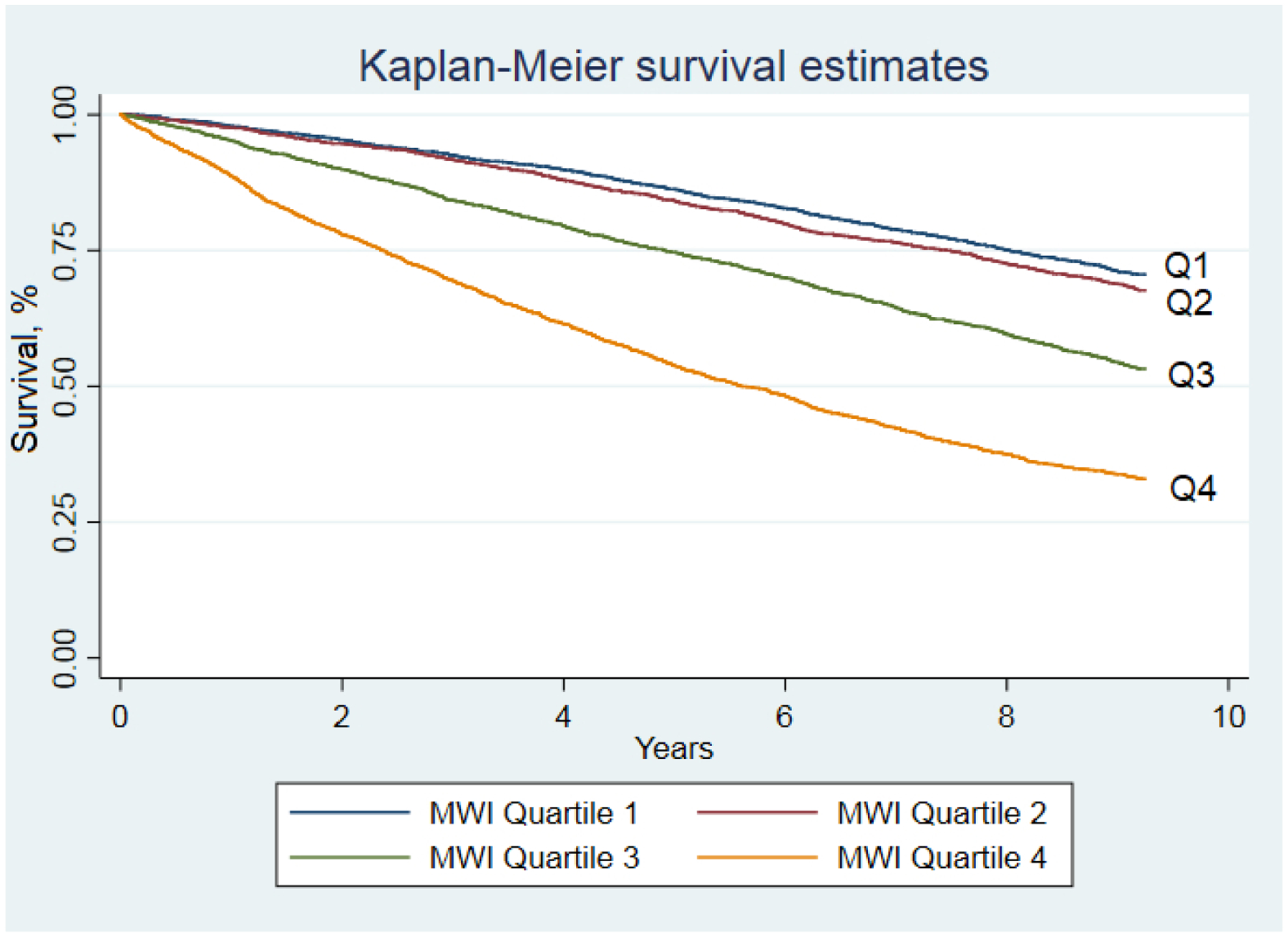
Abbreviations: MWI, multimorbidity-weighted index
The HRs for mortality and C-statistics were similar for MWI-ICD, Elixhauser, Charlson, the NJ Medicare reweighted Charlson, and van Walraven reweighted Charlson. Based on AIC, model fit was worst for simple disease count and HRQOL-CI compared with Charlson, Elixhauser, and MWI-ICD.
When MWI-ICD and disease count were included together in Cox models, the association between MWI-ICD and mortality persisted (HR=2.01, 95%CI: 1.90–2.12) while disease count was actually inversely associated with mortality (HR=0.76, 95%CI: 0.72–0.81). Similarly, the effect of the MWI weights alone (derived from regression of MWI on simple count) were associated with more than double the risk of mortality (HR=2.22, 95%CI: 2.09–2.35).
Multimorbidity and physical functioning
Participants with the highest MWI-ICD had worse future 8-year physical functioning than those with the lowest MWI-ICD (mean 48.6±31.8, Table 3). There were monotonic associations between increasing MWI-ICD and worse physical functioning for all metrics. MWI-ICD and HRQOL-CI had comparable T- and R2 (0.11 and 0.10, respective) values for 8-year physical functioning compared with mortality-based measures and simple disease count. The next best model to explain future physical functioning was Elixhauser (R2=0.08).
Table 3.
Short Form-36 physical functioning by standardized metrics after 8-years of follow-up from baseline, 2006–2014, N=4,950, physical functioning mean 48.6±31.8.
| Model | β coefficient (95% CI) | P Value | T Value | R2 |
|---|---|---|---|---|
| Multimorbidity-Weighted Index-ICD coded conditions, continuous | −12.50 (−13.54, −11.47) | <0.001 | −23.66 | 0.11 |
| Multimorbidity-Weighted Index-ICD coded conditions, quartiles | ||||
| Q4 | −28.5 (−31.1, −25.9) | <0.001 | −21.5 | 0.10 |
| Q3 | −18.2 (−20.5, −15.9) | <0.001 | −15.5 | |
| Q2 | −6.8 (−9.0, −4.6) | <0.001 | −6.1 | |
| Q1 (reference) | 0 | |||
| Simple disease count, continuous | −9.54 (−10.51, −8.56) | <0.001 | −19.13 | 0.07 |
| Simple Disease Count, quartiles | ||||
| Q4 | −21.9 (−24.4, −19.4) | <0.001 | −17.1 | 0.06 |
| Q3 | −12.6 (−15.0, −10.1) | <0.001 | −10.0 | |
| Q2 | −4.6 (−6.9, −2.3) | 0.001 | −3.9 | |
| Q1 (reference) | 0 | |||
| Charlson Index, continuous | −9.42 (−10.52, −8.32) | <0.001 | −16.77 | 0.05 |
| Charlson Index, quartiles | ||||
| Q4 | −20.5 (−22.8, −18.1) | <0.001 | −17.0 | 0.06 |
| Q3 | −12.2 (−14.8, −9.6) | <0.001 | −9.1 | |
| Q2 | −7.4 (−9.6, −5.2) | <0.001 | −6.6 | |
| Q1 (reference) | 0 | |||
| Elixhauser score, continuous | −11.20 (−12.28, −10.13) | <0.001 | −20.48 | 0.08 |
| Elixhauser Score, quartiles | ||||
| Q4 | −25.5 (−28.2, −22.7) | <0.001 | −18.2 | 0.07 |
| Q3 | −14.6 (−17.6, −11.7) | <0.001 | −9.7 | |
| Q2 | −8.1 (−10.6, −5.5) | <0.001 | −6.3 | |
| Q1 (reference) | 0 | |||
| HRQOL-Comorbidity Index, continuous | −11.62 (−12.58, −10.67) | <0.001 | −23.85 | 0.10 |
| HRQOL-Comorbidity Index, quartiles | ||||
| Q4 | −26.34 (−28.87, −23.81) | <0.001 | −20.39 | 0.09 |
| Q3 | −16.74 (−19.22, −14.26) | <0.001 | −13.24 | |
| Q2 | −6.48 (−8.78, −4.18) | <0.001 | −5.52 | |
| Q1 (reference) | 0 |
Abbreviations: HRQOL, health-related quality of life; ICD, International Classification of Diseases Q, Quartile; R2, coefficient of determination
DISCUSSION
In this study of HRS-Medicare adults, we adapted a validated MWI developed from self-reported physician-diagnosed conditions to ICD-codes in Medicare claims. MWI-ICD had desirable distributional characteristics, was as strongly associated with mortality as mortality-focused metrics, and was more strongly associated with future physical functioning. Overall, these results suggest MWI-ICD can be used to quantitate multimorbidity in datasets that rely upon administrative claims to identify morbidity.
This study contributes to the existing literature in several ways. First, to place MWI-ICD in context with the most commonly used comorbidity measures, we compared its distribution, discrimination, and model fit with Charlson, Elixhauser and simple disease count. MWI-ICD had the least left-censoring, widest distribution, and most unique values to characterize multimorbidity than other measures. Using MWI, 5% of Medicare beneficiaries had no multimorbidity, compared with 35% of participants with no Charlson comorbidity and 15% with no Elixhauser comorbidity. While disease count also yielded 5% of participants with a score of 0 and wider distributions than Charlson and Elixhauser, it had the worst model fit for predicting mortality and future physical functioning. MWI-ICD and Elixhauser had the best balance of values using the Gini index measure for dispersion of the multimorbidity distribution.
Compared with other comorbidity measures, MWI-ICD predicted not only mortality but also future 8-year physical functioning. For mortality prediction, discrimination was similar for MWI-ICD, Charlson, Elixhauser, and recalibrated Charlson and Elixhauser measures,3, 4 despite that MWI was not designed to predict mortality as were the others. With advances in medicine, other person-centered health outcomes including physical functioning and HRQOL are of increasing interest. However, these are not routinely captured in clinical settings and administrative databases. MWI-ICD provides both a measure of cumulative disease burden and proxy for physical functioning, given its use of physical functioning to weight disease impact. For future 8-year physical functioning prediction, MWI-ICD outperformed mortality-based measures with superior goodness of fit and was comparable to HRQOL-CI.
Disease count is also a convenient and widely used measure of multimorbidity but had the worst discrimination and goodness-of-fit for predicting mortality and physical functioning. Further, in mutually-adjusted models of MWI-ICD and disease count, the association between MWI-ICD with mortality persisted while disease count and mortality did not, suggesting that the weights from MWI provide useful, meaningful information for mortality prediction beyond that of disease count. Because MWI-ICD can be implemented as easily as disease count in claims data but is more strongly associated with both mortality and quality-of-life outcomes, we see few remaining circumstances in which disease count should be used.
Perhaps most importantly, we created a mapping of ICD-9-CM codes based on self-reported chronic conditions so that our validated MWI may be used by others in claims data (Supplementary Table S1). The ICD-coded conditions were highly correlated with the available self-reported conditions in the HRS. These mappings cover an expansive list of conditions and set the stage for future work to expand MWI by identifying the effects of even more conditions on physical functioning.
Our study has potential limitations. First, it likely underestimates multimorbidity because chronic conditions were not updated longitudinally using repeated measures. This would underestimate the impact of multimorbidity on mortality risk and future physical functioning. Related, our ability to capture conditions depends on coding practices that could vary temporally or geographically. Given small sample sizes in the HRS at the regional level, this study cannot discern whether differences in multimorbidity across regions are due to coding artifact or true differences in the distribution of disease prevalence and risk factors, which varies substantially across the US. Second, additional multimorbidity measures and modifications of Charlson and Elixhauser exist, although Elixhauser and Charlson are currently among the most commonly used metrics for comorbidity risk-adjustment. Two popular recalibrations of Charlson and Elixhauser4, 5 had similar or modestly improved model fit and discrimination compared with the original measures. However, these recalibrations have not yet routinely replaced the original measures published in 1987 and 1992.26, 27 Third, disease count in this study was more comprehensive than versions in other studies since it included all conditions in MWI, rather than typical short, pre-defined lists of common conditions. This would overestimate the performance of disease count compared with other national survey studies. Fourth, this study was limited to ICD-9 codes, which are most useful for its application to the many existing cohorts that predate the introduction of ICD-10. Previous studies report that the performance of Charlson-Deyo and Elixhauser remained largely consistent and unaltered across ICD-9 and ICD-10 coding algorithms.28 Studies are underway to map and validate MWI-ICD to ICD-10 codes for use in claims data after October 2015. Fifth, this study examined mortality and future physical functioning but healthcare cost and utilization warrant study for additional construct validity. Finally, this study is limited to Medicare beneficiaries in the HRS, a nationally-representative sample of older adults. Further studies are needed before generalizing to younger populations.
Our study has important research and health policy implications. MWI-ICD outperformed commonly-used mortality-based metrics for predicting future physical functioning, as expected. It was not designed to predict mortality but had comparable discrimination and model fit. Further, MWI-ICD distinctly had the widest distribution and most unique values to characterize multimorbidity compared with other measures. Given this major strength, MWI-ICD should be considered as a measure for multimorbidity in claims data, and we provide all necessary coding for its implementation (Supplementary Appendices S1, S2, and Table S1). Better quantification of multimorbidity is possible through this readily-implemented multimorbidity-weighted index, enabling the study of this critical and increasingly prevalent construct in various real-world settings.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jamie Luster, MPH and Chiao-Li Chan, MSW for assistance with mapping multimorbidity-weighted index conditions to ICD-9-CM codes.
Sponsor’s role: The sponsor had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Footnotes
This research was presented at the American Geriatrics Society Annual Meeting, May 2019.
Conflict of interest: None to declare.
REFERENCES
- [1].Chronic care: making the case for ongoing care. Anderson G (online). Available at: rwjf.org/en/research-publications/find-rwjf-research/2010/01/chronic-care.html. Accessed July 15, 2018.
- [2].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40: 373–383 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- [3].Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36: 8–27 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- [4].Schneeweiss S, Wang PS, Avorn J, Glynn RJ. Improved comorbidity adjustment for predicting mortality in Medicare populations. Health Serv Res. 2003;38: 1103–1120 10.1111/1475-6773.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47: 626–633 10.1097/MLR.0b013e31819432e5 [DOI] [PubMed] [Google Scholar]
- [6].Greene ME, Rolfson O, Gordon M, Garellick G, Nemes S. Standard Comorbidity Measures Do Not Predict Patient-reported Outcomes 1 Year After Total Hip Arthroplasty. Clin Orthop Relat Res. 2015;473: 3370–3379 10.1007/s11999-015-4195-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wei M, Mukamal KJ. Multimorbidity, Mortality, and Long-Term Physical Functioning in 3 Prospective Cohorts of Community-Dwelling Adults. Am J Epidemiol. 2018;187: 103–112 10.1093/aje/kwx198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wei MY, Kawachi I, Okereke OI, Mukamal KJ. Diverse cumulative impact of chronic diseases on physical health-related quality of life: implications for a measure of multimorbidity. Am J Epidemiol. 2016;184: 357–365 10.1093/aje/kwv456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wei M, Kabeto M, Langa K, Mukamal K. Multimorbidity and physical and cognitive function: performance of a new multimorbidity-weighted index. J Gerontol A Biol Sci Med Sci. 2018;73: 225–232 10.1093/gerona/glx114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wei MY, Levine DA, Zahodne LB, Kabeto MU, Langa KM. Multimorbidity and cognitive decline over 14 years in older Americans. J Gerontol A Biol Sci Med Sci. 2019. 10.1093/gerona/glz147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wei M, Mukamal K. Multimorbidity and Mental Health-Related Quality of Life and Risk of Completed Suicide. J Am Geriatr Soc. 2019;67: 511–519 10.1111/jgs.15678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wei M, Kabeto M, Galecki A, Langa K. Physical Functioning Decline and Mortality in Older Adults With Multimorbidity: Joint Modeling of Longitudinal and Survival Data. J Gerontol A Biol Sci Med Sci. 2019;74: 226–232 10.1093/gerona/gly038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mukherjee B, Ou HT, Wang F, Erickson SR. A new comorbidity index: the health-related quality of life comorbidity index. J Clin Epidemiol. 2011;64: 309–319 10.1016/j.jclinepi.2010.01.025 [DOI] [PubMed] [Google Scholar]
- [14].Heeringa SG, Connor JH. Technical Description of the Health and Retirement Survey Sample Design 1995 10.7826/isr-um.06.585031.001.05.0001.1995 [DOI] [Google Scholar]
- [15].CMS Chronic Conditions Data Warehouse (CCW). Centers for Medicare and Medicaid Services (online). Available at: https://www.ccwdata.org/web/guest/home. Accessed July 18, 2019.
- [16].Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45: 613–619 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- [17].ICD-9-CM Diagnosis and Procedure Codes: Abbreviated and Full Code Titles. Centers for Medicare and Medicaid Services (online). Available at: https://www.cms.gov/Medicare/Coding/ICD9ProviderDiagnosticCodes/codes.html. Accessed Jun 15, 2018.
- [18].Death Information in the Research Identifiable Medicare Data. Research Data Assistance Center (ResDAC) (online). Available at: https://www.resdac.org/articles/death-information-research-identifiable-medicare-data. Accessed Jan 10, 2019.
- [19].Sources and Use of Medicare Enrollment Information. Virnig B (online). Available at: http://resdac.umn.edu/sites/resdac.umn.edu/files/Sources%20and%20Use%20of%20Medicare%20Enrollment%20Information%20(Slides).pdf. Accessed Jan 10, 2019.
- [20].Ware JE Jr. SF-36 health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center Hospitals, Inc., 1993. [Google Scholar]
- [21].Sn robust estimator of scale documentation. (online). Available at: http://rosa.unipr.it/FSDA/Sn.html. Accessed Apr 18, 2019.
- [22].Accurate calculation of a Gini index using SAS and R. Duoba V, MacGibbon N (online). Available at: http://archive.stats.govt.nz/methods/research-papers/working-papers-original/calc-gini-index-17-02.aspx. Accessed Apr 18, 2019.
- [23].Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34: 187–220 [Google Scholar]
- [24].Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med.23: 2109–2123 10.1002/sim.1802 [DOI] [PubMed] [Google Scholar]
- [25].Willett W. Nutritional epidemiology. 3rd ed. New York, NY: Oxford University Press, 2013. [Google Scholar]
- [26].Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173: 676–682 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- [27].Armitage JN, van der Meulen JH. Identifying co-morbidity in surgical patients using administrative data with the Royal College of Surgeons Charlson Score. Br J Surg. 2010;97: 772–781 10.1002/bjs.6930 [DOI] [PubMed] [Google Scholar]
- [28].Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8: 12 10.1186/1472-6963-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


