Abstract
Objective
To assess possible long-term excess mortality and causes of death of patients with chronic subdural hematoma (CSDH).
Methods
A retrospective study (1990–2015) of adult patients (n = 1133, median age = 76 years old, men = 65%) with CSDH identified by ICD-codes and verified by medical records. All patients were followed until death or the end of 2017. Cumulative relative survival ratios and relative excess risks of death (RER) were estimated by comparing patients’ mortality with that in the entire regional matched population. The causes of death were compared with a separate reference group formed by randomly choosing sex, age, and calendar time matched controls (4 controls per each CSDH patient).
Results
The median follow-up time was 4.8 years (range = 0–27 years), and 710 (63%) of the patients died (median age at death = 84 years old). The cumulative excess mortality was 1 year = 9%, 5 years = 18%, 10 years = 27%, 15 years = 37%, and 20 years = 48%. A subgroup of CSDH patients (n = 206) with no comorbidity had no excess mortality. Excess mortality was related to poor modified Rankin score at admission (RER = 4.93) and at discharge (RER = 8.31), alcohol abuse (RER = 4.47), warfarin (RER = 2.94), age ≥ 80 years old (RER = 1.83), non-operative treatment (RER = 1.56), and non-traumatic etiology (RER = 1.69). Hematoma characteristics or recurrence were unrelated to excess mortality. Dementia was the most common cause of death among the CSDH patients (21%) and the third most common cause in the reference group (15%, p < 0.001).
Conclusions
Patients with CSDH have continuous excess mortality up to 20 years after diagnosis. Patient-related characteristics have a strong association with excess mortality, whereas specific CSDH-related findings do not. CSDH patients have an increased risk for dementia-related mortality.
Keywords: Subdural hematoma, chronic; Mortality, excess; Causes of death; Survival; Mortality, excess
Introduction
Chronic subdural hematoma (CSDH) is a common disease in neurosurgical practice among elderly patients [1, 3, 27]. The reported annual incidence of CSDH has ranged widely across studies, from 1.7 to 20.6 per 100,000—and the overall incidence is increasing as the global population becomes progressively older [38]. In a prior study using this same patient cohort, we reported that during a 26-year period between 1990 and 2015, the overall incidence doubled from 8.2 to 17.6/100,000/year in the Pirkanmaa region, Finland [32]. The incidence per 100,000 person-years remained quite stable among adults younger than 70 years, whereas the incidence nearly tripled among the population 80 years or older. The global population of people aged 80 and older is expected to more than triple between 2015 and 2050 [17]. Consequently, CSDH is a condition of growing importance.
CSDH has been considered to be relatively benign, but during the last years, it has been recognized to have worse outcome than earlier assumed [11, 25, 29]. It has been speculated that CSDH may be a sentinel health event, and a harbinger of subsequent morbidity and mortality [3, 11, 29]. Age-related brain degeneration with an enlarging potential subdural space is assumed to be an important risk factor for CSDH [22, 24, 37]. Conversely, CSDH itself has been associated with a significant increase in the degree of brain atrophy post-CSDH [4]. Other well-known risk factors for CSDH are trauma [26], alcohol overuse [28], and antithrombotic therapy [6, 15, 23, 30].
Reported mortality rates after CSDH vary widely across studies, and a 1-year mortality rate of up to 32% has been reported [29]. In general, CSDH patients are from an age group with high baseline expected mortality. It is not possible to draw reliable conclusions on the excess mortality related to CSDH without comparing these patients to a matched sample from the general population. To date, only four studies (Table 1) have compared mortality after CSDH with anticipated survival [11, 16, 25, 29]. All of these studies have shown varied excess mortality, but the follow-up periods have extended only up to 8 years. Furthermore, there are no prior studies reporting long-term mortality in CSDH patients compared with a matched sample from the general population in an unselected, population-based series. Additionally, only two studies have previously reported the causes of death after a diagnosis of CSDH [19, 25].
Table 1.
Summary of studies on long-term excess mortality in chronic subdural hematoma patients
| Authors | Country | Time period | n | Age mean, y (range) | Follow-up period median, y (range for survivors) | Mortality (%) | Control data | Excess mortality | |
|---|---|---|---|---|---|---|---|---|---|
| 6 months | 1 year | ||||||||
| Miranda et al. 20117 | USA | 2000–2008 | 209 | 80.6 (65–96) | 1.45 (N/A-8.3) | 26.3 | 32 | Center of Disease Control and Prevention data |
• Excess mortality up to 1 year beyond diagnosis • Median survival: o CSDH 4.4 year o Anticipated actuarial survival 6 year |
| Dumont et al. 20138 | USA | 1996–2010 | 287 | 75 (55-N/A) | 2.3 (0.5–14) | N/A | 30 | Center of Disease Control and Prevention data |
• 1 year standardized mortality ratio: o 55–64 years:17 o 65–74 years:8.1 o 75–84 years:3.4 o ≥ 85 years:2.9 • Median survival: 4.0 ± 0.5y |
| Manickam et al. 20169 | Australia | 2006–2011 | 155 | 69.3 (18-N/A) | 5.2 (N/A-14.19) | 14.19 | 20.35 | Australian Bureau of Statistics, and the Registry of Births, Deaths and Marriages |
• Excess mortality throughout follow-up. • Average long-term survival: o CSDH: 5.29 ± 0.59 year o Actuarial data: 17.74 ± 1.8 year |
| Guilfoyle et al. 201719 | UK | 2004–2007 | 215 | 78 (35–95) | N/A (8–10) |
Drain group: 8.6 No drain group: 18.1 |
N/A | Cohorts of the general population with the same number of cases and identical age and sex profiles as the drain and no drain groups (Human Mortality Database) |
• 5 year cumulative excess mortality: o Drain group: 10.2% o No drain group: 22.4% |
| Present study | Finland | 1990–2015 | 1133 | 73 (22–99) | 4.8 (2–27) | 9.5 | 13.7 | The population of Pirkanmaa region stratified by sex, age, and calendar year (Statistics Finland) |
• Cumulative excess mortality: o 1 year: 9% o 5 years: 18% o 10 years: 27% o 15 years: 37% o 20 years: 48% |
CSDH = chronic subdural hematoma; N/A = not available
The objective of this study was to examine the possible long-term excess mortality related to CSDH, and the causes of death after a diagnosed CSDH. A large unselected, population-based CSDH patient cohort was compared with the general population from the same region, matched by sex, age, and calendar time. The causes of death of the CSDH patients were compared with a separate matched reference group.
Methods
Material and ethical aspects
The study was conducted in the Department of Neurosurgery at the Tampere University Hospital (Tampere, Finland). All adult patients (≥ 18 years old Pirkanmaa residents) with a diagnosis of CSDH between 1990 and 2015 were retrospectively identified using the hospital’s patient administrative databases. The cases were identified using International Classification of Diseases (ICD) codes for traumatic and non-traumatic subdural hematomas (SDHs). Verified cases were classified by SDH type (acute, subacute, chronic, and hygroma) by reviewing all the medical records. Exclusion criteria were acute or subacute SDH (< 3 weeks after head trauma), hygroma (a collection of subdural cerebrospinal fluid without any signs of blood), and any form of intracranial surgery within 12 months preceding the CSDH diagnosis.
The dates and causes of deaths were obtained from Statistics Finland (Helsinki, Finland). The Finnish official cause of death statistics are, in practice, 100% complete in relation to the cause and date of death. The entire Pirkanmaa population matched by sex, age, and calendar time was used for the excess mortality analysis. For the cause of death comparison, a separate reference group was formed by randomly choosing 4:1 sex, age (± 6 months), and calendar time–matched control subjects from Pirkanmaa for each CSDH patient. The reference group (n = 4532) was obtained from the Statistics Finland.
The Pirkanmaa region is a geographically well-defined area with both rural and urban areas that holds one of Finland’s five neurosurgical departments (Department of Neurosurgery, Tampere University Hospital, Tampere, Finland). All neurosurgical cases of the Pirkanmaa region are referred to the Tampere University Hospital. Over 9% of the Finnish population lives in the Pirkanmaa region. The population increased from 427,223 in 1990 to 506,114 in 2015. The population over 80 years old has almost doubled from 13,565 to 26,417 during the study period.
Data collection
A detailed and structured data collection was performed from medical records. CT scans or MR images were not separately inspected. Patients were stratified into three groups according to age:(1) 18–59 years, (2) 60–79 years, and (3) ≥ 80 years. The age categories were formed a priori on the basis of previous literature, convenience and the ease of presenting the results. The data collection included the following: comorbidities, medication, possible trauma, symptoms, neurological condition assessments based on both the Glasgow Coma Scale (GCS) and the modified Rankin Scale (mRS) score at admission, and for the operative group also at discharge. CSDH-related findings collected were localization (unilateral/bilateral) and hematoma thickness divided into three groups (≤ 15 mm, 16–25 mm, and > 25 mm) selected before the data collection was started. Operation details were collected. CSDH recurrence was defined as an ipsilateral hematoma needing re-operation within 2 years of the original operation. All patients were followed until death or the end of year 2017.
Survival analysis
The variables chosen for survival analysis were sex, age groups, and variables known to be CSDH risk factors (trauma, chronic alcohol abuse, and antithrombotic medication). The effect of neurological condition, treatment group (operative versus non-operative), and hematoma recurrence were analyzed.
Statistical analyses
SPSS (IBM SPSS Statistics for Windows, Version 25.0, Armonk, NY, USA) was used for data analyses. Survival analyses were conducted using the statistical software R (version 3.6.0) with popEpi package (version 0.4.7). Descriptive statistics [frequency (n), percentage, median, interquartile range, range] were used to describe variable and subgroup characteristics. The Chi square test was used to compare differences between groups. The statistical significance level was set at p < 0.05.
The cumulative relative survival ratio (CRSR) summarizes patients’ excess risk of death due to the disease by comparing the survival of patients to that of the matched general population (the population of Pirkanmaa region stratified by sex, age, and calendar year). CRSRs were estimated by using the Ederer II method [13, 34]. To compare differences in relative survival adjusted for age, sex, and follow-up time, we estimated relative excess risk (RER) of death by using Poisson regression [10]. Each model included sex, age at diagnosis (4 groups: 0–59, 60–69, 70–79, and 80+ years), and 5 intervals of follow-up time after diagnosis (0 to < 1 year, 1 to < 5 years, and three 5-year intervals from 5 to 20 years) in addition to a risk factor.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Characteristics
A total of 1133 patients with CSDH were identified, 736 (65%) were men. The median age for CSDH diagnosis was 76 years, and women were older than men (79 vs. 75 years). Median follow-up time was 4.8 years, with a minimum of 0 days and maximum of 27 years. Median follow-up time for survivors was 6.6 years, with a minimum of 2 years. No patients were lost from follow-up.
Of all the patients, 965 (85%) were operated. The indication for surgery was based on imaging and symptoms attributable to the mass effect of the hematoma. Operatively treated patients were slightly younger than non-operatively treated (median age 76 vs. 79 years, p = 0.001). At least one comorbidity was reported by 82% of the patients in both treatment groups. Antithrombotic medication was used by 42% of the patients. Two or more comorbidities were reported by 38% of operative group patients and 48% of non-operative group patients (p = 0.015), three or more by 13% and 15% (p = 0.052), respectively. Non-operatively treated CSDH patients more often had previously diagnosed dementia (16% vs. 8%, p = 0.001). The overall prevalence of dementia in CSDH patients aged 70 years or older was 12%. Significant differences were also noted in admission mRS; operatively treated patients had worse neurological disability [mRS scores of 4–5 were found in 52% of the operative group (n = 506/965) versus 28% of the non-operative group (n = 47/168), p < 0.001]. Operatively treated patients more often had headache or localizing neurological deficits. The characteristics of the entire sample and treatment subgroups are presented in Table 2. We have previously published the details of CSDH patients from this same patient cohort stratified by gender, age groups, and time periods [32].
Table 2.
Characteristics of all chronic subdural hematoma patients and treatment subgroups
| Total sample n = 1133 | Non-operative treatment n = 168 | Operative treatment n = 965 | p value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Median age, years, IQR | 76 | 67–83 | 79 | 68–86 | 76 | 66–82 | 0.001 |
| Sex | |||||||
| Men | 736 | 65.0 | 102 | 60.7 | 634 | 65.7 | 0.21 |
| Traumatic etiology | 672 | 59.3 | 107 | 63.7 | 565 | 58.5 | 0.21 |
| Comorbidity | |||||||
| Cardiovascular disease | 644 | 56.8 | 96 | 57.1 | 548 | 56.8 | 0.93 |
| Diabetes | 178 | 15.7 | 27 | 16.1 | 151 | 15.6 | 0.89 |
| Chronic alcohol abuse | 126 | 11.1 | 20 | 11.9 | 106 | 11.0 | 0.73 |
| Cerebrovascular disease | 124 | 10.9 | 24 | 14.3 | 100 | 10.4 | 0.13 |
| Dementia | 99 | 8.8 | 26 | 15.7 | 73 | 7.6 | 0.001 |
| Pulmonary disease | 68 | 6.0 | 16 | 9.5 | 52 | 5.4 | 0.05 |
| Epilepsy | 32 | 2.8 | 3 | 1.8 | 29 | 3.0 | 0.38 |
| Neurodegenerative disease | 20 | 1.8 | 7 | 4.2 | 13 | 1.3 | 0.01 |
| Hydrocephalus | 9 | 0.8 | 0 | 0 | 9 | 0.9 | 0.21 |
| Medication | |||||||
| Antiplatelet | 268 | 23.7 | 36 | 21.4 | 232 | 24.0 | 0.46 |
| Warfarin | 187 | 16.5 | 26 | 15.5 | 161 | 16.7 | 0.69 |
| Warfarin AND antiplatelet | 23 | 2.0 | 2 | 1.2 | 21 | 2.2 | 0.40 |
| Admission GCS | |||||||
| 13–15 | 1007 | 88.9 | 157 | 93.5 | 850 | 88.1 | 0.04 |
| 9–12 | 91 | 8.0 | 8 | 4.8 | 83 | 8.6 | 0.91 |
| 3–8 | 35 | 3.1 | 3 | 1.8 | 32 | 3.3 | 0.29 |
| Admission mRS 0–3 | 580 | 51.2 | 121 | 72.0 | 459 | 47.6 | < 0.001 |
| Symptoms | |||||||
| Hemiparesis | 444 | 39.2 | 9 | 5.4 | 435 | 45.1 | < 0.001 |
| Vertigo or postural instability | 408 | 36.0 | 35 | 20.8 | 373 | 38.7 | < 0.001 |
| Disorientation/memory impairment | 390 | 34.4 | 54 | 32.1 | 336 | 34.8 | 0.50 |
| General malaise | 367 | 32.4 | 50 | 29.8 | 317 | 32.8 | 0.43 |
| Headache | 340 | 30.0 | 28 | 16.7 | 312 | 32.3 | < 0.001 |
| Aphasia | 245 | 21.6 | 9 | 5.4 | 236 | 24.5 | < 0.001 |
| Seizure | 117 | 10.3 | 28 | 16.7 | 89 | 9.2 | 0.003 |
| Nausea and/or vomiting | 60 | 5.3 | 7 | 4.2 | 53 | 5.5 | 0.48 |
IQR Interquartile range; GCS Glasgow Coma Scale; mRS Modified Rankin Scale
Most operations used local anesthesia (n = 828; 86%) via one burr hole, and the hematoma was removed through irrigation. A subdural drain was inserted in 59 patients (6%). The drain was kept below the head level with no suction for 24–48 h. Subgaleal drains were not used. Only one patient underwent craniotomy as the primary surgery. The patients were actively mobilized directly after the operation. Recurrent hematoma was treated surgically for 273 cases (28%, median age 76 years). The reason for non-operative treatment for majority of the cases was that the CSDH did not cause neurological signs or significant symptoms. Only 7 patients were not offered surgery because they presented in a moribund state, and the analyses have been done also by excluding these patients. Most non-operatively treated patients were not admitted to a neurosurgical clinic. Non-operative treatment included discontinuation of possible antithrombotic medication, active mobilization, and follow-up CT-scans (routinely or for emerging new symptoms).
Mortality after diagnosis of CSDH
By the end of the follow-up period, 710 (63%) of the 1133 patients had died, 449 of men (61%), and 261 of women (66%). Median age at death was 84 years (IQR 76–89 years), 83 years for men, and 86 years for women. Similarly, the median age at death was 84 years in the operative group and 85 years in the non-operative group. The 30-day and 6-month mortalities after diagnosis of CSDH were 3% and 10%, respectively. The overall 1-year and 2-year mortality rates were 14% and 22%, respectively. There was no significant difference in mortality between men and women. One-year mortality was 12% in the operative group (n = 965) and 21% in the non-operative group (n = 168; p = 0.003). After the patients (n = 7) who were not offered surgery, because they presented in a moribund state, were withdrawn from the non-operative group, the non-operative 1-year mortality was 18% (operative vs. non-operative: p = 0.053). One-year mortality was 5% among the patients under the age of 60, and 22% among those aged 80 and older (p < 0.001). A subgroup of patients with no comorbidities had a 1-year mortality of only 3% (n = 206, median age 72 years, IQR 61–78 years). Mortality rates are presented in Table 3. For comparison, the reference group mortality rates also are reported in Table 3.
Table 3.
Mortality after diagnosis of CSDH stratified by sex, age, treatment group, and prevalence of comorbidity. Also shown the mortality of the reference group
| Total sample n = 1133 | Men n = 736 | Women n = 397 | Age group 18–59 years n = 167 | Age group 60–79 years n = 565 | Age group ≥ 80 years n = 401 | Operative n = 965 | Non-operative n = 168 | Non-operative n = 161* | No comorbidity n = 206 | Reference group n = 4532 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cumulative mortality | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| 30 days | 38 | 3.4 | 26 | 3.5 | 12 | 3.0 | 3 | 1.8 | 15 | 2.7 | 20 | 5.0 | 25 | 2.6 | 13 | 7.7 | 8 | 5.0 | 3 | 1.5 | 16 | 0.4 |
| 90 days | 75 | 6.6 | 51 | 6.9 | 24 | 6.0 | 3 | 1.8 | 34 | 6.0 | 38 | 9.5 | 54 | 5.6 | 21 | 12.5 | 15 | 9.3 | 4 | 1.9 | 61 | 1.3 |
| 6 months | 108 | 9.5 | 74 | 10.1 | 34 | 8.6 | 5 | 3.0 | 45 | 8.0 | 58 | 14.4 | 82 | 8.5 | 26 | 15.5 | 20 | 12.4 | 6 | 2.9 | 125 | 2.8 |
| 1 year | 155 | 13.7 | 107 | 14.5 | 48 | 12.1 | 8 | 4.8 | 59 | 10.4 | 88 | 22.0 | 120 | 12.4 | 35 | 20.8 | 29 | 18.0 | 6 | 2.9 | 248 | 5.5 |
| 2 year | 254 | 22.4 | 168 | 22.8 | 86 | 21.7 | 20 | 12.0 | 97 | 17.1 | 137 | 34.2 | 200 | 20.7 | 54 | 32.1 | 47 | 29.2 | 19 | 9.2 | 486 | 10.7 |
*The patients (n = 7) who were not offered surgery (because presenting in a moribund state) were not included
Long-term excess mortality
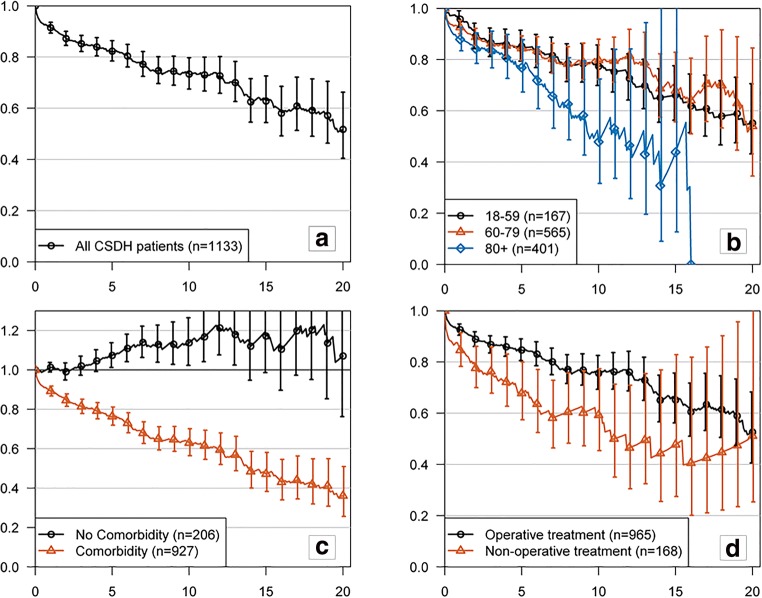
The 1-year cumulative relative survival ratio (CRSR) for all CSDH patients was 0.91 (95% CI 0.89–0.94), implying 9% excess mortality compared with the matched general population. The cumulative excess mortality was 18% in 5 years (CRSR 0.82; 95% CI 0.78–0.86), 27% in 10 years (CRSR 0.73; 95% CI 0.67–0.80), 37% in 15 years (CRSR 0.63; 95% CI 0.54–0.73), and 48% in 20 years (CRSR 0.52; 95% CI 0.40–0.66). The excess mortality rate was highest during the first year of follow-up after diagnosis of CSDH, and it was 2–4% per year during the rest of the follow-up period. The excess mortality seemed to be more pronounced in women, but the difference was not statistically significant. CSDH patients had excess mortality in every age group, and it was more pronounced in the age group of ≥ 80 years and in the non-operatively treated patients. A subgroup of patients with no comorbidities had better survival than the matched general population. The CRSR with 95% confidence intervals are shown in Fig. 1.
Fig. 1.
Excess mortality of chronic subdural hematoma patients. Cumulative relative survival ratios (with 95% confidence intervals) illustrating excess mortality of the CSDH patients compared with the matched general population; entire cohort (a), stratified by age groups (b), by prevalence of comorbidity (c), and by treatment group. The horizontal line at 1.0 represents the survival of the matched general population and curves below that line represent excess mortality of the study population. The vertical line shows follow-up time (years)
Relative excess risk of death
In the age-, gender-, and follow-up time–adjusted regression model for RER, excess mortality was significantly related to (i) poor mRS 4–5 (RER = 4.93) at admission and especially (ii) at discharge (RER = 8.31), (iii) chronic alcohol abuse (RER = 4.47), (iv) warfarin medication (RER = 2.94), (v) age ≥ 80 years old (RER = 1.83), (vi) non-operative treatment (RER = 1.56; moribund patients n = 7 excluded), and (vii) non-traumatic etiology (RER = 1.69). Hematoma localization (unilateral/bilateral), thickness, or recurrence were not related to the excess mortality. Detailed RER results are presented in Table 4.
Table 4.
Relative excess risk of death (RER) estimates and 95% confidence intervals for each subgroup of the 1133 chronic subdural hematoma patients adjusted for age, gender, and follow-up time
| Total sample n = 1133 | RER | 95% CI | ||
|---|---|---|---|---|
| n | % | |||
| Sex | ||||
| Men | 736 | 65.0 | 1 | Ref |
| Women | 397 | 35.0 | 1.17 | 0.82–1.65 |
| Age at CSDH diagnosis, years | ||||
| 18–59 | 167 | 14.7 | 1 | Ref |
| 60–79 | 565 | 49.9 | 1.05 | 0.68–1.61 |
| ≥ 80 | 401 | 35.4 | 1.83 | 1.11–3.02 |
| Chronic alcohol abuse | ||||
| No | 1007 | 88.9 | 1 | Ref |
| Yes | 126 | 11.1 | 4.47 | 2.88–6.95 |
| Traumatic etiology | ||||
| Yes | 672 | 59.3 | 1 | Ref |
| No | 461 | 40.7 | 1.69 | 1.20–2.38 |
| Antithrombotic medication | ||||
| None | 655 | 57.8 | 1 | Ref |
| Antiplatelet | 268 | 23.7 | 1.20 | 0.72–2.01 |
| Warfarin | 187 | 16.5 | 2.94 | 1.91–4.54 |
| Warfarin AND antiplatelet | 23 | 2.0 | 3.24 | 1.35–7.75 |
| Admission GCS | ||||
| 13–15 | 1007 | 88.9 | 1 | Ref |
| 9–12 | 91 | 8.0 | 3.53 | 2.35–5.32 |
| 3–8 | 35 | 3.1 | 5.71 | 3.47–9.41 |
| Admission mRS | ||||
| 0–3 | 580 | 51.2 | 1 | Ref |
| 4–5 | 553 | 48.8 | 4.93 | 3.12–7.80 |
| Hematoma localization | ||||
| Unilateral | 876 | 77.3 | 1 | Ref |
| Bilateral | 257 | 22.7 | 0.74 | 0.46–1.18 |
| Hematoma thickness, mm (missing n = 23) | ||||
| ≤ 15 mm | 376 | 33.2 | 1 | Ref |
| 16–25 | 474 | 41.8 | 0.69 | 0.48–1.01 |
| > 25 | 260 | 22.9 | 0.59 | 0.34–1.01 |
| Operative treatment | ||||
| Yes | 965 | 85.2 | 1 | Ref |
| No | 168 | 14.8 | 1.77 | 1.18–2.65 |
| No1 | 161 | 14.2 | 1.56 | 1.01–2.41 |
| Discharge GCS2 | ||||
| 13–15 | 942 | 97.6 | 1 | Ref |
| 9–12 | 9 | 0.9 | 14.84 | 6.64–33.18 |
| 3–8 | 14 | 1.5 | 98.30 | 39.35–245.56 |
| Discharge mRS2 | ||||
| 0–3 | 727 | 75.3 | 1 | Ref |
| 4–5 | 238 | 24.7 | 8.31 | 5.48–12.58 |
| Recurrent hematoma2 | ||||
| No | 692 | 71.7 | 1 | Ref |
| Yes | 273 | 28.3 | 0.78 | 0.48–1.26 |
RER Relative excess risk of death, CI Confidence interval, Ref Reference, GCS Glasgow Coma Scale score, mRS Modified Rankin Scale score
1The patients (n = 7) who were not offered surgery because they presented in a moribund state were not included
2Includes only operatively treated patients
Causes of death after CSDH
The most frequent causes of death for women were dementia (29%), ischemic cardiac disease (15%), and cerebral ischemia (12%), and the most common causes for men were ischemic cardiac disease (23%), dementia (16%), and cancer (14%). SDH (the hematoma type (chronic, subacute, acute) could not be verified due to the nature of the cause of death data) was the cause of death in 42/710 patients (6%). In the matched reference group (n = 4532), the most common causes of death were ischemic cardiac disease (25%; women 22% and men 26%), cancer (19%; women 15% and men 22%), and dementia (15%; women 18% and men 13%). As a cause of death, dementia was more common in patients with CSDH than in the reference group (21% vs. 15%, p < 0.001). The difference was significant for women (29% vs. 18%, p < 0.001), but not for men (16% vs. 13%, p = 0.12). The cause of death was traumatic in 11% of the CSDH patients, and 3% in the reference group (p < 0.001). SDH was the cause of death in 6% and in 0.5%, respectively (p < 0.001). Causes of death among the CSDH patients and the reference group are presented in Table 5. When stratifying the causes of death by survival time, the incidence of trauma-related death was significantly higher among CSDH patients compared with the reference group during the first 5 years (p < 0.001). In contrast, the incidence of dementia as the cause of death was higher among CSDH patients compared with the reference group after the first year, and increased over time reaching statistical difference from 1 to 10 years (p < 0.001; Fig. 2).
Table 5.
The causes of death until the end of 2017 of the 1133 patients with chronic subdural hematomas between 1990 and 2015 in Pirkanmaa, Finland. The causes of death in the matched reference group between 1990 and 2015 are presented for comparison
| Total sample n = 1133 | Men n = 736 | Women n = 397 | Operative treatment n = 965 | Non-operative treatment n = 168 | Causes of death in the matched reference group n = 4532 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Age, years (median, IQR) | 76 | 67–83 | 75 | 65–81 | 79 | 70–85 | 76 | 66–82 | 79 | 68–86 | 76 | 67–83 |
| Follow-up time, years | ||||||||||||
| Median | 4.8 | 5.1 | 4.6 | 5.2 | 3.3 | 6.4 | ||||||
| Range | 0–27 | 0–26 | 0–27 | 0–27 | 0–21 | 0–28 | ||||||
| No. of deaths | 710 | 62.7 | 449 | 61.0 | 261 | 65.7 | 609 | 62.3 | 101 | 60.1 | 1918 | 42.3 |
| Age at death, median, IQR | 84 | 76–89 | 83 | 74–88 | 86 | 80–91 | 84 | 76–89 | 85 | 79–90 | 84 | 78–88 |
| Ischemic cardiac disease | 143 | 20.1 | 103 | 22.9 | 40 | 15.3 | 122 | 20.0 | 21 | 20.8 | 473 | 24.7 |
| Cerebrovascular disease | 90 | 12.7 | 54 | 12.0 | 36 | 13.8 | 80 | 13.1 | 10 | 9.9 | 216 | 11.3 |
| Cerebral hemorrhage | 18 | 2.5 | 13 | 2.9 | 5 | 1.9 | 13 | 2.1 | 5 | 5.0 | 23 | 1.2 |
| Cerebral ischemia | 72 | 10.1 | 41 | 9.1 | 31 | 11.9 | 67 | 11.0 | 5 | 5.0 | 167 | 8.7 |
| Cancer | 91 | 12.8 | 64 | 14.3 | 27 | 10.3 | 80 | 13.1 | 11 | 10.9 | 370 | 19.3 |
| Dementia and Alzheimer’s disease | 146 | 20.6 | 70 | 15.6 | 76 | 29.1 | 121 | 19.9 | 25 | 24.8 | 278 | 14.5 |
| Pulmonary disease | 34 | 4.8 | 24 | 5.3 | 10 | 3.8 | 29 | 4.8 | 5 | 5.0 | 119 | 6.2 |
| Pneumonia | 16 | 2.3 | 9 | 2.0 | 7 | 2.7 | 15 | 2.5 | 1 | 1.0 | 45 | 2.3 |
| Trauma | 75 | 10.6 | 50 | 11.1 | 25 | 9.6 | 65 | 10.7 | 10 | 9.9 | 58 | 3.0 |
| Accidental falls | 41 | 5.8 | 25 | 5.6 | 16 | 6.1 | 36 | 5.9 | 5 | 5.0 | 33 | 1.7 |
| Subdural hematoma, traumatic | 38 | 5.4 | 25 | 5.6 | 13 | 5.0 | 33 | 5.4 | 5 | 5.0 | 9 | 0.5 |
| Subdural hematoma, non-traumatic | 4 | 0.6 | 4 | 0.9 | 0 | 3 | 0.5 | 1 | 1.0 | 1 | 0.05 | |
| Unknown | 0 | 0 | 0 | 0 | 0 | 20 | 1.0 | |||||
Fig. 2.
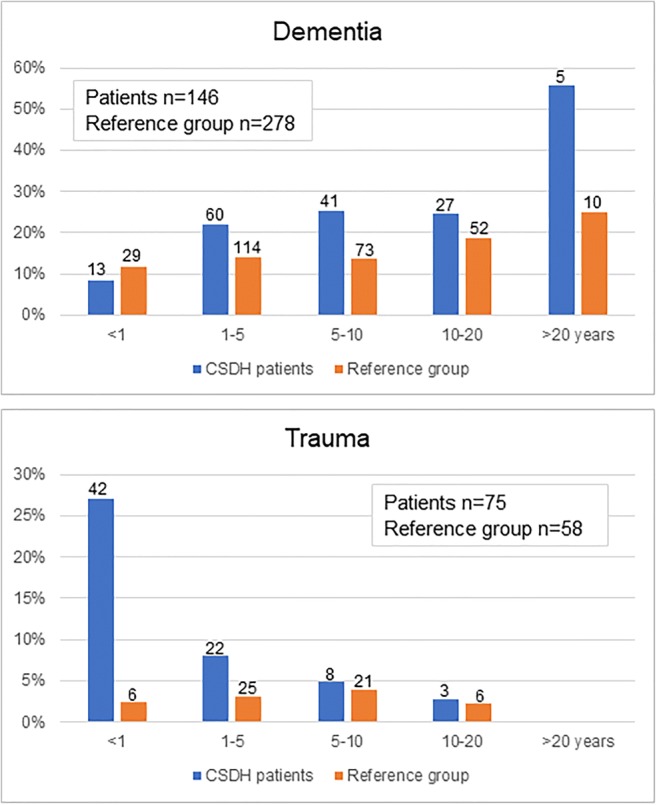
Dementia and trauma as a cause of death stratified by survival time. The CSDH patients are compared with the matched reference group. The percentages represent all the deaths during the time period. The number of deaths 710 among CSDH patients, and 1918 among the reference group
Discussion
Summary of the key findings
In our large population-based cohort, patients with CSDH had excess mortality, which increased over time from 9% at 1 year to 48% at 20 years after CSDH diagnosis. A subgroup of patients with no comorbidities had no excess mortality. The most important factors related to excess mortality were neurological disability at admission and at discharge. Age over 80 years almost doubled, warfarin almost tripled, and chronic alcohol abuse almost quintupled the risk of death after CSDH. Hematoma localization (unilateral/bilateral) or thickness were not relative risk factors nor was hematoma recurrence. In the median follow-up time of 4.8 years, there were 710 deaths, of which 6% were caused by SDH. The most common cause of death was dementia, which was significantly more common as a cause of death among the CSDH patients than in the reference group. As a cause of death, dementia occurred later in CSDH patients than in the reference group.
Comparison of the current findings to prior literature
Miranda et al. observed excess mortality up to 1 year beyond diagnosis, but after that, life expectancy was equivalent with the general population [29]. Treatment group, size or laterality of subdural hematoma, and antithrombotic medication use were not associated with the mortality rate. Dumont and colleagues showed that patients with CSDH had worse survival than expected in every age group, and patients undergoing surgical drainage of CSDH (median survival 5.5 years) had significantly longer survival compared with patients not undergoing surgical drainage (2.3 years) [11]. The authors speculated that there can be selection bias, because the patients most likely to improve from surgery were offered surgical treatment. Mortality after CSDH was highest in the oldest patients over 85 years old, but the standardized mortality ratio was lower than in any other age group. Manickam et al. reported excess mortality continuing throughout a prolonged follow-up (median 5.2 years) as peers lived 12.4 years longer [25]. A prospective, randomized study by Santarius et al. revealed that among operatively treated patients, CSDH drainage significantly reduced 6-month mortality from 18 to 9% [33]. Additionally, a recent 5-year follow-up analysis of the aforementioned study showed a significant survival advantage for drainage as the relative survival in the no drain group was 77.6% compared with 89.8% in the drain group [16].
In our study, CSDH patients had excess mortality in every age group. The excess mortality was more pronounced in the age group of ≥ 80 years. The risk of excess mortality was higher in the non-operative group than in the operative group (RER 1.56) even though the neurological condition at admission was better among non-operatively treated patients, and they were not predisposed to surgical complications. The reason behind this is probably that the burden of comorbidities was somewhat higher among non-operatively treated patients.
In contrast to the study by Santarius, CSDH recurrence was not a risk factor for excess mortality among our study patients. In fact, the patients with recurrence had a lower mortality at least during the first 2 years. We speculate that this might be explained by more frequent medical attention (follow-ups and re-operations) for patients that are in better general health before their first CSDH. Patients with more comorbidities are less likely to undergo a second operation. Even so, our 6-month overall mortality rate (10%) was comparable with the findings by Santarius and colleagues [33]. Also, our relative survival at 5 years was similar (82%) than analyzed by Guilfoyle [16]. The mortality differences between studies are most likely due to differences in case ascertainment, healthcare systems, and population-related life expectancies.
Our study is in line with the previous studies demonstrating that neurological disability at discharge is strongly associated to long-term survival [11, 25, 29]. This is no surprise because it correlates to functional status, which has been recognized to have a great impact on life expectancy in general [20]. However, it is difficult to differentiate the effects of underlying comorbidities from the effects of CSDH on long-term survival. A subgroup of patients (n = 206) with no comorbidities survived better than the matched general population. In addition, hematoma bilaterality, thickness, or recurrence were not relative risk factors. Accordingly, even a large recurring CSDH may not affect the long-term survival by itself. Therefore, the patient-related variables are probably more important than the CSDH itself. Based on this data, it seems likely that the comorbidities are the cause of excess mortality rather than CSDH itself. Some patients are frail due to age-associated brain atrophy and other comorbidities, and CSDH seems to be a sentinel health event, a harbinger of subsequent morbidity and mortality, for this group of patients [3, 11, 29]. In contrast, patients with no comorbidities are probably healthier than the matched general population.
Hence, the excess mortality after diagnosis of CSDH might be reduced by more assertively treating the comorbidities, of which the most common were vascular diseases, diabetes, and chronic alcohol abuse. It is also known, that the hospitalization of older people decreases daily living functioning [7]. For this reason, it has been proposed that already perioperative care should be optimized by a multidisciplinary approach and by promoting early rehabilitation [35].
Antithrombotic drug use is common among CSDH patients and is speculated to attribute to the greater incidence of CSDH among elderly [6, 8, 15, 23, 30]. In our study, the use of warfarin, but not the use of antiplatelets, was a relative risk factor for excess mortality. This could reflect the increased risks of warfarin in the context of CSDH or be explained by the fact that the baseline diseases treated with antiplatelet drugs are not as severe as with warfarin. Similarly, chronic alcohol abuse was a relative risk factor for excess mortality after CSDH, but it is a risk factor for excess mortality also independently [9, 36]. Moreover, non-traumatic etiology was a relative risk factor for excess mortality among CSDH patients. This could be at least partly explained by the more common use of antithrombotic medication by patients with non-traumatic than traumatic etiology (47% vs. 39%), and the underlying medication-suggesting comorbidities. Additionally, CSDH might be a manifestation of degenerative or inflammatory disease rather than trauma [14]. In other words, aging, longstanding and ongoing alcohol abuse, and worse baseline general and neurological health all appear to contribute to greater mortality following CSDH.
The most important reason for the greater incidence of CSDH among the elderly has been speculated to be attributed to brain atrophy [22, 24, 37]. Dementia has been linked to brain atrophy [5]. At the time of diagnosis of CSDH, the prevalence of dementia in our CSDH patients (12% in patients aged 70 years or older) was similar as the prevalence in Western Europe reported by the World Alzheimer report 2015 [31]. As a cause of death, dementia was more prevalent in patients with CSDH than in our reference group. The difference was seen after the first year and was more pronounced in the later years. Additionally, the excess mortality related to CSDH increases with time. Our results support the idea that CSDH may be a risk factor for dementia. This could be explained by Bin Zahid and colleagues’ observation that CSDH is related to a significant increase in the degree of subsequent brain atrophy [4]. It seems that brain atrophy is a risk factor for CSDH, which in turn accelerates neurodegeneration and increases the risk of dementia. Further long-term prospective studies are needed to verify this association.
Strengths and limitations
Our series represents the most extensive non-register-based study of consecutive CSDH cases treated in one neurosurgical department. Although retrospective in nature, the population-based setting makes it less prone to selection bias. Moreover, all the data was collected by one of the authors (M.R.). Our study gives a reliable population-based estimate of the CSDH-associated excess mortality based on the comparison with a matched general population.
This study has several limitations. ICD-codes were used to retrospectively identify all the patients of interest. There is a possibility that some cases were not recognized due to incomplete or incorrect ICD-coding. It is likely that all the patients undergoing surgery were identified, but the ICD-coding can be incomplete among the non-operatively treated patients, as a neurosurgeon has only been consulted on these cases. Neuroimaging was not reviewed, and there can be inconsistencies in reporting the hematoma thickness. In addition, the distinction between subacute and chronic SDH is not always obvious, both in relation to time and neuroradiological characteristics. No definition of CSDH is universally accepted [18]. Subacute SDHs were excluded from this study, because this hematoma subtype is considered to represent an entity of its own [2, 12, 21]. Additionally, autopsies were not performed on all of the deceased patients, and some of the causes of deaths might not be correct. Also, adjustments to the statistical analyses were limited because we did not have access to comorbidity data from the matched general population. However, it is reasonable to assume that controls and patients had similar comorbidities.
Future CSDH research should focus on preventive measures that take into account prior health conditions and fall-related injury risk factors that predispose to CSDH. Frailty, functionality, dependency, and comorbidity should be of special interest as these issues are prognosticators of general disability, hospital readmission, and mortality.
Conclusions
Patients with CSDH have long-term excess mortality, which is evident up to at least 20 years after diagnosis. Patient-related characteristics, especially chronic alcohol abuse, antithrombotic medication use, and neurological disability both at admission, and at discharge, have a strong association with excess mortality, whereas specific CSDH-related findings do not. A subgroup of patients with no comorbidities had no excess mortality. The most common cause of death was dementia, which was more common as a cause of death in patients with CSDH than in the reference group after the first year. Consequently, there can be a two-way correlation between CSDH and dementia.
Funding information
Pirkanmaa Hospital District and the Maire Taponen Foundation provided financial support in the form of a personal research scholarship (M.R.).
Compliance with ethical standards
Conflict of interest
Dr. Luoto has received funding from the Government’s Special Financial Transfer tied to academic research in Health Sciences (Finland), the Emil Aaltonen Foundation, and Finnish Medical SocietyDuodecim. Dr. Iverson acknowledges unrestricted philanthropic support from the Mooney-Reed Charitable Foundation, Heinz Family Foundation, ImPACT Applications, Inc., and Spaulding Research Institute. He serves as a strategic scientific advisor for BioDirection, Inc. The other authors report nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (the Ethics Committee of the Pirkanmaa Hospital District, approval code R12082) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All data was collected retrospectively without contacting the patients. For this type of study formal consent is not required.
Disclaimer
The sponsors had no role in the design or conduct of this research.
Footnotes
This article is part of the Topical Collection on Brain trauma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adhiyaman V, Asghar M, Ganeshram KN, Bhowmick BK. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002;78:71–75. doi: 10.1136/pmj.78.916.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves JL, Santiago JG, Costa G, Mota Pinto A. A standardized classification for subdural hematomas- I. Am J Forensic Med Pathol. 2016;37:174–178. doi: 10.1097/PAF.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 3.Bartek J, Jr, Sjavik K, Stahl F, Kristiansson H, Solheim O, Gulati S, Sagberg LM, Forander P, Jakola AS. Surgery for chronic subdural hematoma in nonagenarians: a Scandinavian population-based multicenter study. Acta Neurol Scand. 2017;136:516–520. doi: 10.1111/ane.12764. [DOI] [PubMed] [Google Scholar]
- 4.Bin Zahid A, Balser D, Thomas R, Mahan MY, Hubbard ME, Samadani U (2018) Increase in brain atrophy after subdural hematoma to rates greater than associated with dementia. J Neurosurg 1–9 [DOI] [PubMed]
- 5.Chapleau M, Aldebert J, Montembeault M, Brambati SM. Atrophy in Alzheimer’s disease and semantic dementia: an ALE meta-analysis of voxel-based morphometry studies. J Alzheimers Dis. 2016;54:941–955. doi: 10.3233/JAD-160382. [DOI] [PubMed] [Google Scholar]
- 6.Connolly BJ, Pearce LA, Hart RG. Vitamin K antagonists and risk of subdural hematoma: meta-analysis of randomized clinical trials. Stroke. 2014;45:1672–1678. doi: 10.1161/STROKEAHA.114.005430. [DOI] [PubMed] [Google Scholar]
- 7.Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 8.De Bonis P, Trevisi G, de Waure C, Sferrazza A, Volpe M, Pompucci A, Anile C, Mangiola A. Antiplatelet/anticoagulant agents and chronic subdural hematoma in the elderly. PLoS One. 2013;8:e68732. doi: 10.1371/journal.pone.0068732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 10.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 11.Dumont TM, Rughani AI, Goeckes T, Tranmer BI. Chronic subdural hematoma: a sentinel health event. World Neurosurg. 2013;80:889–892. doi: 10.1016/j.wneu.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Echlin F. Traumatic subdural hematoma; acute, subacute and chronic; an analysis of 70 operated cases. J Neurosurg. 1949;6:294–303. doi: 10.3171/jns.1949.6.4.0294. [DOI] [PubMed] [Google Scholar]
- 13.Ederer F HH (1959) Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. Bethesda, MD end results Eval sect Natl Cancer Inst 1959
- 14.Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017;14:108-017-0881-y. doi: 10.1186/s12974-017-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaist D, Garcia Rodriguez LA, Hellfritzsch M, Poulsen FR, Halle B, Hallas J, Pottegard A. Association of antithrombotic drug use with subdural hematoma risk. JAMA. 2017;317:836–846. doi: 10.1001/jama.2017.0639. [DOI] [PubMed] [Google Scholar]
- 16.Guilfoyle MR, Hutchinson PJ, Santarius T. Improved long-term survival with subdural drains following evacuation of chronic subdural haematoma. Acta Neurochir. 2017;159:903–905. doi: 10.1007/s00701-017-3095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He W, Goodkind D, Kowal P. U.S. Census Bureau, International Population Reports, P95/16–1, An Aging World: 2015. Washington, DC: U.S. Government Publishing Office; 2016. [Google Scholar]
- 18.Iorio-Morin C, Touchette C, Levesque M, Effendi K, Fortin D, Mathieu D. Chronic subdural hematoma: toward a new management paradigm for an increasingly complex population. J Neurotrauma. 2018;35:1882–1885. doi: 10.1089/neu.2018.5872. [DOI] [PubMed] [Google Scholar]
- 19.Jones S, Kafetz K. A prospective study of chronic subdural haematomas in elderly patients. Age Ageing. 1999;28:519–521. doi: 10.1093/ageing/28.6.519. [DOI] [PubMed] [Google Scholar]
- 20.Keeler E, Guralnik JM, Tian H, Wallace RB, Reuben DB. The impact of functional status on life expectancy in older persons. J Gerontol A Biol Sci Med Sci. 2010;65:727–733. doi: 10.1093/gerona/glq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kpelao E, Beketi KA, Moumouni AK, Doleagbenou A, Ntimon B, Egbohou P, Mouzou T, Tomta K, Sama DH, Abalo A, Walla A, Dossim A. Clinical profile of subdural hematomas: dangerousness of subdural subacute hematoma. Neurosurg Rev. 2016;39:237–240. doi: 10.1007/s10143-015-0669-4. [DOI] [PubMed] [Google Scholar]
- 22.Lee KS. Chronic subdural hematoma in the aged, trauma or degeneration? J Korean Neurosurg Soc. 2016;59:1–5. doi: 10.3340/jkns.2016.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindvall P, Koskinen LO. Anticoagulants and antiplatelet agents and the risk of development and recurrence of chronic subdural haematomas. J Clin Neurosci. 2009;16:1287–1290. doi: 10.1016/j.jocn.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, Wang J, Hu X. Aging of cerebral white matter. Ageing Res Rev. 2017;34:64–76. doi: 10.1016/j.arr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manickam A, Marshman LA, Johnston R. Long-term survival after chronic subdural haematoma. J Clin Neurosci. 2016;34:100–104. doi: 10.1016/j.jocn.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637–645. doi: 10.3171/jns.1981.54.5.0637. [DOI] [PubMed] [Google Scholar]
- 27.Maurice-Williams RS. Chronic subdural haematoma: an everyday problem for the neurosurgeon. Br J Neurosurg. 1999;13:547–549. doi: 10.1080/02688699943033. [DOI] [PubMed] [Google Scholar]
- 28.Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci. 2018;50:7–15. doi: 10.1016/j.jocn.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 29.Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: not a benign disease. J Neurosurg. 2011;114:72–76. doi: 10.3171/2010.8.JNS10298. [DOI] [PubMed] [Google Scholar]
- 30.Nathan S, Goodarzi Z, Jette N, Gallagher C, Holroyd-Leduc J. Anticoagulant and antiplatelet use in seniors with chronic subdural hematoma: systematic review. Neurology. 2017;88:1889–1893. doi: 10.1212/WNL.0000000000003918. [DOI] [PubMed] [Google Scholar]
- 31.Prince M, Wimo A, Ali G, Wu Y, Prina M. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 32.Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Ohman J, Helen P (2019) The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg 1–11 [DOI] [PubMed]
- 33.Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, Richards HK, Marcus H, Parker RA, Price SJ, Kirollos RW, Pickard JD, Hutchinson PJ. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374:1067–1073. doi: 10.1016/S0140-6736(09)61115-6. [DOI] [PubMed] [Google Scholar]
- 34.Seppa K, Hakulinen T, Laara E, Pitkaniemi J. Comparing net survival estimators of cancer patients. Stat Med. 2016;35:1866–1879. doi: 10.1002/sim.6833. [DOI] [PubMed] [Google Scholar]
- 35.Shapey J, Glancz LJ, Brennan PM. Chronic subdural haematoma in the elderly: is it time for a new paradigm in management? Curr Geriatr Rep. 2016;5:71–77. doi: 10.1007/s13670-016-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield JB, Heath AC, Madden PAF, Landers JG, Martin NG. Effects of high alcohol intake, alcohol-related symptoms and smoking on mortality. Addiction. 2018;113:158–166. doi: 10.1111/add.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang AI, Balser DS, Mikheev A, Offen S, Huang JH, Babb J, Rusinek H, Samadani U. Cerebral atrophy is associated with development of chronic subdural haematoma. Brain Inj. 2012;26:1731–1736. doi: 10.3109/02699052.2012.698364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang W, Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am. 2017;28:205–210. doi: 10.1016/j.nec.2016.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.