Abstract
Titanium dioxide (TiO2) nanostructures are one of the most plentiful compounds that have emerged in various fields of technology such as medicine, energy and biosensing. Various TiO2 nanostructures (nanotubes [NTs] and nanowires) have been employed in photoelectrochemical (PEC) biosensing applications, greatly enhancing the detection of targets. TiO2 nanostructures, used as reinforced material or coatings for the bare surface of titanium implants, are excellent additive materials to compensate titanium implants deficiencies—like poor surface interaction with surrounding tissues—by providing nanoporous surfaces and hierarchical structures. These nanostructures can also be loaded by diversified drugs—like osteoporosis drugs, anticancer and antibiotics—and used as local drug delivery systems. Furthermore, TiO2 nanostructures and their derivatives are new emerging antimicrobial agents to overcome human pathogenic microorganisms. However, like all other nanomaterials, toxicity and biocompatibility of TiO2 nanostructures must be considered. This review highlights recent advances, along with the properties and numerous applications of TiO2-based nanostructure compounds in nano biosensing, medical implants, drug delivery and antibacterial fields. Moreover, in the present study, some recent advances accomplished on the pharmaceutical applications of TiO2 nanostructures, as well as its toxicity and biocompatibility, are presented.
Keywords: titanium dioxide nanomaterial, drug release, antibacterial, implants, biosensors, nanotoxicity
Introduction
Nanotechnology, as one of the most interesting areas of science, gives scientists the ability to create, control and use materials in the nanometer scale.1–4 Biotechnology has helped to transfer the knowledge of biological systems to industry. Furthermore, the distinctive physicochemical and biological properties of nanomaterials resulted in the use of nanotechnology in different health care areas.4–9 Nanobiotechnology is one of the latest emerging fields of science, which is the interface between biology and nanotechnology, analyzing and creating novel functionalized nano biosystems.8 This multidisciplinary research field has great potential in the development of improved medical engineering.1,10
Several nanostructures with unique properties have been fabricated by nanotechnology for biotechnological applications,1,2,6,8,9,11-14 among which, nano-sized titanium dioxide (TiO2) has been widely used. The element of titanium was first discovered in 1791 by William Gregor. TiO2 is a white and poorly soluble material with numerous applications in biomedical fields like cosmetics, medicines and pharmaceutical products.5,15,16 It consists of two crystalline forms, Rutile and Anatase, which have important industrial applications. It has also been proven that mixed polymorphs of TiO2 (for example, Anatase 80% and Rutile 20%) are more efficient for biomedical use compared to the presence of one crystal. Anatase form of TiO2 is more active than the Rutile form in regard to photocatalytic and cytotoxic properties.16 However, most of the metal-oxide nanoparticles (NPs) are costly for industrial scale-up, and some are toxic.7,13 TiO2 is not only cost-effective but is mostly a non-toxic substance,17 which has been approved by the American Food and Drug Administration (FDA) for utilization in food and drug-related products.18
Nanostructured TiO2 has broad potential applications due to their nanosized features, low toxicity, good biocompatibility, intrinsic properties and versatile fabrication techniques.11,12
For example, nanostructured arrays composed of TiO2 nanotube wall, including Ag2O and Cu NPs, are ideal candidates for biomedical applications such as antibacterial surface coating. Additionally, they have good cytocompatibility, and are useful for osteoblast cell spreading, proliferation and differentiation.19,20 Furthermore, the drug delivery system, bone/drug implant, self-cleaning substrate and antibacterial agent, for example, are wide spectrum applications of TiO2 nanostructures.
In recent years, various types of nanostructured systems have been developed for delivery of chemotherapeutic agents in an effort to destroy tumor cells effectively, yet most of these delivery systems are still not able to target tumor cells specifically, and their drug release process is poorly controlled, leading to serious side effects.21,22 Thus, the ability to obtain specific accumulation of drug at the tumor site is still an important challenge. Therefore, focusing on design of an ideal drug delivery system, which has combination of abilities for stimuli-triggered drug release and cancer cell targeting, is required. Porous TiO2 nanostructures offer the potential to solve this problem, owing to their remarkable properties, such as high functional surface and photocatalytic activity.23
TiO2 nanostructures, like titania nanotubes (NTs), are desirable structures to be used for surface coating of implants, and compared to polymer based implants, TiO2 NTs have good stability against disintegration and swelling. More importantly, TiO2 nanostructures are able to be used as a smart delivery system to control drug release in implants. These aspects of TiO2 nanostructures address the most important challenges of the previously devised implant.24
On the other hand, the porous structure of TiO2 NTs enhances bone regeneration and repair, which is favorable in bone tissue growth and implant biological fixation.12,25-29 Nowadays, the increasing risk of device-related infections in medicine result in a serious health problem. Furthermore, the spread of antibiotic resistant bacteria in biomedical devices is considered a large threats to human health. Therefore, much attention is focused on newly developed anti-microbial strategies for medical implements used in clinic. Among different strategies, the idea of coating device surfaces with antimicrobial active metals are considered one of the essential strategies. However, the bimetallic corrosion is unavoidable during the service. In this regard, photocatalytic TiO2 nanomaterials with anatase form provide more advantages for antimicrobial purposes.30 Titania photocatalytic activity of TiO2 under the UV irradiation exposure results in disinfecting properties, mostly related to the generation of reactive oxygen species (ROS).31
Sensitive and accurate detection of biological analytes at low concentrations is another applicability of TiO2 nanostructures that is very beneficial for biomedical research, as well as clinical diagnosis. In recent years, there has been considerable interest in the sensing application of TiO2 for use in biosensors.12,27
According to literature, nanostructured TiO2 appears to be an inert and safe material when exposed to human body. A deep understanding of nanoscale phenomena will pave the way for fabrication of novel biomedical devices and constructing the antibacterial surfaces, implants devices and more. As TiO2 nanomaterials have received remarkable attention in different fields, this review focuses on the recent advances of its biomedical application, discussing its nanotoxicity and most important factors affecting its biocompatibility, as well as future challenges in these regards.
TiO2-Based Drug Delivery Systems for Cancer Therapy
Nowadays, nanotechnology in cancer treatment and diagnosis is more popular as a result of the severe side effects of traditional chemotherapeutic agents due to their cytotoxicity on normal cells.1,6,13,14,32 The most challenging aim of nanotechnology research in cancer therapy is discovering nanostructures for delivery and release of drugs in a way that enhances the therapeutic effect and decreases the side effects.9 Targeted drug delivery and controlled drug release are the main strategies to achieve this aim. TiO2 nanostructures—due to the high biocompatibility, tunable drug releasing ability and low toxicity—are recognized as an appropriate candidate to increase the clinical therapeutic effect of conventional chemotherapeutic agents through targeted delivery and controlled release.
TiO2-based nanostructures are potent systems for both targeted delivery and controlled release of cytotoxic anticancer agents. In the following, we review the important studies of TiO2 nanostructures in targeted drug delivery and different controlled release systems.33
Targeted Drug Delivery
Several lines of studies have shown that targeted drug delivery of cytotoxic chemo agents loaded on TiO2-based nanostructures could increase the therapeutic efficiency of agents in cancer treatment. For the first time, Li et al investigated the potential application of one-dimensional TiO2 whiskers for drug delivery in cancer therapy and its synergistic effect on internalization and accumulation of daunorubicin in SMMC-7721 cells.34 Likewise, it has been reported that conjugation of doxorubicin (DOX) to TiO2 NPs caused synergistic response in breast cancer cell lines.35
In a recent study, ZnS quantum dots were deposited onto TiO2 nanotube surface, then the drug release profile was studied using 5-fluorouracil (5-FU), indicating that TiO2/ZnS NTsare of value as drug delivery systems.36 Targeted drug delivery using surface-modified NPs increases the performance of the anticancer drugs by specific delivery of them to cancer cells, minimizing the toxicity of anticancer drugs.1,13,32 In 2013, Venkatasubbu et al observed the anticancer activity of paclitaxel attached to modified hydroxyapatite (Hap) and TiO2 NPs in diethylnitrosamine (DEN)-induced hepatocarcinoma in animal models. Surface modification of NPs conducted by polyethylene glycol (PEG) as a non-immunogenic polymer and folic acid as a tumor marker. The hematological and biochemical results of research showed the higher anticancer activity of the surface-modified paclitaxel attached to the Hap and TiO2 NPs in comparison to pure paclitaxel.37 In this manner, the treatment of ovarian cancer cell line with cisplatin loaded on hyaluronic acid-TiO2 NPs enhanced drug accumulation in cancer cells compared to free cisplatin.38 In addition to the mentioned advantages, nanostructure mediated targeted drug delivery in cancer cells can enhance bioavailability and release time of drug.1,13,14,37
Controlled Drug Release in Cancer Therapy
Among new strategies for cancer treatment, light is one of interesting external stimulus for releasing of a drug from delivery system due to its capability for providing a spatial and temporal control over the release of anticancer agents. TiO2 nanostructures also attract great attention as photoactive drug delivery carriers. In addition to photoactivity, TiO2-based nanostructures have wide range of valuable properties including high surface area, stability, availability, and possibility for surface modification making them as a suitable carrier for attachment of various drugs.39 In 2014, Wang et al reported a new multifunctional porous TiO2 NPs loaded with paclitaxel, which had the potential of ultraviolet (UV)-triggered drug release. They modified TiO2 NPs with polyethyleneimine (PEI) as a cancer-targeting agent. The authors believed that this development could be more informative for light-controlled drug release and skin cancer treatment.23
In another study, a mesoporous TiO2 shell for Near-Infrared-Triggered drug delivery was prepared by loading DOXto prepare a porous TiO2 shell core-shell structures, and HA capping was performed then the drug release was studied. The results indicated that the cell viability decreased clearly at lower drug doses, making it a promising method for cancer therapy.40 In a recentstudy, colloidal TiO2 NPs were used as carrier for light-controlled delivery of ruthenium complex (metallo-drug) to the melanoma cancer cell line. This system showed fast release profile upon UV light compared with visible light illumination. Besides, the cell death increased by UV light as compared to red light. The authors proposed that both components of system are photosensitizer and could induce cell death by generating ROS.39
It has been reported that the elevated level of oxidative stress by exogenous agents, due to the elevation of ROS in tumor environment, can be more destructive in cancer cells.41 Prior studies have shown that induction of ROS production in a tumor environment triggers apoptosis, which is the appropriate type of cell death in cancers.42 Thus, the exposure of cancer cells to increased level of ROS can be a new potential treatment to selectively induce cell death in cancer cells without affecting normal cells.41
Similar to light, ultrasound is another external stimuli for induction of drug release in TiO2-based nanomaterials. Several studies have confirmed that ultrasound is more effective than UV-light due to the deeper penetration in tumor cells. Besides that, ultrasound causes no harm to the host cells. In this procedure, ultrasound stimulates TiO2 as a sonosensitizer and generates ROS to kill tumor cells. TiO2 NPs have indicated enhanced antitumor activity against different cancer cell lines after stimulation with ultrasonic.33 In an investigation conducted on hydrophilized TiO2 (H TiO2) NPs in sonodynamictherapy, HTiO2 NPs showed long circulating time and more resistance against degradation.43
Radiofrequency is an excellent noninvasive strategy, which can be used to trigger drug release in TiO2 NT based-delivery systems. AuNPs are one of the most efficient thermal transducers for transferring radiofrequency energy to induce drug release from TiO2 NTs.44 This approach has demonstrated significant potential in the inhibition of hepatocellular and human gastrointestinal cancer cells.45
TiO2 NT platforms can be used as the most promising carriers for controlled drug release of anticancer agents in local drug delivery systems. It has been shown that dimensions and surface functionalization of TNTs plays important role in controlling drug release from TiO2 NTs. In a study completed involving TiO2 NTs with different lengths and sizes, higher lengths of tubes provided higher total drug elution of paclitaxel.46
Stimuli-Controlled Drug Release
In recent years, new developed cancer therapy systems pose both function of controlled release and targeted delivery of drug. In a study, multifunctional porous TiO2 NPs were functionalized with PEI to provide photocatalytic property for TiO2, in order to become a UV- controlled drug release system. In such a system, the loaded antitumor agent was not released in the blood and normal tissues during circulation due to the blocking role of PEI. Additionally, to further target cancer cells, which overexpress folate receptor, folic acid was conjugated covalently to the surface of TiO2 NPs.23
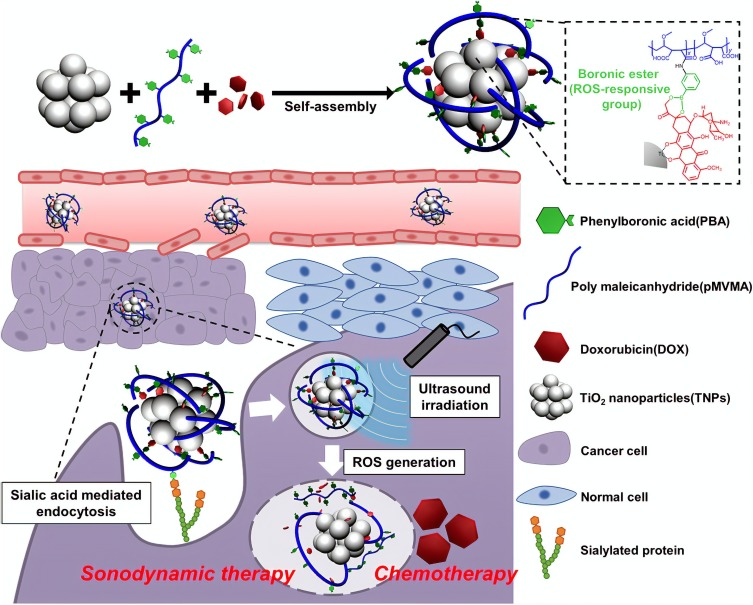
In another study, DOX loaded TiO2 NPs, which were coated with polymeric phenyboronic acid (pPBA) through boronic ester bond, demonstrated high tumor targeting capability due to the specific interaction of PBA with sialylated epitope of tumor cells. On the other hand, ultrasound irradiation through ROS generation could release DOX from NP via cleavage of boronic ester bonding (Figure 1).47
Figure 1.
A schematic image indicates a TiO2 based-targeted delivery of an anticancer agent and controlled drug release induced by ultrasound irradiation.
Notes: Reprinted from: Kim S, Im S, Park EY, et al. Drug-loaded titanium dioxide nanoparticle coated with tumor targeting polymer as a sonodynamic chemotherapeutic agent for anti-cancer therapy. Nanomedicine. 2020;24:102110. Copyright © 2019 Elsevier Inc. All rights reserved. With permission from Elsevier.47
TiO2-Based Antibacterial Devices for Prevention and Treatment of Infections
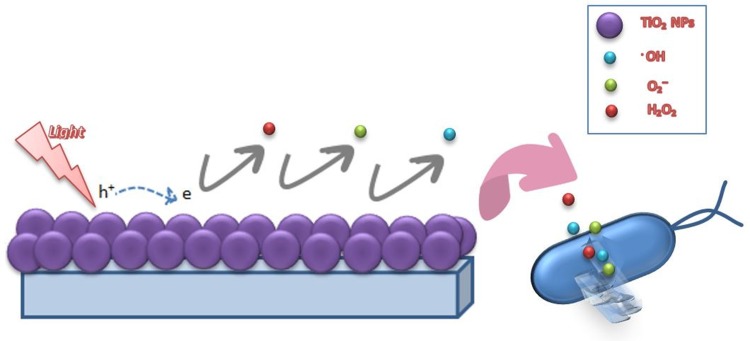
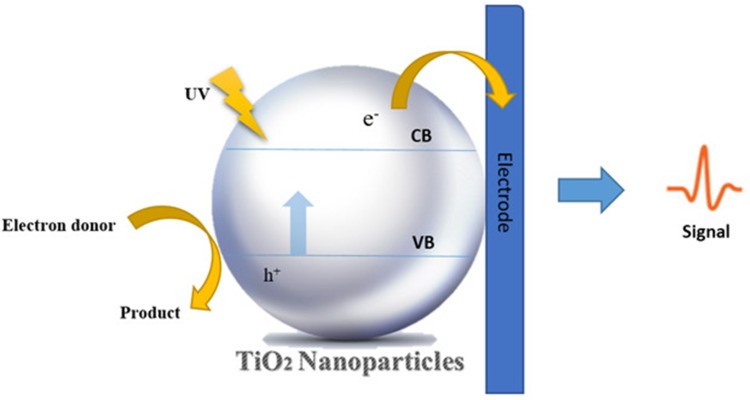
With the advent of nanotechnology, materials, such as metals and metal oxides, have been noticed as effective microbicide agents, which have the advantage of improved safety and stability compared to organic antimicrobial agents.17,48,49 A large number of studies show the inhibitory activity of TiO2 because of photocatalytic action, which is due to ROS—such as O2, OH and H2O2—being generated.31 ROS is the product of redox reactions occurring between an adsorbent (water or oxygen) and electrons of TiO2 when illuminated by UV light at wavelength of shorter than 385 nm.31,50 Figure 2 shows the schematic diagram of bacterial destruction by illuminated TiO2. The most important drawback to TiO2 is its necessity for UV light irradiation for activation of photocatalyst. However, recent studies have solved this problem by using visible light-activated photocatalysts, like Ag/AgBr/TiO2, and doped-TiO2 nanostructures.51–53
Figure 2.
The bacterial destruction by UV-illuminated TiO2 NPs.
The first use of TiO2 as a photocatalytic micro biocide was reported by Matsunaga et al (1985), when they investigated TiO2 antibacterial activity towards Lactobacillus acidophilus, Saccharomyces cerevisiae and E. coli.54 Various studies have explained that the photocatalytic activity of TiO2 in water is efficient against broad range of organisms, such as bacteria (Gram-negative and Gram-positive), fungi, viruses, algae, protozoa and bacterial toxins.31 TiO2 thin films are as potent as the suspended form, and a major advantage of them is easy separation of catalyst from liquid at the end of process. The pioneering study by Matsunaga et al proved that TiO2-Pt thin film inhibits microbial cell viability in water and in exposure of UV-light.54
Nowadays, controlling inhabitant bacterial species on medical devices and implants is a serious problem in biomedicine due to the high prevalence of antimicrobial resistance and biofilm generation. Increasing microorganism resistance is one of the most important challenges in many industries. In this regard, capability of TiO2 as an antimicrobial agent has been studied in several biomedical researches. This part of the review aims to focus on newly developed research studies in biomedical application of TiO2 antibacterial properties. Table 1 summarizes the antibacterial applications of different TiO2 nanostructures.
Table 1.
Antibacterial Properties of TiO2 Nanostructures in Biomedical Applications
| Application | Component | Additional Properties | Antibacterial Activity | Condition | Ref |
|---|---|---|---|---|---|
| Dentistry Implants | TiO2 NTs+ Zn | Cytocompatible | S. mutans, Porphyromonas gingivalis | – | 58 |
| Heat(NH400NTs (NH400) | Osteoblast viability | Porphyromonas gingivalis | – | 63 | |
| Glass ionomer cement (GIC) + TiO2 NPs | Long-lasting restoration | S. mutans | Direct contact with GIC + TiO2 NPs | 58 | |
| TiO2+ PMMA/PEEK nanocomposite | Cytocompatible | E. coli, S. aureus | Under dark condition | 60 | |
| Ag-doped TiO2 NTs(TNT) | – | S. aureus | - | 70 | |
| Orthopedic Implants | TNTs decorated with TiO2 NPs | Stem cell osteogenic | S. mutans, Porphyromonas gingivalis, | Direct contact | 58 |
| Ti-160 | Osteoblast proliferation |
S. aureus,P. aeruginosa Amp-resistant E. coli |
Direct contact (16 hours at 37°C) on to the material surface | 74 | |
| TiO2 +micro-arc oxidation (TM) composites coated on Mg alloy | in vivo degradation behavior and cytocompatible | E.coli | Direct contact | 71 | |
| Medical and Hospital Devices | PLA-TiO2 nanocomposite films | Water absorption properties | K. pneumoniae, S. aureus | Direct contact | 55 |
| Sulfur-doped TiO2 | – | E.coli | Hospital lighting conditions. | 79 | |
| TiO2-coated silicon catheters | – | E. coli K-12 | Exposure of UV light | 78 | |
| TiO2-coated catheters | – | E. coli | Exposure of UV light | 76 |
Antibacterial Application in Dentistry
Titanium is widely applied in dental implants because of its suitable chemical and mechanical properties. However, infection of Ti-based dental implants still creates additional problems that can result in implant failure. Controlling infection and acquiring another technique for placing implants into bones are prominent for long-term dental implant success. Modifying implant surface is one of the effective ways to enhance the Ti implants properties. In this case, TiO2 NTs not only provide antibacterial activity, but also could enhance osteogenic activity of implants.56,57 This approach has been used by Liu et al to confirm that TiO2 NTs, along with Zn, increase implant efficacy due to cytocompatibility and antibacterial functions at a proper concentration. This study depicted a novel surface modification in dental implant applications.58 Furthermore, it has been found that modification of Ti-based dental implants with multilayer TiO2 nano-network can increase cell adhesion, proliferation and mobility.59
Peri-implantitis is the devastating inflammation affecting the soft and hard tissues around dental implants, a process that results in bone loss. Therefore, inhibition of initial adhesion of bacteria on the implant is essential to avoid implant-associated infections.60–62 In a recently published research from 2017, the antibacterial activity and osteoblast viability of heat and plasma treated TiO2 NTs are evaluated. The study was conducted in four groups—including the polished titanium, TiO2 NTs, heat-treated (at 300ºC) TiO2 NT and heat-treated (at 400ºC)—TiO2 NT and also in four groups based on plasma treatment. The results showed that plasma treatment reduces the adhesion of Porphyromonas gingivalis, but it does not affect osteoblast activity. Overall, the heat-treated group at 400°C was chosen as the most suitable for dental implants because of optimum osteoblast and antibacterial activity.63 In another study, hydrothermal treatment of nano-structures on Ti implants at 225ºC for 5 hours presented a new topography with nanoflower structure that showed antibacterial effect against methicillin resistant Staphylococcus aureus. Besides that, these Ti implants did not exhibit cytotoxicity against mammalian cells during the 14 days and improved calcium deposition from osteoblast.64 All the evidence shows that changing treatment parameters—such as pressure, temperature and time—can result in different crystalline phase and topographies.
Tooth decay or dental caries are a common problem that occurs when acid-producing bacteria in the mouth dissolves the outer layers of the teeth. To avoid this, dental disinfectants based on metal oxide nanostructures have shown beneficial in compared to conventional disinfectant such as chlorhexidine.65 According to a previously published study, TiO2-containing nanomaterials have demonstrated antibacterial effect toward the oral pathogenic species of Streptococcus mutants.66 Moreover, the use of TiO2 NPs in dental composites, cement, sealants, bases, liners and adhesive materials yielded a strong antibacterial effect. In a research conducted by Garcia-Contreras et al, it was proposed that glass ionomer cement (GIC) incorporated with TiO2 NPs is a promising dental material for its antibacterial capability and long-lasting restoration against mastication force.67
Antibacterial Application in Orthopedic Implants
A bone infection, which is also known as osteomyelitis, is a consequence of bacteria or fungi invasion. Bone infections are mostly caused by the S. aureus, Staphylococcus epidermidis (S. epidermidis), Enterobacteriaceae and Pseudomonas aeruginosa bacteria.68 Typically, osteomyelitis occurs after bone surgery or bone fracture repaired by implants. In this condition, a prolonged and specific antibiotic treatment is necessary. Therefore, preventing wound and bone infection by self-sterilized implants would be helpful. As mentioned previously in this report, modification of implant surfaces with TiO2 nanostructures is a new approach in this regard.69
Hou et al studied Ag-doped TiO2 NTs to reveal that surface modification of Ti-based implants provides antibacterial properties for TiO2.70 This strategy decreased bacterial adhesion onto the surface of TiO2 NTs as compared to bare Ti surfaces and unloaded TiO2 NTs. In recentwork, TiO2 incorporated micro-arc oxidation (TM) composites coated on Mg alloy introduced as a bioactive material, which possesses antibacterial properties against E.coli, in vivo degradation behavior and cytocompatibility for orthopedic applications.71
In orthopedic fractures, metal pins and wires are employed as a fixation device, and these devices are susceptible to bacterial colonization of S. aureus, S. epidermidis and E.coli.72 It has been suggested that coating a pin or wire with a TiO2-containing material is effective, since photo-killing activity of TiO2 will influence the bacteria more than the host tissue. However, more cytotoxic observation is required before clinical application.
In addition to antibacterial properties of TiO2 modified surfaces, being non-poisonous and environmentally safe are the most important priority for modification of implant surfaces by TiO2 nanostructures.58
Furthermore, TiO2 NPs are able to promote osteogenic differentiation.73 In this manner, for the first time, Liu et al simultaneously investigated antibacterial and stem cell osteogenic properties of TiO2 NTs decorated with TiO2 NPs. This investigation proved that applying TiO2 NPs on TiO2 NTs increases surface area and photocatalysis action of TiO2 NTs. This phenomenon lowered the numbers of S. mutans and Porphyromonas gingivalis on the TiO2 NTs-TiO2 NPs as compared to pure Ti and TiO2 NTs after the first seven days.58 The current research applied the electrophoretic deposition method to produce two distinct surface nanotopographies, Ti-160 and Ti-120. Surfaces treated with Ti-160 showed significant reduction of S. aureus (95.6%), Pseudomonas aeruginosa (P. aeruginosa) (90.2%) and ampicillin-resistant E. coli (81.1%), which was active against both Gram-negative and Gram-positive bacteria. Ti-120 treated surface also decreased the bacterial colonization and, further, caused osteoblast proliferation for up to 5 days.74
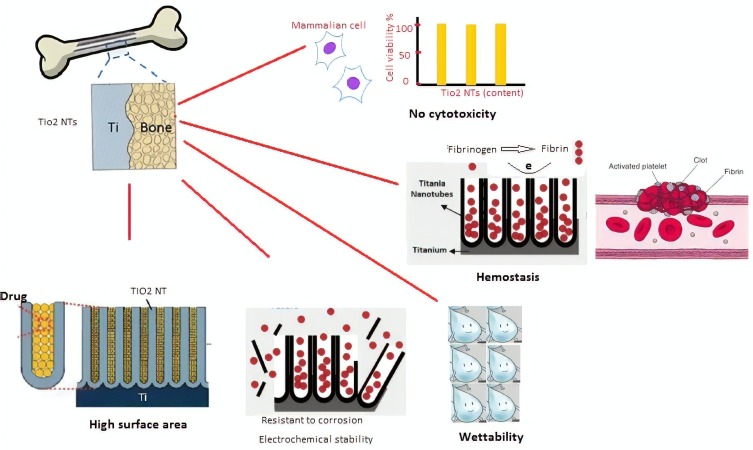
Applying TiO2 NTs arrays as a carrier for drugs—such as antibiotics—is a new approach to overcome infection-related problems, which is discussed in next section.68 Another vital aspect of TiO2 is its biocompatibility, which is clinically proven. This property has also been discussed further in the following section. Furthermore, as demonstrated in Figure 3, the most important aspect in which these nanobiomaterials are used for implant are high surface area, good wettability, resistance to corrosion, homeostasis and low toxicity.
Figure 3.
The main properties of TiO2 nanostructure make it suitable for implant and other in vivo applications.
Antibacterial Application in Medical and Hospital Devices
Microbial biofilms are one of the most serious challenges in the field of medical devices. S. epidermidis and S. aureus are the most common bacteria to form a biofilm on medical devices and cause an infection. Antibacterial properties of TiO2-coated surfaces could be employed in the hospital industry because traditional approaches of disinfection methods are not as effective as photocatalytic methods.75 Bonetta et al reported the application of TiO2 in the coating of Petri dishes and ceramic tiles surfaces to show that TiO2 coated surface is the best in deactivation of E. coli, S. aureus, Pseudomonas putida and Listeria innocua in exposure of UV-light.18
TiO2-coated catheters are another promising antibacterial application of TiO2 in biomedicine due to their safety and potential of light-induced disinfection for clinical use. Sekiguchi et al developed TiO2-coated catheters for clean intermittent catheterization (CIC) and improved the antibacterial activity of the TiO2 catheter against E. coli. As a result, the number of E. coli declined to an insignificant level within 60 min exposure to UV light.76 Besides, TiO2-coated silicone catheters and medical tubes showed bactericidal effect on E. coli cells.77
In clinical and medical devices, sterile needles, such as lancets, are demanded and applied as a self-monitoring device for blood glucose (SMBG) in diabetes. In the progression of needles, the safe-sterilizing capability is one of the serious issues. Up until now, different sterilizing methods have been suggested, among which Gamma-ray irradiation is widely employed. The main problem regarding γ-ray irradiation is equipment costs and γ-ray leakage. Therefore, the antibacterial properties of TiO2—due to its photocatalytic activity—have been examined. Nakamura et al developed a self-sterilizing lancets coated with an annealed TiO2 layer. Their observation determined that these coated lancets had antibacterial properties against E. coli K-12 suspension under UV-illumination.78 Besides, sulfur-doped TiO2 could also be applied for antimicrobial purposes on the surfaces of medical devices under hospital lighting conditions. This study performed by Dunnill et al confirmed that 99.5% of E. coli death occurs after 24 hours of irradiation.79
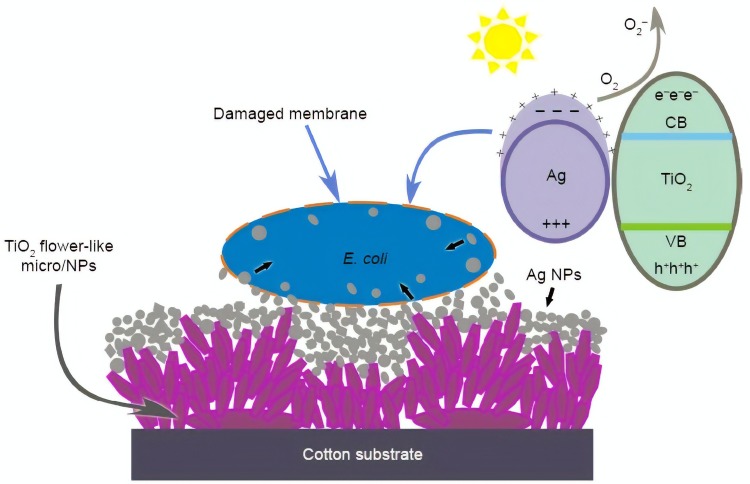
In addition, Li et al,80 fabricated a multifunctional textiles with biocidal activity (composite of TiO2 and Ag NPs) against common Gram-positive and Gram-negative bacteria, along with proposed antibacterial activity (Figure 4). The risk of microbial infection is one of the important factors in producing antimicrobial wound healing materials. On the other hand, uncontrolled bleeding from wound is the main cause of infection in most of accident and surgery cases. Therefore, developing effective materials with hemostatic and antimicrobial property is required.
Figure 4.
The suggested antibacterial activity mechanism of flower-like nanostructures on cotton surface coated with TiO2 and Ag NPs.
Notes: Reproduced with permission from Li S, Zhu T, Huang J, Guo Q, Chen G, Lai Y. Durable antibacterial and UV-protective Ag/TiO2@fabrics for sustainable biomedical application. Int J Nanomedicine. 2017;12:2593-2606. © 2017 Li et al; Creative Commons Attribution – Non Commercial (unported, v3.0) License (http://creativecommons.org/licenses/by-nc/3.0/).80
Several lines of studies have demonstrated that the combination of metal oxides, such as TiO2, with natural polymers can be a promising approach for the control of infection and hemorrhage.47 In this regard, nanocomposites based on TiO2 and chitosan NPs have mostly studied. In 2013, Archana et al developed nanocomposited films consisting of TiO2 NPs loaded with chitosan-pectin, which have high antibacterial activity against a broad range of bacteria and improved hemostatic ability due to the presence of TiO2. Moreover, the introduced nanocomposite showed no toxicity in in vivo and in vivo studies.81 The most important application of these findings could be the design of wound healing bandages, which protect wound from microbial infection and hemorrhage.
Other research evaluated the effects of TiO2 doped films into polyester and polyethylene to inactivate and kill bacteria. The TEM images of damaged E. coli reveal that, after 120 min, outer layers of bacterial cell wall demonstrate discontinuities in some regions.82 In turn, cell bacteria inactivation leads to changes in cell morphology. As the cell membrane regulates the material flow and osmotic pressure into and out of the cell, leakage of internal components of cell caused by the cell wall damage grow on the TiO2 nanostructured film.
TiO2-Based Implants
Due to the nanotopographical characteristics of TiO2 nanostructures, they have successfully been incorporated into medical applications as implants to cover some of the titanium implants’ deficiencies. Here, the application of these valuable nanostructures in bone, dental and drug release implants are going to be discussed.
Bone Implants
Bone is one of the most common transplant tissues.83 There are three main approaches to overcome bone defects. The first is an autologous bone graft, in which bone is harvested from the patient’s own body. Although this method is the gold standard, it suffers from some downsides—like short-term instability, insignificant defects, and limited supply. Bone allografts, or bone transplanted from a donor, is the second method that partly compensates for the drawbacks of the first method. However, the clinical failure of this method is overwhelming (30–60% over ten years). The third consists of employing synthetic implants and engineering of new bone to replace damaged bone; this method is exponentially growing to overcome the limits of previous methods. However, this method also faces some deficiencies, like insufficient mechanical strength necessary to carry load.83,84
There are some key factors regarding the integration of implants and tissue engineering materials with living bone, such as surface properties—micro and nanotopography—and composition. To this end, a bioactive, cell-friendly and artificial implant material was fabricated by researchers.85 In this study, nanotubular structure (from titanate materials) enhanced cellular behaviors and apatite formation ability of cells on this substrate. They claim that these nanostructures made from titanate biomaterials are promising candidates for biomedical application and bone implants.
Fabrication of material surfaces that provide osseointegration and support an implant for our body is one of the key challenges in orthopedic biomaterials. A critical principle to be considered in both bone and all tissue engineering is to provide cell support and guide tissue formation through using 3D, and usually porous, scaffolds. Among metals—such as stainless steel, cobalt alloys, titanium alloys, etc.—nanostructured TiO2 continues to be considered one of the most interesting materials due to their outstanding features, such as low toxicity, flexibility, high corrosion resistance and high tensile strength.86 A qualified scaffold must also possess some essential characteristics, including being biocompatible87 and porous with interconnection porosity to transfer nutrient and metabolic waste. In addition, it must have a unique surface pattern for cell adhesion, growth and proliferation in all types of tissues; in the case of bone-implants, it should stimulate osteogenesis.83,87 Lastly, it is also important that mechanical properties of scaffold be similar to host tissue and that its degradation rate is as fast as the tissue formation rate.
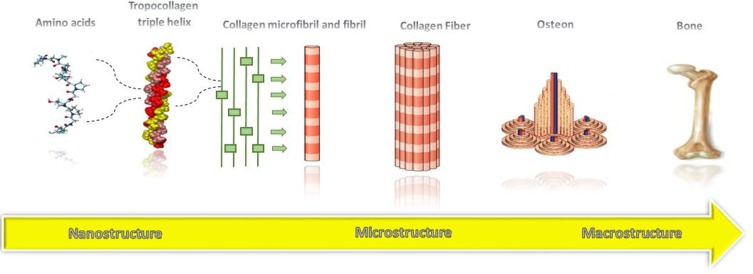
Bone is a natural inorganic-organic composite, including an organic matrix composed of collagen fibrils and an inorganic reinforcement made of Hap nanocrystals.88 As shown in Figure 5, natural bone gains its unique mechanical properties from hierarchical structures assembled in a highly organized way, expanded from nanoscale (collagen fibers) to macroscopic dimensions (dense cortical bone and a macroporous network of bone).83,87 This hierarchical structure of bone provides not only tensile strength but also the toughness of bone. Thus, a successful bone scaffold must simulate the hierarchical structure of bone by bringing together multiple length scale materials with various mechanical properties. Also, the important role of surface characteristics in the interaction of extracellular matrix with the implant and osseointegration is indispensable.29,83
Figure 5.
The hierarchical structure of natural bone.
Osseointegration is defined as a direct structural and functional connection between ordered, living bone and the surface of a load-carrying implant. In other words, osseointegration is a development of newly formed bone around the artificial implant. In this regard, the interaction between tissue and implant at the interface is the crucial factor of implant success or failure. Nanotopography of the scaffold, which promotes cell function, is one of the most important criteria in osseointegration. In this regards, titania nanostructures have attracted much attention.29
Titanium and its different alloys have been used as the main material in bone implants because of their high specific strength, satisfactory stability, low elastic modules that perfectly match with elastic modules of tissue, and ability to form a thin and stable oxide (TiO2) layer on the surface that is resistant to corrosion. However, disadvantages include reduced interaction with surrounding tissue and their bioactivity.88 One of the approaches to overcome these problems is to coat the surface of this metal family with biocompatible compounds.89
In 2015, the Chung group coated the Ti surface with hydroxyl apatite-TiO2 (HAp-TiO2) and immobilized BMP-2 on the surface to construct a uniform nanoporous structure. BMP-2 (bone morphogenetic protein-2) is the group of growth factors that are unique because of their influence on cell differentiation of osteoblasts. Furthermore, HAp-TiO2, with a proper porous structure, encourages cell growth and adhesion. All of these features lead to a reduction in bone healing time at a bone-implant surface.90
Mohammadi et al completed an experiment to compare the impact of SiO2, TiO2 and SiC on enhancement of setting time, mechanical strength and hydraulic reaction of calcium phosphate bone cement. Their results indicated that TiO2 NPs are an excellent addition to enhance mechanical strength of calcium phosphate cement in the short-term.91 The nanometric thickness increased surface area and porous structure of TiO2 NTs coatings, accelerating cell adhesion and improving bone capabilities.92
Even the diameter of TiO2 NTs is one of the important factors in cell adhesion and osseointegration. In 2013, the influence of different diameters prepared by anodization TiO2 NTs on the adsorption of collagen type I was investigated.93 Collagen type I (Col-I) was adsorbed with a higher outcome and faster speed on TiO2 NTs with diameter around 100 nm compared to 30 nm, in which the molecular dynamics simulations confirmed that Van der Waals forces and the hydrogen bond between Col-I molecules and TiO2 NTs were the main driving force in adsorption mechanism. Thus, not only does TiO2 NTs dimension have an essential role in the regulation of biological functions of cells, but also—as this research demonstrated—it influences the adsorption capacity of collagen on this family of nanostructures.93
In a recently developed study, an efficient method was described to enhance the adhesion of TiO2 NTs. This utilized high-pressure torsion treatment to encourage grain refinement on the basic Ti layer. This process also improved the osseointegration and biocompatibility of TiO2 NTs due to the increased surface elastic modulus. In this research, it has been shown that TiO2 NTs with length of 0.4 µm, because of less interfacial stress, have high adhesion strength as compared to the longer NTs with length of 2 µm.94
Employing an innovative method, Alves et al managed to fabricate a collection of 50–90 nm diameter TiO2 NTs, which simulated the morphology of micron structure of natural bone, and filled them with bioactive elements (calcium and phosphorous) to construct a more efficient bio-functional implant surface. The results demonstrated an enhancement in osteoblastic cell function and adhesion at the interface, along with displaying an improved corrosion behavior for TiO2 NTs.95 Not only do they participate in the bone-implant interface, but TiO2 NPs could also play an important role in improving the quality of implants. Polymethylmethacrylate (PMMA) is bone cement with the primary function of transferring forces from bone to prosthesis. PMMA has been used in surgical fixation of artificial joints for a long time.96 Khaled et al studied the physical and mechanical properties of PMMA bone cement that was reinforced with nano-sized titania fiber fillers (0–2%). The results revealed significant improvement in fracture toughness (63%), flexural strength (20%) and modulus (20%), and the optimal composition contained 1% wt nanostructured TiO2 fibers compared to control cement.97
Dental Implants
Organ loss due to disorders, disease or injuries leads to the need for organ restoration—for example, joints or teeth—which is conventionally performed by replacement with artificial material. These artificial materials should be connected to surrounding tissues via fibrous connective tissues in order to efficiently perform their biological functions.98,99 Like other organ replacements, the long-term success of dental implants largely depends on rapid healing with safe integration into the jaw bone and surface. Among the few characteristics that define a dental implant system, the surface treatment, topography, macro design and geometry of implant are the main parameters for both short- and long-term durability of dental implants.100,101
As a significant effective factor on osseointegration, implant surfaces—which are critical for implant stability—have been studied and developed further in recent decades to provide a faster and more adequate osseointegration process in newly formed bone. Surface characteristics of the dental implant are defined at the macro-, micro-, and nano-scale. The most common form of nano features encountered in dental implant surfaces is nanoroughness.91,102 In other words, along with chemistry of dental implants, surface topography is crucial to achieving suitable osseointegration. The porous surface has a crucial role in this area. A porous surface provides more substantial space for drug loading and, with better biocompatibility, TiO2 NTs arrays are desirable candidates to cover the surface of the dental implant. A study in which TiO2 NTs were grown on dental implant surfaces by anodic oxidation method confirmed abovementioned fact. TiO2 NTs with a diameter of 60 nm and length of 10 μm that were loaded with BMP-2 showed improved osseointegration.103
The interaction between implants and blood play a vital role in peri-implant healing. In some cases, immunological interaction due to the exposure of implantable titanium causes inflammation or fibrosis, leading to implant failure. It has been demonstrated that TiO2 NTs have good blood-compatibility, indicating their high potential for surface modification of blood-contacting implants.46 Even immobilizing some biological molecules—like Gly-Arg-Gly-Asp-Ser peptide—on TiNTs may improve osseointegration in dental implants.104
A 20–160 nm mixed nano-/submicron-scale TiO2 network layer successfully enhanced human bone marrow stem cell adhesion on titanium dental implants. The result was significantly positive.105 TiO2 mesh layer on a polished Ti surface with averaged lateral mesh size (between 34 and 93 nm) showed a better blood response, since size scale of the nano-mesh TiO2 layer was similar to that of blood proteins. All these actions led to promoted cell growth in the application of dental implants.106 Also, the increment in blood compatibility is associated with minimizing the activation of both blood platelets and blood clotting cascade on the implant surface in the plasma. Huang et al investigated the effects of different constructions of the TiO2 NT on blood platelet behaviors and adhesion. The results of their research suggested that surface chemistry, structure, wettability and crystalline phase had a collegial effect on the platelet-rich plasma responses, where amorphous TiO2 NTs with larger diameter could reduce platelet adhesion and activation. Additionally, it has been reported that combination of several properties of TiO2 NTs—such as wettability, surface topography and chemistry—can improve cell adhesion, migration, proliferation and differentiation. In this case, development of high contrast wettability patterns on surface of TiO2 nanostructures have been a promising approach to achieve more applicable biomedical applications, such as implant scaffolds.107,108
Several lines of studies have demonstrated that crystalline phase plays a key role in cell behaviors and blood compatibility of TiO2 NTs. TiO2 NTs with mixed crystalline phases could cause a decline in platelet adhesion and fibrin network formation phenomena.109 It has been proposed that rutile TiO2 NTs provide highest protein adsorption of collagen and fibronectin. While crystalline phase of TiO2 NTs has no significant effect on cell proliferation and differentiation.110
In conclusion, the nanotopography of dental implant surface results in better osseointegration, cell adhesion and blood response of dental implants, which are very important in the success of the implant.
Like bone implants, incorporation of some biological compounds can promote TiO2 biocompatibility in dental implants, too. Biocompatibility of TiO2/HA nanocomposites with surroundings tissue has been evaluated by Youn et al. They coated the surface of commercial titanium implants with nano TiO2/HA composite bioceramic utilizing sol-gel route. In order to examine the nerve regeneration, cultured Schwann cells were used. SEM, MTT assay, total protein content, leakage of cytosolic lactate dehydrogenase (LDH) activity and measurement of the amount of brain-derived neurotrophic factor (BDNF) secreted by Schwann by enzyme-linked immunosorbent assay (ELISA) was performed to evaluate the response of the cells to the coating. The observed results emphasized the good biocompatibility of TiO2/HA nanocomposite bioceramic coating with Schwann cells for nerve regeneration around dental implants.111 A novel multiform TiO2 nano-network was introduced for dental implants by Yang and Huang.112 The authors highlighted that the hydrophilicity and protein adsorption ability were improved on nano-network structures leading to enhance cell adhesion, mobility, proliferation and osteogenic differentiation.
Drug-Releasing Implants
After implant surgery, the patient receives drugs—such as antibiotics, anti-inflammatories and growth factors—to prevent infection, inflammation and induce suitable integration of implant with natural tissue, respectively. These medicines are delivered by four different delivery routes, namely oral, intravenous, intramuscular and topical. The uptake of antibiotics is only provided by intravenous, intramuscular and topical routes because this group of drugs travels to the liver, where it is then inactivated. Although growth factors are often delivered intravenously and topically, systematic growth factor delivery is not effective enough, as the drug cannot reach the interface of implant and tissue. Systemic doses increase is not a solution to this problem because it leads to cellular toxicity.113 Therefore, the need for development of a more suitable directed drug delivery system to the site of implantation is unavoidable. This strategy has the advantage of minimizing the side effects of systematic administration by decreasing the drug concentration.114 In the following, two novel local drug release systems based on TiNTs, and TiO2 mesoporous films is introduced.114
TiNTs arrays, fabricated by electrochemical method with pore diameter of 10 to 300 nm, thickness of 0.5 to 500 μm, high pore volume of more than 20 m3/g, high surface area of 180 to 250 m2/g, high aspect ratio of ~1000–2000 and NT density of ~1010 NTs/cm2, have been investigated for application in local drug delivery. The system can be described as a layer of tightly packed, vertically aligned, ordered NT structures with hexagonal arrangement that grows perpendicularly to the Ti surface. TiNTs have also attracted considerable attention because of their NT structure, high surface area and loading capacity, tunable drug release capability, tailorable surface chemistry, both chemical stability and mechanical rigidity, terrific biocompatibility, and also the ability to grow on the surface of current implants. Logic and his research group have done fabulous work in this area and provided most of the comprehensive reviews on this topic, so it will not be covered further in this work.68
Mesoporous TiO2 films not only provide a chemically inert mesoporous surface to combine with either active low molecular weight hydrophobic or hydrophilic substance, but also they promote osseointegration as they supply nanotopography on the surface. A 200 nm-thick mesoporous TiO2 thin film with 6 nm-sized pores has been developed and applied to the surface of titanium implants. Then, it was loaded with renowned osteoporosis drugs, Alendronate (ALN) and Raloxifene (RLX). The drug-loaded implant was compared to the implant without drugs regarding implant fixation and de novo bone growth. Early bone-implant fixation by both drugs has been improved by the ex vitro evaluation, and locally delivered RLX got the most surprising results.114
The mesoporous TiO2 thin film also can be loaded by antibacterial agents, as they can provide a high surface area in order to load high doses of drugs. In 2014, a mesoporous thin film fabricated using the previously mentioned method was loaded by four different antibacterial agents, and their antibacterial effect was evaluated against five different bacteria species. Additionally, silver NPs and minocycline—as two antibacterial agents—were loaded together to be evaluated for their synergic bactericidal effect. The combination of these two antibacterial agents affected all five types of bacteria. Because of the high capability of releasing the antibacterial agent, this device could be applied to the field of dental and orthopedic implants to prevent bacterial infections.115
Osteoporosis drugs and antibiotics are not the only agents able to be loaded on nanoTiO2 coated surfaces. Magnesium (Mg), an important element in proliferation and metabolism of osteoblast cells in the human body, can also be incorporated onto these surfaces. It is a critical element for protein formation and growth factor expression, while also aiding bone mineral deposition on the surface of implant.116,117 Mesoporous TiO2 thin films loaded with Mg have been used to evaluate the impact of Mg on osseointegration, osteoconductivity and osteoblast differentiation. The results demonstrated that local release of Mg from mesoporous TiO2 thin film coating causes higher expression of OC, RUNX2 and IGF1 genes, which are involved in osteoblast differentiation and bone formation.118 Also, new bone formation occurred around all surfaces, and there was an observation of a tendency toward more bone-to-implant contact.116 The newly formed bone additionally displayed a similar bone mineral density to that natural bone.118
Strontium (Sr) is a promising mineral that can take part in decreasing bone fracture risk in osteoporotic patients. It has attracted much attention after the development of an anti-osteoporotic drug, Strontium Ranelate (SR).119 An increase in osteoblast replication and mineralization of bone matrix result of Sr is achieved via the calcium-sensing receptor (CaR) dependent mechanism. In 2013, an experiment conducted by Zhao et al examined the influence of Sr on osteogenic effects and properties. They loaded different amounts of Sr in TiO2 NTs with different diameters. The results determined the positive effects of NT10-Sr3 in inducing differentiation of osteogenesis, bone morphogenetic protein, mesenchymal stem cells and adsorption of favorable proteins for cell function.119
Polyetheretherketone (PEEK) is an FDA approved thermoplastic biopolymer that has been used extensively in orthopedic applications because of excellent mechanical, chemical and biocompatible properties. The inert nature of PEEK is the most critical impediment. Lately, it has been demonstrated that titanium film improves this property as a bioactive coating and could be directly used onto PEEK implants by e-beam evaporation. In a study, Roy et al increased in vitro biocompatibility of PEEK by designing nanoporous TiO2 surface that allowed in vivo osteoconductivity of implant by enhancing immobilization and delivery of BMP-2.72
TiO2 coatings on implant surfaces not only deliver a drug locally to the target but also reduce organ toxicity. TiO2 NTs have a high capability to be applied to the Ti implants, because they increase biocompatibility and surface area of the implant, however, uncontrolled drug release and poor mechanical properties are two crucial disadvantages with TiO2 NTs. From this aspect of view, Jia and Kerr engineered a drug carrier for ibuprofen by using biocompatible PLGA (polylactic-co-glycolic acid)/TiO2 NTs instead of pure TiO2 NTs. Ibuprofen is an anti-inflammatory drug with very short plasma half-life (1–3 h). Developing polymer/TiO2 NTs increased the time of ibuprofen drug release up to 5 days for low-molecular-weight PLGA and nine days for high-molecular-weight PLGA.120
Moreover, Gulati et al attempted to enhance drug release by coating TiO2 NTs with different thicknesses of chitosan or PLGA. TiO2 NTs were produced through a two-step anodization procedure. The result revealed that thin PLGA coatings through one dip-coating cycle yielded 57 ± 1% burst drug release during 6 hours, which is faster than thin chitosan, yielding 40 ± 2%. In a contrary manner, thin PLGA and chitosan coatings obtained after five dip-coating cycles released 12 ± 2% and 35± 2% of the drug during 6 hours, respectively. These results confirmed that the type and thickness of polymer are two critical factors to control drug release quantity. Furthermore, the result of this study confirmed that polymer-coated TiO2 NTs exhibit higher osteoblast adhesion and proliferation compared to non-coated TiO2 NTs.121 Likewise, another study presented chitosan coated TiO2 NTs, which were used for controlled drug release of gentamicin. These NTs demonstrated simultaneous antibacterial properties and osteoblast adhesion.122 Development of such strategies can overcome essential problems in orthopedic implants related with bacterial infections and bone cell promotion.
Designing a sustained drug releasing system for veterinary medicine is another area of research that significantly decreases the harmful side effect and improves the therapeutic effect for the animal disease. The Enrofloxacin Hydrochloride (Enro) is a veterinary antibiotic with the lowest minimum inhibitory concentrations (MICs) mostly used in poultry. Lai et al developed a long-term sustained releasing system for Enro drug. This group used hydrothermal reaction method for concocting a drug by TiO2 NTs, which have a high specific area (246.784 m2/g) and desirable controlled release ability. in vivo drug delivery of Enro-TiNTs showed drug release rate of 32.65% in the PBS solution, while the desired release profile was achieved in low temperatures.123
Biosensors Based on TiO2
In recent years, nanotechnology has made considerable progress in the development of biosensors.6,14 Due to their unique properties (e.g., small size and big surface area-to-volume ratio), nanomaterial-based biosensors provide sensitive and rapid biological detection. Although different kinds of nanomaterials have been used in medical biosensors, this section of the paper is exclusively confined to nanobiosensors based on TiO2 nanostructures.124–126
Recent interest in novel hybrid systems based on biomolecules and TiO2 nanostructures has led to considerable success in the fabrication of bio-nano hybrid devices, such as biomolecule-sensitized solar cells (BSSCs) and photoelectrochemical cells (PECs).127 Apart from these above-mentioned devices, interfacing of biomolecules and TiO2 nanostructured thin film has opened up a route for further developments in this area, including biosensors. Sensitive detection of biological analytes provides opportunities for further investigation into the process involved in monitoring patient response to medical or surgical therapy. To be commercially viable, a biosensor should be inexpensive, user-friendly, sensitive and accurate, fast, and easily manufactured with high select rates.25,128,129
Furthermore, TiO2 nanostructures have been employed to construct various detecting devices, such as humidity, oxygen and hydrogen sensors. These nano-sized semiconductors have proven to be an excellent electrode material in biosensors due to their special characteristics, such as porous structure, large specific surface area and excellent biocompatibility. TiO2 can be used as immobilizing matrix, which reacts with the amine and carboxyl groups of enzymes and maintains their biocatalytic activity.130,131
TiO2-based nanocomposites have attracted considerable interest for use in the biosensor. In 2015, Wang et al used DNA functionalized nanocomposite containing electro-reduced graphene oxide, TiO2 nanowires and chitosan to detect the specific title gene sequence from Vibrio parahaemolyticus. The presence of TiO2 nanowires increases the interface area of nanocomposite.132
TiO2-based biosensing of target analytes is commonly carried out by electrochemical (e.g., amperometric and potentiometric process) or PEC techniques. Electrochemical biosensors are usually based on amperometric and potentiometric detection. When the bio-recognition elements—such as antibodies, enzymes, aptamers or cells—bind to or react with target molecules, the conductive properties of a medium between electrodes changes, resulting in a detectable current signal (amperometric), a detectable potential or charge accumulation (potentiometric).
Among various electrochemistry-based biosensors developed so far, the PEC biosensors that are based on the photoelectric phenomena have attracted a great deal of attention. The PEC assay has the advantage of enabling both optical and electrochemical detection. Because of the significant scientific and technological interest in recent years, here, we focus on PEC biosensors.
Depending on the intended manner of biosensing, the construction of biosensors can be different.12 In general, a typical TiO2-based PEC biosensor comprises three main components: a nanostructured TiO2 film deposited onto a conductive substrate as the working electrode (WE), which is responsible for generating and transporting electrons and holes under illumination; a counter electrode (CE) coated with some electrocatalyst, and an electrolyte placed between the two electrodes to shuttle the holes to the CE.
The PEC biosensors are based on the PEC process in which photon-to-electricity conversion occurs. The general mechanism of the PEC process involves photon absorption by the semiconductor, generation and separation of electron (e−)-hole (h+) pairs, and transfer of electrons to the WEand holes to the electrolyte. The presence of target analyte changes the photocurrent generated by the system; this change in photocurrent is proportional to concentration of target in the sample (Figure 6).
Figure 6.
The schematic diagram of the biosensor PEC process.
Various biomolecules, such as enzymes and DNA, have been used as bio-recognition elements. For instance, in the case that DNA is conjugated to TiO2, the presence of target DNA leads to formation or cleavage of double-stranded DNA resulting in change in light-harvesting performance of the WE directly or indirectly. So, this causes a change in charge separation and, subsequently, the generated photocurrent.133 The material used as a transducer in TiO2-based PEC biosensors can be made of (a) TiO2 alone, (b) TiO2 hybrid with some inorganic semiconductors, or (c) composite of TiO2 with other materials.
Narrow-band gaps quantum dots can sensitize TiO2 NPs and achieve energy band modulation. CdS NPs with a bandgap as narrow as 2.4 eV and a broad excitation spectrum are ideal sensitizing materials. Excitation of the CdS causes charge separation and prompts transfer of conduction band (CB) electrons to that of TiO2.
Recently, CdS QDs/TiO2 NTs hybrid has been used for sensitive detection of prostate-specific antigen (PSA), which is important as a tumor marker for prostate cancer.134 The CdS QDs loaded TiO2 NTs were used as transducers. The sensitization of TiO2 with CdS can expand the wavelength range of excitation and improve the photoelectric performance of TiO2 electrodes. Also, the coupling of CdS and TiO2 can reduce the recombination of the photo-induced electrons and holes; so, these phenomena result in higher conversion efficiency.135
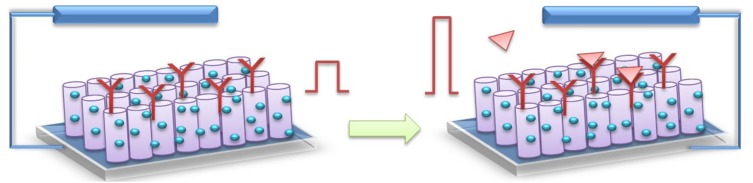
In the presence of PSA, through an immune-sandwich assembly, immune-gold labeled alkaline phosphatase (ALP) was attached to the CdS QDs/TiO2 NTs electrode (Figure 7). ALP is able to catalyze ascorbic acid 2-phosphate (AAP) hydrolysis in situ in order to generate AA for efficient electron-donating. Results indicate that increased PSA concentration caused improved photocurrent responding.
Figure 7.
Schematic demonstration of PEC biosensors based on TiO2 NTs. The signal is enhanced in the presence of target molecules.
Recently, a PEC biosensor based on CdS sensitized Fe-doped TiO2 has been reported by Fan et al for the detection of squamous cell carcinoma antigen. Iron doping of TiO2 improves the absorption of composite in visible region. In addition, rough surfaces of Fe-TiO2 NPs are suitable for sufficient binding of carboxyl functionalized CdS NPs.136 PEC biosensors can be integrated with other detection methods to create biosensors with desired properties and behaviors. For example, Bernacka-Wojcik et al developed a disposable biosensor by combining dye-sensitized solar cells (DSSCs), in which TiO2 nanoparticulate films were used as a photoanode and colorimetric DNA detection method for sensing specific DNA sequence from Mycobacterium tuberculosis.137
The mechanism of the biosensor is described as follows. A source of light with certain wavelength is adjusted to shine directly on the solution containing DNA functionalized gold NPs and passes through it. Finally, a DSSC, placed after the solution, absorbs passed light and generates photocurrent. Since the dye adsorbed on the surface of TiO2 nanoparticulate film has the maximum absorbance at the wavelength of shined light, any obstacle in the absorption of light at this wavelength leads to a decline in photocurrent.
Target DNA can bind to complementary strands of target DNA attached to the Au NPs, leading to their aggregation. Monodispersed and aggregated Au NPs have different absorbance peaks. In the presence of target DNA in Au NPs solution, the absorbance peak of solution at the wavelength similar to those of the shined light decreases, so the DSSC can capture more light at this wavelength to increase generated photo-current. The biosensor was able to detect the aggregation of Au NPs concentrations as low as 1.0 nM.
In another work, a nanocomposite of g-C3N4 and TiO2 nanosheets was used to fabricate a PEC biosensor that was able to detect glucose within a detection limit of only 0.01 mM.138 Two-dimensional TiO2 nanosheets with a high specific surface area showed promising potential to accommodate a substantial load of glucose oxidase. Besides, in order to excite PEC biosensor by visible irradiation and avoid possible deactivation by UV light, g-C3N4 was used to minimize the bandgap of the nanocomposite.
In conclusion, nanobiosensors based on TiO2 nanostructures (such as NPs, nanorods, and NTs) are developed for sensing of wide range of biomarkers (including DNA, RNA, proteins, lipids, metabolites, etc.).139 In recent years, PEC biosensors based on TiO2 nanostructures have attracted much attention due to their desirable sensitivity and great analytical performance.140,141 However, further research is needed to provide more sensitive and selective TiO2-based biosensors to be used in clinical applications.
Toxicity and Biocompatibility of TiO2 Nanostructures
In recent years, nanoscience has been more widely is used as an interdisciplinary field of medical science and engineering.1,13 Nanomaterials with unique physicochemical properties, such as large surface area to volume ratios, led to being enormous reactive compounds compared with bulk materials and, thus, can be potentially hazardous.142,143 Undoubtedly, considering the wide range of nanomaterial applications throughout our daily lives—like health, food, medicine, industry, cosmetics, agriculture, etc.—the nanotoxicity evaluation and safety assessment of nanomaterials is an important area, given the limited information available in this regard.144–146
Understanding the toxicity mechanism of nanomaterials will be useful for predicting the potential toxicity of nano-sized particles on human health. In this regard, several in vivo and in vitro studies have been completed to assess ROS generation, changes in activity of antioxidant enzymes, lipid peroxidation, damaged DNA bases, protein carbonyl content, total glutathione concentration, superoxide dismutase activity, catalase activity and total antioxidant potential.143,145,147
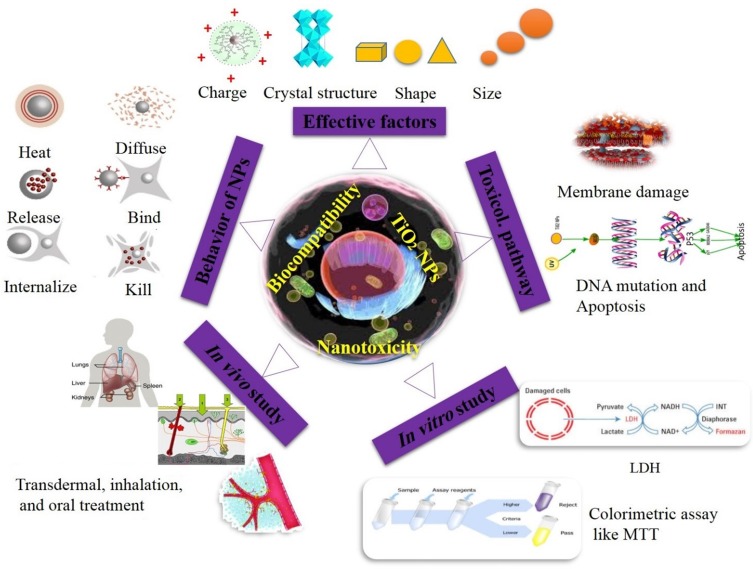
Nano-sized materials that are directly related to human health must have no allergic or inflammation effects. Therefore, design and fabrication of nontoxic materials that have no hazardous potential for human health are one of the nanotoxicology research aims.145 NPs can interact with macromolecules and cell organs for a long time in different ways, such as circulation, extravasation, diffusing, binding, internalizing, degrading, releasing, reacting, killing, regulation of expression, remodeling and normalizing (Figure 8).148 Considering the small size of nanomaterials, these particles can penetrate and undergo uptake into human cells and tissue through ingestion, skin penetration, injections and inhalation.149 Besides, nanostructures toxicity commonly demonstrate effects through membrane damage, dysfunction of protein, DNA mutation, cell apoptosis, proliferation behavior and more (Figure 8).
Figure 8.
Key parameters affecting the toxicity of TiO2 nanostructures and methods of assessing their toxicity.
The primary mechanism of NP toxicity is ROS and subsequent production of oxidative stress. The dangerous aspect of this mechanism eventually ends up in amyotrophic lateral sclerosis, cardiovascular, pulmonary, neurodegenerative, arthritis, renal or cancer diseases.147,149 ROS production is one of the main mechanisms of nanotoxicity, which could trigger a series of oxidative stress that affects some physiological redox-regulated functions and leads to cell disruption. Therefore, it causes several toxic mainstream events, including occurrence of apoptosis, genotoxicity, uncontrolled cell signaling, change in cell motility, cytotoxicity and cancer initiation.13,32 In addition, oxidative stress at a higher level could lead to intracellular Ca2+ release and change in mitochondrial membrane structure.147,150 Rallo et al recommended that oxidative stress induced by NPs is associated with cell signaling changes in three stages named low (nrf2 transcription factor defense against oxidative stress),151 high (activation of inflammation signaling pathway via NFкB) and very high level (activation of cell self-destruction pathways and necrosis) of oxidative stress.152
As mentioned, TiO2 NPs are one of the most commonly used nanomaterials with medical, industrial, and commercial applications.146,147,153,154 The aim of drug carrier systems is to enhance efficacy and decrease adverse reactions of drug molecules.144 Nevertheless, the TiO2 particles in the nanometer-scale have unknown hazardous effects.153 Therefore, before using each NPs—especially TiO2, we need to know about its biocompatibility and toxicity in advance. Generally speaking, toxicity of a nano-sized material is mostly dependent on the NP type, concentration, time of exposure, physico-chemical properties of the particles (size, shape, particle surface, surface ± charge and surface-containing groups), aggregation, mode of interaction with cells, NPs ion release, dissolution, crystal structure (Ru or An), hydrophobicity, bandgap, pH of medium, surface coating group and functionalization. These properties of NPs can affect cellular uptake and generate adverse results (Figure 8).147,148,155,156 For instance, accumulation of NPs with high aspect ratios (NPs shape) in macrophages is slower than ones with low aspect ratios. This, in turn, leads to reducing the time of clearance and efficiency of removal from the blood.148 As described, characteristic properties of nanostructures influence their biological interaction with surrounding molecules.149 It should be mentioned that cells can only uptake drug nanocarriers smaller than 300 nm. Therefore, size is a key factor of toxicity for a drug carrier system to be easily uptaken by cells and tissues.1,2,7,13,154
On one hand, as previously mentioned, size is one of the significant determinants that affect interaction of NPs with cells and proteins and is a key factor in evaluation of toxicity of NPs. On the other hand, size and surface area to volume ratio affect the ability of nano-sized particles to penetrate cell membranes and other biological barriers, leading to several side effects.144,156 Palaniappan et al pointed out that the structural conformation of proteins is dependent upon particle size of TiO2.142 Apart from size, the particle shapes—such as rode, spherical, fibers or tubes—are a crucial factor to determine the toxicity and have different toxicity potential because of easier or faster endocytosis uptake, or perhaps different contact area with the cell membrane.
Surface charge is one of the other most important factors of NPs toxicity, as it influences the hydrophobicity of NPs, adsorption of ions, interaction of NPs with physiological environments, can affect the NPs and protein interactions, all of which may change bio-distribution of NPs. However, toxicity and undesirable effects of NPs can be reduced by coating, which affects the stability of NPs and inhibits their agglomeration.149,156 Crystalline structure is one of the other factors of ultrafine particle toxicity. The TiO2 crystal structure effect (Anatase: TiO2–An with <25 nm and Rutile: TiO2–Ru with<100 nm) was evaluated on the genotoxicity responses in human hepatoma HepG2 cells. The results showed that the genotoxicity potential of TiO2 NPs varies based on crystal structure, so that genotoxicity of TiO2–An crystal structure (with smaller size) is more inducing than TiO2–Ru crystal structure, whereas both crystal structures caused ROS production, DNA damage and up-regulated mRNA expression of p53. This study recommended that not only size but also crystalline structure influence the NP toxicity.157
Suh et al studied toxicity by several different metal oxide NPs using cell membrane damage assays, and among the metal oxides, TiO2 tends to be less hazardous to organisms than other nanomaterials (TiO2<Al2O3<SiO2).158 Titanium oxide is a harmless metal oxide and is non-toxic; therefore, regarding their good biocompatibility, titanium NTs can be used as drug carriers.143,153,154 Shokuhfar et al introduced a biocompatible nanocarrier in 2013 for anti-inflammatory drug molecules delivery based on TiO2 NTs and reported that this system can be applied to many other drug systems.154 Wang et al developed a pH-controlled antitumor drug release system of TiO2–NTs. They reported that TiO2–NTs carriers have good biocompatibility and excellent drug delivery properties.159 A comparative study of ZnO and TiO2 NPs nanotoxicity was conducted by De Angelis et al (based on NPs size, number of particles, surface area, surface charge, agglomeration state, impurities, chemical composition and ion-release; results showed that ZnO NPs produced more significant cytotoxicity than TiO2. They found that TiO2 NPs were not cytotoxic, but both ZnO and TiO2 induced ROS generation. In addition, they suggested that due to spherical shape of these TiO2 NPs, higher cell interaction of TiO2 was observed in comparison to ZnO NPs, which consequently leads to higher cellular uptake. Also, the ROS production was lower after 24 h exposure to TiO2 NPs than that observed after 6 h. For this reason, they suggested that maintaining antioxidant potential of cells after 24 h TiO2 NPs exposure is the reason for low level ROS production.160
One of the most important issues which must be considered to select NPs type in medicine and nanomaterials-based products is toxicity or safety of nanomaterials. Toxicity of TiO2-based nanostructures have been widely evaluated as in previous researches. These nanostructures are safe (or at least less toxic than other types of NPs), user-, and eco-friendly than other nanostructures.
Summary and Perspectives
TiO2 nanostructures—including NPs, NTs and nanorods—and their composites have attracted further attention in the field of medicine due to their unique properties, such as non-toxicity, biocompatibility and affordability.1,11,12,161,162 Biomedical applications of these fascinating nanomaterials can be categorized into four main groups: biosensing, drug delivery, antibacterial activity and implant applications. The potential application of TiO2 in electrochemical and PEC biosensors for detection of various analytes has been widely investigated in recent years. Possessing the two methods for excitation (light) and detection (current), PEC biosensors are considered to be very sensitive technique with low background signals and good analytical performance. However, this method also faces some deficiencies. TiO2, with a wide bandgap (3.2 eV for anatase, 3.0 eV for rutile phase), absorbs only a small portion of the solar spectrum in the UVregion.163,164 In addition, the photogenerated holes formed at illuminated TiO2 can have a destructive effect on biomolecules.165 It is very important to investigate toxicity of special nanostructures used in biomedical applications. We reviewed toxicity of TiO2 nanostructures in various manners, such as drug delivery, implant application and more.
Moreover, the effective factors in toxicity, toxicological pathways and different in vivo and in vivostudies are mentioned. A growing body of research demonstrates that TiO2 nanostructures are safer than many other nanomaterials for biomedical applications. However, much more information and careful evaluation of toxicity and clinical outcome of TiO2 is required before applying it to clinical practice. The development of bacterial resistance to common antibacterial agents and necessity to perform self-sterilizing conditions highlights the need for robust materials to control bacterial infections. It has been demonstrated that TiO2 NPs can fulfill this role effectively.
Despite powerful antibacterial activity of nano-sized TiO2, its light dependent property has been reported as its drawback. However, this property has made TiO2 a smart nanomaterial for both antibacterial and drug delivery uses. Recent advances have also confirmed that TiO2 NPs can have light-control drug release. This phenomenon can be a compelling aspect for most cancer therapy strategies, such as radiotherapy of skin cancers. Besides, modifying chemotherapy agents by TiO2 NPs improve bioavailability and release time of the drug for clinical applications.
The unique properties of TiO2 nanomaterials, like nontoxicity and nanotopographical characteristics, have attracted more and more attention to the fabrication of implants. Some of the titanium implants’ deficiencies can be covered by Titania. The literature has investigated the potential of TiO2 as bone, dental and drug release implants. Considering all of these aspects, it can be stated that nanomaterials based on TiO2 offer the potential to develop valuable materials for biomedical applications. Finally, in order to properly develop and use TiO2 nanomaterials in medicine, further studies will undoubtedly be necessary.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Darvishi MH, Nomani A, Hashemzadeh H, Amini M, Shokrgozar MA, Dinarvand R. Targeted DNA delivery to cancer cells using a biotinylated chitosan carrier. Biotechnol Appl Biochem. 2017;64(3):423–432. doi: 10.1002/bab.1497 [DOI] [PubMed] [Google Scholar]
- 2.Razavi H, Janfaza S. Ethosome: a nanocarrier for transdermal drug delivery. J Paramed Sci. 2015;6(2):2008–4978. [Google Scholar]
- 3.Hashemzadeh H, Allahverdi A, Sedghi M, et al. PDMS nano-modified scaffolds for improvement of stem cells proliferation and differentiation in microfluidic platform. Nanomaterials. 2020;10:688. doi: 10.3390/nano10040668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esfandyari J, Shojaedin-Givi B, Hashemzadeh H, Mozafari-Nia M, Vaezi Z, Naderi-Manesh H. Capture and detection of rare cancer cells in blood by intrinsic fluorescence of a novel functionalized diatom. Photodiagnosis Photodyn Ther. 2020;101753. doi: 10.1016/j.pdpdt.2020.101753 [DOI] [PubMed] [Google Scholar]
- 5.Garimella R, Eltorai AE. Nanotechnology in orthopedics. J Orthop. 2017;14(1):30–33. doi: 10.1016/j.jor.2016.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janfaza S, Nojavani MB, Nikkhah M, Alizadeh T, Esfandiar A, Ganjali MR. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Mikrochim Acta. 2019;186(3):137. doi: 10.1007/s00604-019-3241-z [DOI] [PubMed] [Google Scholar]
- 7.Razavi H, Darvishi MH, Janfaza S. Silver sulfadiazine encapsulated in lipid-based nanocarriers for burn treatment. J Burn Care Res. 2018;39(3):319–325. [DOI] [PubMed] [Google Scholar]
- 8.Hashemzadeh H, Allahverdi A, Ghorbani M, et al. Gold nanowires/fibrin nanostructure as microfluidics platforms for enhancing stem cell differentiation: bio-AFM study. Micromachines. 2020;11(1):50. doi: 10.3390/mi11010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashemzadeh H, Javadi H, Darvishi MH. Study of structural stability and formation mechanisms in dspc and dpsm liposomes: a coarse-grained molecular dynamics simulation. Sci Rep. 2020;10(1):1837. doi: 10.1038/s41598-020-58730-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jha RK, Jha PK, Chaudhury K, Rana SV, Guha SK. An emerging interface between life science and nanotechnology: present status and prospects of reproductive healthcare aided by nano-biotechnology. Nano Rev. 2014;5:22762. doi: 10.3402/nano.v5.22762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naseri N, Janfaza S, Irani R. Visible light switchable br/tio2 nanostructured photoanodes for bio-inspired solar energy conversion. RSC Adv. 2015;5(24):18642–18646. doi: 10.1039/C4RA16188B [DOI] [Google Scholar]
- 12.Molaeirad A, Janfaza S, Karimi-Fard A, Mahyad B. Photocurrent generation by adsorption of two main pigments of halobacterium salinarum on tio2 nanostructured electrode. Biotechnol Appl Biochem. 2015;62(1):121–125. doi: 10.1002/bab.1244 [DOI] [PubMed] [Google Scholar]
- 13.Hashemzadeh H, Allahverdi A, Peter E, N-M H. Comparison between three-dimensional spheroid and two-dimensional monolayer in a549 lung cancer and pc9 normal cell lines under treatment of silver nanoparticles. Modares J Biotechnol. 2019;10(4):573–580. [Google Scholar]
- 14.Esfandyari J, Shojaedin-Givi B, Mozafari-Nia M, Hashemzadeh H, Naderi-Manesh H. Diatom biosilica shell manipulation with gold, spion nanoparticles and trastuzumab with aims of diagnostics of her2 cells. Modares J Biotechnol. 2019;10(4):581–588. [Google Scholar]
- 15.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006;7(4):295. doi: 10.1016/S1470-2045(06)70651-9 [DOI] [PubMed] [Google Scholar]
- 16.Bavykin DV, Friedrich JM, Walsh FC. Protonated titanates and tio2 nanostructured materials: synthesis, properties, and applications. Adv Mater. 2006;18(21):2807–2824. doi: 10.1002/adma.200502696 [DOI] [Google Scholar]
- 17.McCullagh C, Robertson JM, Bahnemann DW, Robertson PK. The application of tio2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: a review. Res Chem Intermed. 2007;33(3–5):359–375. doi: 10.1163/156856707779238775 [DOI] [Google Scholar]
- 18.Bonetta S, Bonetta S, Motta F, Strini A, Carraro E. Photocatalytic bacterial inactivation by tio2-coated surfaces. AMB Express. 2013;3(1):59. doi: 10.1186/2191-0855-3-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao A, Hang R, Huang X, et al. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials. 2014;35(13):4223–4235. doi: 10.1016/j.biomaterials.2014.01.058 [DOI] [PubMed] [Google Scholar]
- 20.Hang R, Gao A, Huang X, et al. Antibacterial activity and cytocompatibility of cu–ti–o nanotubes. J Biomed Mater Res A. 2014;102(6):1850–1858. doi: 10.1002/jbm.a.34847 [DOI] [PubMed] [Google Scholar]
- 21.Lammers T, Subr V, Ulbrich K, Hennink WE, Storm G, Kiessling F. Polymeric nanomedicines for image-guided drug delivery and tumor-targeted combination therapy. Nano Today. 2010;5(3):197–212. doi: 10.1016/j.nantod.2010.05.001 [DOI] [Google Scholar]
- 22.Zahednezhad F, Zakeri-Milani P, Shahbazi Mojarrad J, Valizadeh H. The latest advances of cisplatin liposomal formulations: essentials for preparation and analysis. Expert Opin Drug Deliv.2020;17:523–541. doi: 10.1080/17425247.2020.1737672 [DOI] [PubMed] [Google Scholar]
- 23.Wang T, Jiang H, Wan L, et al. Potential application of functional porous tio2 nanoparticles in light-controlled drug release and targeted drug delivery. Acta Biomater. 2015;13:354–363. doi: 10.1016/j.actbio.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 24.Xu Z, Jiang X. Rapid fabrication of tio2 coatings with nanoporous composite structure and evaluation of application in artificial implants. Surf Coat Technol. 2020;381:125094. doi: 10.1016/j.surfcoat.2019.125094 [DOI] [Google Scholar]
- 25.Weetall HH. Biosensor technology what? Where? When? And why? Biosens Bioelectron. 1996;11(1–2):i–iv. doi: 10.1016/0956-5663(96)83729-88639278 [DOI] [Google Scholar]
- 26.Razavi H, Janfaza S. Medical nanobiosensors: a tutorial review. Nanomed J. 2015;2(2):74–87. doi: 10.7508/nmj.2015.02.001 [DOI] [Google Scholar]
- 27.Abdullah M, Kamarudin S. Titanium dioxide nanotubes (tnt) in energy and environmental applications: an overview. Renew Sust Energ Rev. 2017;76:212–225. doi: 10.1016/j.rser.2017.01.057 [DOI] [Google Scholar]
- 28.Viter R, Tereshchenko A, Smyntyna V, et al. Toward development of optical biosensors based on photoluminescence of tio 2 nanoparticles for the detection of salmonella. Sens Actuators B Chem. 2017;252:95–102. doi: 10.1016/j.snb.2017.05.139 [DOI] [Google Scholar]
- 29.Zhao L, Mei S, Chu PK, Zhang Y, Wu Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials. 2010;31(19):5072–5082. doi: 10.1016/j.biomaterials.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 30.Chung CJ, Lin HI, Tsou HK, Shi ZY, He JL. An antimicrobial tio2 coating for reducing hospital‐acquired infection. J Biomed Mater Res B. 2008;85(1):220–224. doi: 10.1002/jbm.b.30939 [DOI] [PubMed] [Google Scholar]
- 31.Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90(6):1847–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabarjadi A, Hashemzadeh H, Taravat E. Telomere, chromosome end, and telomerase enzyme as a cancer biomarker. Genet Third Millennium. 2013;11(1):3018–3027. [Google Scholar]
- 33.Çeşmeli S, Biray Avci C. Application of titanium dioxide (tio2) nanoparticles in cancer therapies. J Drug Target. 2019;27(7):762–766. doi: 10.1080/1061186X.2018.1527338 [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Wang X, Lu X, et al. The incorporation of daunorubicin in cancer cells through the use of titanium dioxide whiskers. Biomaterials. 2009;30(27):4708–4715. doi: 10.1016/j.biomaterials.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 35.Akram MW, Raziq F, Fakhar-e-Alam M, et al. Tailoring of au-tio2 nanoparticles conjugated with doxorubicin for their synergistic response and photodynamic therapy applications. J Photochem Photobiol a Chem. 2019;384:112040. doi: 10.1016/j.jphotochem.2019.112040 [DOI] [Google Scholar]
- 36.Faria HAM, de Queiroz AAA. A novel drug delivery of 5-fluorouracil device based on tio2/zns nanotubes. Mater Sci Eng C. 2015;56:260–268. doi: 10.1016/j.msec.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 37.Venkatasubbu GD, Ramasamy S, Reddy GP, Kumar J. in vivo and in vivo anticancer activity of surface modified paclitaxel attached hydroxyapatite and titanium dioxide nanoparticles. Biomed Microdevices. 2013;15(4):711–726. doi: 10.1007/s10544-013-9767-7 [DOI] [PubMed] [Google Scholar]
- 38.Liu E, Zhou Y, Liu Z, et al. Cisplatin loaded hyaluronic acid modified tio2 nanoparticles for neoadjuvant chemotherapy of ovarian cancer. J Nanomater. 2015;2015:1–8. [Google Scholar]
- 39.Nešić M, Žakula J, Korićanac L, et al. Light controlled metallo-drug delivery system based on the tio2-nanoparticles and ru-complex. J Photochem Photobiol a Chem. 2017;347:55–66. doi: 10.1016/j.jphotochem.2017.06.045 [DOI] [Google Scholar]
- 40.Yin M, Ju E, Chen Z, Li Z, Ren J, Qu X. Upconverting nanoparticles with a mesoporous tio2 shell for near‐infrared‐triggered drug delivery and synergistic targeted cancer therapy. Chem Eur J. 2014;20(43):14012–14017. doi: 10.1002/chem.201403733 [DOI] [PubMed] [Google Scholar]
- 41.Chu X, Mao L, Johnson O, et al. Exploration of tio2 nanoparticle mediated microdynamic therapy on cancer treatment. Nanomedicine. 2019;18:272–281. doi: 10.1016/j.nano.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 42.Jafari S, Lavasanifar A, Hejazi MS, Maleki-Dizaji N, Mesgari M, Molavi O. Stat3 inhibitory stattic enhances immunogenic cell death induced by chemotherapy in cancer cells. DARU. 2020;28(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada A, Ono M, Yuba E, Kono K. Titanium dioxide nanoparticle-entrapped polyion complex micelles generate singlet oxygen in the cells by ultrasound irradiation for sonodynamic therapy. Biomater Sci. 2013;1(1):65–73. doi: 10.1039/C2BM00066K [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Huang J-Y, Li H-Q, et al. Tio2 nanotube platforms for smart drug delivery: a review. Int J Nanomedicine. 2016;11:4819. doi: 10.2147/IJN.S108847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnology. 2008;6(1):2. doi: 10.1186/1477-3155-6-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Y, Yang H, Yang Y, et al. Progress in tio 2 nanotube coatings for biomedical applications: a review. J Mater Chem B. 2018;6(13):1862–1886. doi: 10.1039/C8TB00149A [DOI] [PubMed] [Google Scholar]
- 47.Kim S, Im S, Park EY, et al. Drug-loaded titanium dioxide nanoparticle coated with tumor targeting polymer as a sonodynamic chemotherapeutic agent for anti-cancer therapy. Nanomedicine. 2020;24:102110. doi: 10.1016/j.nano.2019.102110 [DOI] [PubMed] [Google Scholar]
- 48.Calamak S, Shahbazi R, Eroglu I, Gultekinoglu M, Ulubayram K. An overview of nanofiber-based antibacterial drug design. Expert Opin Drug Discov. 2017;12(4):391–406. doi: 10.1080/17460441.2017.1290603 [DOI] [PubMed] [Google Scholar]
- 49.Kaviyarasu K, Geetha N, Kanimozhi K, et al. in vivo cytotoxicity effect and antibacterial performance of human lung epithelial cells a549 activity of zinc oxide doped tio2 nanocrystals: investigation of bio-medical application by chemical method. Mater Sci Eng C. 2017;74:325–333. doi: 10.1016/j.msec.2016.12.024 [DOI] [PubMed] [Google Scholar]
- 50.Ravishankar Rai V, Jamuna Bai A. Nanoparticles and their potential application as antimicrobials In: Méndez-Vilas A, editor. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances Badajoz: Formatex. 2011:197–209. [Google Scholar]
- 51.Nagay BE, Dini C, Cordeiro JM, et al. Visible-light-induced photocatalytic and antibacterial activity of tio2 codoped with nitrogen and bismuth: new perspectives to control implant-biofilm-related diseases. ACS Appl Mater Interfaces. 2019;11(20):18186–18202. doi: 10.1021/acsami.9b03311 [DOI] [PubMed] [Google Scholar]
- 52.Liao C, Li Y, Tjong SC. Visible-light active titanium dioxide nanomaterials with bactericidal properties. Nanomaterials. 2020;10(1):124. doi: 10.3390/nano10010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu W, Qi M, Li X, et al. Tio2 nanotubes modified with au nanoparticles for visible-light enhanced antibacterial and anti-inflammatory capabilities. J Electroanal Chem. 2019;842:66–73. doi: 10.1016/j.jelechem.2019.04.062 [DOI] [Google Scholar]
- 54.Matsunaga T, Tomoda R, Nakajima T, Wake H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett. 1985;29(1–2):211–214. doi: 10.1111/j.1574-6968.1985.tb00864.x [DOI] [Google Scholar]
- 55.Dural-Erem A, Erem HH, Ozcan G, Skrifvars M. Anatase titanium dioxide loaded polylactide membranous films: preparation, characterization, and antibacterial activity assessment. J Text Inst. 2015;106(6):571–576. doi: 10.1080/00405000.2014.929274 [DOI] [Google Scholar]
- 56.Azzawi ZG, Hamad TI, Kadhim SA, Naji GA-H. Osseointegration evaluation of laser-deposited titanium dioxide nanoparticles on commercially pure titanium dental implants. J Mater Sci Mater Med. 2018;29(7):96. [DOI] [PubMed] [Google Scholar]
- 57.Souza JC, Sordi MB, Kanazawa M, et al. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019;94:112–131. doi: 10.1016/j.actbio.2019.05.045 [DOI] [PubMed] [Google Scholar]
- 58.Liu W, Su P, Chen S, et al. Antibacterial and osteogenic stem cell differentiation properties of photoinduced tio2 nanoparticle-decorated tio2 nanotubes. Nanomedicine. 2015;10(5):713–723. doi: 10.2217/nnm.14.183 [DOI] [PubMed] [Google Scholar]
- 59.Choi S-H, Jang Y-S, Jang J-H, Bae T-S, Lee S-J, Lee M-H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J Appl Biomater Funct Mater. 2019;17(3):2280800019847067. doi: 10.1177/2280800019847067 [DOI] [PubMed] [Google Scholar]
- 60.Chen S, Yang J, Li K, Lu B, Ren L. Carboxylic acid-functionalized tio2 nanoparticle-loaded pmma/peek copolymer matrix as a dental resin for 3d complete denture manufacturing by stereolitographic technique. Int J Food Prop. 2018;21(1):2557–2565. doi: 10.1080/10942912.2018.1534125 [DOI] [Google Scholar]
- 61.Shirai R, Miura T, Yoshida A, et al. Antimicrobial effect of titanium dioxide after ultraviolet irradiation against periodontal pathogen. Dent Mater J. 2016;35(3):511–516. doi: 10.4012/dmj.2015-406 [DOI] [PubMed] [Google Scholar]
- 62.Jia L, Qiu J, Du L, Li Z, Liu H, Ge S. Tio2 nanorod arrays as a photocatalytic coating enhanced antifungal and antibacterial efficiency of ti substrates. Nanomedicine. 2017;12(7):761–776. doi: 10.2217/nnm-2016-0398 [DOI] [PubMed] [Google Scholar]
- 63.Ji M-K, Oh G, Kim J-W, et al. Effects on antibacterial activity and osteoblast viability of non-thermal atmospheric pressure plasma and heat treatments of tio2 nanotubes. J Nanosci Nanotechnol. 2017;17(4):2312–2315. doi: 10.1166/jnn.2017.13328 [DOI] [PubMed] [Google Scholar]
- 64.Vishnu J, Manivasagam VK, Gopal V, et al. Hydrothermal treatment of etched titanium: a potential surface nano-modification technique for enhanced biocompatibility. Nanomedicine. 2019;20:102016. doi: 10.1016/j.nano.2019.102016 [DOI] [PubMed] [Google Scholar]
- 65.Panpaliya NP, Dahake PT, Kale YJ, et al. in vivo evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dent J. 2019;31(1):76–83. doi: 10.1016/j.sdentj.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Besinis A, De Peralta T, Handy RD. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on streptococcus mutans using a suite of bioassays. Nanotoxicology. 2014;8(1):1–16. doi: 10.3109/17435390.2012.742935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Sakagami H, Morales-Luckie RA, Nakajima H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl Oral Sci. 2015;23(3):321–328. doi: 10.1590/1678-775720140496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Losic D, Aw MS, Santos A, Gulati K, Bariana M. Titania nanotube arrays for local drug delivery: recent advances and perspectives. Expert Opin Drug Deliv. 2015;12(1):103–127. doi: 10.1517/17425247.2014.945418 [DOI] [PubMed] [Google Scholar]
- 69.Parnia F, Yazdani J, Javaherzadeh V, Dizaj SM. Overview of nanoparticle coating of dental implants for enhanced osseointegration and antimicrobial purposes. J Pharm Pharm Sci. 2017;20:148–160. doi: 10.18433/J3GP6G [DOI] [PubMed] [Google Scholar]
- 70.Hou X, Mao D, Ma H, et al. Antibacterial ability of ag–tio2 nanotubes prepared by ion implantation and anodic oxidation. Mater Lett. 2015;161:309–312. doi: 10.1016/j.matlet.2015.08.125 [DOI] [Google Scholar]
- 71.Bakhsheshi-Rad H, Hamzah E, Ismail A, et al. in vivo degradation behavior, antibacterial activity and cytotoxicity of tio2-mao/znha composite coating on mg alloy for orthopedic implants. Surf Coat Technol. 2018;334:450–460. doi: 10.1016/j.surfcoat.2017.11.027 [DOI] [Google Scholar]
- 72.Roy AS, Parveen A, Koppalkar AR, Prasad MA. Effect of nano-titanium dioxide with different antibiotics against methicillin-resistant staphylococcus aureus. J Biomater Nanobiotechnol. 2010;1(01):37. doi: 10.4236/jbnb.2010.11005 [DOI] [Google Scholar]
- 73.Jin Z, Yan X, Shen K, et al. Tio2 nanotubes promote osteogenic differentiation of mesenchymal stem cells via regulation of lncrna ccl3-as. Colloids Surf B Biointerfaces. 2019;181:416–425. doi: 10.1016/j.colsurfb.2019.05.041 [DOI] [PubMed] [Google Scholar]
- 74.Bhardwaj G, Webster TJ. Reduced bacterial growth and increased osteoblast proliferation on titanium with a nanophase tio2 surface treatment. Int J Nanomedicine. 2017;12:363. doi: 10.2147/IJN.S116105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Velasco E, Baldovino-Medrano VG, Gaigneaux EM, Giraldo SA. Development of an efficient strategy for coating tio 2 on polyester–cotton fabrics for bactericidal applications. Top Catal. 2016;59(2–4):378–386. doi: 10.1007/s11244-015-0429-2 [DOI] [Google Scholar]
- 76.Sekiguchi Y, Yao Y, Ohko Y, et al. Self‐sterilizing catheters with titanium dioxide photocatalyst thin films for clean intermittent catheterization: basis and study of clinical use. Int j Urol. 2007;14(5):426–430. doi: 10.1111/j.1442-2042.2007.01743.x [DOI] [PubMed] [Google Scholar]
- 77.Ohko Y, Utsumi Y, Niwa C, et al. Self‐sterilizing and self‐cleaning of silicone catheters coated with tio2 photocatalyst thin films: a preclinical work. J Biomed Mater Res. 2001;58(1):97–101. doi: [DOI] [PubMed] [Google Scholar]
- 78.Nakamura H, Tanaka M, Shinohara S, Gotoh M, Karube I. Development of a self-sterilizing lancet coated with a titanium dioxide photocatalytic nano-layer for self-monitoring of blood glucose. Biosens Bioelectron. 2007;22(9):1920–1925. doi: 10.1016/j.bios.2006.08.018 [DOI] [PubMed] [Google Scholar]
- 79.Dunnill CW, Aiken ZA, Kafizas A, et al. White light induced photocatalytic activity of sulfur-doped tio 2 thin films and their potential for antibacterial application. J Mater Chem. 2009;19(46):8747–8754. doi: 10.1039/b913793a [DOI] [Google Scholar]
- 80.Li S, Zhu T, Huang J, Guo Q, Chen G, Lai Y. Durable antibacterial and UV-protective Ag/TiO2@fabrics for sustainable biomedical application. Int J Nanomedicine. 2017;12:2593-2606. doi: 10.2147/IJN.S132035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Archana D, Dutta J, Dutta P. Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int J Biol Macromol. 2013;57:193–203. doi: 10.1016/j.ijbiomac.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 82.Kiwi J, Rtimi S, Sanjines R, Pulgarin C. Tio2 and tio2-doped films able to kill bacteria by contact: new evidence for the dynamics of bacterial inactivation in the dark and under light irradiation. Int J Photoenergy. 2014;2014(1):785037. doi: 10.1155/2014/785037 [DOI] [Google Scholar]
- 83.Saiz E, Zimmermann EA, Lee JS, Wegst UG, Tomsia AP. Perspectives on the role of nanotechnology in bone tissue engineering. Dental Mater. 2013;29(1):103–115. doi: 10.1016/j.dental.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haugen HJ, Monjo M, Rubert M, et al. Porous ceramic titanium dioxide scaffolds promote bone formation in rabbit peri-implant cortical defect model. Acta Biomater. 2013;9(2):5390–5399. doi: 10.1016/j.actbio.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 85.Wang H, Lai Y-K, Zheng R-Y, Bian Y, Zhang K-Q, Lin C-J. Tuning the surface microstructure of titanate coatings on titanium implants for enhancing bioactivity of implants. Int J Nanomedicine. 2015;10:3887. doi: 10.2147/IJN.S75999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–2953. doi: 10.1016/j.biomaterials.2008.04.023 [DOI] [PubMed] [Google Scholar]
- 87.Jones JR. New trends in bioactive scaffolds: the importance of nanostructure. J Eur Ceram Soc. 2009;29(7):1275–1281. doi: 10.1016/j.jeurceramsoc.2008.08.003 [DOI] [Google Scholar]
- 88.Bosco R, Van Den Beucken J, Leeuwenburgh S, Jansen J. Surface engineering for bone implants: a trend from passive to active surfaces. Coatings. 2012;2(3):95–119. doi: 10.3390/coatings2030095 [DOI] [Google Scholar]
- 89.Saleh MM, Touny A, Al-Omair MA, Saleh M. Biodegradable/biocompatible coated metal implants for orthopedic applications. Biomed Mater Eng. 2016;27(1):87–99. doi: 10.3233/BME-161568 [DOI] [PubMed] [Google Scholar]
- 90.Chen H-T, Shu H-Y, Chung C-J, He J-L. Assessment of bone morphogenic protein and hydroxyapatite–titanium dioxide composites for bone implant materials. Surf Coat Technol. 2015;276:168–174. doi: 10.1016/j.surfcoat.2015.06.056 [DOI] [Google Scholar]
- 91.Mohammadi M, Hesaraki S, Hafezi-Ardakani M. Investigation of biocompatible nanosized materials for development of strong calcium phosphate bone cement: comparison of nano-titania, nano-silicon carbide and amorphous nano-silica. Ceram Int. 2014;40(6):8377–8387. doi: 10.1016/j.ceramint.2014.01.044 [DOI] [Google Scholar]
- 92.Brammer KS, Frandsen CJ, Jin S. Tio 2 nanotubes for bone regeneration. Trends Biotechnol. 2012;30(6):315–322. doi: 10.1016/j.tibtech.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 93.Yang W, Xi X, Ran Q, Liu P, Hu Y, Cai K. Influence of the titania nanotubes dimensions on adsorption of collagen: an experimental and computational study. Mater Sci Eng C. 2014;34:410–416. doi: 10.1016/j.msec.2013.09.042 [DOI] [PubMed] [Google Scholar]
- 94.Hu N, Wu Y, Xie L, et al. Enhanced interfacial adhesion and osseointegration of anodic tio2 nanotube arrays on ultra-fine-grained titanium and underlying mechanisms. Acta Biomater. 2020;106(1):360–375. doi: 10.1016/j.actbio.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 95.Alves SA, Patel SB, Sukotjo C, et al. Synthesis of calcium-phosphorous doped tio 2 nanotubes by anodization and reverse polarization: a promising strategy for an efficient biofunctional implant surface. Appl Surf Sci. 2017;399:682–701. doi: 10.1016/j.apsusc.2016.12.105 [DOI] [Google Scholar]
- 96.Fomin A, Steinhauer A, Rodionov I, et al. Nanostructure of composite bioactive titania coatings modified with hydroxyapatite in medical titanium implants. Biomed Eng. 2013;47(3):138. doi: 10.1007/s10527-013-9353-6 [DOI] [PubMed] [Google Scholar]
- 97.Khaled S, Charpentier PA, Rizkalla AS. Physical and mechanical properties of pmma bone cement reinforced with nano-sized titania fibers. J Biomater Appl. 2011;25(6):515–537. doi: 10.1177/0885328209356944 [DOI] [PubMed] [Google Scholar]
- 98.Oshima M, Inoue K, Nakajima K, et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep. 2014;4:6044. doi: 10.1038/srep06044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larsson L, Decker A, Nibali L, Pilipchuk S, Berglundh T, Giannobile W. Regenerative medicine for periodontal and peri-implant diseases. J Dent Res. 2016;95(3):255–266. doi: 10.1177/0022034515618887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shibli A, Ehrenfest DD. In dental implant surfaces, nanowar has begun … but nanoquest is still at stake! Poseido. 2013;1(3):131–140. [Google Scholar]
- 101.Richard C Innovative surface treatments of titanium alloys for biomedical applications. Paper presented at: Materials Science Forum 2017. [Google Scholar]
- 102.Anil S, Anand P, Alghamdi H, Jansen J. Dental implant surface enhancement and osseointegration. Implant Dentistry-a Rapidly Evolving Practice. 2011;83–108. [Google Scholar]
- 103.Shim SC, Choe BH, Jang IS, et al. Effect of tio2 nanotube arrays on osseointegration for dental implant. Paper presented at: Advanced Materials Research 2014. [Google Scholar]
- 104.Kim G-H, Park S-W, Lee K, et al. Evaluation of osteoblast-like cell viability and differentiation on the gly-arg-gly-asp-ser peptide immobilized titanium dioxide nanotube via chemical grafting. J Nanosci Nanotechnol. 2016;16(2):1396–1399. doi: 10.1166/jnn.2016.11916 [DOI] [PubMed] [Google Scholar]
- 105.Yang W-E, Hsu M-L, Lin M-C, Chen Z-H, Chen L-K, Huang -H-H. Nano/submicron-scale tio 2 network on titanium surface for dental implant application. J Alloys Compd. 2009;479(1):642–647. doi: 10.1016/j.jallcom.2009.01.021 [DOI] [Google Scholar]
- 106.Huang HH, Chen JY, Lin MC, Wang YT, Lee TL, Chen LK. Blood responses to titanium surface with tio2 nano‐mesh structure. Clin Oral Implants Res. 2012;23(3):379–383. doi: 10.1111/j.1600-0501.2010.02152.x [DOI] [PubMed] [Google Scholar]
- 107.Li H, Lai Y, Huang J, et al. Multifunctional wettability patterns prepared by laser processing on superhydrophobic tio 2 nanostructured surfaces. J Mater Chem B. 2015;3(3):342–347. doi: 10.1039/C4TB01814A [DOI] [PubMed] [Google Scholar]
- 108.Lai Y, Lin L, Pan F, et al. Bioinspired patterning with extreme wettability contrast on tio2 nanotube array surface: a versatile platform for biomedical applications. Small. 2013;9(17):2945–2953. doi: 10.1002/smll.201300187 [DOI] [PubMed] [Google Scholar]
- 109.Huang Q, Yang Y, Zheng D, et al. Effect of construction of tio2 nanotubes on platelet behaviors: structure-property relationships. Acta Biomater. 2017;51:505–512. doi: 10.1016/j.actbio.2017.01.044 [DOI] [PubMed] [Google Scholar]
- 110.Li Y, Dong Y, Zhang Y, et al. Synergistic effect of crystalline phase on protein adsorption and cell behaviors on tio 2 nanotubes. Appl Nanosci. 2019;9:1–13. [Google Scholar]
- 111.Yuan Q, He G, Tan Z, et al. Biocompatibility of nano-tio2/ha bioceramic coating for nerve regeneration around dental implants. Paper presented at: Key Engineering Materials; 2007. [Google Scholar]
- 112.Yang W-E, Huang -H-H. Multiform tio2 nano-network enhances biological response to titanium surface for dental implant applications. Appl Surf Sci. 2019;471:1041–1052. doi: 10.1016/j.apsusc.2018.11.244 [DOI] [Google Scholar]
- 113.Popat KC, Eltgroth M, LaTempa TJ, Grimes CA, Desai TA. Titania nanotubes: a novel platform for drug‐eluting coatings for medical implants? Small. 2007;3(11):1878–1881. doi: 10.1002/smll.200700412 [DOI] [PubMed] [Google Scholar]
- 114.Harmankaya N, Karlsson J, Palmquist A, et al. Raloxifene and alendronate containing thin mesoporous titanium oxide films improve implant fixation to bone. Acta Biomater. 2013;9(6):7064–7073. doi: 10.1016/j.actbio.2013.02.040 [DOI] [PubMed] [Google Scholar]
- 115.Park SW, Lee D, Choi YS, et al. Mesoporous tio 2 implants for loading high dosage of antibacterial agent. Appl Surf Sci. 2014;303:140–146. doi: 10.1016/j.apsusc.2014.02.111 [DOI] [Google Scholar]
- 116.Galli S, Naito Y, Karlsson J, et al. Osteoconductive potential of mesoporous titania implant surfaces loaded with magnesium: an experimental study in the rabbit. Clin Implant Dent Relat Res. 2015;17(6):1048–1059. doi: 10.1111/cid.12211 [DOI] [PubMed] [Google Scholar]
- 117.Cecchinato F, Xue Y, Karlsson J, et al. in vivo evaluation of human fetal osteoblast response to magnesium loaded mesoporous tio2 coating. J Biomed Mater Res A. 2014;102(11):3862–3871. doi: 10.1002/jbm.a.35062 [DOI] [PubMed] [Google Scholar]
- 118.Galli S, Naito Y, Karlsson J, et al. Local release of magnesium from mesoporous tio 2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014;10(12):5193–5201. doi: 10.1016/j.actbio.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 119.Zhao L, Wang H, Huo K, et al. The osteogenic activity of strontium loaded titania nanotube arrays on titanium substrates. Biomaterials. 2013;34(1):19–29. doi: 10.1016/j.biomaterials.2012.09.041 [DOI] [PubMed] [Google Scholar]
- 120.Jia H, Kerr LL. Sustained ibuprofen release using composite poly (lactic‐co‐glycolic acid)/titanium dioxide nanotubes from ti implant surface. J Pharm Sci. 2013;102(7):2341–2348. doi: 10.1002/jps.23580 [DOI] [PubMed] [Google Scholar]
- 121.Gulati K, Ramakrishnan S, Aw MS, Atkins GJ, Findlay DM, Losic D. Biocompatible polymer coating of titania nanotube arrays for improved drug elution and osteoblast adhesion. Acta Biomater. 2012;8(1):449–456. doi: 10.1016/j.actbio.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 122.Kumeria T, Mon H, Aw MS, et al. Advanced biopolymer-coated drug-releasing titania nanotubes (tnts) implants with simultaneously enhanced osteoblast adhesion and antibacterial properties. Colloids Surf B Biointerfaces. 2015;130:255–263. doi: 10.1016/j.colsurfb.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 123.Lai S, Zhang W, Liu F, et al. Tio2 nanotubes as animal drug delivery system and in vivo controlled release. J Nanosci Nanotechnol. 2013;13(1):91–97. doi: 10.1166/jnn.2013.7086 [DOI] [PubMed] [Google Scholar]
- 124.Ma F, Zhang Q, Zhang C-Y. Nanomaterials-based biosensors for DNA methyltransferase assay. J Mater Chem B. 2020. doi: 10.1039/C9TB02458A [DOI] [PubMed] [Google Scholar]
- 125.Sibel AO, Shah A, editors. Nanomaterials-based enzyme biosensors for electrochemical applications: recent trends and future prospects In: New Developments in Nanosensors for Pharmaceutical Analysis. Elsevier; 2019:381–408. [Google Scholar]
- 126.Su S, Sun Q, Gu X, et al. Two-dimensional nanomaterials for biosensing applications. Trends Analyt Chem. 2019;119:115610. doi: 10.1016/j.trac.2019.07.021 [DOI] [Google Scholar]
- 127.Mahyad B, Janfaza S, Hosseini ES. Bio-nano hybrid materials based on bacteriorhodopsin: potential applications and future strategies. Adv Colloid Interface Sci. 2015;225:194–202. doi: 10.1016/j.cis.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 128.Estevez MC, Otte MA, Sepulveda B, Lechuga LM. Trends and challenges of refractometric nanoplasmonic biosensors: a review. Anal Chim Acta. 2014;806:55–73. doi: 10.1016/j.aca.2013.10.048 [DOI] [PubMed] [Google Scholar]
- 129.Sang S, Wang Y, Feng Q, Wei Y, Ji J, Zhang W. Progress of new label-free techniques for biosensors: a review. Crit Rev Biotechnol. 2015; 1–17. doi: 10.3109/07388551.2014.991270 [DOI] [PubMed] [Google Scholar]
- 130.Kennedy JF, Kay IM. Hydrous titanium oxides-new supports for the simple immobilisation of enzymes. J Chem Soc Perkin 1. 1976;3:329–335. doi: 10.1039/P19760000329 [DOI] [PubMed] [Google Scholar]
- 131.Kurokawa Y, Sano T, Ohta H, Nakagawa Y. Immobilization of enzyme onto cellulose–titanium oxide composite fiber. Biotechnol Bioeng. 1993;42(3):394–397. doi: 10.1002/bit.260420318 [DOI] [PubMed] [Google Scholar]
- 132.Wang X, Li G, Liu L, et al. Application of titanium dioxide nanowires and electroreduced graphene oxide modified electrodes for the electrochemical detection of specific tlh gene sequence from vibrio parahaemolyticus. Anal Methods. 2015;7(6):2623–2629. doi: 10.1039/C4AY02932A [DOI] [Google Scholar]
- 133.Tokudome H, Yamada Y, Sonezaki S, et al. Photoelectrochemical deoxyribonucleic acid sensing on a nanostructured tio2 electrode. Appl Phys Lett. 2005;87(21):213901. doi: 10.1063/1.2135392 [DOI] [Google Scholar]
- 134.Zhao -W-W, Ma Z-Y, Yan D-Y, Xu -J-J, Chen H-Y. In situ enzymatic ascorbic acid production as electron donor for cds quantum dots equipped tio2 nanotubes: a general and efficient approach for new photoelectrochemical immunoassay. Anal Chem. 2012;84(24):10518–10521. doi: 10.1021/ac3028799 [DOI] [PubMed] [Google Scholar]
- 135.Lee Y-L, Lo Y-S. Highly efficient quantum-dot-sensitized solar cell based on co-sensitization of cds/cdse. Adv Funct Mater. 2009;19(4):604–609. doi: 10.1002/adfm.200800940 [DOI] [Google Scholar]
- 136.Fan D, Wu D, Cui J, et al. An ultrasensitive label-free immunosensor based on cds sensitized fe–tio2 with high visible-light photoelectrochemical activity. Biosens Bioelectron. 2015;74:843–848. doi: 10.1016/j.bios.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 137.Bernacka-Wojcik I, Senadeera R, Wojcik PJ, et al. Inkjet printed and “doctor blade” tio2 photodetectors for DNA biosensors. Biosens Bioelectron. 2010;25(5):1229–1234. doi: 10.1016/j.bios.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 138.Liu P, Huo X, Tang Y, Xu J, Liu X, Wong DK. A tio2 nanosheet-g-c3n4 composite photoelectrochemical enzyme biosensor excitable by visible irradiation. Anal Chim Acta. 2017;984:86–95. doi: 10.1016/j.aca.2017.06.043 [DOI] [PubMed] [Google Scholar]
- 139.Atchudan R, Muthuchamy N, Edison TNJI, et al. An ultrasensitive photoelectrochemical biosensor for glucose based on bio-derived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens Bioelectron. 2019;126:160–169. doi: 10.1016/j.bios.2018.10.049 [DOI] [PubMed] [Google Scholar]
- 140.Ge L, Liu Q, Hao N, Kun W. Recent developments of photoelectrochemical biosensors for food analysis. J Mater Chem B. 2019;7(46):7283–7300. doi: 10.1039/C9TB01644A [DOI] [PubMed] [Google Scholar]
- 141.Zhang X, Peng J, Song Y, Chen Y, Lu F, Gao W. Porous hollow carbon nanobubbles@ zncds multi-shelled dodecahedral cages with enhanced visible-light harvesting for ultrasensitive photoelectrochemical biosensors. Biosens Bioelectron. 2019;133:125–132. doi: 10.1016/j.bios.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 142.Palaniappan PR, Pramod KS. Ftir study of the effect of ntio2 on the biochemical constituents of gill tissues of zebrafish (danio rerio). Food Chem Toxicol. 2010;48(8–9):2337–2343. doi: 10.1016/j.fct.2010.05.068 [DOI] [PubMed] [Google Scholar]
- 143.Dubey A, Goswami M, Yadav K, Chaudhary D. Oxidative stress and nano-toxicity induced by tio2 and zno on wag cell line. PLoS One. 2015;10(5):e0127493. doi: 10.1371/journal.pone.0127493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sadeghnia HR, Zoljalali N, Hanafi-Bojd MY, Nikoofal-Sahlabadi S, Malaekeh-Nikouei B. Effect of mesoporous silica nanoparticles on cell viability and markers of oxidative stress. Toxicol Mech Methods. 2015;25(6):433–439. doi: 10.3109/15376516.2015.1070229 [DOI] [PubMed] [Google Scholar]
- 145.Hutchison GR, Malone EM. Nanotoxicity. Betasys: Springer; 2011:419–434. [Google Scholar]
- 146.Buettner KM, Valentine AM. Bioinorganic chemistry of titanium. Chem Rev. 2012;112(3):1863–1881. doi: 10.1021/cr1002886 [DOI] [PubMed] [Google Scholar]
- 147.Fu PP, Xia Q, Hwang H-M, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22(1):64–75. doi: 10.1016/j.jfda.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hauert S, Bhatia SN. Mechanisms of cooperation in cancer nanomedicine: towards systems nanotechnology. Trends Biotechnol. 2014;32(9):448–455. doi: 10.1016/j.tibtech.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharifi S, Behzadi S, Laurent S, Laird Forrest M, Stroeve P, Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41(6):2323–2343. doi: 10.1039/C1CS15188F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sabella S, Carney RP, Brunetti V, et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale. 2014;6(12):7052–7061. doi: 10.1039/C4NR01234H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sies H. On the history of oxidative stress: concept and some aspects of current development. Curr Opin Toxicol. 2018. doi: 10.1016/j.cotox.2018.01.002 [DOI] [Google Scholar]
- 152.Rallo R, France B, Liu R, et al. Self-organizing map analysis of toxicity-related cell signaling pathways for metal and metal oxide nanoparticles. Environ Sci Technol. 2011;45(4):1695–1702. doi: 10.1021/es103606x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li S-Q, Zhu -R-R, Zhu H, et al. Nanotoxicity of tio2 nanoparticles to erythrocyte in vivo. Food Chem Toxicol. 2008;46(12):3626–3631. doi: 10.1016/j.fct.2008.09.012 [DOI] [PubMed] [Google Scholar]
- 154.Shokuhfar T, Sinha-Ray S, Sukotjo C, Yarin AL. Intercalation of anti-inflammatory drug molecules within tio2 nanotubes. RSC Adv. 2013;3(38):17380–17386. doi: 10.1039/C3RA42173B [DOI] [Google Scholar]
- 155.Pierzchała K, Lekka M, Magrez A, Kulik AJ, Forró L, Sienkiewicz A. Photocatalytic and phototoxic properties of tio2-based nanofilaments: esr and AFM assays. Nanotoxicology. 2012;6(8):813–824. doi: 10.3109/17435390.2011.625129 [DOI] [PubMed] [Google Scholar]
- 156.Wang Y, Santos A, Evdokiou A, Losic D. An overview of nanotoxicity and nanomedicine research: principles, progress and implications for cancer therapy. J Mater Chem B. 2015;3(36):7153–7172. doi: 10.1039/C5TB00956A [DOI] [PubMed] [Google Scholar]
- 157.Petković J, Žegura B, Stevanović M, et al. DNA damage and alterations in expression of DNA damage responsive genes induced by tio2 nanoparticles in human hepatoma hepg2 cells. Nanotoxicology. 2011;5(3):341–353. doi: 10.3109/17435390.2010.507316 [DOI] [PubMed] [Google Scholar]
- 158.Suh WH, Suslick KS, Stucky GD, Suh Y-H. Nanotechnology, nanotoxicology, and neuroscience. Prog Neurobiol. 2009;87(3):133–170. doi: 10.1016/j.pneurobio.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Y, Yuan L, Yao C, Fang J, Wu M. Cytotoxicity evaluation of ph-controlled antitumor drug release system of titanium dioxide nanotubes. J Nanosci Nanotechnol. 2015;15(6):4143–4148. doi: 10.1166/jnn.2015.9792 [DOI] [PubMed] [Google Scholar]
- 160.De Angelis I, Barone F, Zijno A, et al. Comparative study of zno and tio2 nanoparticles: physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology. 2013;7(8):1361–1372. doi: 10.3109/17435390.2012.741724 [DOI] [PubMed] [Google Scholar]
- 161.Mello MG, Taipina MO, Rabelo G, Cremasco A, Caram R. Production and characterization of tio2 nanotubes on ti-nb-mo-sn system for biomedical applications. Surf Coat Technol. 2017;326(PartA):126–133. doi: 10.1016/j.surfcoat.2017.07.027 [DOI] [Google Scholar]
- 162.Ratnakar A, Hari Prasad K, Vivekananthan S, Karthika PC, Kumar A. Synthesis and characterization of polyurethane – titanium dioxide – hydroxyapatite nanocomposite for biomedical applications. Mater Today. 2016;3(10, Part B):4052–4057. doi: 10.1016/j.matpr.2016.11.072 [DOI] [Google Scholar]
- 163.Radecka M, Rekas M, Trenczek-Zajac A, Zakrzewska K. Importance of the band gap energy and flat band potential for application of modified tio2 photoanodes in water photolysis. J Power Sources. 2008;181(1):46–55. doi: 10.1016/j.jpowsour.2007.10.082 [DOI] [Google Scholar]
- 164.Bosc F, Ayral A, Albouy P-A, Guizard C. A simple route for low-temperature synthesis of mesoporous and nanocrystalline anatase thin films. Chem Mater. 2003;15(12):2463–2468. doi: 10.1021/cm031025a [DOI] [Google Scholar]
- 165.Wang G-L, Xu -J-J, Chen H-Y, Fu S-Z. Label-free photoelectrochemical immunoassay for α-fetoprotein detection based on tio 2/cds hybrid. Biosens Bioelectron. 2009;25(4):791–796. doi: 10.1016/j.bios.2009.08.027 [DOI] [PubMed] [Google Scholar]