Abstract
Background
The impact of coronavirus disease 2019 on pregnant women is incompletely understood, but early data from case series suggest a variable course of illness from asymptomatic or mild disease to maternal death. It is unclear whether pregnant women manifest enhanced disease similar to influenza viral infection or whether specific risk factors might predispose to severe disease.
Objective
To describe maternal disease and obstetrical outcomes associated with coronavirus disease 2019 in pregnancy to rapidly inform clinical care.
Study Design
This is a retrospective study of pregnant patients with a laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection from 6 hospital systems in Washington State between Jan. 21, 2020, and April 17, 2020. Demographics, medical and obstetrical history, and coronavirus disease 2019 encounter data were abstracted from medical records.
Results
A total of 46 pregnant patients with a severe acute respiratory syndrome coronavirus 2 infection were identified from hospital systems capturing 40% of births in Washington State. Nearly all pregnant individuals with a severe acute respiratory syndrome coronavirus 2 infection were symptomatic (93.5%, n=43) and the majority were in their second or third trimester (43.5% [n=20] and 50.0% [n=23], respectively). Symptoms resolved in a median of 24 days (interquartile range, 13–37). Notably, 7 women were hospitalized (16%) including 1 admitted to the intensive care unit. A total of 6 cases (15%) were categorized as severe coronavirus disease 2019 with nearly all patients being either overweight or obese before pregnancy or with asthma or other comorbidities. Of the 8 deliveries that occurred during the study period, there was 1 preterm birth at 33 weeks’ gestation to improve pulmonary status in a woman with class III obesity, and 1 stillbirth of unknown etiology.
Conclusion
Severe coronavirus disease 2019 developed in approximately 15% of pregnant patients and occurred primarily in overweight or obese women with underlying conditions. Obesity and coronavirus disease 2019 may synergistically increase risk for a medically indicated preterm birth to improve maternal pulmonary status in late pregnancy. These findings support categorizing pregnant patients as a higher-risk group, particularly those with chronic comorbidities.
Key words: asthma, coronavirus disease 2019, fetal death, infection, maternal morbidity, obesity, overweight, preterm birth, pregnancy, respiratory insufficiency, severe acute respiratory syndrome coronavirus 2, stillbirth
Click Video under article title in Contents at ajog.org
Coronavirus disease 2019 (COVID-19) has led to the largest and deadliest pandemic since the 1918 influenza pandemic. The first reported case of COVID-19 in the United States was in Washington State on January 21, 2020; the United States now has the highest rates of COVID-19 prevalence and mortality worldwide.1 , 2 COVID-19 is caused by severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2), which results in a spectrum of disease ranging from asymptomatic and mild cases to respiratory failure, shock, multiorgan dysfunction, and death.3
AJOG at a Glance.
Why was this study conducted?
This study was performed to determine the impact of coronavirus disease 2019 (COVID-19) on the health of pregnant women in Washington State.
Key findings
In this case series of 46 pregnant women with a laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, severe COVID-19 developed in approximately 15% of pregnant patients and occurred primarily in overweight or obese women with underlying conditions.
What does this add to what is known?
These findings support categorizing pregnant patients as a higher-risk group, particularly those with obesity and chronic comorbidities.
The clinical course of COVID-19 in pregnant women is incompletely understood and there is concern for enhanced disease in some pregnant women and an increased risk for spontaneous abortion, preterm birth, or morbidity or mortality in the fetus and the neonate.4, 5, 6, 7, 8 Several case series have reported a variable course of illness in the antepartum, intrapartum, and postpartum periods.9, 10, 11, 12, 13, 14 Limited reports suggesting vertical transmission underscore the potential vulnerability of the fetus and neonate.15, 16, 17, 18 Furthermore, the relationship between timing of infection in pregnancy and the long-term impacts on neurodevelopmental and neuropsychiatric outcomes in the children are unknown.19 , 20 Many questions remain unanswered, such as whether pregnancy is a high-risk state for enhanced disease in some circumstances and the impact of infection on the developing fetus and neonate.
Washington State has been on the forefront of the national COVID-19 response. It was among the first states to confirm community transmission21 and to declare a state of emergency.22 In response to the pandemic, the Washington State COVID-19 in Pregnancy Collaborative was established to investigate cases among pregnant patients at major tertiary referral centers and community hospitals disproportionately affected by the pandemic. This study aimed to describe maternal and obstetrical outcomes associated with COVID-19 disease in pregnancy to rapidly inform clinical care.
Materials and Methods
Study design and study population
We identified pregnant women (≥18 years old) with laboratory-confirmed SARS-CoV-2 infections from 6 hospital systems in Washington State between Jan. 21, 2020, and April 17, 2020. All pregnant patients with a positive SARS-CoV-2 test result during any trimester of pregnancy, regardless of symptoms, were included. All testing was performed using a polymerase chain reaction (PCR) test, which varied in assay design and source by institution. Participating institutions were part of the Washington State COVID-19 in Pregnancy Collaborative, representing 16 hospitals from the Seattle-Tacoma-Bellevue metropolitan area, Bellingham, Spokane, and their surrounding areas. Sites included the University of Washington Hospital system (Montlake, Northwest, and Harborview campuses), Swedish Medical Center (First Hill, Ballard, Issaquah and Edmonds campuses), University of Washington Valley Medical Center, MultiCare Health System (Auburn Medical Center, Covington Medical Center, Tacoma General Hospital, Good Samaritan Hospital, Valley Hospital, and Deaconess Hospital), EvergreenHealth Medical Center, and PeaceHealth St. Joseph’s Medical Center. These sites have 34,000 deliveries annually, which represent 40% of the ∼86,000 deliveries each year in Washington State.23
Patient identification, data collection, and statistical analysis
Eligible subjects were identified at collaborating institutions by site-specific team members through electronic medical records searches using the 10th revision of the International Classification of Diseases diagnostic codes and site-specific algorithms. Deidentified data were abstracted from the electronic medical records by a primary abstractor and entered into a REDCap database (Research Electronic Data Capture software, Vanderbilt University, Nashville, TN) managed by the coordinating team at the University of Washington. All data entries were confirmed by a secondary abstractor. Abstractors included University of Washington School of Medicine students, University of Washington Department of Obstetrics and Gynecology resident physicians, attending obstetricians, maternal-fetal medicine specialists, and an obstetrical nurse. Data collected included demographics, medical and obstetrical history, SARS-CoV-2 testing, and clinical encounters such as symptoms, laboratory results, pulmonary imaging, and hospitalization, when applicable. For patients who delivered by the time of chart abstraction, we collected data on delivery characteristics and complications. Data were summarized using proportions and medians (interquartile range [IQR]). A Kaplan–Meier curve was generated to estimate days from COVID-19-associated respiratory symptom onset to resolution. Patients with ongoing symptoms were censored at the last report of symptoms in a clinical encounter.
Coronavirus disease 2019 categories
We used criteria for COVID-19 disease severity previously defined in nonpregnant adults24 and subsequently applied to pregnant women.25 Categories were defined as follows: (1) mild (nonpneumonia or mild pneumonia); (2) severe (dyspnea, respiratory rate of ≥30 breaths/min, percutaneous oxygen saturation of ≤93% on room air at rest, arterial oxygen tension over inspiratory oxygen fraction of <300 mm Hg, or lung infiltrates >50% within 24 to 48 hours; and (3) critical (severe respiratory distress, respiratory failure requiring mechanical ventilation, shock, or multiple organ dysfunction or failure). Normal laboratory reference ranges in each trimester of pregnancy are listed in Supplemental Table 1.26
Placental and fetal histopathology
In one case, a fetal autopsy was performed with gross and histopathologic evaluation of fetal tissues and the placenta. PCR testing of multiple fetal and placental tissues was performed for SARS-CoV-2 RNA and cytomegalovirus DNA using established clinical assays at the University of Washington.
Ethics statement
This multisite medical records review was approved by the Institutional Review Boards (IRB) at the University of Washington (STUDY# 00009701, approved March 06, 2020) and Swedish Medical Center (STUDY #2020000172, approved March 19, 2020). All remaining sites entered into reliance agreements with the University of Washington IRB for study approval. Patient consent and Health Insurance Portability and Accountability Act authorization were waived by the IRBs for this study using deidentified data. Consent to publish information associated with the fetal autopsy was obtained through a study approved by the Seattle Children’s Hospital IRB.
Results
Patient demographics, comorbidities, and pregnancy history
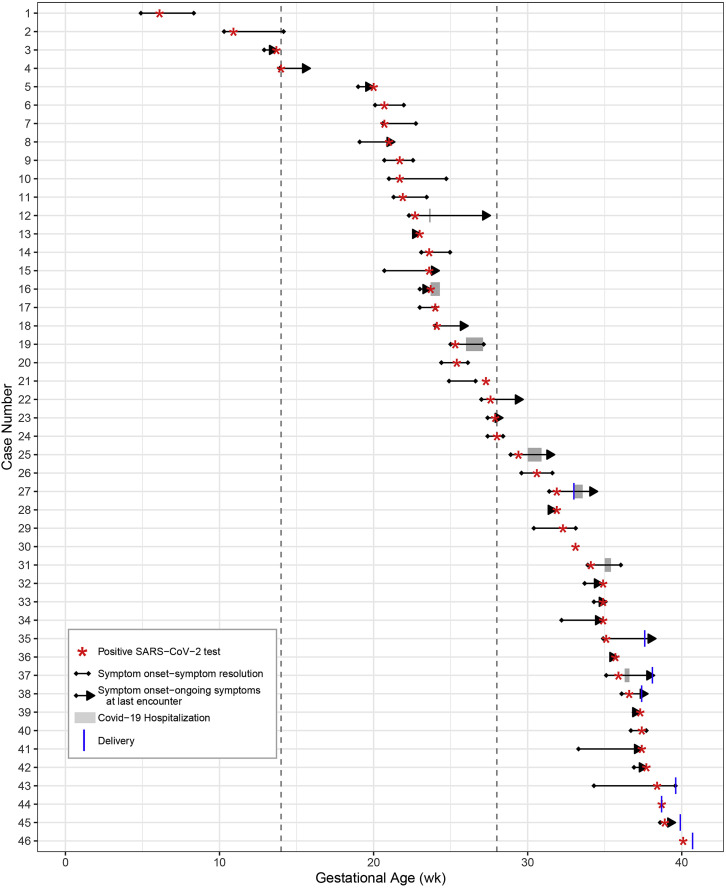
A total of 46 pregnant patients with SARS-CoV-2 infections were identified during the study period with a median age of 29 years (IQR, 26–34) and 26.1% (n=12) were nulliparous (Table 1). One woman was pregnant with twins and the remainder had singleton pregnancies. The majority were white (60.9%, n=28) and had private insurance (58.7%, n=27). Positive SARS-CoV-2 test results were identified predominantly in second- (43.5%, n=20) and third-trimester (50.0%, n=23) pregnancies; only 3 cases were detected in first-trimester pregnancies (6.5%; Figure ). Approximately two-thirds of patients were either overweight (28.6%, n=12) or obese (35.7%, n=15) by their prepregnancy body mass index (BMI); 2 women met the criteria for class III obesity (BMI≥40 kg/m2). Although the majority of patients were healthy, 26.1% (n=12) had underlying health conditions, such as type 2 diabetes (n=3), asthma (n=4), hypothyroidism (n=2), hypertension (n=2), and several less common conditions (eg, Crohn’s disease treated with immunosuppressive medication, history of seizure disorder). Although no patients reported smoking cigarettes during pregnancy, 1 reported marijuana use and 1 endorsed alcohol use.
Table 1.
Demographics, comorbidities, and pregnancy history of 46 pregnant patients with severe acute respiratory syndrome coronavirus 2 infection
| Characteristics | Patients (n=46)a |
|---|---|
| Demographics | |
| Age | 29 (26–34) |
| Race | |
| Asian | 2 (4.3) |
| Native Hawaiian or other Pacific Islander | 1 (2.2) |
| Black or African American | 3 (6.5) |
| White | 28 (60.9) |
| Multiracial | 1 (2.2) |
| Other | 2 (4.3) |
| Unknown or not reported | 9 (19.6) |
| Ethnicity | |
| Hispanic or Latino | 11 (23.9) |
| Not Hispanic or Latino | 33 (71.7) |
| Unknown or not reported | 2 (4.3) |
| Type of insurance at diagnosis | |
| Public | 18 (39.1) |
| Private | 27 (58.7) |
| Unknown | 1 (2.2) |
| Prepregnancy existing comorbidities | |
| Type 2 diabetes mellitus | 3 (6.5) |
| Asthma | 4 (8.7) |
| Hypothyroidism | 3 (6.5) |
| Hypertension | 2 (4.3) |
| Other comorbiditiesb | 5 (10.9) |
| Prepregnancy BMIc, kg/m2 | |
| Underweight (<18.5) | 1 (2.4) |
| Normal (18.5–24.9) | 14 (33.3) |
| Overweight (25.0–29.9) | 12 (28.6) |
| Obese (≥30.0) | 15 (35.7) |
| Pregnancy history | |
| Gravidity | 2.0 (2.0–5.0) |
| Parity | 1.0 (0.0–2.0) |
| History of preterm birth | 3 (6.5%) |
For 1 patient, weight at 14 weeks’ gestation was used to calculate prepregnancy BMI.
BMI, body mass index, IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
Characteristics summarized as n (%) or median (IQR).
Other comorbidities included Crohn’s disease with immunosuppressive therapy (n=1), heart valve repair (n=1), papillary thyroid carcinoma with thyroidectomy (n=1), and seizure disorder (n=2).
Only available for 42 patients. Prepregnancy weight or weight before 12 weeks’ gestation was used if prepregnancy weight was not available.
Figure.
Timeline of symptom onset and resolution, laboratory testing, COVID-19 hospital admission, and delivery for 46 pregnant patients
Time is indicated on the x-axis and is measured by gestational age in weeks. Each line of the y-axis reflects an individual patient. Gestational age of the first positive SARS-CoV-2 test (red star), length of symptoms (black lines), gestational age at symptom onset (black dot), gestational age or days postpartum at symptom resolution (black dot if resolved and a black arrow if ongoing at last encounter [censoring]), length of COVID-19 hospitalizations (gray bar), and gestational age at delivery (blue vertical line) are indicated for each patient, as applicable. Three patients were asymptomatic. Of the 7 patients hospitalized for COVID-19–associated respiratory concerns, 6 were severe (Table 3).
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
Severe acute respiratory syndrome coronavirus 2 testing and symptoms
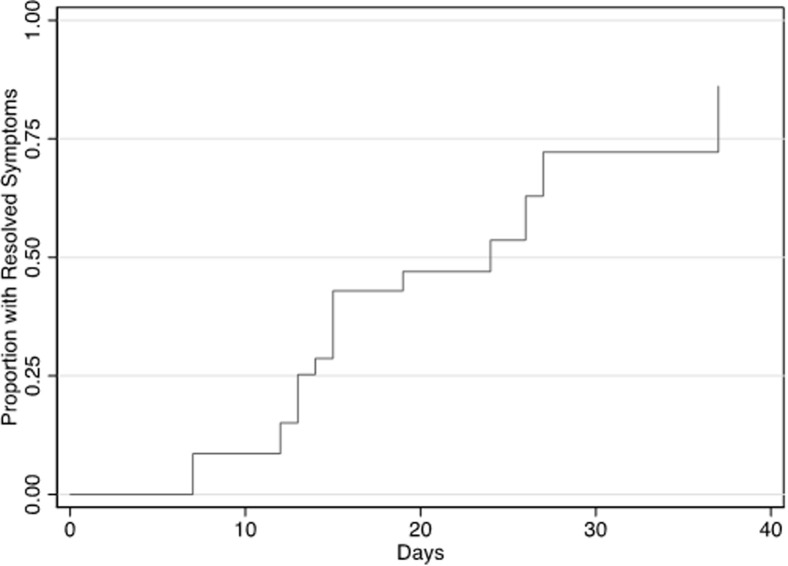
SARS-CoV-2 testing became increasingly available over the study period starting with facility-based and outpatient “drive through” testing stations for symptomatic individuals and expanding to universal screening on labor and delivery at several medical centers. Approximately all pregnant patients (93.5%, n=43) were tested owing to COVID-19-related symptoms (Table 2 ). The remaining 3 patients were asymptomatic but tested because of known exposure. Women reported a median of 2 symptoms (IQR, 1–5), which most commonly included cough (69.8%, n=30), fever or chills (51.2%, n=22), nasal congestion (48.8%, 21), and shortness of breath (44.2%, n=19) (Table 2). Loss of taste or smell was reported in 30.2% (n=13) of cases. Median time to symptom resolution was 24 days (IQR, 13–37) (Figure and Supplemental Figure). In one case, a woman with a prolonged symptomatic course of at least 37 days sought care in the emergency room 3 times and was hospitalized once for respiratory symptoms. Follow-up data on symptoms were not available for 3 women who were asymptomatic at SARS-CoV-2 testing. No coinfections were detected in 7 patients (15.2%) tested for other respiratory viruses (ie, influenza and respiratory syncytial viruses).
Table 2.
Coronavirus disease 2019 symptoms at the first positive severe acute respiratory syndrome coronavirus 2 test
| Characteristics | Patients (n=46)a |
|---|---|
| Symptomatic before (or at) the first positive test | 43 (93.5%) |
| Among symptomatic (n=43): | |
| Gestational age at symptom onset, wk | 27.0 (21.0–33.9) |
| Number of symptoms reported | 2 (1–5) |
| Reported symptomsb: | |
| Cough | 30 (69.8%) |
| Subjective fever or chill | 22 (51.2%) |
| Nasal congestion | 21 (48.8%) |
| Shortness of breath or dyspnea | 19 (44.2%) |
| Headache | 14 (32.6%) |
| Loss of taste or smell | 13 (30.2%) |
| Myalgia | 13 (30.2%) |
| Fatigue | 12 (27.9%) |
| Sore throat | 12 (27.9%) |
| Other symptomc | 10 (23.3%) |
| Nausea or vomiting | 5 (11.6%) |
| Diarrhea | 3 (7.0%) |
| Days between symptom onset to resolutiond | 24 (13, 37) |
IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
Characteristics summarized as n (%) or median (IQR).
No significant difference (P<.05) by trimester of infection for any reported symptom. One patient had missing symptom data for the day of positive testing, but symptom data were available and included for a subsequent COVID-19-associated encounter.
Chest pain or tightness (n=5), dizziness (n=1), night sweats (n=1), tachycardia (n=1), epigastric pain (n=1), and right upper quadrant pain (n=1).
Estimated by generating a Kaplan–Meier curve to incorporate censoring. Pregnant patients with severe COVID-19 were non-Hispanic white (n=4), Hispanic race unknown (n=1), and race or ethnicity unknown (n=1).
Supplemental Figure.
Days to symptom resolution
This Kaplan–Meier curve depicts days to symptom resolution in 43 symptomatic pregnant patients with data on gestational age at symptom onset. Median time to symptom resolution was 24 days (IQR, 13–37). Symptoms were reported by patients at each clinical encounter (virtual, outpatient, admissions) for SARS-CoV-2 testing and follow-up. Patients with ongoing symptoms were censored at the last report of symptoms (no resolution data available).
IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
Coronavirus disease 2019 disease course, imaging, medical management, and hospital admission
The majority of cases were managed as outpatients for either mild in severity (78.3%, 36/46) or asymptomatic (6.5%, 3/46) presentations. Although few outpatients underwent pulmonary imaging (12.8%, n=5/39), 2 women had pneumonia, but were not admitted. An additional 7 pregnant patients (15.2%) were hospitalized for COVID-19, 1 of whom was admitted to the intensive care unit (Table 3 ). A total of 6 of the 7 hospitalized patients met the criteria for severe COVID-19 disease.24 The majority of patients with severe disease were overweight or obese before pregnancy (80%, 4/5 with data) and half had asthma and obesity-associated conditions (eg, hypertension). Three (42.9%) hospitalized patients received COVID-19-directed medications such as hydroxychloroquine and remdesivir (n=1) or remdesivir alone (n=2). In addition, 2 patients received azithromycin without concomitant hydroxychloroquine; 1 for possible community-acquired pneumonia and 1 in the setting of asthma exacerbation.
Table 3.
Clinical features of pregnant patients with coronavirus disease 2019–associated hospital admissions
| Characteristics | Case number |
||||||
|---|---|---|---|---|---|---|---|
| 12 | 37 | 27 | 25 | 19 | 16 | 31 | |
| Medical history | |||||||
| Age group, ya | 30–35 | 30–35 | 30–35 | 20–25 | 20–25 | 30–35 | 30–35 |
| Existing comorbidities | None | Previous smoker | Asthma, hypertension, hypothyroidism, Crohn’s disease on immunosuppressive medication | Asthma | Asthma, hypertension | Type 2 diabetes mellitus, hypertension | None |
| Pregnancy complications | Overweight | Asymmetrical IUGR (concern before COVID-19) | Class III obesity | Overweight | Class II obesity | Class III obesity | None |
| Prepregnancy BMI | 26.3 | 26.2b | 48.9 | 26.2 | 35.7 | 42.4 | 23.1 |
| SARS-CoV-2 testing | |||||||
| Gestational age at symptom onset, wk | 22.3 | 35.1 | 31.4 | 28.9 | 25 | 23 | 33.9 |
| Gestational age at the first positive test, wk | 22.7 | 35.9 | 31.9 | 29.4 | 25.3 | 23.7 | 34.1 |
| Hospitalization | |||||||
| Timing | |||||||
| gestational age at hospitalization | 23.6c | 36.3 | 33.0 | 30.0 | 26.0 | 23.7 | 35.0 |
| Number of days hospitalized | 1 | 2 | 4 | 6, ICU for 3 days | 8 | 4 | 3 |
| Vital signs | |||||||
| Highest respiratory rate, breaths/min | 20 | 22 | 32 | 49 | 28 | 28 | 32 |
| Lowest oxygen saturation, % | 96 | 94 | 96 | 82 | 92 | 95 | 92 |
| Highest temperature, °C | 37.0 | 38.0 | 37.2 | 37.3 | 38.4 | 38.8 | 37.7 |
| Severity | |||||||
| Severe case? | Yes, dyspnea | Yes, dyspnea and infiltrates on CXR | Yes, RR≥30 | Yes, RR≥30, oxygen saturation≤93% | Yes, oxygen saturation≤93% | No | Yes, RR≥30, oxygen saturation≤93% |
| Pulmonary imaging | Chest CT at GA 26.1 (normal) | CXR at GA 36.3 weeks with pulmonary infiltrates | CXR at GA 33.0 weeks with bilateral consolidations | CXR at GAs 29.4 and 30.0 weeks with bilateral consolidations, linear opacities | CXR at GAs 25.3 (normal), 26.1, and 26.4 weeks with bilateral consolidations | CXR at GA 23.7 weeks with unilateral consolidation | CXR at GA 35.0 weeks with patchy opacities |
| COVID-19 treatment | None | None | Remdesivir | Remdesivir, hydroxychloroquine | Azithromycin and oral prednisone (asthma) | Remdesivir, azithromycin, and ceftriaxone (pneumonia) | Pulmonary vasodilator |
| Respiratory support | Nasal cannula | Nasal cannula | None | High-flow nasal cannula | Nasal cannula | Nasal cannula | None |
| Delivery status at discharge? | Pregnant | Pregnant | Delivered by CD at GA 33.0 weeks; worsening respiratory status | Pregnant | Pregnant | Pregnant | Pregnant |
| Laboratory results during admission | |||||||
| Lowest hematocrit, % | 34.0 | 34.0 | 30.0 | 30.7 | 31.5 | 31.8 | 32.0 |
| Lowest platelets, 103 μL | 196 | 128 | 118 | 171 | 241 | 112 | 197 |
| Lowest WBC count, 103 μL | 8.2 | 5.7 | 4.6 | 4.5 | 6.1 | 2.8 | 5.2 |
| Highest WBC count, 103 μL | 9.6 | 6.4 | 8.1 | 10 | 10.2 | 3.4 | 5.2 |
| Lowest neutrophils, 103 μL | 5.5 | 4.3 | 2.8 | 2.7 | 3.6 | 1.1 | 3.8 |
| Lowest lymphocytes, 103 μL | 3.3 | 1.1 | 0.9 | 0.6 | 1.5 | 0.8 | 18.4 |
| Highest AST, units/L | 12 | 12 | 46 | 29 | 22 | 45 | |
| Highest ALT, units/L | 7 | 8 | 40 | 32 | 27 | 46 | |
| Highest D-dimer, μg/mL | 0.2 | 4.08 | 0.25 | 0.31 | |||
| Highest CRP, mg/L | 1.6 | 9.3 | 5.8 | 5.2 | 9.9 | ||
| Highest creatinine, mg/dL | 0.51 | 0.47 | 0.66 | 0.6 | 0.77 | 0.51 | |
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; CD, cesarean delivery; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; CT, computed tomography; CXR, chest X-ray; GA, gestational age; ICU, intensive care unit; IUGR, intrauterine growth restriction; RR, respiratory rate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cells.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
Age group (5-year increments) is presented to make it less likely that a patient might be identifiable.
Prepregnancy BMI not available. This value represents BMI at SARS-CoV-2 diagnosis, which was at 14 weeks’ gestation.
This patient had 3 emergency department visits for respiratory concerns, 1 of which prompted this hospitalization.
Laboratory testing was performed in 24 women, who were either hospitalized for COVID-19 (n=7) or managed as an outpatient (n=17). Due to multiple encounters, including the delivery admission, laboratory test results were evaluated from the time of COVID-19 diagnosis until delivery (Table 3 and Supplemental Table 2). Of the 24 patients with white blood cell measurements, 8 received a diagnosis of leukopenia (33%) based on pregnancy-specific laboratory reference ranges (<5.6×103/μL) (Supplemental Table 1); half of these patients (4/8) were managed as outpatients. Neither creatinine nor C-reactive protein was elevated in patients who were tested (creatinine, 0/21; C-reactive protein, 0/6) (Supplemental Table 1). Notably, 7 patients had a mildly elevated aspartate aminotransferase, that is, 5 managed as outpatients (31.3%, 5/16) and 2 who were hospitalized (33.3%, 2/6). Finally, a markedly elevated D-dimer was detected in 1 of 5 patients (20%) in which the test was ordered (case 25, 4.08 ng/mL) (Table 3).
The patient admitted to the intensive care unit was a young woman (20–25 years old) at 30 weeks’ gestation, who presented with a 1-week history of fever and cough. She was overweight before pregnancy (BMI, 26.2 kg/m2) and had asthma. She was admitted to the intensive care unit owing to acute respiratory failure with a percutaneous oxygen saturation of 82% on room air and a respiratory rate of 49 breaths/min. She received remdesivir (6 doses), hydroxychloroquine, and high-flow oxygen. She was transferred out of the intensive care unit at day 3 and discharged home at day 6 (case 25, Table 3).
Maternal-fetal outcomes
During the study period, 8 (17.4%) patients delivered, that is, 7 live births and 1 stillbirth (Table 4 ). The median number of days between a positive SARS-CoV-2 test and delivery was 7.5 days (IQR, 5.0–11.5). The median gestational age at delivery was 38.4 weeks (IQR, 37.5–39.8). In one case, worsening respiratory status and multiple comorbidities, such as class III obesity, led to the decision to deliver the patient preterm at 33 weeks’ gestation (case 27, Table 3). Of the 8 deliveries, 5 (62.5%) were vaginal and 3 (37.5%) were cesarean delivery. Two of the 3 cesarean deliveries were performed, in part, to improve maternal respiratory status owing to COVID-19 disease. During the delivery admission, postpartum preeclampsia with severe features developed in 2 women within 1 day of delivery; both women had elevated blood pressure, but no preeclampsia-associated laboratory abnormalities. In these 2 cases, intravenous antihypertensive medications were administered, but magnesium sulfate was not given because of concern for exacerbating pulmonary edema.
Table 4.
Maternal, pregnancy, and neonatal outcomes for 8 deliveries among severe acute respiratory syndrome coronavirus 2–infected pregnant patients
| Characteristics | Deliveries (n=8)a |
|---|---|
| Delivery characteristics | |
| Gestational age at delivery | 38.4 (37.5–39.8) |
| Preterm birth | 1 (12.5) |
| Labor | |
| None | 2 (25.0) |
| Spontaneousb | 2 (25.0) |
| Inducedc | 4 (50.0) |
| Outcome | |
| Live birth | 7 (87.5) |
| Stillbirth | 1 (12.5) |
| Delivery route | |
| Vaginal | 5 (62.5) |
| Cesareand | 3 (37.5) |
| Complications | |
| Pregnancy | |
| Gestational diabetese | 1 (12.5) |
| Gestational hypertensionf | 2 (25.0) |
| Cholestasis | 1 (12.5) |
| Delivery | |
| Placental abruption | 1 (12.5) |
| Nonreassuring fetal status or fetal distress | 3 (37.5) |
| Postpartum | |
| Postpartum preeclampsia with severe featuresg | 2 (25.0) |
| SARS-CoV-2 testing | |
| Days between positive test and delivery | 7.5 (5.0–11.5) |
COVID-19, coronavirus disease 2019; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
Characteristics summarized as n (%) or median (IQR).
One patient with spontaneous onset of labor had labor subsequently augmented.
Reasons for inductions included fetal demise (n=1), premature rupture of membranes (n=1), diabetes mellitus (n=1), hypertensive disorders of pregnancy (n=1), growth restrictions (n=1), and scheduled induction (n=2). No inductions of labor were performed to improve maternal lung function.
Cesarean delivery indications included (multiple indications in some cases) repeat cesarean delivery (n=2), nonreassuring fetal status (n=1), diabetes mellitus (n=1), respiratory compromise (n=1), second-stage arrest (n=1), malpresentation (n=1), COVID-19 (n=2) (decision in the context of COVID-19 and other comorbidities [n=1] and worsening respiratory status [n=1]), and others (n=1) (cholestasis, history of shoulder dystocia, fetal macrosomia in the current pregnancy).
Treated with insulin.
Diagnosed concurrently with (n=1) or after positive SARS-CoV-2 test (n=1).
Both cases were defined as severe by blood pressure criteria.
Details of the case resulting in a stillbirth at 38.7 weeks’ gestation are described in the Appendix. Postmortem examination of the placenta revealed severe chronic villitis, but no viral inclusions. Qualitative PCR testing of placental and fetal tissues was negative for SARS-CoV-2 and cytomegalovirus; notably, there was a delay between fetal demise and RNA preservation for PCR analysis, which can lead to inaccurate PCR results. The etiology in this case was unclear.
Comment
Principal findings
This case series of 46 pregnant patients with COVID-19 represents all known cases across 6 large hospital systems in Washington State from a time period when patients were mainly tested based on symptoms. Notably, 1 in 7 pregnant patients was hospitalized for respiratory concerns and 1 in 8 had severe COVID-19 disease. Pregnant patients with severe COVID-19 were nearly all overweight or obese before pregnancy and many had additional comorbidities such as asthma and hypertension. Obesity as a risk factor for severe COVID-19 in pregnancy is particularly concerning as the national prevalence of obesity was 39.7% among women of reproductive age (20–39 years old) in 2017–2018.27 Obesity is known to impair lung function through both mechanical and inflammatory pathways.28 A synergistically detrimental impact on maternal lung function may occur in the setting of multiple factors such as COVID-19 pneumonia, obesity, asthma, and the added mechanical stress of an enlarged uterus in late pregnancy; this combination may also increase the risk for a medically indicated preterm birth to improve maternal respiratory status.
Results in the context of what is known
Similar to the nonpregnant population, descriptions of the clinical course of COVID-19 disease in pregnancy have been variable.7 , 17 , 25 A systematic review of early case series was notable for a low rate of admission to the intensive care unit (3%), no maternal deaths, and only 1 neonatal death and 1 intrauterine fetal demise.6 In a recent and larger case series from the Hubei province in China, the rate of severe pneumonia (7%–8%) in pregnant women was not higher than the general population (15%).7 Newer reports have highlighted critical cases in pregnant women involving respiratory failure, mechanical ventilation, maternal death, and obstetrical complications such as preterm birth and intrauterine fetal demise.8 , 17 , 29, 30, 31, 32 Our population-based case series of pregnant patients with COVID-19 from counties in Washington State with the highest burden of disease offers a unique insight into the disease course in pregnancy and identifies potential risk factors associated with severe disease. Obesity, asthma, and hypertension seemed to be overrepresented in pregnant patients with severe disease in our cohort, which is similar to studies in nonpregnant adults.33 , 34
Clinical implications
Although outpatient management of COVID-19 may be safe for most pregnant patients, the risks of COVID-19 for maternal health remain incompletely defined. There is evidence of COVID-19-associated coagulopathy and whether pregnant women would benefit from thromboprophylaxis is unknown.35 In our case series, the markedly elevated D-dimer (>4.0 μg/mL) in a pregnant woman with severe COVID-19 is important, because levels greater than 1.0 and 2.0 μg/mL have been linked to an increased risk for COVID-19-associated mortality.36, 37, 38 Pregnant women are known to have an elevated D-dimer during pregnancy, which may be as high as 3.3 μg/mL in the second and third trimesters39 and could predispose pregnant women to an even greater risk for COVID-19-associated thrombogenic events and mortality. Laboratory testing of D-dimer should be considered for pregnant women with COVID-19.
Pregnant women typically represent a unique and vulnerable group to infectious diseases, not only because they often have enhanced disease (ie, influenza and hepatitis E viruses)40 but also because of the detrimental impact on obstetrical course and neonatal outcomes. In our series, the timing of delivery for 1 in 4 women was influenced by the impact of COVID-19 pneumonia on maternal lung function; in one case, this necessitated preterm delivery at 33 weeks’ gestation. COVID-19 disease in the mother can pose a maternal-fetal dilemma, because an intervention that would benefit her (ie, delivery to improve maternal lung function) might result in morbidity or mortality to the neonate if delivered prematurely. The rate of medically indicated preterm birth is a critically important feature contributing to the vulnerability of pregnant women to COVID-19.
The impact of COVID-19 on resource utilization across all sites was considerable and not captured by this data. Outpatient adjustments included changes such as daily symptom screening, daily calls to patients with COVID-19, notification of new visitation policies, rescheduling of appointments, and conversion to new telemedicine platforms. In the hospital, limitations on the number of people providing labor support, development of new practices for universal screening before or upon admission, new construction of negative pressure rooms, and frequent care coordination between obstetrics and intensive care unit teams. All of these changes resulted in increases in time, supplies, and staffing that created challenges to delivery of the usual standard of maternity care. Furthermore, the impact of quarantine on women’s lives, stress, mental health, bonding, breastfeeding, and child development is a critically important outcome not captured in our case series.
Research implications
Rigorous population-based studies are needed to identify risk factors for severe disease and the rate of adverse outcomes in pregnancy and to ascertain whether risks are increased in late pregnancy similar to influenza.41, 42, 43 Whether vertical transmission occurs remains unknown, but several case reports seem suspicious.16, 17, 18 We must also conduct follow-up studies of children exposed to SARS-CoV-2 infections in pregnancy to determine the risk for COVID-19 disease in the immediate newborn period. Both preterm birth and maternal infections may pose short- and long-term risks for the child such as mortality, prematurity-related complications, and neuropsychiatric disease as an adult.19 , 20 , 44 , 45 Finally, we must determine the impact of quarantine and mother-newborn separation on maternal health so that we can better support women in the postpartum period.
None of the pregnant women in our case series, who received medications for COVID-19 (eg, remdesivir), were enrolled in a clinical trial, despite recommendations from the Infectious Disease Society of America that treatment of hospitalized patients with COVID-19 occurs in the context of a clinical trial.46 Pregnant and breastfeeding individuals are almost universally excluded from COVID-19 clinical treatment trials, such as the World Health Organization–sponsored SOLIDARITY trial (ISRCTN83971151).47 Currently, pregnant women can access both remdesivir through compassionate use and convalescent plasma (NCT04338360) through an expanded access clinical trial if they have confirmed severe COVID-19.46 , 48 In general, trials that allow inclusion of pregnant and breastfeeding women focus on outpatient treatment trials (NCT04354428 and NCT043558068) or postexposure prophylaxis trials (NCT04308668 and NCT04328961), with the exception of a convalescent plasma trial for patients hospitalized for COVID-19 (NCT04348656). It is imperative to enroll pregnant women in clinical trials testing COVID-19 therapeutics to enable development of evidence-based treatment guidelines.
Strengths and limitations
The main strength of this study is the inclusion of multiple health systems across Washington State representing counties with the highest burden of COVID-19 cases and approximately 40% of annual deliveries in the state. We also included both symptomatic and asymptomatic cases and infections from all trimesters, allowing us to better describe the full range of infection in pregnancy. Finally, all data were initially abstracted and reviewed for accuracy by clinical obstetrical providers. An important study limitation is that some cases could have been missed despite the use of multiple methods of case detection at most sites. It is also likely that our case series underestimated the prevalence of asymptomatic cases because testing resources were primarily directed toward symptomatic cases during this study period. This biased our study toward inclusion of patients with worse clinical outcomes. Finally, other studies seem to have found more severe respiratory complications when infection occurred during the peripartum period, which was not well captured in our study because delivery outcomes were only available in 8 women.6 , 25 , 49 , 50
Conclusion
In this population-based case series from Washington State, 15% of pregnant patients with COVID-19 were hospitalized with severe disease. Nearly all women were overweight or obese before pregnancy and had other important comorbidities, such as asthma and hypertension. Our data suggest that pregnant women who have common health conditions such as obesity and asthma may be at a greater risk for severe COVID-19 and medically indicated preterm delivery to improve lung function. Larger population-based studies are needed to determine whether pregnant individuals are at higher risk for severe COVID-19 compared with nonpregnant adult women and to what extent obesity and other comorbidities may enhance risk.51 Taken together, pregnant women should be considered a high-risk population for severe COVID-19, particularly for women in the second and third trimesters that began pregnancy overweight or obese.
Acknowledgments
We would like to acknowledge several individuals for their support of this project. We thank Ms Adrienne Meyer and Dr Emily Guthrie from the University of Washington IRB for organizing reliance agreements with multiple study sites. Ms Emily Begnel graciously volunteered her time to help build the REDCap database. We are thankful for the assistance provided by Ms Bethann M. Pflugeisen for implementing site-specific algorithms to identify pregnant patients with COVID-19 in the MultiCare Health System. Ms Nicole Wothe provided administrative assistance with project management and manuscript submission. We thank Dr Benjamin T. Bradley for providing advice associated with the fetal autopsy.
Footnotes
This work was primarily funded by the Department of Obstetrics and Gynecology, University of Washington, and gift funds. This work was also supported by the National Institute of Allergy and Infectious Diseases (grant numbers AI133976, AI145890, AI144938, and AI143265 to K.A.W. and AI120793 to S.M.L.). Study data were managed using a research electronic data capture (REDCap) tool hosted by the Institute of Translational Health Sciences at the University of Washington, which was supported by the National Center for Advancing Translational Sciences (UL1TR002319). The funding sources were not involved in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflict of interest. We note that A.K. has received honoraria for her work on maternal immunization through Pfizer and GlaxoSmithKline, which are outside the scope of this manuscript.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health or other funders.
Cite this article as: Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol 2020;223:911.e1-14.
Appendix
Case description: stillbirth case
A 38-year-old G6P2123 at 38 5/7 weeks’ gestation presented with decreased fetal movements for 2–3 weeks and vaginal bleeding. The patient’s partner had a laboratory-confirmed diagnosis of COVID-19 25 days before the patient’s presentation. She denied symptoms except for a 2-day history of rhinorrhea. She had no fever, shortness of breath, or cough. Upon admission, she received a diagnosis of an intrauterine fetal demise by ultrasound evaluation and COVID-19 by PCR testing. Her pregnancy was complicated by a marginal umbilical cord insertion noted by ultrasonography results. A sonographic estimate of the fetal weight was in the 75th percentile at 28 6/7 weeks’ gestation. She had a history of a preterm delivery in a previous pregnancy at 36 1/7 weeks’ gestation with a cytomegalovirus infection reported in that pregnancy. Mildly elevated blood pressures were noted at the time of admission after diagnosis of fetal demise and were intermittently elevated in labor and postpartum period. She had no symptoms of preeclampsia and normal preeclampsia labs. She did not receive magnesium sulfate. Her white blood cell count was 13.1×103 μL. Serologic testing result was negative for rubella immunoglobulin M (IgM), cytomegalovirus IgM, toxoplasma immunoglobulin G (IgG) and IgM, and parvovirus IgM. Parvovirus IgG serology result was positive. A hypercoagulability workup revealed low protein S activity at 55% (normal 60%–140%), normal protein C activity at 105 IU/dL (70–180 IU/dL), normal antithrombin III activity at 105% (80%–120%), no factor V Leiden gene mutation, and negative results for antiphospholipid antibody testing (anticardiolipin IgM and IgG, lupus anticoagulant, and anti–beta-2-glycoprotein antibody). The partial thromboplastin time-lupus anticoagulant screen was elevated at 47 s (normal<40 s) and dilute Russell’s viper venom (dRVVT) screen was elevated at 48 s (normal<45 s); although the dRVVT test result was positive, the corrected mixing study suggested this was a false positive. A Kleihauer–Betke test result was negative. Additional laboratory test results were normal such as prothrombin time at 13.5 s, partial thromboplastin time at 33.6 s, thyroid-stimulating hormone at 0.9 mIU/L, and a hemoglobin A1c at 4.9%. The test result of a group B Streptococcus culture of the rectovaginal space was negative. The result of a urine toxicology screen for common drugs of abuse was negative.
The patient remained afebrile during her hospital stay with no respiratory symptoms and was discharged home at postpartum day 1. At the time of delivery, no nuchal cord or knot in the cord was noted. A foul odor was present in the placenta. A culture of the chorioamniotic membranes revealed only mixed genital flora. A microarray test result of the fetus was normal. Autopsy revealed a term fetus with measurements appropriate for gestational age. No dysmorphic features or congenital anomalies were present. There were changes of acute hypoxia or systemic failure, such as serous pleural and pericardial effusions, petechial hemorrhages, and bilateral acute intraventricular hemorrhage. Meconium staining and aspiration into the lungs was present. The placenta was small for gestational age (<5%) with acute chorioamnionitis, mild funisitis, and severe chronic villitis. No viral cytopathic changes were seen and the result of an immunostain for cytomegalovirus was negative. The qRT-PCR results testing the placental parenchyma and fetal nasopharynx for SARS-CoV-2 were negative.
Supplemental Table 1.
Normal laboratory reference ranges in pregnant women by trimester
| First trimester | Second trimester | Third trimester | Threshold used as abnormala | |
|---|---|---|---|---|
| White blood cell count, 103/μL | 5.7–13.6 | 5.6–14.8 | 5.9–16.9 | ≤5.6 |
| Aspartate aminotransferase, units/L | 3–23 | 3–33 | 4–32 | ≥33 |
| Troponinb, ng/mL | Not reported | Not reported | 0–0.06a | ≥0.06 |
| D-dimer, μg/mL | 0.2–1.2 | 0.4–3.3 | 0.6–3.3 | ≥3.3 |
| C-reactive protein, mg/L | Not reported | 0.4–20.3 | 0.4–8.1 | ≥20.3 |
| Creatinine, mg/dL | 0.4–0.7 | 0.4–0.8 | 0.4–0.9 | >0.9 |
| Prothrombin time, s | 9.7–13.5 | 9.5–13.4 | 9.6–12.9 | >13.5 |
The normal laboratory reference ranges by trimester were extracted from a comprehensive review of maternal laboratory values in uncomplicated pregnancies derived from approximately 70 references1 with the exception of D-dimer. For D-dimer values, we used a higher laboratory reference range2 to take a more conservative approach to determination of abnormal values in pregnancy.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
We applied a single threshold to identify abnormal values, which reflected either the lowest or highest value in the reference range from the second and third trimesters, as appropriate for evaluating the severity of COVID-19 disease.
Troponin level in the third trimester denotes a reference range for an intrapartum value.
Supplemental Table 2.
Select laboratory results
| Laboratory testa | Patients with COVID-19 managed as outpatients (n=39) |
Hospitalized patients with COVID-19 (n=7) |
||
|---|---|---|---|---|
| n | Median (range) or n (%) | n | Median (range) or n (%) | |
| White blood cell count, 103/μL | 17 | 7 | ||
| Lowest white blood cell count | 7.2 (3.9–13.1) | 5.2 (2.8–8.2) | ||
| Lymphopenia (≤5.6×103/μL blood) | 4 (23.5) | 4 (57.1) | ||
| AST, units/L | 16 | 6 | ||
| Highest AST | 20.5 (12–82) | 25.5 (12–46) | ||
| Elevated AST (≥33 units/L) | 5 (31.3) | 2 (33.3) | ||
| D-dimer (μg/mL) | 1 | 5 | ||
| Highest D-dimer | 1.5 | 0.31 (0.2–4.1) | ||
| Elevated D-dimer (≥3.3 μg/L) | 0 | 1 (20.0) | ||
| CRP, mg/L | 2 | 6 | ||
| Highest CRP | 0.45 (0.4–0.5) | 7.6 (1.6–9.9) | ||
| Elevated CRP (≥20.3) | 0 | 0 | ||
| Creatinine, mg/dL | 15 | 6 | ||
| Highest creatinine | 0.56 (0.42–0.81) | 0.58 (0.47–0.77) | ||
| Elevated creatinine (>0.9 mg/dL) | 0 | 0 | ||
AST, aspartate transaminase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein.
Lokken et al. COVID-19 in pregnant women in Washington State. Am J Obstet Gynecol 2020.
Some patients had labs drawn across multiple encounters during follow-ups such as the delivery admission. The lowest or highest (as appropriate) ever measurement taken is included in these results. Not all patients had laboratory testing.
Supplementary Data
References
- 1.Patel A., Jernigan D.B., 2019-nCoV CDC Response Team Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak—United States, December 31, 2019–February 4, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:140–146. doi: 10.15585/mmwr.mm6905e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus Disease 2019 (COVID-19) situation report–83. Geneva. WHO. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200412-sitrep-83-covid-19.pdf?sfvrsn=697ce98d_4 Available at: Accessed April 17, 2020.
- 3.Bhatraju P.K., Ghassemieh B.J., Nichols M. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Mascio D., Khalil A., Saccone G. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100107. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13867. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan J., Guo J., Fan C. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baud D., Greub G., Favre G. Second-trimester miscarriage in a pregnant woman With SARS-CoV-2 infection. JAMA. 2020 doi: 10.1001/jama.2020.7233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N., Han L., Peng M. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu N., Li W., Kang Q. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25789. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breslin N., Baptiste C., Miller R. COVID-19 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100111. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng H., Xu C., Fan J. Antibodies in infants born to mothers With COVID-19 pneumonia. JAMA. 2020 doi: 10.1001/jama.2020.4861. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong L., Tian J., He S. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020 doi: 10.1055/s-0040-1710050. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamouroux A., Attie-Bitach T., Martinovic J., Leruez-Ville M., Ville Y. Evidence for and against vertical transmission for SARS-CoV-2 (COVID-19) Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.039. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Haddad B.J.S., Jacobsson B., Chabra S. Long-term risk of neuropsychiatric disease After exposure to infection in utero. JAMA Psychiatry. 2019;76:594–602. doi: 10.1001/jamapsychiatry.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Haddad B.J.S., Oler E., Armistead B. The fetal origins of mental illness. Am J Obstet Gynecol. 2019;221:549–562. doi: 10.1016/j.ajog.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC COVID-19 Response Team Geographic differences in COVID-19 cases, deaths, and incidence—United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:465–471. doi: 10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proclamation by the governor. Olympia, Washington 20-05. State of Washington Office of the Governor. 2020. Available at: https://www.governor.wa.gov/sites/default/files/20-05%20Coronavirus%20%28final%29.pdf?utm_medium=email&utm_source=govdelivery. Accessed April 17, 2020
- 23.Washington State Department of Health Washington State Vital Statistics 2018 Highlights. https://www.doh.wa.gov/Portals/1/Documents/Pubs/422-099-2018-2010-VitalStatHighlights.pdf Available at: Accessed June 8, 2020.
- 24.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Breslin N., Baptiste C., Gyamfi-Bannerman C. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020:100118. doi: 10.1016/j.ajogmf.2020.100118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbassi-Ghanavati M., Greer L.G., Cunningham F.G. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114:1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- 27.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. NCHS Data Brief; 2020. Prevalence of obesity and severe obesity among adults: United States, 2017–2018; p. 360. [PubMed] [Google Scholar]
- 28.Dixon A.E., Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A. Maternal death due to COVID-19 disease. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karami P., Naghavi M., Feyzi A. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020:101665. doi: 10.1016/j.tmaid.2020.101665. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirshberg A., Kern-Goldberger A.R., Levine L.D. Care of critically ill pregnant patients with COVID-19: a case series. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce-Williams R.A.M., Burd J., Felder L. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM. 2020:100134. doi: 10.1016/j.ajogmf.2020.100134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring) 2020 doi: 10.1002/oby.22818. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Di Renzo G.C., Giardina I. Coronavirus disease 2019 in pregnancy: consider thromboembolic disorders and thromboprophylaxis. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L., Yan X., Fan Q. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutiérrez García I., Pérez Cañadas P., Martínez Uriarte J., García Izquierdo O., Angeles Jódar Pérez M., García de Guadiana Romualdo L. D-dimer during pregnancy: establishing trimester-specific reference intervals. Scand J Clin Lab Invest. 2018;78:439–442. doi: 10.1080/00365513.2018.1488177. [DOI] [PubMed] [Google Scholar]
- 40.Kourtis A.P., Read J.S., Jamieson D.J. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuzil K.M., Reed G.W., Mitchel E.F., Simonsen L., Griffin M.R. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 42.Dodds L., McNeil S.A., Fell D.B. Impact of influenza exposure on rates of hospital admissions and physician visits because of respiratory illness among pregnant women. CMAJ. 2007;176:463–468. doi: 10.1503/cmaj.061435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schanzer D.L., Langley J.M., Tam T.W. Influenza-attributed hospitalization rates among pregnant women in Canada 1994–2000. J Obstet Gynaecol Can. 2007;29:622–629. doi: 10.1016/s1701-2163(16)32559-2. [DOI] [PubMed] [Google Scholar]
- 44.Behrman R.E., Butler A.S. National Academies Press; Washington, DC: 2007. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes, eds. Preterm birth: causes, consequences, and prevention. [PubMed] [Google Scholar]
- 45.Ward R.M., Beachy J.C. Neonatal complications following preterm birth. BJOG. 2003;110(Suppl 20):8–16. doi: 10.1016/s1470-0328(03)00012-0. [DOI] [PubMed] [Google Scholar]
- 46.Bimraj A., Morgan R.L., Shumaker A.H. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 2020. 2020. https://www.idsociety.org/COVID19guidelines Available at: Accessed April 12, 2020. [DOI] [PMC free article] [PubMed]
- 47.LaCourse S.M., John-Stewart G., Adams Waldorf K.M. Importance of inclusion of pregnant and breastfeeding women in COVID-19 therapeutic trials. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa444. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joyner M. Expanded access to convalescent plasma for the treatment of patients With COVID-19. 2020. https://www.uscovidplasma.org/pdf/COVID-19%20Plasma%20EAP.pdf Available at: Accessed June 9, 2020.
- 49.Rasmussen S.A., Jamieson D.J., Bresee J.S. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14:95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong S.F., Chow K.M., Leung T.N. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buekens P., Alger J., Bréart G., Cafferata M.L., Harville E., Tomasso G. A call for action for COVID-19 surveillance and research during pregnancy. Lancet Glob Health. 2020 doi: 10.1016/S2214-109X(20)30206-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplemental References
- 1.Abbassi-Ghanavati M., Greer L.G., Cunningham F.G. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114:1326–1331. doi: 10.1097/AOG.0b013e3181c2bde8. [DOI] [PubMed] [Google Scholar]
- 2.Gutiérrez García I., Pérez Cañadas P., Martínez Uriarte J., García Izquierdo O., Angeles Jódar Pérez M., García de Guadiana Romualdo L. D-dimer during pregnancy: establishing trimester-specific reference intervals. Scand J Clin Lab Invest. 2018;78:439–442. doi: 10.1080/00365513.2018.1488177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.