Abstract
Patients with Duchenne muscular dystrophy (DMD) develop skeletal muscle weakness and cardiomyopathy. Validated skeletal muscle outcome measures are limited to ambulatory patients, but most DMD patients in cardiac trials are non-ambulatory. New objective functional assessments are needed. This study’s objective was to assess the correlation and longitudinal change of two measures: quantitative muscle testing (QMT) and accelerometry. Patients with DMD were prospectively enrolled and underwent QMT and wore wrist and ankle accelerometers for seven days at baseline, 1-, and 2-years. QMT measures were indexed to age. Accelerometer recordings were total vector magnitudes (VM) and awake VM. Correlations were assessed using a Spearman correlation, and longitudinal change was evaluated using a paired t-test or a Wilcoxon signed rank test. Forty-eight participants were included. QMT and accelerometry measures had a moderate or strong correlation, particularly indexed arm QMT with total wrist VM (rho=0.85, p<0.001), total indexed QMT with total wrist VM (rho=0.8, p<0.001) and indexed leg QMT with total ankle VM (rho=0.69, p<0.001). QMT and accelerometry measures declined significantly over time. Accelerometry correlates with QMT and indexed QMT in boys with DMD. A combination of QMT and accelerometry may provide a complementary assessment of skeletal muscle function in non-ambulatory boys with DMD.
Keywords: Duchenne muscular dystrophy, Skeletal muscle strength, Quantitative muscle testing, Accelerometry
1. Introduction
Duchenne Muscular Dystrophy (DMD) is an X-linked recessive disease affecting 1 in 4700 male births (1). A mutation in the dystrophin gene leads to progressive skeletal and cardiac muscle dysfunction (2). Most patients lose the ability to ambulate between ages 10 and 13 years and subsequently develop progressive cardiac disease in late teenage years (3, 4). Life has been prolonged for patients with DMD with noninvasive ventilatory support (5). Current standard of care includes corticosteroids, which have been shown to improve strength and pulmonary function (6, 7). New gene-based therapies focused on extending ambulation, such as stop codon readthrough agents and exonskipping agents, are currently either available or undergoing clinical trials (6). One of the main barriers facing these therapeutic trials is limited noninvasive outcome measures beyond ambulation. There is also a need to better assess functional status over time since DMD progression for the individual patient is not necessarily linear with age. Such an assessment needs to be clinically meaningful as well as objective, sensitive, easily reproducible, and reflective of the functional status of the patient (8, 9).
Traditional functional measurements in patients with DMD include the supine to stand (STS) time, the 10-meter walk or run, the four-stair climb, and the six-minute walk test (6MWT) (10). These assessments are limited to ambulatory patients, effectively excluding almost all patients with DMD in their second and third decades of life. An alternative measurement for non-ambulatory patients is quantitative muscle testing (QMT), which has been demonstrated as a sensitive and reliable measure of skeletal muscle strength in patients with DMD (11). QMT can detect small variations in muscle strength as well as variations among individual muscle groups. QMT has been shown to correlate well with functional assessments like the 6MWT in longitudinal studies (11). However, both QMT and traditional functional measurements assume maximal patient effort and only measure a single movement in time. The question remains whether these tests are sensitive for measurement of day-to-day changes in functional capacity and endurance of patients with DMD.
Wearable accelerometers have commonly been used to assess daily physical activity in healthy children and in children with various diseases such as cerebral palsy (12). Accelerometry has been used to predict energy expended in activities performed at various levels of intensity and duration (13). The accelerometer records movement continuously, and the results could be considered a global assessment of functional physical activity in daily life. The raw accelerometry data can be transformed into commonly used physical activity categories such as sleep, sedentary behaviors, and light, moderate, and vigorous physical activity intensities (13).
Reports from studies measuring physical activity in youth with DMD are scarce, and none to our knowledge has evaluated the relationship between QMT and accelerometry. Thus, the main objective of this study was to examine the association of accelerometry as a measure of functional status with QMT as a measure of muscle strength in patients with DMD. We hypothesized that the total amount of movement measured using accelerometers will be significantly correlated with QMT and that both measures will decline over time.
2. Methods
2.1. Participants
The Vanderbilt Institutional Review Board approved this prospective study. All patients underwent appropriate consent or assent. Participants with DMD confirmed with clinical phenotype and diagnosis by either muscle biopsy or genetic testing were prospectively enrolled. Patients were recruited from the Neuromuscular Cardiology Clinic and the Muscular Dystrophy Association Clinic. The study consisted of the baseline study visit and two subsequent annual visits (visit 2 and visit 3). Demographics, past medical history, current and previous medications, and ambulatory status were obtained from the electronic medical record at each visit. Participants were excluded from this analysis if they had no analyzable accelerometry or QMT data at any visit.
2.2. Quantitative Muscle Testing
The QMT assessment was performed at each visit using a handheld myometer as previously described (14). Arm QMT score (pounds) was the sum of flexion and extension values for both elbows. Leg QMT was the sum of flexion and extension values for both knees. Total QMT score was the sum of values for elbows and knees. Skeletal muscle strength in healthy males increases in a relatively linear pattern with age until 20–25 years of age, but it often plateaus or decreases in patients with DMD (15, 16). While normative data has been reported, we were unable to adequately calculate QMT z-scores for all ages, so we adapted our previously reported correction. Participants under 20 years old were indexed to age, and for participants >20 years old, we divided QMT scores by 20 (17). Indexed QMT score accounts for progressive loss of strength with age in patients with DMD in contrast to progressive gain observed in children without DMD (13).
2.3. Total Amount of Movement
The total amount of movement (physical activity) was measured at each visit using triaxial accelerometers (GT3X+, ActiGraph, Pensacola, FL). Participants were asked to maintain their normal free-living activities and were instructed to wear the accelerometers for 7 consecutive days and 24 hours per day, including when sleeping, napping, and during water-related activities such as bathing and showering. Accelerometry data recording started immediately after the study visit during which the QMT was measured. The monitors were worn on the ankle of a dominant leg and on the wrist of a dominant hand to detect both ambulation and movements of a leg and an arm, respectively.
Raw accelerometry data collected at a sampling frequency of 30 Hz (30 observations per second for each axis) were integrated into 15-s epochs and converted to vector magnitude (VM) counts calculated as a square root of the sum of squared recordings from the accelerometer axes using Actilife software (ActiGraph, Pensacola, FL). Wear and non-wear were assessed by using Choi’s algorithm (18). A recording was considered valid if it included ≥3 valid days with ≥2 weekdays and ≥1 weekend day, each with ≥6 hours of monitor wearing from 7:00am to 10:00pm. Awake status was assessed using a validated algorithm for bedrest/wake identification (11).
2.4. Statistical Analysis
Descriptive statistics are presented as means with standard deviations for continuous variables along with frequencies and proportions (%) for categorical variables. QMT results were paired with accelerometry data from that same clinic visit. Spearman rank correlation tests were used to determine the relationships between QMT and accelerometry, indexed QMT and accelerometry, QMT with age, and accelerometry with age. A subset analysis in only the non-ambulatory participants was also performed. Longitudinal change was assessed using a paired t-test for QMT and indexed QMT and Wilcoxon signed rank for accelerometry given non-normal distribution. Statistical analysis was performed using STATA, version 15 (StatCorp LLC, College Station, TX) software. All tests were 2-sided and a p-value < 0.05 was considered significant. The following cut-offs were used to determine strength of relationship for the coefficient correlations: r ≤ 0.35, weak, r = 0.36 to 0.69, moderate; r = 0.7 to 1.0, strong. Study data was managed using REDCap (Research Electronic Data Capture) tools (19).
3. Results
Forty-nine participants were enrolled, but one participant with no analyzable accelerometry or QMT data was removed from the analysis. Of these 48 participants, 46 underwent baseline QMT and 43 had valid baseline accelerometry data. Demographics are shown in Table 1.
Table 1.
Demographics and baseline clinical characteristics
| Mean ± SD or n (percent) | |
|---|---|
| Age (years) | 13.0 ± 4.3 |
| Height (cm) | 145 ± 18 |
| Weight (kg) | 50 ± 20 |
| Body surface area (kg per m2) | 1.4 ± 0.3 |
| Race | |
| Caucasian | 43 (90%) |
| Black | 3 (6%) |
| Corticosteroids | 33 (69%) |
| Median duration corticosteroids (years) | 5.5 IQR (3,7) |
| Ambulatory | 16 (34%) |
SD = standard deviation
IQR = interquartile range
QMT and accelerometry data are presented in Table 2. Wrist VM counts were higher than ankle.
Table 2.
Baseline quantitative muscle testing (QMT) and accelerometry
| Mean ± SD | N | |
|---|---|---|
| Median (IQR) | ||
| Arm QMT (lbs) | 23 ± 12 | 46 |
| 21 (16, 30) | ||
| Leg QMT (lbs) | 43 ± 21 | 46 |
| 40 (29, 59.5) | ||
| Total QMT (lbs) | 66 ± 32 | 46 |
| 60 (42.5, 92) | ||
| Indexed arm QMT (lbs/year) | 2.0 ± 1.2 | 46 |
| 1.8 (0.94, 3.1) | ||
| Indexed leg QMT (lbs/year) | 3.7 ± 2.2 | 46 |
| 3.2 (1.9, 4.9) | ||
| Indexed total QMT (lbs/year) | 5.6 ± 3.4 | 46 |
| 4.9 (2.8, 8.0) | ||
| Total wrist VM (counts/min) | 1320 ± 770 | 43 |
| 1240 (630, 1930) | ||
| Awake wrist VM (counts/min) | 6180 ± 26900 | 40 |
| 1960 (1140, 2590) | ||
| Total ankle VM (counts/min) | 317 ± 446 | 38 |
| 89 (30, 559) | ||
| Awake ankle VM (counts/min) | 448 ± 597 | 34 |
| 228 (52, 719) |
SD = standard deviation
IQR = interquartile range
lbs = Pounds
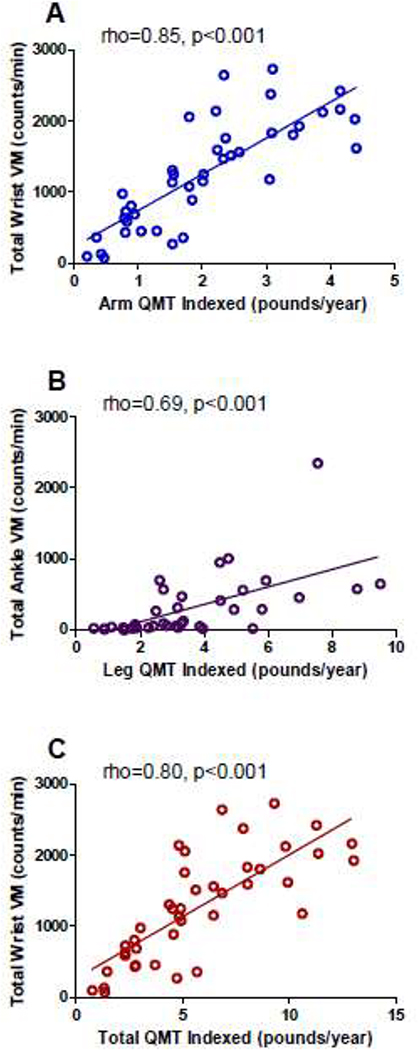
All accelerometry VM measures had moderate to strong correlations with QMT (Table 3). Total arm QMT and total QMT had moderate correlations with wrist VM values. Similarly, total leg QMT and total QMT had moderate correlations with ankle VM values. Indexed QMT had moderate to strong correlations with accelerometry. For the arm measurements, the strongest correlation was between total wrist VM and indexed arm QMT (rho = 0.85, P < 0.001) and indexed total QMT (rho = 0.80, p < 0.001). For the leg measurements, total ankle VM had the strongest correlations with indexed leg QMT (rho = 0.69, p < 0.001) and indexed total QMT (rho = 0.71, p < 0.001). Representative scatterplots are in Figure A.1. A subset analysis performed only in non-ambulatory participants also demonstrated strong correlations between indexed QMT and total wrist measures with moderate correlation between indexed QMT and total ankle measures (Table A.1).
Table 3.
Correlation between quantitative muscle testing (QMT) and accelerometry vector magnitudes (VM) counts
| Total Wrist VM | Awake wrist VM | Total ankle VM | Awake ankle VM | |
|---|---|---|---|---|
| Total arm QMT | Rho=0.69 P<0.001 (n=41) |
Rho=0.50 p=0.002 (n=38) |
||
| Indexed arm QMT | Rho=0.85 p<0.001 (n=41) |
Rho=0.69 p<0.001 (n=38) |
||
| Total leg QMT | Rho=0.53 p<0.001 (n=37) |
Rho=0.44 P=0.009 (n=33) |
||
| Indexed leg QMT | Rho=0.69 p<0.001 (n=37) |
Rho=0.66 p<0.001 (n=33) |
||
| Total QMT | Rho=0.62 p<0.001 (n=41) |
Rho=0.41 p=0.001 (n=38) |
Rho=0.56 p<0.001 (n=37) |
Rho=0.46 p=0.007 (n=33) |
| Indexed total QMT | Rho=0.80 p<0.001 (n=41) |
Rho=0.64 p<0.001 (n=38) |
Rho=0.71 p<0.001 (n=37) |
Rho=0.68 p<0.001 (n=33) |
As expected, age and QMT had a moderate inverse correlation as the strength of children with DMD begins to decline as they age in contrast to children without DMD. Accelerometry measures were also moderately and negatively correlated with age (Table 4).
Table 4.
Correlation of age with quantitative muscle testing (QMT) and accelerometry
| Rho | |
|---|---|
| p-value (N) | |
| Total arm QMT | −0.44 |
| 0.002 (46) | |
| Total leg QMT | −0.36 |
| 0.012 (46) | |
| Total QMT | −0.39 |
| 0.007 (46) | |
| Total wrist VM | −0.71 |
| <0.001 (43) | |
| Awake wrist VM | −0.56 |
| <0.001 (40) | |
| Total ankle | −0.62 |
| <0.001 (38) | |
| Awake ankle VM | −0.56 |
| <0.001 (34) |
VM = vector magnitude
Longitudinal data demonstrates a progressive weakening of participants with DMD at 1- and 2-years (Table 5). DMD participants also demonstrated a progressive decrease in physical activity at 1- and 2-years for wrist accelerometry and at 2-years for ankle accelerometry.
Table 5.
Quantitative muscle testing (QMT) and accelerometry change over time
| Measure | N | Baseline mean ± SD and Visit 2 mean ± SD | p-value | N | Baseline mean ± SD and Visit 3 mean ± SD | p-value |
|---|---|---|---|---|---|---|
| Arm QMT (lbs) | 40 | 24 ± 12 | 0.002 | 28 | 26 ± 12 | <0.001 |
| 21 ± 11 | 19 ± 9 | |||||
| Indexed arm QMT (lbs/year) | 40 | 2.0 ± 1.2 | <0.001 | 28 | 2.2 ± 1.2 | <0.001 |
| 1.6 ± 1.0 | 1.4 ± 0.8 | |||||
| Leg QMT (lbs) | 40 | 44 ± 26 | <0.001 | 28 | 48 ± 21 | <0.001 |
| 37 ± 17 | 36 ± 16 | |||||
| Indexed leg QMT (lbs/year) | 40 | 3.7 ± 2.2 | <0.001 | 28 | 4.1 ± 2.3 | <0.001 |
| 2.9 ± 1.6 | 2.6 ± 1.3 | |||||
| Total QMT (lbs) | 40 | 67 ± 31 | <0.001 | 28 | 74 ± 32 | <0.001 |
| 58 ± 27 | 55 ± 24 | |||||
| Indexed total QMT (lbs/year) | 40 | 5.7 ± 3.3 | <0.001 | 28 | 6.3 ± 3.4 | <0.001 |
| 4.5 ± 2.5 | 4.0 ± 2.1 | |||||
| Total wrist VM (counts/min) | 36 | 1240 (644, 2000) | 0.040 | 20 | 1210 (648, 1760) | 0.004 |
| 1210 (598, 1680) | 967 (436, 1350) | |||||
| Awake wrist VM (counts/min) | 33 | 2010 (1140, 2590) | 0.020 | 22 | 1860 (1090, 2410) | 0.009 |
| 1710 (1050, 2290) | 1360 (1060, 1730) | |||||
| Total ankle VM (counts/min) | 27 | 77 (30, 471) | 0.080 | 16 | 202 (51, 563) | 0.049 |
| 94 (46, 250) | 73 (38, 160) | |||||
| Awake ankle VM (counts/min) | 24 | 102 (52, 692) | 0.068 | 14 | 253 (61, 716) | 0.016 |
| 130(52, 356) | 73 (38, 160) |
SD = standard deviation
IQR = interquartile range
lbs = pounds
VM = vector magnitude
4. Discussion
The major finding of this study is that in participants with DMD, total daily movement measured using accelerometers is significantly correlated with skeletal muscle strength, as measured by QMT. We also demonstrated a progressive decrease in daily movement and QMT over time. This finding is important because current validated measures of skeletal muscle assessment are limited in non-ambulatory patients with DMD and these two measures can be performed in both ambulatory and non-ambulatory patients. A better assessment of physical activity and QMT is critical for determining the impact of therapies on physical activity in patients with DMD. This study is the first of which we are aware to evaluate accelerometry comprehensively in a large population of patients with DMD, to correlate these values with QMT, and to assess longitudinal change of both measures.
We demonstrated a moderate to strong correlation between accelerometry and both QMT and indexed QMT in patients with DMD. These correlations were similar in the subset of non-ambulatory patients, though the Rho values were less given the smaller numbers. QMT is known as a reliable and reproducible measure of functional status in DMD patients. However, QMT is a single measurement of skeletal muscle strength that is taken in the clinic, is dependent on a patient’s effort, and does not assess a patient’s endurance for physical activity. Accelerometry is a continuous recording of physical activity during a pre-specified period of time and can provide a metric of total amount of movement in an effort-independent manner. We postulate that accelerometry and QMT provide a complementary assessment of skeletal muscle function in both ambulatory and non-ambulatory patients with DMD.
Similar to this study, Kimura et al found a correlation between wrist accelerometry and measures of skeletal muscle strength in boys with DMD (20). They reported a moderate correlation with the six-minute walk test in 7 patients with DMD and with a less comprehensive measure of muscle strength (knee extension only) in 22 patients with DMD (20). Prior work has also demonstrated an inverse correlation between age and both accelerometry and QMT (11, 20). Our longitudinal analysis showing that both accelerometry and QMT results significantly decreased at each visit is consistent with a well-known progressive decline in both strength and functional status in patients with DMD (9, 19, 20). This decline was present despite a majority of our patient population receiving corticosteroids, which have been shown to increase physical activity (7). Further studies are needed to assess the role of corticosteroids in functional status over time.
Other studies have demonstrated a correlation between accelerometry and commonly used metrics of skeletal muscle function in ambulatory DMD boys, such as the 6MWT and the North Star Ambulatory Assessment (21–23). Our study assessed skeletal muscle strength in both ambulatory and non-ambulatory patients with DMD, with a focus on non-ambulatory patients. Non-ambulatory patients with DMD comprise an ever-increasing portion of DMD patients, especially in studies aimed at improving DMD cardiomyopathy. It is critical that skeletal muscle outcome measures are included in these studies. Accelerometers have the advantages of portability, tolerability, and the ability to measure daily physical activity. This study demonstrated relatively good compliance with the accelerometer, particularly the wrist accelerometer. The device itself is reasonably priced and can be re-used.
Of note, all timepoints demonstrated significantly more minutes of wear time on the wrist than on the ankle (baseline 1200min ± 600 vs 400min ± 500, p<0.001; 1000min ± 600 vs 99min ± 90, p<0.001; and 840min ± 400 vs 57min ± 37, p<0.001). It is unclear if this difference represents better compliance with the wrist monitor or if it is due to a near lack of motion in older boys, which may make assessment of true wear time difficult. In the latter case, it would skew our data by underestimating the decline in physical activity as patients become more sedentary.
Assessing patterns of physical activity and energy expenditure of physical activity were beyond the scope of this study. We found that the amount of movement per minute was generally higher at baseline than at visits 2 and 3. In a study of 5 ambulatory patients with DMD, Jeannet et al. characterized their daily physical activity as short bursts of activity rather than sustained efforts (24). Pande et al showed that traditional models of energy expenditure cannot be used in patients with DMD or other disabilities and that specific models in DMD are much more accurate (25). In the absence of a practical gold standard criterion method for assessing physical activity that can be used with patients with DMD in daily life, we consider accelerometry as a potentially useful approach for assessing how physical activity patterns influence disease progression.
4.1. Limitations
Although this study is limited by a relatively small sample size, it is the largest study of which we are aware to evaluate accelerometry in DMD patients. It is a single center study, which may make results less generalizable, though our Neuromuscular Cardiology and Muscular Dystrophy Clinics draw patients from a large catchment area. We included both ambulatory and non-ambulatory boys in order to provide a broader patient population for baseline accelerometry measures. Since most patients were non-ambulatory, we did not evaluate the association of standard metrics of ambulatory function with accelerometry. However, accelerometry has been shown to correlate with both the six-minute walk and North Star Ambulatory Assessment in prior studies. Future studies should include a larger patient population and multiple centers to further delineate the role for accelerometry in DMD patients.
5. Conclusions
Total amount of movement measured with wrist- and ankle-worn accelerometers is strongly correlated with QMT-measured muscle strength in patients with DMD. A combination of QMT and accelerometry may provide a complementary assessment of skeletal muscle function in both ambulatory and non-ambulatory patients with DMD.
Highlights.
In boys with Duchenne muscular dystrophy, accelerometry can measure daily activity.
Accelerometry measures correlate with quantitative muscle testing in boys with DMD.
Accelerometry and quantitative muscle testing showed progressive decline.
The two measures may be complementary to assess skeletal muscle function in DMD.
Acknowledgments
Funding:
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Award Number K23HL123938 (Bethesda, MD) (Soslow). Additional funds were provided by UL1 award TR002243 from the National Center for Advancing Translational Sciences (NCATS) and grants DK058404 and DK20593 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The project described was supported by CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
This project was supported by the Fighting Duchenne Foundation and the Fight DMD/Jonah & Emory Discovery Grant (Nashville, TN) (Markham/Soslow).
The sponsors and funders had no role in the design and conduct of the study or in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Appendix A
Table A.1.
Correlation between quantitative muscle testing (QMT) and accelerometry vector magnitude (VM) counts in non-ambulatory subjects.
| Total wrist VM | Awake wrist VM | Total ankle VM | Awake ankle VM | |
|---|---|---|---|---|
| Total arm QMT | Rho=0.57 p=0.002 (n=26) |
Rho=0.24 p=0.242 (n=23) |
||
| Indexed arm QMT | Rho=0.76 p<0.001 (n=26) |
Rho=0.49 p=0.017 (n=23) |
||
| Total leg QMT | Rho=0.53 p=0.049 (n=25) |
Rho=0.24 p=0.295 (n=21) |
||
| Indexed leg QMT | Rho=0.56 p=0.004 (n=25) |
Rho=0.55 p=0.01 (n=21) |
||
| Total QMT | Rho=0.55 p=0.003 (n=26) |
Rho=0.19 p=0.194 (n=23) |
Rho=0.45 p=0.025 (n=25) |
Rho=0.29 p=0.195 (n=21) |
| Indexed total QMT | Rho=0.76 p<0.001 (n=26) |
Rho=0.48 p=0.02 (n=23) |
Rho=0.56 p=0.003 (n=25) |
Rho=0.54 p=0.01 (n=21) |
Figure A.1.
Correlation of accelerometry and indexed quantitative muscle testing (QMT)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dooley J, Gordon KE, Dodds L, MacSween J. Duchenne muscular dystrophy: a 30-year population-based incidence study. Clin Pediatr. 2010;49(2):177–9. [DOI] [PubMed] [Google Scholar]
- [2].Hoffman EP, Jr. RHB, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28. [DOI] [PubMed] [Google Scholar]
- [3].Boland BJ, Silbert PL, Groover RV, Wollan PC, Silverstein MD. Skeletal, cardiac, and smooth muscle failure in Duchenne muscular dystrophy. Pediatr Neurol. 1996;14(1):7–12. [DOI] [PubMed] [Google Scholar]
- [4].Nigro G, Comi LI, Politano L, Bain RJI. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26(3):271–7. [DOI] [PubMed] [Google Scholar]
- [5].Eagle M, Baudouin SV, Chandler C, Giddings DR, Bullock R, Bushby K. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12(10):926–9. [DOI] [PubMed] [Google Scholar]
- [6].Reinig AM, Mirzaei S, Berlau DJ. Advances in the treatment of Duchenne Muscular Dystrophy: new and emerging pharmacotherapies. Pharmacotherapy. 2017;37(4):492–9. [DOI] [PubMed] [Google Scholar]
- [7].Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2016(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shieh P Duchenne muscular dystrophy: clinical trials and emerging tribulations. Curr Opin Neurol. 2015;28(5):542–6. [DOI] [PubMed] [Google Scholar]
- [9].Domingos J, Muntoni F. Outcome measures in Duchenne muscular dystrophy: sensitivity to change, clinical meaningfulness, and implications for clinical trials. Dev Med Child Neurol. 2018;60(2):117. [DOI] [PubMed] [Google Scholar]
- [10].Barnard AM, Willcocks RJ, Finanger EL, Daniels MJ, Triplett WT, Rooney WD, et al. Skeletal muscle magnetic resonance biomarkers correlate with function and sentinel events in Duchenne muscular dystrophy. PLoS ONE. 2018;13(3):e0194283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lerario A, Bonfiglo S, Sormani M, Tettamanti A, Marktel S, Napolitano S, et al. Quantitative muscle strength assessment in Duchenne muscular dystrophy: longitudinal study and correlation with functional measures. BMC Neurol. 2012;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gorter JW, Noorduyn SG, Obeid J, Timmons BW. Accelerometry: a feasible method to quantify physical activity in ambulatory and nonambulatory adolescents with cerebral palsy. Int J Pediatr. 2012;2012:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tracy DJ, Xu Z, Choi L, Acra S, Chen KY, Buchowski MS. Separating bedtime rest from activity using waist or wrist-worn accelerometers in youth. PLoS ONE. 2014;9(4):e92512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Posner AD, Soslow JH, Burnette WB, Bian A, Shintani A, Sawyer DB, et al. The correlation of skeletal and cardiac muscle dysfunction in Duchenne muscular dystrophy. J Neuromuscl Dis. 2016;3(1):91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McKay M, Baldwin J, Ferreira P, Simic M, Vanicek N, Burns J, et al. Normative reference values for strength and flexibility of 1,000 children and adults. Neurology. 2017;88(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Escobar R, Munoz K, Dominguez A, Banados P, Bravo M. Maximal isometric muscle strength values obtained by hand-held dynamometry in children between 6 and 15 years of age. Muscle Nerve. 2017;55(1):16–22. [DOI] [PubMed] [Google Scholar]
- [17].Soslow JH, Xu M, Slaughter JC, Crum K, Chew JD, Burnette WB, et al. The role of matrix metalloproteinases and tissue inhibitors of metalloproteinases in Duchenne muscular dystrophy cardiomyopathy. J Card Fail. 2019;25(4):259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kimura S, Ozasa S, Nomura K, Yoshioka K, Endo F. Estimation of muscle strength from actigraph data in Duchenne muscular dystrophy. Pediatr Int. 2014;56:748–2. [DOI] [PubMed] [Google Scholar]
- [21].Davidson ZE, Ryan MM, Kornberg A, Walker KZ, Truby H. Strong correlation between the 6-minute walk test and accelerometry functional outcomes in boys with Duchenne muscular dystrophy. J Child Neurol. 2015;30(3):357–63. [DOI] [PubMed] [Google Scholar]
- [22].McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve. 2010;41(4):500–10. [DOI] [PubMed] [Google Scholar]
- [23].Mazzone E, Martinelli D, Berardinelli A, Messina S, D’Amico A, Vasco G, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2010;20(11):712–6. [DOI] [PubMed] [Google Scholar]
- [24].Jeannet P-Y, Arminian K, Bloetzer C, Najafi B, Paraschiv-Ionescu A. Continuous monitoring and quantification of multiple parameters of daily physical activity in ambulatory Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2011;15:40–7. [DOI] [PubMed] [Google Scholar]
- [25].Pande A, Mohapatra P, Micorici A, Han JJ. Machine learning to improve energy expenditure estimation in children with disabilities: a pilot study in Duchenne muscular dystrophy. JMIR Rehabil Assist Technol 2016;3(2):e7. [DOI] [PMC free article] [PubMed] [Google Scholar]