Abstract
The association between body mass index (BMI) and non-cardia gastric cancer (NCGC) risk remains controversial. The purpose of this study was to examine the association of BMI with NCGC risk with consideration of Helicobacter pylori (HP) biomarkers. This international nested case-control study, composed of 1,591 incident NCGC cases and 1,953 matched controls, was established from 8 cohorts in China, Japan, and Korea, where the majority of NCGCs are diagnosed worldwide. HP antibody biomarkers were measured in blood collected at cohort enrollment by multiplex serology. The NCGC risk according to baseline BMI was estimated using logistic regression to produce odds ratios (ORs) and 95% confidence intervals (CIs). We found a U-shaped association between BMI category and NCGC risk. Compared to those with reference BMI (22.6–25.0 kg/m2), those with lower and higher BMI had an increased NCGC risk (BMI <18.5 kg/m2, OR=1.56, 95% CI=1.04–2.34; BMI >27.5 kg/m2, OR=1.48, 95% CI=1.15–1.91; adjusted for age, sex, and smoking). The U-shaped association was persistent among subjects with HP infection and high-risk biomarkers (HP+CagA+: BMI <18.5 kg/m2, OR=1.60, 95% CI=1.00–2.55; BMI >27.5 kg/m2, OR=1.59, 95% CI=1.21–2.11; and Omp+HP0305+: BMI <18.5 kg/m2, OR=1.88, 95% CI=1.04–3.42; BMI >27.5 kg/m2, OR=1.70, 95% CI=1.20–2.42, respectively). Our study provides evidence of significantly increased NCGC risk among individuals with low or high BMI, including in subjects with high-risk HP biomarkers (HP+CagA+, Omp+HP0305+) in the high-risk area of East Asia.
Keywords: Body mass index, Stomach neoplasms, Helicobacter pylori, Biomarkers, Non-cardia gastric cancer
INTRODUCTION
Gastric cancer (GC) is the fifth most common cancer, with an estimated annual occurrence of almost 1 million incident cases worldwide, of which over half occur in East Asia.1 Specifically, GC cases in Eastern Asia accounted for 60% (619,226 cases) of all GC cases worldwide in 2018. GC deaths in East Asia were responsible for 58% (453,513 deaths) of GC deaths worldwide (782,685 deaths).1. GC is classified into cardia gastric cancer (CGC) and non-cardia gastric cancer (NCGC) according to the topographical subsites. CGC rates are typically lower than NCGC rates, however composition of these two GC histologic types differs according to regional area. In general, NCGC incidence rate greatly exceeds CGC incidence rate in Eastern and Southeastern Asia (age-standardized rates: 21.7 per 100,000 for NCGC vs. 8.7 per 100,000 for CGC in males; 9.5 per 100,000 for NCGC vs. 2.4 per 100,000 for CGC in females). Whereas, incidence of NCGC and CGC are relatively comparable in parts of Northern and Western Europe and Oceania. Several countries in Central Asia even have a higher incidence rate of CGC than that of NCGC.2
Concerned with the outcomes associated with the increase in obesity worldwide, a large number of studies have examined the health impact of obesity on many non-communicable diseases, including cancer. Specifically, a number of studies have sought to evaluate the association between body mass index (BMI) and GC, however the findings have been inconsistent, especially for NCGC. Four meta-analyses on the association between BMI and GC were published between 2006 and 2013.3–6 Two of these meta-analyses were based on case-control and cohort studies and focused only on CGC and esophageal cancer.4,5 The remaining two meta-analyses included only cohort studies and considered both CGC and NCGC.3,6 The increased total GC risk in obese subjects was reported in two meta-analyses.3,6 However, these meta-analyses found inconsistent results for the association between overweight or obesity and overall GC risk according to ethnic group.3,6 In the meta-analysis by Yang et al., a higher risk of total GC among individuals with a BMI of ≥ 25 kg/m2 was found only in Western populations.6 Whereas, another meta-analysis by Chen et al. reported a significantly increased total GC risk in overweight or obese group only in Asian populations.3 For CGC alone, these meta-analyses from 2006 to 2013 found that overweight is associated with a 1.2–1.4 times increased CGC risk, and obesity is related to 1.5–2.1 times elevated CGC risk.3–6 These results were based on populations which consist primarily of Westerners.3–6 Thus, there is inconsistency in the association between obesity and topographic subtype of GC according to ethnic group; but, moreover, obesity is primarily related with an increased CGC risk, and there is no consistent evidence supporting the association between obesity and NCGC risk in Westerners or Asians.3–6
Additionally, most of the previous studies have not considered Helicobacter pylori (HP) infection status of study subjects in the analysis. Based on previous studies reporting positive associations between BMI and HP infection,7–9 there exists the possibility that HP plays a role as a confounder in the association of BMI and GC risk. However, it is not known whether the association between BMI and GC risk is due to the independent effect of BMI or the confounding effect of HP infection. We therefore conducted this study to investigate the independent effect of BMI on NCGC risk, with adjustment for HP infection status. We also sought to assess whether the risk of NCGC by BMI levels is persistent in stratified analyses by HP infection. For this purpose, we used data from the HP Biomarker Cohort Consortium (HpBCC), an international nested case-control consortium with data from 8 cohort studies10–16 in China, Japan, and Korea, whose initial purpose was to evaluate a novel HP biomarker panel for NCGC risk.17
METHODS
Study design and subjects
Study participants arose from the HpBCC, composed of 1,608 incident NCGC cases and 1,962 matched controls. Among HpBCC participants, we excluded those without information on BMI at baseline, thus 1,591 cases and 1,953 matched controls were included in this study. The outcome, NCGC, was defined following the International Classification of Diseases for Oncology (ICD-O) code system (ICD-O C16.1–16.6, C16.8, or C16.9). NCGC cases for all of the cohorts, except Linxian Nutrition Intervention Trial (NIT), were matched to controls according to sex, date of birth, and date of blood collection. In case of the NIT, frequency matching by sex was used. There were no differences overall by age between cases and controls. Details on individual cohorts’ recruitment, outcome ascertainment methods, and the sampling frame in selection as nested case-control participants were described in our prior paper.17 In the analysis of the association between BMI and NCGC risk, we excluded the Korea National Cancer Center (KNCC) population due to the following three reasons. First, the KNCC did not have information on the potential confounder of total caloric intake. Second, the median follow-up time in the KNCC was very short, at 0.7 years, compared to the average follow-up in the other cohort studies of 2.4–7.6 years.17 Third, the source population in the KNCC (participants in KNCC cancer screening program) was different from that of the other cohort studies (general health examinees from communities, public health center, or health examination center). These individuals who visited the cancer center for cancer screening may be more likely to have cancer-related risk factors and symptoms, and consequently more likely to be diagnosed as early cancer than the general population.
We also performed sensitivity analyses removing individuals with overlapping gastric cancer (C16.8, n=5 cases) and with unspecified gastric cancer (C16.9, n=116 cases) and no changes in the results were found (data not shown).
Consortium-wide analytic variables including age, sex, smoking status, educational attainment, previous diagnosis of gastritis, family history of GC, aspirin use, and fruit and vegetable intake (where available) were collected at the time of baseline cohort enrollment.
This study was approved by the Institutional Review Boards of Vanderbilt University (Nashville, TN, USA); Duke University (Durham, NC, USA); German Cancer Research Center (Heidelberg, Germany); Shanghai Cancer Institute (Shanghai, China); National Cancer Center (Tokyo, Japan); Yonsei University (Seoul, Korea). Written informed consent was provided by all participants in the study.
Data Availability
To request access to the data, please contact corresponding author Meira Epplein, who is the principal investigator of the study and responsible for the consortium dataset.
Baseline BMI assessment
Height and weight measured at cohort enrollment was used to calculate the baseline BMI by following the definition: weight (kg) / square of height (m2). BMI was classified into 6 groups using the scheme utilized by the Asia Cohort Consortium, to which the cohorts in this study also belong, in their seminal paper on BMI and risk of death in over one million Asians,18 after combining the highest two categories due to low numbers in our study population: <18.5, 18.5–20.0, 20.1–22.5, 22.6–25.0 (reference), 25.1–27.5, and ≥27.5, all in kg/m2.
H. pylori (HP) multiplex serology
Serum samples of all HpBCC participants were sent to the German Cancer Research Center (DKFZ, Heidelberg, Germany). Human HP IgA, IgM, and IgG antibodies to 15 recombinantly expressed fusion proteins (UreA, Catalase, GroEL, NapA, CagA, CagM, Cagδ, HP0231, VacA, HpaA, Cad, HyuA, Omp, HcpC, and HP0305) were assessed using HP multiplex serology based on a glutathione S-transferase capture immunosorbent assay combined with fluorescent bead technology (Luminex).19,20 Calculation of antigen-specific cutoff points [mean median reporter fluorescence intensity (MFI) plus three times standard deviation (SD), excluding positive outliers] was done using 17 HP- sera previously classified for HP status run within the same experiment. Defining HP+ (sero-positivity) as reactivity with at least 4 proteins has shown good agreement (k = 0.70) with commercial serologic assay, resulting in 89% sensitivity and 82% specificity.19 Test reliability of the assay for 12 replicate samples from two pooled samples (for a total of twenty-four quality control samples) was 98%.
HP infection status were assessed by incorporation of HP antibody serotypes (HP- vs. HP+) and HP biomarkers (CagA, Omp and HP0305) which were most strongly associated with GC risk in this population [17]. In detail, antibody reactivity to HP and its virulence factor, CagA (HP-, compared to HP+ and CagA-, and HP+ and CagA+), and the biomarkers Omp and HP0305 (in three categories: Omp- and HP0305- compared to either Omp+ or HP0305+, and Omp+ and HP0305+) were used.
Statistical analysis
Basic characteristics between NCGC cases and controls or among BMI categories (<23.0, 23.0–24.9, ≥25.0 kg/m2) were compared by conducting the Pearson’s Chi-square test for categorical variables and Student t-test or analysis of variance (ANOVA) for numerical variables. To calculate odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) for each BMI category in relation to NCGC risk, we used several conditional logistic regression models: initial ORs (95% CIs) were calculated from logistic regression models stratified by cohort (Model 1); adjusted ORs (95% CIs) were calculated from the models stratified by cohort and adjusted for age, sex, and smoking status (Model 2); and expanded adjusted ORs (95% CIs) were calculated from the models with same stratification and adjusting variables plus additional variables such as HP or its biomarkers (Omp- and HP0305- compared to Omp+ or HP0305+, or Omp+ and HP0305+ [Model 3]; HP- vs. HP+ [Model 4]; and HP- compared to HP+ and CagA, or HP+ and CagA+ [Model 5]). We also performed spline regression analysis of five models with adjustment of covariates within the Model 1 – Model 5 to evaluate a non-linear association between BMI and NCGC risk. Specifically, a restricted cubic spline function was used to calculate P values for curve trend tests and to make Figure 1. Given a sample size of 2917 we used 3 knots with percentile of BMI below in the calculation: 5% (BMI=18.90), 50% (BMI=23.04) and 95% (BMI=29.05).
Figure 1.
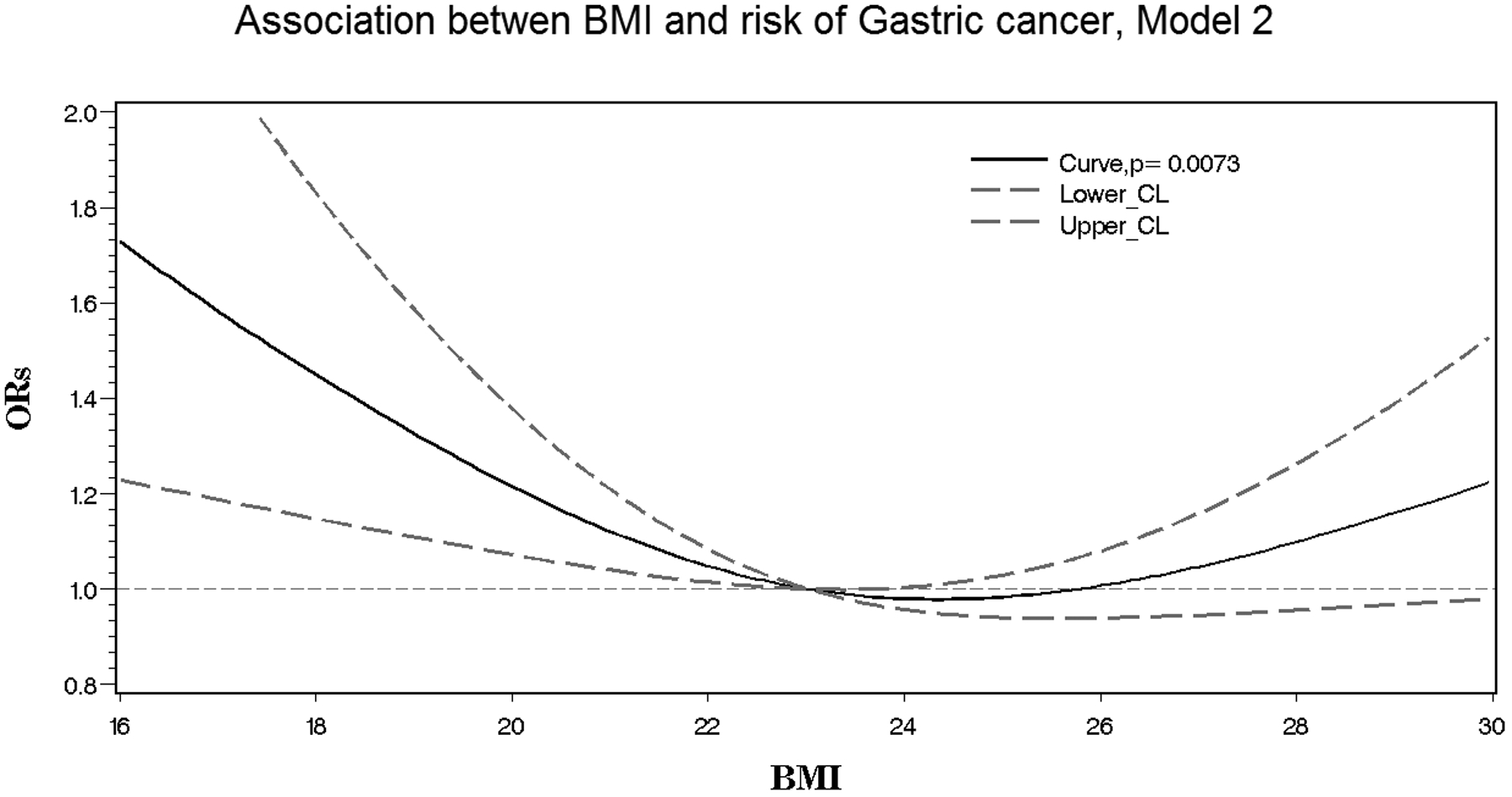
Non-linear association between body mass index and non-cardia gastric cancer risk within the H. pylori Biomarker Cohort Consortium (HpBCC). Presented is the spline regression model with adjustment for age, sex, and smoking status.
We then stratified by HP or its biomarker status, and again performed logistic regression (Model 2), stratified by cohort and additionally adjusted for age, sex, and smoking to examine potential differences in the BMI-NCGC association by HP or its biomarker status. Interaction between BMI and HP or its biomarker status on NCGC risk was also assessed with the addition of a multiplicative interaction term in the model.
Because weight loss is a common symptom among cancer patients, there is chance of reverse causation among cohort participants who are diagnosed with NCGC soon after cohort enrollment. Hence, we performed a sensitivity analysis according to period from BMI measurement to cancer diagnosis.
Additionally, we performed stratified analyses by smoking status (never smokers vs. ever smokers; never smokers, and former smokers vs. current smokers, respectively) to assess NCGC risk according to BMI in each non-smoker and smoker group. Finally, we explored the effect of adjustment for education and diet among those subsets of individuals not missing these variables.
All statistical analysis was performed using the SAS software, version 9.4 (SAS institute, Cary, NC, USA).
RESULTS
This study included 1,591 prospectively ascertained NCGC cases and the 1,953 controls who had information on BMI at baseline. The mean age at study entry was 58·5 years for cases and 58·2 years for controls (Supplementary Table 1), dates of enrolment varied from 1985 to 2010, and the average follow-up time from blood collection to diagnosis was 5·7 years. A slightly greater percentage of cases were male (57.1%) as compared to the controls (50.3%) even though cases and controls were matched on sex, due to the different proportion of women versus men who had two controls matched to every case (the Shanghai Men’s Health Study contributed 66 cases and 132 controls; the Shanghai Women’s Health Study contributed 295 cases and 579 controls).
Subjects having lower BMI levels (< 22.6 kg/m2) were more likely to be male, and current smokers, and to have less formal education. HP infection and dual HP+CagA+ status were found to be more common in subjects with higher BMI levels (> 25 kg/m2) (Table 1).
Table 1.
Characteristics of study population with information on body mass index, the H. pylori Biomarker Cohort Consortium (HpBCC)
| Body mass index, kg/m2 | ||||
|---|---|---|---|---|
| <22.6 (N=1,464) |
22.6–25.0 (N=1,010) |
>25.0 (N=1,070) |
||
| Median (IQR) | Median (IQR) | Median (IQR) | P | |
| Age, years | 57.5 (49.3, 64.6) | 58.1 (51.0, 65.3) | 59.4 (52.1, 65.2) | <0.01 |
| N (%) | N (%) | N (%) | P1 | |
| Gender | ||||
| Female | 613 (41.87) | 459 (45.45) | 582 (54.39) | |
| Male | 851 (58.13) | 551 (54.55) | 488 (45.61) | <0.01 |
| Smoking | ||||
| Never smoker | 810 (55.33) | 582 (57.62) | 657 (61.40) | |
| Former smoker | 133 (9.08) | 173 (17.13) | 183 (17.10) | |
| Current smoker | 521 (35.59) | 255 (25.25) | 230 (21.50) | <0.01 |
| Education | ||||
| ≤Elementary school | 642 (43.85) | 303 (30.00) | 358 (33.46) | |
| Junior high school | 254 (17.35) | 213 (21.09) | 241 (22.52) | |
| High school | 220 (15.03) | 214 (21.19) | 186 (17.38) | |
| ≥Professional education | 157 (10.72) | 150 (14.85) | 187 (17.48) | |
| Missing | 191 (13.05) | 130 (12.87) | 98 (9.16) | <0.01 |
| History of gastritis | ||||
| Yes | 882 (60.25) | 603 (59.70) | 693 (64.77) | |
| No | 187 (12.77) | 118 (11.68) | 150 (14.02) | |
| Missing | 395 (26.98) | 289 (28.61) | 227 (21.21) | 0.74 |
| H. pylori infection status | ||||
| Negative | 229 (15.64) | 128 (12.67) | 130 (12.15) | |
| Positive | 1235 (84.36) | 882 (87.33) | 940 (87.85) | 0.02 |
| H. pylori | ||||
| H. pylori and CagA status | H. pylori- | H. pylori+ and CagA- | H. pylori+ and CagA+ | |
| No. | 487 | 192 | 2865 | |
| BMI (mean±SD) | 23.3±3.2 | 23.3±3.2 | 23.7±3.2 | 0.01 |
| Omp and HP0305 status | Omp− and HP0305- | Omp+ or HP0305+ | Omp+ and HP0305+ | |
| No. | 595 | 967 | 1982 | |
| BMI (mean±SD) | 23.5±3.3 | 23.8±3.2 | 23.5±3.1 | 0.04 |
Abbreviations: IQR, interquartile range.
Chi-square test was done within those non-missing subjects.
Post-hoc test: between H. pylori- and H. pylori+ and CagA+, p<0.05
Post-hoc test: between Omp+ or HP0305+ and Omp+ and HP0305+, p<0.05
A non-linear relationship between BMI and NCGC risk was observed as a U-shaped pattern in the spline analysis irrespectrive of variables included in the model for adjustment (p-curve = 0.007) (see Model 2 as shown in Figure 1).
In the pooled analysis of the consortium participants, the risk of NCGC was lowest in the reference group with BMI level of 22.6–25.0 kg/m2, and risk gradually increased as BMI increased or decreased from this reference group, regardless of the type or the number of confounding variables included for adjustment. Compared to the reference group (BMI of 22.6–25.0 kg/m2), individuals in the underweight group (< 18.5 kg/m2) had a 1.59-fold higher NCGC risk (95% CI, 1.05–2.41 in Model 5); and those with higher BMI (> 27.5 kg/m2) had a 1.46-fold higher NCGC risk (95% CI, 1.13–1.89 in Model 5) (Table 2). This U-shaped pattern was persistent no matter which BMI categorization scheme was used (Supplementary Table 2).
Table 2.
The association of body mass index and non-cardia gastric cancer risk, the H. pylori Biomarker Cohort Consortium (HpBCC)
| BMI (kg/m2) | Cases N (%) |
Controls N (%) |
OR (95% CI)1 | OR (95% CI)2 | OR (95% CI)3 | OR (95% CI)4 | OR (95% CI)5 |
|---|---|---|---|---|---|---|---|
| < 18.5 | 59 (4.1) | 51 (2.8) | 1.63 (1.09, 2.44) | 1.56 (1.04, 2.34) | 1.70 (1.12, 2.58) | 1.56 (1.03, 2.36) | 1.59 (1.05, 2.41) |
| 18.5–20.0 | 145 (10.1) | 153 (8.5) | 1.34 (1.03, 1.76) | 1.31 (1.00, 1.71) | 1.30 (0.98, 1.71) | 1.33 (1.01, 1.76) | 1.33 (1.01, 1.76) |
| 20.1–22.5 | 477 (33.4) | 496 (27.7) | 1.37 (1.14, 1.66) | 1.37 (1.13, 1.65) | 1.34 (1.10, 1.62) | 1.39 (1.15, 1.69) | 1.38 (1.14, 1.68) |
| 22.6–25.0 | 368 (25.7) | 557 (31.1) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25.1–27.5 | 215 (15.1) | 340 (19.0) | 1.02 (0.82, 1.27) | 1.00 (0.81, 1.25) | 0.99 (0.79, 1.24) | 1.01 (0.80, 1.26) | 1.00 (0.80, 1.25) |
| >27.5 | 166 (11.6) | 195 (10.9) | 1.53 (1.19, 1.97) | 1.48 (1.15, 1.91) | 1.52 (1.17, 1.97) | 1.48 (1.14, 1.91) | 1.46 (1.13, 1.89) |
Conditional logistic regression stratified by cohort
Conditional logistic regression stratified by cohort, and additionally adjusted for age, sex, smoking status (within those without missing values of smoking).
Conditional logistic regression stratified by cohort, and additionally adjusted for age, sex, smoking status, and dual Omp/HP0305 sero-positivity
Conditional logistic regression stratified by cohort, and additionally adjusted for age, sex, smoking status, and H. pylori infection (+ vs. −)
Conditional logistic regression stratified by cohort, and additionally adjusted for age, sex, smoking status, and H. pylori and CagA status (HP-, HP+CagA-, and HP+CagA+)
Because there is the possibility of reverse causation between low BMI and NCGC risk, we performed sensitivity analyses according to period from BMI measurement to NCGC diagnosis. Specifically, we excluded NCGC patients who had been diagnosed within 1, 2, 3, or 5 years from BMI measurement to NCGC diagnosis. Increased NCGC risks in low and high BMI were constantly found regardless of GC patients exclusion, suggesting no reverse causation in this study (Supplementary Table 3).
In the stratified analysis by HP biomarkers, the U-shaped relationship between BMI and GC risk appeared stronger in subjects who were HP+, HP+ and CagA+, or Omp+ and HP0305+ than those with HP- or Omp- and HP0305-. However, the 95% CIs of the lowest BMI (< 18.5 kg/m2) group and that of the highest BMI (> 27.5 kg/m2) group were wide and overlapping between two strata of HP- and HP+ (or HP+ and CagA+) or between the strata of Omp- and HP0305- and Omp+ and HP0305+ (Table 3), leading to no significant interactions by HP status.
Table 3.
The association of body mass index and non-cardia gastric cancer risk by H. pylori infection, the H. pylori Biomarker Cohort Consortium (HpBCC)
| A. Stratification analysis by H. pylori antibody status3 | ||||||
|---|---|---|---|---|---|---|
| BMI (kg/m2) | H. pylori − | H. pylori + | H. pylori +CagA+ | |||
| Ca/Co1 | OR (95% CI)2 | Ca/Co1 | OR (95% CI)2 | Ca/Co1 | OR (95% CI)2 | |
| < 18.5 | 4/11 | 0.88 (0.24, 3.22) | 55/40 | 1.63 (1.05, 2.53) | 49/35 | 1.60 (1.00, 2.55) |
| 18.5–20.0 | 18/30 | 1.69 (0.77, 3.71) | 127/123 | 1.28 (0.95, 1,72) | 123/109 | 1.37 (1.01, 1.86) |
| 20.1–22.5 | 37/115 | 0.97 (0.52, 1.78) | 440/381 | 1.45 (1.19, 1,78) | 420/353 | 1.47 (1.19, 1.81) |
| 22.6–25.0 | 26/97 | 1.00 (reference) | 342/460 | 1.00 (reference) | 322/424 | 1.00 (reference) |
| 25.1–27.5 | 15/55 | 1.15 (0.55, 2.44) | 200/285 | 1.00 (0.79, 1.27) | 188/267 | 0.98 (0.77, 1.25) |
| >27.5 | 7/32 | 0.84 (0.32, 2.20) | 159/163 | 1.55 (1.18, 2.03) | 155/151 | 1.59 (1.21, 2.11) |
| B. Stratification analysis by H. pylori Omp and HP0305 antibody biomarker4 | ||||||
| BMI (kg/m2) | Omp− and HP0305- | Omp+ or HP0305+ | Omp+ and HP0305+ | |||
| Ca/Co1 | OR (95% CI)2 | Ca/Co1 | OR (95% CI)2 | Ca/Co1 | OR (95% CI)2 | |
| < 18.5 | 7/16 | 1.19 (0.43, 3.30) | 18/16 | 1.70 (0.81, 3.56) | 34/19 | 1.88 (1.04, 3.42) |
| 18.5–20.0 | 19/35 | 1.77 (0.85, 3.67) | 26/41 | 1.05 (0.59, 1.86) | 100/77 | 1.34 (0.94, 1.92) |
| 20.1–22.5 | 30/127 | 0.72 (0.40, 1.31) | 107/126 | 1.41 (0.97, 2.07) | 340/243 | 1.49 (1.16, 1.91) |
| 22.6–25.0 | 32/127 | 1.00 (reference) | 94/165 | 1.00 (reference) | 242/265 | 1.00 (reference) |
| 25.1–27.5 | 15/73 | 1.01 (0.50, 2.05) | 63/100 | 1.15 (0.76, 1.74) | 137/167 | 0.95 (0.71, 1.27) |
| >27.5 | 14/50 | 1.24 (0.59, 2.60) | 44/62 | 1.45 (0.90, 2.33) | 108/83 | 1.70 (1.20, 2.42) |
N of cases / N of controls
Conditional logistic regression stratified by cohort, and adjusted for age, sex, and smoking.
p value for interaction between BMI and H.pylori is 0.3361
p value for interaction between BMI and H. pylori Omp/HP0305 status is 0.2628
Although we adjusted for smoking status in the logistic regression model, we further conducted stratified analysis by smoking status to assess the association between BMI and GC risk for this potential effect modifier. The U-shaped association were remained in both never smokers and ever smokers (Supplementary Table 4).
DISCUSSION
In this Asian consortium study of risk factors for non-cardia gastric cancer, we found a U-shaped association of BMI with NCGC risk, regardless of BMI categories and adjustment for confounding variables, including HP status.
Most of the prior meta-analyses have not reported the U-shaped association between BMI and GC risk,3–6 regardless of ethnicity or location of GC. Previous studies reporting no association between underweight and GC risk may be due to statistically insufficient power because of the low incidence of GC and the low proportion of underweight in Western populations, as well as the fact that most studies do not examine underweight as a potential risk factor.3 However, a recent study based in the United Kingdom reported a non-linear association, suggesting a U-shape or reverse J-shape, including a significant increased risk for underweight, between BMI and GC risk when they set BMI of 22 kg/m2 as the reference BMI.21 Particularly in smokers, the reverse-J-shaped association of GC risk with obesity and underweight was strongly observed. The reverse-J-shaped association between obesity or underweight and GC risk was persistent but attenuated in non-smokers.21 Unfortunately, the UK biobank study did not provide specific information on location of GC (cardia versus non-cardia). However, in the EPIC cohort study, including a part of the UK biobank, a suggestion of a U-shaped association between BMI and NCGC and BMI was found, although it was statistically insignificant.22 These results suggest that underweight people in Western populations may also have higher risks of GC similar to underweight subjects in Asian populations.
In addition, a recent study among participants in the Korean Multicenter Cancer Cohort (KMCC), some of whom (N=356, 10% of HpBCC participants) overlapped with those included from this cohort in the consortium of East Asian studies that is the HpBCC, also reported evidence of a U-shaped association between BMI and GC risk. Low BMI (<23.0 kg/m2) and high BMI (≥ 25.0 kg/m2) were significantly associated with increased GC risk. However, unlike the present study, the association was strongest in the HP never infected group, but this stratified analysis was hampered by the fact that only one cancer case developed among the reference population (BMI of 23.0–24.9 kg/m2) in this overall low-risk group.23
The U-shaped association, which is a bi-directional increase in GC risk at low and high BMI relative to the middle level of BMI, may be biologically plausible. Although the exact mechanisms responsible for NCGC risk in individuals with higher BMI are unclear to date, two mechanisms can be proposed to explain the association between BMI and NCGC development. The first potential explanation is based on the high incidence or prevalence of precursors of GC. The proposed gastric precancerous cascade after HP infection is through chronic gastritis, gastric atrophy, intestinal metaplasia, and gastric dysplasia.24 Gastric atrophy is a major cancer precursor of NCGC24 and is initiated from HP infection. Chronic inflammation derived from infection contributes to chronic gastritis. Chronic gastritis then can convert into gastric atrophy, intestinal metaplasia, and dysplasia, and finally transformed into GC.24 Previous studies reported that overweight and obesity were associated with dysplasia25 and intestinal metaplasia,26 and it is possible that the pro-inflammatory effects of obesity may then contribute to the progression of these inflammatory states toward cancer. Other studies found associations between underweight and intestinal metaplasia27 and an inverse relationship between BMI and atrophic gastritis, whereby low BMI may then be reflecting malabsorption from the condition of severe gastritis.28 It is not clear whether high or low BMI is the cause of or consequence of these precancerous lesions. However, previous studies indirectly indicate that high and low BMI levels may reflect the precancerous status of GC, and this may partly account for the U-shape association between BMI and the GC.
The second mechanism is based on the relationship between low BMI and smoking. Smoking increases the likelihood of being underweight regardless of ethnicity, and the likelihood is even higher for heavy smokers.29,30 Cigarette smoking is a risk factor for GC and contributes to induce a microenvironment suitable for HP infection.31 Smoking mainly contributes to conversion from gastric atrophy to GC by acting as a primary carcinogen on its own and by making conditions susceptible to carcinogenesis through interaction with carcinogen-metabolizing enzyme, DNA repair enzymes and their coding genes.31 Thus, the association between underweight and NCGC may be the result of the interaction of three factors, such as underweight by smoking, carcinogenicity of smoking, and more vulnerable carcinogenic environment at underweight, or it may also be partly confounded by smoking. In our analyses we were able to adjust for smoking crudely, in three categories (never, former, current), and thus there may be residual confounding. However, the impact of smoking on the association between BMI and GC risk may not be immense, considering the similar U-shaped pattern with GC risk related to BMI that we found in both never smokers and ever smokers in this study.
The use of serology to determine H. pylori status has some inherent limitations, which include inability to distinguish current versus past infection as well as the potential disappearance of the evidence of the evidence of infection as one progressed through the cascade of events leading to gastric cancer. It would also have been of interest to examine if potential biological mediators, including inflammatory markers, varied by BMI and might help to explain the U-shaped association found, but such markers were not available in the cohort datasets. To note, although we have previously found that high fruit intake reduces gastric cancer risk in this population,32 it did not confound the BMI-gastric cancer association in the present analyses (see Supplementary Table 5B). And, although we had significant missing values for fruit or vegetable intake (41%) and history of gastritis (29%), most variables, including smoking status, were collected from almost all subjects,17 allowing us to at least partially control the confounding effects of these variables on GC risk. In addition, the U-shaped association was persistent among those without missing values in education or without missing values in total energy intake and fruit intake (Supplementary Table 5). Our study population had an overall mean follow-up time of over 5 years between blood collection and cancer incidence. We were able to perform stratification analyses by HP biomarker status and combined analysis for HP biomarker status and BMI thanks to the large number of cases and controls (1,591 and 1,953, respectively). Therefore, we analyzed the independent effects of BMI on NCGC according to HP biomarker status and found that the combined effect of HP infection and high-risk of BMI (low and high BMI) increases the risk of GC. However, the 95% confidence interval of GC risk for high-risk BMI was wide due to the low prevalence of HP non-infected subjects, and thus a significant difference by HP status was not found.
In conclusion, our study found a U-shaped association between BMI and NCGC, using a nested case-control design matched by age, sex, and cohort, from 8 cohort studies in 3 countries in East Asia (China, Japan, and Korea) with the highest incidence of GC. It is the largest nested case-control study, with HP biomarkers assessed, to date. In this study, we identified the independent effects of BMI on NCGC risk after fully adjusting for HP infection. The U-shaped association was persistent in individuals with HP infection or positive dual biomarkers (Omp+ and HP0305+). However, we did not find a statistically significant difference in the association of BMI with GC risk between the HP infected population and that in HP non-infected population due to the small number of HP non-infected population. Future studies with a large number of HP non-infected individuals are needed to substantiate the HP-dependent findings of the effect of BMI on NCGC risk. The results of this study will also contribute to help identifying individuals at highest risk of gastric cancer for future prevention strategies.
Supplementary Material
Novelty and Impact.
The association between body mass index (BMI) and risk of non-cardia gastric cancer (GC) remains controversial. Thus, we conducted this nested case-control study to evaluate the association between BMI and non-cardia GC with consideration of Helicobacter pylori (HP) infection in a consortium of East Asian prospective cohorts. We found a U-shaped association between BMI and non-cardia GC risk, including among subjects with high-risk HP biomarkers, in the high-incident region of East Asia.
ACKNOWLEDGEMENT
This work was supported by the National Cancer Insitute at the National Institutes of Health (R01 CA174853 to M.E.). The sponsors had no role in study design; the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Dr. Epplein had full access to the data in the study. And Dr. Epplein and Dr. Park had the final responsibility for the decision to submit for publication.
This study was also partly supportd by the UM1 grant mecahnism (CA173640 and CA182910) for the SWHS and SMHS, respectively, and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2016R1A2B4014552).
Abbreviations:
- BMI
Body mass index
- NCGC
Non-cardia gastric cancer
- HP
Helicobacter pylori
- ORs
Odds ratios
- CIs
Confidence intervals
- GC
Gastric cancer
- CGC
Cardia gastric cancer
- NCGC
Non-cardia gastric cancer
- HpBCC
H. pylori Biomarker Cohort Consortium
- ICD-O
International Classification of Diseases for Oncology
- NIT
Nutrition Intervention Trial
- KNCC
Korea National Cancer Center
- WHO
World health organization
- MFI
Mean median reporter fluorescence intensity
- SD
Standard deviation
- ANOVA
Analysis of variance
- KMCC
Korean Multicenter Cancer Cohort
Footnotes
DECLARATION OF INTERESTS
The authors have no coflicts of interest to declare.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64(12):1881–1888. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Liu L, Wang X, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(8):1395–1408. [DOI] [PubMed] [Google Scholar]
- 4.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(5):872–878. [DOI] [PubMed] [Google Scholar]
- 5.Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(3):609–617. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. European journal of cancer. 2009;45(16):2867–2873. [DOI] [PubMed] [Google Scholar]
- 7.Al-Zubaidi AM, Alzobydi AH, Alsareii SA, Al-Shahrani A, Alzaman N, Kassim S. Body Mass Index and Helicobacter pylori among Obese and Non-Obese Patients in Najran, Saudi Arabia: A Case-Control Study. Int J Environ Res Public Health. 2018;15(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu C, Yan M, Sun Y, et al. Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese population. Helicobacter. 2014;19(6):437–442. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Du T, Chen X, Yu X, Tu L, Zhang C. Association between Helicobacter pylori infection and overweight or obesity in a Chinese population. J Infect Dev Ctries. 2015;9(9):945–953. [DOI] [PubMed] [Google Scholar]
- 10.Jo J, Nam CM, Sull JW, et al. Prediction of Colorectal Cancer Risk Using a Genetic Risk Score: The Korean Cancer Prevention Study-II (KCPS-II). Genomics & informatics. 2012;10(3):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J Cancer screenee cohort study of the National Cancer Center in South Korea. Epidemiology and health. 2014;36:e2014013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Taylor PR, Li JY, et al. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Annals of epidemiology. 1993;3(6):577–585. [DOI] [PubMed] [Google Scholar]
- 13.Shu XO, Li H, Yang G, et al. Cohort Profile: The Shanghai Men’s Health Study. International journal of epidemiology. 2015;44(3):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44(9):777–782. [DOI] [PubMed] [Google Scholar]
- 15.Yoo KY, Shin HR, Chang SH, et al. Korean Multi-center Cancer Cohort Study including a Biological Materials Bank (KMCC-I). Asian Pacific journal of cancer prevention : APJCP. 2002;3(1):85–92. [PubMed] [Google Scholar]
- 16.Zheng W, Chow WH, Yang G, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. American journal of epidemiology. 2005;162(11):1123–1131. [DOI] [PubMed] [Google Scholar]
- 17.Cai H, Ye F, Michel A, et al. Helicobacter pylori blood biomarker for gastric cancer risk in East Asia. International journal of epidemiology. 2016;45(3):774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. The New England journal of medicine. 2011;364(8):719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel A, Waterboer T, Kist M, Pawlita M. Helicobacter pylori multiplex serology. Helicobacter. 2009;14(6):525–535. [DOI] [PubMed] [Google Scholar]
- 20.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51(10):1845–1853. [DOI] [PubMed] [Google Scholar]
- 21.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steffen A, Huerta JM, Weiderpass E, et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. International journal of cancer Journal international du cancer. 2015;137(3):646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang J, Cho EJ, Hwang Y, et al. Association between Body Mass Index and Gastric Cancer Risk According to Effect Modification by Helicobacter pylori Infection. Cancer Res Treat. 2019;51(3):1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YK, Kang WM, Ma ZQ, Liu YQ, Zhou L, Yu JC. Body mass index, serum total cholesterol, and risk of gastric high-grade dysplasia: A case-control study among Chinese adults. Medicine (Baltimore). 2016;95(35):e4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joo YE, Park HK, Myung DS, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia: a nationwide multicenter prospective study in Korea. Gut Liver. 2013;7(3):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HJ, Yoo TW, Park DI, et al. Influence of overweight and obesity on upper endoscopic findings. Journal of gastroenterology and hepatology. 2007;22(4):477–481. [DOI] [PubMed] [Google Scholar]
- 28.Watabe H, Mitsushima T, Derakhshan MH, et al. Study of association between atrophic gastritis and body mass index: a cross-sectional study in 10,197 Japanese subjects. Digestive diseases and sciences. 2009;54(5):988–995. [DOI] [PubMed] [Google Scholar]
- 29.Lohse T, Rohrmann S, Bopp M, Faeh D. Heavy Smoking Is More Strongly Associated with General Unhealthy Lifestyle than Obesity and Underweight. PloS one. 2016;11(2):e0148563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q. Smoking and body weight: evidence from China health and nutrition survey. BMC Public Health. 2015;15:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamajima N, Naito M, Kondo T, Goto Y. Genetic factors involved in the development of Helicobacter pylori-related gastric cancer. Cancer science. 2006;97(11):1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Cai H, Sasazuki S, et al. Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: A pooled analysis of prospective studies in China, Japan, and Korea. International journal of cancer Journal international du cancer. 2017;140(3):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To request access to the data, please contact corresponding author Meira Epplein, who is the principal investigator of the study and responsible for the consortium dataset.
Baseline BMI assessment
Height and weight measured at cohort enrollment was used to calculate the baseline BMI by following the definition: weight (kg) / square of height (m2). BMI was classified into 6 groups using the scheme utilized by the Asia Cohort Consortium, to which the cohorts in this study also belong, in their seminal paper on BMI and risk of death in over one million Asians,18 after combining the highest two categories due to low numbers in our study population: <18.5, 18.5–20.0, 20.1–22.5, 22.6–25.0 (reference), 25.1–27.5, and ≥27.5, all in kg/m2.
H. pylori (HP) multiplex serology
Serum samples of all HpBCC participants were sent to the German Cancer Research Center (DKFZ, Heidelberg, Germany). Human HP IgA, IgM, and IgG antibodies to 15 recombinantly expressed fusion proteins (UreA, Catalase, GroEL, NapA, CagA, CagM, Cagδ, HP0231, VacA, HpaA, Cad, HyuA, Omp, HcpC, and HP0305) were assessed using HP multiplex serology based on a glutathione S-transferase capture immunosorbent assay combined with fluorescent bead technology (Luminex).19,20 Calculation of antigen-specific cutoff points [mean median reporter fluorescence intensity (MFI) plus three times standard deviation (SD), excluding positive outliers] was done using 17 HP- sera previously classified for HP status run within the same experiment. Defining HP+ (sero-positivity) as reactivity with at least 4 proteins has shown good agreement (k = 0.70) with commercial serologic assay, resulting in 89% sensitivity and 82% specificity.19 Test reliability of the assay for 12 replicate samples from two pooled samples (for a total of twenty-four quality control samples) was 98%.
HP infection status were assessed by incorporation of HP antibody serotypes (HP- vs. HP+) and HP biomarkers (CagA, Omp and HP0305) which were most strongly associated with GC risk in this population [17]. In detail, antibody reactivity to HP and its virulence factor, CagA (HP-, compared to HP+ and CagA-, and HP+ and CagA+), and the biomarkers Omp and HP0305 (in three categories: Omp- and HP0305- compared to either Omp+ or HP0305+, and Omp+ and HP0305+) were used.


