Abstract
Acute Otitis Media (AOM) is a multifactorial disease occurring mostly in young children who are immunologically naïve to AOM pathogens. This review focuses on work from Rochester NY, USA over the past 12 years among young children who had AOM infections microbiologically-confirmed by tympanocentesis, so called “stringently-defined”. Among stringently-defined otitis prone children deficiencies in fundamental immune defense mechanisms have been identified that contribute to the propensity of young children to experience recurrent AOM. Dysfunction in innate immune responses that cause an immunopathological impact in the nasopharynx have been discovered including inadequate proinflammatory cytokine response and poor epithelial cell repair. Adaptive immunity defects in B cell function and immunologic memory resulting in low levels of antibody to otopathogen-specific antigens allows repeated infections. CD4+ and CD8+ T cell function and memory defects significantly contribute. The immune profile of an otitis prone child resembles that of a neonate through the first year of life. Immunologic deficits in otitis prone children cause them to be unusually vulnerable to viral upper respiratory infections and respond inadequately to routine pediatric vaccines.
Keywords: acute otitis media, otitis prone, innate immunity, adaptive immunity, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, antibody, CD4+ T cells, B cells, antigen presenting cells
Acute otitis media (AOM) is extremely common, drives antibiotic use, emergence of antibiotic resistant bacteria and is costly. Temporary hearing loss is the most common complication; rarely there are intracranial complications.1 The World Health Organization estimates that 51,000 deaths/year are attributable to AOM in children younger than 5 years old and that chronic otitis media (occurring in 65-330 million people) is the major cause of hearing loss in developing countries.2,3 Each episode of AOM is typically followed by 4-12 weeks of otitis media with effusion (OME) during which time the child has diminished hearing and this often leads to temporary delayed speech and language development and can be associated with permanent hearing loss.4-6 In the US alone, the economic burden of otitis media exceeds $6 billion/year in medical treatment, surgical management, and loss of income for working parents.7
For several decades the underlying pathogenesis of AOM in children has been attributed to Eustachian tube dysfunction as most important.8 Children demonstrate a diminished propensity to develop AOM over time and this change has long been thought due to the Eustachian tube anatomy of children transitioning to more “adultlike” by age three to five years old to account for the frequency of AOM infections being outgrown. Direct evidence for this explanation of otitis proneness (OP) is lacking in very young children since Eustachian tube functional testing has not been reported in children below age three years old.9,10 Evidence supporting the role of Eustachian tube dysfunction derives mostly from studies in older children and adults11 although persistent dysfunction occurs after children have outgrown their propensity for recurrent AOM12 which argues against a causative link. OM has been described to be a heritable condition based on a twin study13 and single nucleotide polymorphisms involving immune mediating cytokines have been described in OP children.14-17 However, a clinical condition occurring as frequently as OP likely has other underlying causes.
Our work over the past decade brings forward an immunologic explanation for OP susceptibility and our work in Rochester, NY USA is the focus of this review. In our studies, clinically diagnosed children with AOM and confirmed by tympanocentesis were defined as stringently OP (sOP) if they experienced 3 separate AOM infections within a 6 month or 4 AOM infections within a 12 month time span. Children experiencing fewer AOM infections, confirmed by tympanocentesis, were defined as non-otitis prone (NOP). Other groups studying immunological characteristics of OP chldren have most often found deficiencies 8-21 but those studies did not restrict the definition of OP to cases where microbiologic confirmation occurred.1 It is probable that a requirement for microbiologic proof of AOM refines the study population to allow clearer outcome differences during immunologic studies. Indeed, with a strict definition, we have identified multiple deficiencies in innate and adaptive immunity among young children who are sOP and introduced the term “prolonged neonatal-like immune profile (PNIP) because of striking similarities in immune responses seen in sOP children that resemble neonatal immunity.22,23 Specifically, we observed that peripheral blood mononuclear cells (PBMCs) from sOP children between the ages of 6 and 12 months display a general skewing away from Th1 and Th17 immunity and toward Th2 and Treg dominance and a lack of enhanced inflammatory cytokine production by antigen presenting cells (APCs).24,25 We found that the immune problems of sOP children were largely outgrown by age three years old coinciding with the epidemiologic observation of diminishing AOM in that age time frame. In previous studies, sOP children were not diagnosed by otolaryngologists. The clinical diagnoses were made by referring primary care physicians. Unfortunately, if 50% of the children entering those prior studies did not have recurrent AOM and were not authentic OP children then those previous studies may have missed the immune deficiencies we identified because their study populations were significantly contaminated by non-otitis prone (NOP) children. The innovation of our work has been to apply stringent diagnostic criteria and microbiologic verification prospectively in a longitudinal study design that eliminates children who are misdiagnosed.
The significance of our investigations extends beyond application to OP children. We have shown that sOP children are prone to more frequent clinically-diagnosed, (a subset confirmed by virus-specific detection) viral upper respiratory infections26 and 23% of sOP children respond with sub-protective antibody levels after routine pediatric vaccinations.22,27
In this review we describe the accumulated results from 2006-2018, of studies involving 796 children, from whom we have prospectively collected about 20,000 samples that included blood, peripheral blood mononuclear cells (PBMCs), nasal swabs, nasal washes and/or oropharyngeal swabs during 3837 healthy planned visits at ages 6, 9, 12, 15, 18, 24, and 30-36 months of age and 1276 AOM visits, all before child age 36 months. 75 (~10%) children met the sOP definition. Children that did not meet the sOP criteria we classify as NOP. There are many factors that may contribute to children with recurrent AOM28 but our work suggests that primary among those factors are immunologic deficiencies described here (Table 1 summarizes the findings).
Table 1.
Immune Measures in sOP vs NOP Children
| MUCOSAL (NP & MEF) | |||
|---|---|---|---|
| sOP:NOP | Visit Type(s) | Ref | |
| Innate Responses | |||
| Immune modulators-NP | |||
| MIP-1β | ↓ | AOM | 30 |
| CXCL5 | ↓ | AOM | 30 |
| IL-8 | ↓, NSD | AOM | 26,30 |
| IL-6 | ↓ | AOM, URI@Healthy | 24,26,30 |
| EGF | ↓ | AOM | 30 |
| EGFR | ↓ | AOM | 30 |
| Angiogenin | ↓ | AOM | 30 |
| ICAM-1 | ↓ | AOM | 30 |
| IL-7 | ↓ | AOM | 31 |
| IGFBP-4 | ↓ | AOM | 31 |
| IL-23 | ↓ | Healthy | 24 |
| TNFα, IL-6, IL-10, RANTES, | ↓ | URI@Healthy | 26 |
| MCP-1 | NSD | AOM | 26,30 |
| IL-2 | ↑ | AOM | 26,31 |
| 26 | |||
| IL-2 | NSD | URI@Healthy | 26 |
| IL-17a | NSD | AOM, URI@Healthy | 26,31 |
| IFN-γ | NSD | AOM, URI@Healthy | 26,31 |
| IL-1β, IL-8, MIP-1α | NSD | URI@Healthy | 26 |
| TLR2/4 (RNA) | ↑ | AOM | 30 |
| TLR2 (RNA) (Epithelials & Neutropils) | ↑ | AOM | 30 |
| ICAM-1 | ↓ | AOM | 30 |
| Immune modulators-MEF | |||
| IL-2 (RNA) | ↑ | AOM | 31 |
| IL-8 (RNA) | ↑ | AOM | 67 |
| SLPI (RNA) | ↑ | AOM | 67 |
| MIP-1α (RNA) | ↑ | AOM | 67 |
| RANTES (RNA) | ↓ | AOM | 67 |
| IFNα1 (RNA) | ↓ | AOM | 67 |
| IRF7 (RNA) | ↓ | AOM | 67 |
| MAPK8 (RNA) | ↓ | AOM | 67 |
| TICAM2 (RNA) | ↓ | AOM | 67 |
| ZBP1 (RNA) | ↓ | AOM | 67 |
| Adaptive Responses | |||
| Antibodies | |||
| IgG to Spn & Mcat Ags | ↓ | Healthy | 33,68 |
| IgA to Spn & Mcat Ags | ↓ | Healthy | 33,68 |
| Colonization | |||
| Spn, NTHi, Mcat | ↑ | Healthy, URI@Healthy, AOM | 26,33,68 |
| Viral Infection | |||
| RSV | ↑ | AOM | 48 |
| URIs | ↑ | Healthy | 26 |
| BLOOD | |||
| sOP:NOP | Visit Type(s) | Ref | |
| Baseline Responses | |||
| PBMC (unstimulated) | |||
| IL-2 | ↓ | AOM | 31 |
| IFN γ | ↓ | AOM | 31 |
| BAFF, APRIL | NSD | Healthy | 42 |
| APCs (unstimulated) | |||
| Total Monocytes & cDCs | ↑ | Healthy | 25 |
| MHC II | ↓ | Healthy & AOM | 69 |
| PBMC (R848 stimulated) | |||
| IL-1β, IL-6, IL-8, IL-10, IL-12, IFN-α, TNF-α, IFN-γ, CCL2, CCL4, CCL5, CXCL10 | NSD | Healthy | 25 |
| APCs (R484 stimulated) | |||
| Intracellular IL-12, TNF-α, IFN-α | NSD | Healthy | 25 |
| Innate & Adaptive Responses | |||
| PBMC (HK-Spn stimulated) | |||
| IL-2, IL-4, IL-6, IL-10, IL-13 | NSD | Healthy | 24 |
| IFN-γ | ↓ | Healthy | 24 |
| IL-17A | ↓ | Healthy | 24 |
| IL-21 | ↓ | Healthy | 24 |
| IL-23 | ↓ | Healthy | 24 |
| IL-2 (RNA) | ↓ | Healthy | 24 |
| IL-13 (RNA) | ↓ | Healthy | 24 |
| IL-17A (RNA) | ↓ | Healthy | 24 |
| IL-23 (RNA) | ↓ | Healthy | 24 |
| TGF-β (RNA) | ↓ | Healthy | 24 |
| IFN-γ (RNA) | ↓ | Healthy | 24 |
| TBX21 (RNA) | ↓ | Healthy | 24 |
| RORC (RNA) | ↓ | Healthy | 24 |
| FOXP3 (RNA) | ↑ | Healthy | 24 |
| Adaptive Responses | |||
| Antibodies | |||
| IgG to Spn, NTHi, Mcat Ags | ↓ | Healthy & AOM | 34-36,40,43 |
| Antibodies to pediatric vaccine Ags (DTaP, Hib, Polio, Spn PSs) | ↓ | Healthy | 22,41,42 |
| IgG to RSV | ↓ | Healthy | 48 |
| B cells | |||
| Total B cells | NSD | Healthy | 41 |
| BAFFR, TACI | ↓ | Healthy | 42 |
| BCMA | NSD | Healthy | 42 |
| BAFFR, TACI, B7-1, B7-2 (RNAs) | ↓ | Healthy | 42 |
| BCMA, CD40L (RNAs) | NSD | Healthy | 42 |
| %Memory B cells | ↓ | Healthy | 41,42 |
| %Memory B cells response to DTaP | ↓ | Healthy | 40,41 |
| %Switched memory | ↓ | Healthy | 41 |
| %Plasma cells | ↓ | Healthy | 42 |
| Spn Ag-specific ASCs | ↓ | Healthy | 42 |
| CD4+ T cells | |||
| Total CD4+ T-cells | NSD | Healthy | 43 |
| Naïve CD4+ T-cells | NSD | Healthy | 43 |
| Memory CD4+ T-cells | NSD | Healthy | 43 |
| CD4+ T-cells (HK-Spn stimulated) | |||
| pSTAT3 | ↓ | Healthy | 24 |
| pSTAT3 (+Th17 cytokines) | NSD | Healthy | 24 |
| %CD4+ T-cells (HK-Spn stimulated) | |||
| IFN γ | NSD | Healthy | 24 |
| IL-2 | ↓ | Healthy | 24 |
| IL-17A | ↓ | Healthy | 24 |
| TNF-α | ↓ | Healthy | 24 |
| %Naive CD4+ T-cells (HK-Spn stimulated) | |||
| IL-2 | ↓ | Healthy | 24 |
| TNF-α | ↓ | Healthy | 24 |
| %Memory CD4+ T-cells (HK-Spn stimulated) | |||
| IFN γ | ↓ | Healthy | 24 |
| IL-2 | ↓ | Healthy | 24 |
| IL-17A | ↓ | Healthy | 24 |
| TNF-α | ↓ | Healthy | 24 |
| %Memory CD4+ T-cells (Spn or NTHi antigen stimulated) | |||
| IFN γ | ↓ | Healthy | 43 |
| IL-2 | ↓ | Healthy | 43 |
| IL-4 | ↓ | Healthy | 43 |
| IL-17A | ↓ | Healthy | 43 |
| %Memory CD4+ T-cells (SEB stimulated) | |||
| IFN γ, IL-2, IL-4, IL-17a | NSD | Healthy | 43 |
NSD: No statistical difference detected
AOM: Child with acute otitis media (AOM).
Healthy: Child at a normal healthy visit checkup.
URI@Healthy: Child came in during a normal healthy visit checkup but was diagnosed with an upper respiratory infection (URI).
NP: Nasopharyngeal
MEF: Middle ear fluid
PBMC: Peripheral blood mononuclear cells
Spn: S. pneumoniae; HK-Spn: heat killed Spn; NTHi: nontypeable H. influenzae; Mcat: Moraxella catarrhalis
MUCOSAL NASOPHARYNGEAL COMPARTMENT (Figure 1)
Figure 1. Nasopharynx and middle ear responses in sOP versus NOP children.
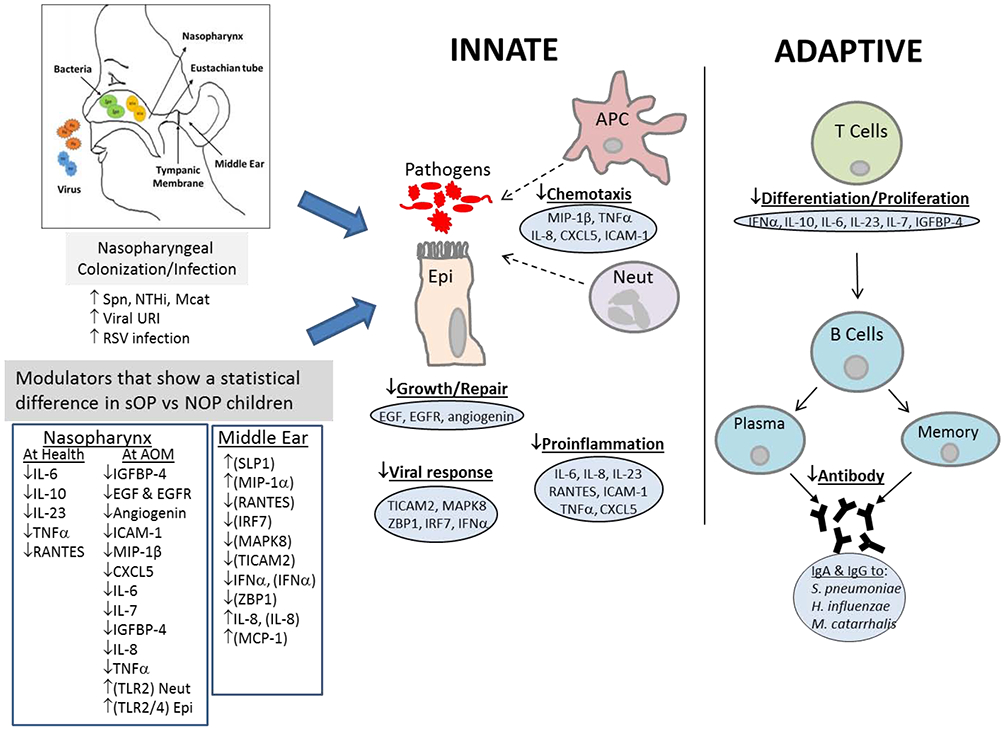
Relative levels that show a statistical difference between sOP versus NOP children are depicted with up or down arrows. Modulator measurements are of proteins except those in brackets are of mRNA.
Innate Responses
Neutrophils
Neutrophil activation and bacterial ingestion represent part of the innate immune response. In a study of NP samples, we found that the neutrophil count increased roughly 600-fold during AOM in sOP and NOP children.29 We further conducted studies of neutrophil recruitment and inflammation in the NP in children during health, viral URI and AOM in the presence or absence of NP colonization by Streptococcus pneumoniae (Spn).29 We found no evidence of dysfunctional neutrophil recruitment or bactericidal ROS production by Neutrophils.30 We compared difference of gene expression of inflammatory effectors from neutrophils of sOP and NOP children during AOM. Real-Time PCR (RT-PCR) was used to assess gene expression of inflammatory effectors. We found that sOP children had decreased mRNA expression of cytokine IL-1β from neutrophils.29
Epithelial and cytokines
We studied the role of epithelial and innate mucosal responses to NP colonization in our sOP child population during a viral URI that proceeded to cause AOM due to Spn.30 We found significantly lower epidermal growth factor, epidermal growth factor receptor and angiogenin concentrations in the NP of sOP compared with NOP children suggesting lower capacity for epithelial repair in sOP children. We also found significantly lower pro inflammatory neutrophil chemoattractants such as MIP-1β, IL-8 and CXCL5 (ENA-78 in the NP of sOP children at onset of AOM. These findings of lower epitheliall repair and impaired proinflammatory response provide other mechanisms as to why sOP children succumb to repeated AOM events.
In a separate study, we investigated whether compartmental responses differed by comparing the cytokine levels in NP, MEF and peripheral blood of sOP and NOP children during an AOM event caused by Spn.31 Nasal washes from sOP children contained significantly higher levels of proinflammatory cytokine IL-2 but lower levels of IL-7 and insulin-like growth factor-4 (IGFBP-4), both involved in proliferation and T-cell homeostasis, but similar expression levels of IFN-γ and IL-17a, both of which are normally protective responses to an infection from innate and adaptive cells. We compared the RNA expression of IFN-γ, IL-2 and IL-17a from the MEF. We found significantly heightened transcription of IL-2 in sOP children and no significant difference in IFN-γ or IL-17a transcript levels. However, plasma IFN-γ and IL-2 levels were significantly lower in sOP children while IL-17a was too low to measure in both cohorts. These results show that the middle ear cytokine response mirrored those of the nasal mucosa versus the peripheral blood, suggesting that proximal mucosal sites may better predict the quality of the middle ear response than peripheral blood. Our results also highlight the differences between local and systemic immune responses that could co-ordinate anti-bacterial immune responses in young children. In addition, this data further highlights that the sOP child has immune deficits that fail to increase recall responses after additional exposure to otopathogens upon additional infections.
In another study NOP children had significantly higher levels of IL-6, IL-10, INF-γ, TNF-α, IL-1β, MCP-1, RANTES, IL-2 and IL-17 during viral URIs versus AOMs following the URIs, when compared to sOP children.26 In that same study, we analyzed the relationship between the NP cytokine/chemokine level and sOP rate using a logistic regression analysis, generalized additive model, we found that sOP children had significantly lower nasal pro-inflammatory levels of IL-6, IL-10, TNF-α and RANTES than NOP children during viral URIs.
Taken together, sOP children have more frequent viral URIs than NOP children, due to deficient antiviral nasopharyngeal pro-inflammatory cytokine and chemokine responses, and this facilitates the development of AOMs. Our findings of lower epidermal repair processes and lower inflammatory response in the nasal mucosa of sOP versus NOP children provide another possible mechanism which might contribute to the predisposition of the sOP child to repeated AOM events.
We studied mucosal antibodies levels in the NP to Spn. Specifically, we investigated mucosal IgG and IgA levels in the NP and MEF from children to three Spn antigens (PhtD, PcpA and Ply).32 We showed that higher naturally acquired mucosal antibody levels to these antigens was associated with reduced AOM caused by Spn. We then sought to correlate the mucosal antibody levels in sOP children to those same pneumococcal proteins with Spn NP colonization and the occurrence of AOM.33 We found that sOP children had significantly higher colonization frequency by Spn (p< 0.0001) and significantly lower IgG and IgA levels to all 3 Spn proteins compared with NOP children except IgG to Ply D1. Spn colonization in NOP children led to 2-fold to 5-fold increase in mucosal IgG and IgA levels to all 3 proteins, whereas Spn colonization in sOP children generally failed to elicit antibody responses. Taken together, these data on mucosal antibody supports our hypothesis that sOP children have an immunological defect in responding to natural immunization by NP colonization and AOM.
SYSTEMIC BLOOD COMPARTMENT (Figure 2)
Figure 2. PBMC and serum immune responses in sOP versus NOP children.
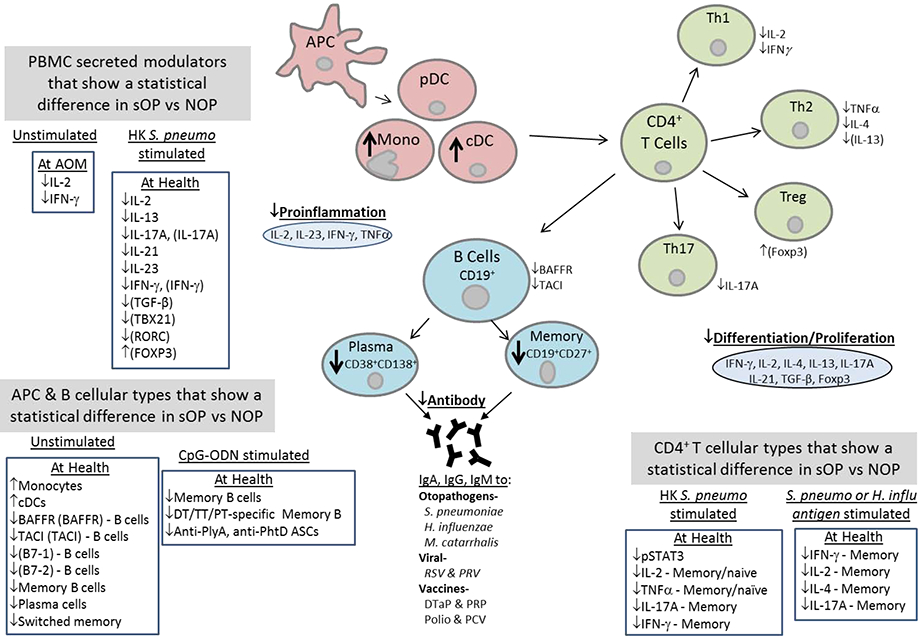
Relative levels that show a statistical difference between sOP versus NOP children are depicted with up or down arrows. Modulator measurements are of proteins except those in brackets are of mRNA.
Professional antigen presenting cells (APCs)
APCs bridge the innate immune system to the adaptive immune system by facilitating presentation of antigens to B cells and T cells. Therefore we sought to determine whether there might be defects in numbers, phenotype and/or function of APCs in the peripheral blood of sOP infants.25 APC phenotypic counts, MHC II expression and intracellular cytokine levels were determined in response to TLR 7/8 stimulation using R848. Innate immune mRNA expression was measured using RT-PCR and cytokines were measured using Luminex technology. We found significantly higher numbers of monocytes and conventional DCs but not plasmacytoid DCs in sOP children even when healthy compared to NOP age-matched infants. The presence of increased numbers of monocytes and cDCs in the blood we hypothesized was consistent with the existence of a heightened pro-inflammatory state in sOP children between recurrent AOMs. However, we found that sOP and NOP infants produce similar levels of innate associated cytokines upon PBMC stimulation with a TLR agonist (TLR7/8). Baseline cytokine/chemokine levels, as well as expression levels of TLRs and intracellular signaling molecules from PBMCs in response to TLR stimulation were similar among sOP and NOP children, suggesting that sOP APC function might not be a major contributor to sOP immune hyporesponsiveness.
Adaptive Responses
Antibody
The three major otopathogens causing recurrent OM in our study children have been Spn, nontypeable H. influenzae (NTHi) and Moraxella catarrhalis (Mcat). We have shown that nasopharyngeal (NP) colonization is not only a necessary first step toward infection, it also is a natural immunizing event. 34,35 We had hypothesized that sOP children would have reduced levels of antibodies to the principal otopathogens resulting in there being less adaptive immunity to control the growth and eventual spread of the bacterium from the NP to the middle ear (ME). To test that hypothesis, we determined serum IgG titers against Spn proteins PhtD, PhtE, LytB, PcpA, PlyD1 rather than serotype-specific capsule polysaccharides.34 We found that sOP children had significantly lower IgG titers to PhtD, PhtE, LytB, PlyD1 than NOP children at healthy visits with asymptomatic NP colonization and at onset of AOM, supporting our hypothesis.
In a similar study, we investigated antibody levels to NTHi protein antigens (P6, D, OMP26). Antibody levels to the three antigens measured longitudinally during NP colonization between age 6 and 24 months showed <2-fold increases over time in sOP children compared to >4-fold increases in NOP children.35 Similar to our findings for protein vaccine candidates of Spn and NTHi, sOP children displayed a later and a significantly lower peak of serum IgG antibody rise than NOP children for Mcat protein antigens (OppA, OMP CD, Hag5-9) during NP colonization of Mcat.36 However, at time of AOM caused by Mcat, only serum IgG antibodies to OppA or Hag5-9 were significantly higher for NOP compared to sOP children.
Although these studies do not inform regarding protective levels of antibody, the fact that sOP children have significantly lower adaptive antibody levels to Spn, NTHi and Mcat antigens from infections by these otopathogens support the finding as to why they also have significantly higher colonization frequency by Spn, NTHi and Mcat than NOP children.33
To extend the findings regarding antibody hypo-responsiveness37-39 we sought to determine whether sOP children would also have lower antibody responses to pediatric vaccine immunizations. We analyzed sera collected from sOP and age-matched NOP children age 6–24 months for IgG concentrations to the DTaP antigens (diphtheria toxoid (DT), tetanus toxoid (TT), pertussis toxoid (PT), filamentous hemagglutinin (FHA), and pertactin (PRN)), polio, hepatitis B, H. influenzae type b capsule polyribosyl-ribitol-phosphate (PRP) and Spn capsular polysaccharide conjugate vaccine. sOP children were significantly more likely to have non-protective responses against DT, TT, hepatitis B, polio 3, and Spn 23F, but not polio 1 and 2, PRP, or Spn 6B and 14.22 Lower putative protective responses to PT, FHA, PRN pertussis antigens were also observed. A high percentage of these sOP children had non-protective antibody titers to the pediatric vaccines tested and sub-protective levels persisted until 24 months of age in many sOP children despite routine vaccination boosters. Currently these vulnerable sOP children do not show evidence of higher rates of vaccine-preventable infections. However, we speculated that they are protected by herd immunity and in the United States and other countries where parent refusal of vaccines has increased or immunizations are limited, herd immunity may become threatened.
Memory B cells
Knowing that a high percentage of sOP children develop lower levels of antibody to otopathogenproteins during natural exposure via the mucosal route, we sought to determine the percentages of memory B cells to Spn antigens compared to NOP children.40 We found that sOP children had significantly lower frequencies of antigen-specific memory B cells against 3 Spn protein antigens (PhtD, PhtE, and Ply). Additionally, these frequencies correlated positively with serum IgG levels to the same antigens. Since sOP children failed to develop protective antibody levels to standard pediatric vaccines when given parenterally, we examined memory B cell responses to the DTaP vaccine antigens (DT, TT, and PT) in healthy sOP and NOP children.41 We found the frequency of total memory CD19+ CD27+ B cells was significantly lower in sOP children. Further, sOP children had significantly fewer memory B cells specific for DT, TT, and PT, and antigen-specific B cell frequencies correlated with serum IgG titers as in the earlier study.
We further analyzed specific aspects of the B-cells in sOP children. We found fewer switched memory B-cells as measured by CD19, CD27, IgG and IgM surface markers.42 The B-cells of sOP children also showed reduced levels of expression of co-stimulatory molecules and TNF family receptors: transmembrane activator and calcium modulating cyclophilin-ligand interactor (TACI), together with B cell maturation antigen (BCMA) and B cell activating factor receptor (BAFFR).
Overall, our data indicate that sOP children have reduced memory B-cells to otopathogen antigens (and vaccines), reduced switched memory B-cells with IgM and IgG receptors and poor expression of TNF family receptors compared to NOP children which may lead to failure in generating Spn and NTHi specific or vaccine antigen specific antibody generation.
CD4 T cells
We hypothesized that alterations in CD4 T-cell subsets in sOP children might also contribute to immune hyporesponsiveness, as compared to NOP children. Using 6 Spn and 3 NTHi protein antigens, we enumerated Spn- and NTHi- specific functional CD4 T-helper memory cell subsets in the peripheral blood of cohorts of sOP and NOP children with AOM or NP colonized with either Spn or NTHi.43 We found significantly reduced percentages of functional CD45RALow memory CD4 T cells producing cytokines (IFN-γ, IL-2, IL-4 and IL-17A) in sOP children following AOM and NP colonization with either Spn or NTHi. Thus we showed that sOP children also have a diminished ability to generate memory T cell responses after NP colonization and AOM. These data had particular importance with regard to IL-17A since data from mouse NP colonization models suggest that protection against Spn carriage is dependent on IL-17 expressing CD4 T cells by a mechanism involving IL-17A increasing Spn killing by neutrophils.44,45 However, the percentage of functional memory CD4 T-cells was similar for sOP and NOP when stimulated with SEB ruling out an intrinsic defect in CD4 T-cells of sOP children.43
Peripheral blood mononuclear cell (PBMC) cytokine production and T cell skewing
Proinflammatory cytokines are known to be critical for protective immunity against Spn infection. Therefore, we compared extracellular cytokine levels from PBMCs of sOP and NOP children in response to Spn stimulation.24 sOP children produced significantly lower levels of IFN-γ, IL-17A, IL-21 and IL-23 than NOP children. RNA analysis of Spn stimulated PBMCs also showed significant lower expression levels of IFN-γ, IL-2, IL-13, IL-17A, IL-23 and TGF-β. Furthermore, Th1 and Th17 fate-determining transcription factors T-box transcription factor 21 (TBX21) and RAR-related orphan receptor C (RORC) showed significantly lower expression levelsin samples from sOP children compared with NOP children while Foxp3 (Treg cell) expression was significantly higher from sOP children.
In a subsequent study, we heat killed Spn stimulation for in vitro stimulation of T cells.24 We found significantly diminished memory CD4 (CD69+CD45RA−) T-cells producing IL-2, TNF-α and IL-17 in sOP children. Addition of exogenous Th17-promoting cytokines (IL- 6, −1β, −23 and TGF-β) restored CD4+ Th17 cell function in cells from sOP children to levels measured in NOP children. We also observed that both CD4 naïve (CD69+CD45RA+) and memory populations were significantly less activated in sOP children. In additional unpublished experiments we stimulated T-cells with TCR stimulating reagents mimicking DC activation (anti-CD3/CD28 Dynabeads), and found sOP children (age 6-9 months) had significantly impaired responses as measured by CD69 expression. Taken together our results indicate that sOP children generate fewer memory T cells and their T cells have deficiencies in their T cell receptors or T cell signaling after antigen priming.
Viral URI co-pathogenesis with otopathogens
A risk factor known to be associated with AOM is a preceding or concurrent viral upper respiratory tract infection (URI).46 In our studies, at onset of AOM, 93% of the children had clinical signs of a viral URI.47 We examined the differential impact of respiratory syncytial virus (RSV) and parainfluenza virus (PIV) URIs on the frequency of AOM caused by Spn and NTHi in sOP and NOP children as a potential mechanism to explain increased susceptibility to AOM.48 RSV was substantially more likely to contribute to AOM in sOP than in NOP children, and additionally sOP children were significantly more likely to be infected by RSV. This corresponded with significantly lower serum antibody titers against RSV in sOP children. PIV infections did not differentially affect AOM events in sOP and NOP children. To investigate the interface of a diminished neutralizing antibody response to virus and the correlative heightened viral replication we detected with neutrophil phagocytic function during AOM, an ex vivo phagocytic assay was developed. RSV impaired the phagocytic activity of neutrophils isolated from infected children significantly more than PIV. These data suggested that a failure to neutralize RSV could disrupt the capacity of neutrophils to engulf the bacterial otopathogen, facilitating the development of AOM. Taken together, the data show that lower innate and adaptive immune responses to RSV in sOP children allowed for viral interference with innate antibacterial immune responses, thus contributing to increased frequency of AOMs.
Correlation between sOP and Neonatal immunity
The immune system of sOP children clearly differs in many ways from that of NOP children. A terminology of “prolonged neonatal-like immune profile” was proposed to provide context to the overarching theme of the immune differences in sOP vs. NOP children.22,23,27,42 The neonatal immune system is required to handle the abrupt transition to multiple, simultaneous antigenic stimulations from microbial colonization, exposure to food antigens, etc., so the responses must be muted through mechanisms of immune suppression. Thus the neonatal immune response tends to be anti-inflammatory rather than pro-inflammatory, resulting in predominance of anti-inflammatory innate cytokines. In neonates responses to TLR signals are dampened49. Newborn APCs are relatively lower in number with low basal levels of MHC-II surface expression and a decreased ability to produce cytokines.39,50,51 Neonatal CD4+ T cells show preferential differentiation of Th2 cells over Th1 cells and higher numbers of T reg cells.39,52,53 Defects in plasma cell differentiation occur due to limited T cell help54. Neonates produce lower levels of antibody following antigenic stimulation39. The ability of B cells to proliferate and differentiate into plasma cells and memory cells influences the levels of antibody and recall responses on antigen re-exposure, respectively. Neonatal B cells have decreased TNF family receptors55. TNF super family members expressed by B cells include BAFF and APRIL and 3 receptors: BAFFR, BCMA and TACi. Preterm newborn neonatal B cells show reduced proliferation in response to BAFF and anti-IgM and express less TACI, BCMA and BAFFR than adult B cells.55,56 Taken together, these multiple features of neonatal immunity that have been identified in the sOP child provide the foundation for the proposed prolonged neonatal-like immune profile moniker.
Another conceptualization of the sOP child immune system might focus on immunosuppression and a chronic hyper-inflammatory state in the nasopharynx due to recurrent viral URI and otopathogens colonization. Early in life, the NP of children is colonized with multiple bacterial species, including potential otopathogens that occupy the nasal epithelial surface. NP colonization is the initial, necessary step in the pathogenesis of AOM. During viral URI, the major bacterial otopathogens can disseminate to the middle ear. Despite similar clinical care and demographic factors, we have shown that sOP children are more susceptible to bacterial AOM during viral URI than NOP children.26 This might be viewed as a failure of commensal-immune equilibrium. Both human and mouse studies have demonstrated that colonization is an immunizing event, generating both B and T cell memory to the colonizing bacteria. Accumulating data suggests adaptive immunity to NP colonizers requires boosting from successive encounters to reach a threshold of protection.57 This may indeed be the reason for reduced colonization rates in healthy adults compared to children. Th1 and Th17 memory cells that promote neutrophilia are protective against otopathogens, with an additional role played by antibodies raised against capsular and protein antigens.58-62 When high levels of anti-capsular antibodies are generated via vaccination (as is the case with pneumococcal conjugate vaccine), the B cell response alone is sufficient to prevent future colonization with the immunizing serotypes.63 Cross-serotype protection is the ideal immune response and has mostly been associated with T cell memory.59,61 Overall, these data suggest both B and T cell responses are important for protection, and development of immune memory over successive childhood infections plays an important role in future protection from both colonization and subsequent AOM infections.
In addition, the epithelial surface is the physical surface which bacteria utilize to colonize the NP, often forming biofilms to occupy a niche in this space. Epithelial cells provide the physical barrier, via tight and adherens junctions, that prevents otopathogens colonization. In addition, epithelial-derived cytokines and chemokines are central initiators and modulators of the local innate immune response64. Therefore, in addition to pathogen-specific memory T and B cells, epithelial barrier and immune function are likely a critical factor in determining the establishment and density of NP bacterial colonization, a necessary step in AOM pathogenesis. Taken together, observations from sOP children demonstrate an immunosuppressed state with its downstream effects on adaptive immune responses/immune memory and inadequate innate immune responses in the NP along with poor NP epithelial cell repair.
Conclusions
All infants are born immunologically naïve to respiratory viruses and potential bacterial otopathogens. All have Eustachian tube anatomy that favors reflux of nasopharyngeal secretions containing viruses and bacteria into the middle ear to cause AOM. Environmental risk to experience viral URI and NP colonization by potential bacterial otopathogens can be delayed by avoidance of exposure to others who harbor and can transmit the organisms. However, infants and young children cannot avoid their parents or siblings and interactions with the wider family group and others in society eventually occurs. AOM infection risk may be mitigated somewhat by avoidance of day care and pacifier use and providing breast feeding.65 Nevertheless, when children are exposed to respiratory viruses leading to occasional or recurrent viral URI, a subset consequently experience AOM and about 10% of children experience recurrent AOM if stringently diagnosed. These children account for approximately half of all AOM cases observed, suggesting a high impact for improved understanding of the immune mechanisms contributing to cause susceptibility in this population of children. Infection history of a child early in life is known to have profound influences on later immune development66. We recently found that.sOP children experience NP colonization by Spn, NTHi, and Mcat at a substantially greater rate and at earlier ages than NOP children (unpublished). However, the immune responses against these pathogens is reduced at 6 months of age before onset of AOM, with lower bacterial otopathogens-specific antibody titers and weaker responses by T and B cells upon stimulation with bacterial antigens in vitro, Thus, the events that predispose children to experience recurrent AOM may occur earlier than we have studied them. Microbiome modulation of the immune response commencing shortly after birth is currently under investigation by our group.
Highlights.
AOM cases, when microbiologically-confirmed, reveal a unique otitis prone cohort.
Otitis proneness in children may result from multiple, transient immune dysfunction.
Nasopharyngeal and systemic immune compartments may display dysfunction.
Innate and adaptive immune systems may display dysfunction.
The immune profile of young otitis prone children may resemble that of a neonate.
Acknowledgements
Scientists who contributed to experiments cited in this review include: Anthony Almudevar, Saleem Basha, Anthony Campagnari, Janet Casey, Linlin Chen, Laura Filkins, Ravinder Kaur, M. Nadeem Khan, Eric Lafontaine, Kyl Liu, Alexandra Livingstone, Nichole Luke-Marshall Matthew Morris, Tim Mosmann, Tim Murphy, Monica Nesselbush, Emily Newman, Ted Nicolosi, Dabin Ren, Sharad Sharma, Naveen Surendran, David Verhoeven, and Qingfu Xu,, Dr. Robert Zagursky assisted in manuscript preparation..
Funding
Study was supported by NIH NIDCD R01 08671.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. The Lancet infectious diseases 2010; 10(3): 195–203. [DOI] [PubMed] [Google Scholar]
- 2.Acuin J Chronic suppurative otitis media. Clinical evidence 2003; (9): 546–64. [PubMed] [Google Scholar]
- 3.Berman S Otitis media in developing countries. Pediatrics 1995; 96(1 Pt 1): 126–31. [PubMed] [Google Scholar]
- 4.Pichichero ME. Otitis media. Pediatric clinics of North America 2013; 60(2): 391–407. [DOI] [PubMed] [Google Scholar]
- 5.Mittal R, Parrish JM, Soni M, Mittal J, Mathee K. Microbial otitis media: recent advancements in treatment, current challenges and opportunities. Journal of medical microbiology 2018; 67(10): 1417–25. [DOI] [PubMed] [Google Scholar]
- 6.Schilder AG, Chonmaitree T, Cripps AW, et al. Otitis media. Nature reviews Disease primers 2016; 2: 16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan B, Wandstrat TL, Cunningham JR. Overall cost in the treatment of otitis media. The Pediatric infectious disease journal 1997; 16(2 Suppl): S9–11. [DOI] [PubMed] [Google Scholar]
- 8.Bluestone CD. Pathogenesis of otitis media: role of eustachian tube. The Pediatric infectious disease journal 1996; 15(4): 281–91. [DOI] [PubMed] [Google Scholar]
- 9.Casselbrant ML, Mandel EM, Seroky JT, Swarts JD, Doyle WJ. A pilot study of the ability of the forced response test to discriminate between 3-year-old children with chronic otitis media with effusion or with recurrent acute otitis media. Acta oto-laryngologica 2011; 131(11): 1150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swarts JD, Casselbrant ML, Teixeira MS, et al. Eustachian tube function in young children without a history of otitis media evaluated using a pressure chamber protocol. Acta oto-laryngologica 2014; 134(6): 579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swarts JD, Bluestone CD. Eustachian tube function in older children and adults with persistent otitis media. International journal of pediatric otorhinolaryngology 2003; 67(8): 853–9. [DOI] [PubMed] [Google Scholar]
- 12.Mandel EM, Casselbrant ML, Richert BC, Teixeira MS, Swarts JD, Doyle WJ. Eustachian Tube Function in 6-Year-Old Children with and without a History of Middle Ear Disease. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2016; 154(3): 502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casselbrant ML, Mandel EM, Fall PA, et al. The heritability of otitis media: a twin and triplet study. Jama 1999; 282(22): 2125–30. [DOI] [PubMed] [Google Scholar]
- 14.Casselbrant ML, Mandel EM, Jung J, et al. Otitis media: a genome-wide linkage scan with evidence of susceptibility loci within the 17q12 and 10q22.3 regions. BMC medical genetics 2009; 10: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito S, Marchisio P, Orenti A, et al. Genetic Polymorphisms of Functional Candidate Genes and Recurrent Acute Otitis Media With or Without Tympanic Membrane Perforation. Medicine 2015; 94(42): e1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hafren L, Einarsdottir E, Kentala E, et al. Predisposition to Childhood Otitis Media and Genetic Polymorphisms within the Toll-Like Receptor 4 (TLR4) Locus. PLoS One 2015; 10(7): e0132551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilia S, Goulielmos GN, Samonis G, Galanakis E. Polymorphisms in IL-6, IL-10, TNF-alpha, IFN-gamma and TGF-beta1 Genes and Susceptibility to Acute Otitis Media in Early Infancy. The Pediatric infectious disease journal 2014; 33(5): 518–21. [DOI] [PubMed] [Google Scholar]
- 18.Seppanen E, Tan D, Corscadden KJ, et al. Evidence of functional cell-mediated immune responses to nontypeable Haemophilus influenzae in otitis-prone children. PloS one 2018; 13(4): e0193962–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkham LS, Wiertsema SP, Corscadden KJ, et al. Otitis-Prone Children Produce Functional Antibodies to Pneumolysin and Pneumococcal Polysaccharides. Clin Vaccine Immunol 2017; 24(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton RB, Kirkham L-AS, Corscadden KJ, et al. Australian Aboriginal Children with Otitis Media Have Reduced Antibody Titers to Specific Nontypeable Haemophilus influenzae Vaccine Antigens. Clinical and vaccine immunology : CVI 2017; 24(4): e00556–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Gier C, Granland CM, Pickering JL, et al. PCV7- and PCV10-Vaccinated Otitis-Prone Children in New Zealand Have Similar Pneumococcal and Haemophilus influenzae Densities in Their Nasopharynx and Middle Ear. Vaccines (Basel) 2019; 7(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. The Pediatric infectious disease journal 2013; 32(11): 1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichichero ME. Ten-Year Study of the Stringently Defined Otitis-prone Child in Rochester, NY. The Pediatric infectious disease journal 2016; 35(9): 1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basha S, Kaur R, Mosmann TR, Pichichero ME. Reduced T-Helper 17 Responses to Streptococcus pneumoniae in Infection-Prone Children Can Be Rescued by Addition of Innate Cytokines. The Journal of infectious diseases 2017; 215(8): 1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surendran N, Nicolosi T, Kaur R, Pichichero ME. Peripheral blood antigen presenting cell responses in otitis-prone and non-otitis-prone infants. Innate immunity 2016; 22(1): 63–71. [DOI] [PubMed] [Google Scholar]
- 26.Ren D, Xu Q, Almudevar AL, Pichichero ME. Impaired Proinflammatory Response in Stringently Defined Otitis-prone Children During Viral Upper Respiratory Infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2019; 68(9): 1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichichero ME, Casey JR, Almudevar A, et al. Functional Immune Cell Differences Associated With Low Vaccine Responses in Infants. The Journal of infectious diseases 2016; 213(12): 2014–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyd JM, Hotomi M, Kono M, et al. Panel 5: Immunology. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery 2017; 156(4_suppl): S63–s75. [DOI] [PubMed] [Google Scholar]
- 29.Morris MC, Pichichero ME. Streptococcus pneumoniae burden and nasopharyngeal inflammation during acute otitis media. Innate immunity 2017; 23(8): 667–77. [DOI] [PubMed] [Google Scholar]
- 30.Verhoeven D, Nesselbush M, Pichichero ME. Lower nasopharyngeal epithelial cell repair and diminished innate inflammation responses contribute to the onset of acute otitis media in otitis-prone children. Medical microbiology and immunology 2013; 202(4): 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhoeven D, Pichichero ME. Divergent mucosal and systemic responses in children in response to acute otitis media. Clinical and experimental immunology 2014; 178(1): 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q, Casey JR, Pichichero ME. Higher levels of mucosal antibody to pneumococcal vaccine candidate proteins are associated with reduced acute otitis media caused by Streptococcus pneumoniae in young children. Mucosal immunology 2015; 8(5): 1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q, Casey JR, Newman E, Pichichero ME. Otitis-Prone Children Have Immunologic Deficiencies in Naturally Acquired Nasopharyngeal Mucosal Antibody Response after Streptococcus pneumoniae Colonization. The Pediatric infectious disease journal 2016; 35(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 34.Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. The Pediatric infectious disease journal 2011; 30(8): 645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine 2011; 29(5): 1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren D, Almudevar AL, Murphy TF, et al. Serum antibody response to Moraxella catarrhalis proteins in stringently defined otitis prone children. Vaccine 2017. [DOI] [PMC free article] [PubMed]
- 37.PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nature immunology 2011; 12(3): 189–94. [DOI] [PubMed] [Google Scholar]
- 38.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. American journal of perinatology 2013; 30(2): 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Review of Clinical Immunology 2014; 10(9): 1171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma SK, Casey JR, Pichichero ME. Reduced serum IgG responses to pneumococcal antigens in otitis-prone children may be due to poor memory B-cell generation. The Journal of infectious diseases 2012; 205(8): 1225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basha S, Pichichero ME. Poor memory B cell generation contributes to non-protective responses to DTaP vaccine antigens in otitis-prone children. Clinical and experimental immunology 2015; 182(3): 314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basha S, Pichichero ME. Decreased TNF family receptor expression on B-cells is associated with reduced humoral responses to Streptococcus pneumoniae infections in young children. Cellular immunology 2017; 320: 11–9. [DOI] [PubMed] [Google Scholar]
- 43.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. The Journal of infectious diseases 2011; 204(4): 645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. The Journal of clinical investigation 2009; 119(7): 1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffitt KL, Gierahn TM, Lu YJ, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell host & microbe 2011; 9(2): 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. Journal of leukocyte biology 2010; 87(2): 213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Q, Almudervar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerging infectious diseases 2012; 18(11): 1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verhoeven D, Xu Q, Pichichero ME. Differential Impact of Respiratory Syncytial Virus and Parainfluenza Virus on the Frequency of Acute Otitis Media Is Explained by Lower Adaptive and Innate Immune Responses in Otitis-Prone Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014; 59(3): 376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37(5): 771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Human immunology 2007; 68(10): 813–22. [DOI] [PubMed] [Google Scholar]
- 51.Moingeon P Adjuvants for allergy vaccines. Human vaccines & immunotherapeutics 2012; 8(10): 1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. American journal of reproductive immunology 2013; 69(4): 346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. Journal of immunology 2006; 176(10): 5741–8. [DOI] [PubMed] [Google Scholar]
- 54.Wood N, Siegrist CA. Neonatal immunization: where do we stand? Current opinion in infectious diseases 2011; 24(3): 190–5. [DOI] [PubMed] [Google Scholar]
- 55.Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood 2007; 110(8): 2948–54. [DOI] [PubMed] [Google Scholar]
- 56.Lucena JM, Burillo Sanz S, Nunez-Roldan A, Sanchez B. Incidence of the C104R TACI Mutation in Patients With Primary Antibody Deficiency. Journal of investigational allergology & clinical immunology 2015; 25(5): 378–9. [PubMed] [Google Scholar]
- 57.Ramos-Sevillano E, Ercoli G, Brown JS. Mechanisms of Naturally Acquired Immunity to Streptococcus pneumoniae. Frontiers in immunology 2019; 10: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmid P, Selak S, Keller M, et al. Th17/Th1 biased immunity to the pneumococcal proteins PcsB, StkP and PsaA in adults of different age. Vaccine 2011; 29(23): 3982–9. [DOI] [PubMed] [Google Scholar]
- 59.Li W, Zhang X, Yang Y, et al. Recognition of conserved antigens by Th17 cells provides broad protection against pulmonary Haemophilus influenzae infection. Proceedings of the National Academy of Sciences of the United States of America 2018; 115(30): E7149–E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q, Bernatoniene J, Bagrade L, et al. Regulation of production of mucosal antibody to pneumococcal protein antigens by T-cell-derived gamma interferon and interleukin-10 in children. Infection and immunity 2006; 74(8): 4735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuipers K, Jong WSP, van der Gaast-de Jongh CE, et al. Th17-mediated cross protection against pneumococcal carriage by vaccination with a variable antigen. Infection and immunity 2017; 85(10): e00281–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinberger DM, Dagan R, Givon-Lavi N, Regev-Yochay G, Malley R, Lipsitch M. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. The Journal of infectious diseases 2008; 197(11): 1511–8. [DOI] [PubMed] [Google Scholar]
- 63.Kaur R, Casey JR, Pichichero ME. Emerging Streptococcus pneumoniae Strains Colonizing the Nasopharynx in Children After 13-valent Pneumococcal Conjugate Vaccination in Comparison to the 7-valent Era, 2006-2015. The Pediatric infectious disease journal 2016; 35(8): 901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the 'epimmunome'. Nat Immunol 2010; 11(8): 656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rovers MM. The burden of otitis media. Vaccine 2008; 26 Suppl 7: G2–4. [DOI] [PubMed] [Google Scholar]
- 66.Restori KH, Srinivasa BT, Ward BJ, Fixman ED. Neonatal Immunity, Respiratory Virus Infections, and the Development of Asthma. Frontiers in immunology 2018; 9: 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaur R, Casey J, Pichichero M. Differences in innate immune response gene regulation in the middle ear of children who are otitis prone and in those not otitis prone. American journal of rhinology & allergy 2016; 30(6): 218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ren D, Murphy TF, Lafontaine ER, Pichichero ME. Stringently Defined Otitis Prone Children Demonstrate Deficient Naturally Induced Mucosal Antibody Response to Moraxella catarrhalis Proteins. Frontiers in immunology 2017; 8: 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma SK, Pichichero ME. Cellular immune response in young children accounts for recurrent acute otitis media. Current allergy and asthma reports 2013; 13(5): 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]


