Abstract
An increasing interest in the development of products of natural origin for crop disease and pest control has emerged in the last decade. Here we introduce a new family of strawberry acyl glycosides (SAGs) formed by a trisaccharide (GalNAc-GalNAc-Glc) and a monounsaturated fatty acid of 6 to 12 carbon atoms linked to the glucose unit. Application of SAGs to Arabidopsis thaliana (hereafter Arabidopsis) plants triggered a transient oxidative burst, callose deposition and defense gene expression, accompanied by increased protection against two phytopathogens, Pseudomonas viridiflava and Botrytis cinerea. SAGs-induced disease protection was also demonstrated in soybean infected with the causal agent of target spot, Corynespora cassiicola. SAGs were shown to exhibit important antimicrobial activity against a wide-range of bacterial and fungal phytopathogens, most probably through membrane destabilization, and the potential use of SAGs as a biofungicide for postharvest disease protection was demonstrated on lemon fruits infected with Penicillium digitatum. Plant growth promotion by application of SAGs was shown by augmented primary root elongation, secondary roots development and increased siliques formation in Arabidopsis, whereas a significant increment in number of seed pods was demonstrated in soybean. Stimulation of radicle development and the induction of an auxin-responsive reporter system (DR5::GUS) in transgenic Arabidopsis plants, suggested that SAGs-stimulated growth at least partly acts through the auxin response pathway. These results indicate that strawberry fatty acid glycosides are promising candidates for the development of environmental-friendly products for disease management in soybean and lemon.
Subject terms: Field trials, Effectors in plant pathology, Plant signalling
Introduction
The excessive use of synthetic pesticides and fertilizers brought about by the green revolution has in many cases had a detrimental effect on the environment and has caused important negative effects on human and animal health. As a consequence stricter regulations have been implemented in many countries to reduce the number of synthetic agrochemicals available in agriculture to promote a more sustainable production system. Due to these recent changes in the limitation of the use of chemicals in agricultural production there is an urgent need to find effective and more environmental-friendly alternatives to significantly reduce and/or replace these products1,2. One sustainable strategy that has gained increasing interest in the last decades has been the development of integrated crop management programs where a combined use of non-chemical tools, including crop rotation, planting time, tillage, use of trap crops etc. and biological products are implemented3–5.
A lot of attention has been focused on the development of biostimulants and biofertilizers, supplied to the crop soil or applied directly on the plant, to improve crop yield size and quality by enhancing plant nutrition, reduce abiotic stress impact and promote plant growth4,6,7. In addition, biocontrollers or biopesticides have been developed for pest and disease control in many crops8,9. All these products of biological origin are more readily degradable and less persistent than chemical fertilizers and pesticides, which make them amenable as replacement for traditional agrochemicals in a sustainable crop production system.
Biocontrol products based on plant extracts with antimicrobial activity or microbial antagonists of pathogens have been implemented as an alternative method in integrated pest management for some time. More recently, defense elicitors that induce an incomplete, systemic resistance to a broad range of plant pathogens10–13, have gained a lot of interest. There is a vast variety of elicitors described both of natural and synthetic origin, which have been classified into two major groups; molecules that are or mimic phytohormones and compounds that mock the presence of a plant enemy14. Recently, various products have reached the market for disease control represented by both these groups of molecules15.
Fatty acyl glucosides are amphipathic compounds composed of a glycosyl moiety linked to one or more hydroxyl fatty acids or to one carboxyl group of a fatty acid by an ester linkage. These compounds are mainly produced by bacteria, yeast, fungi, marine invertebrates, and plants16. Because of their physicochemical and biological properties, they are used as surfactants, antibiotics and drugs in the oil, food, cosmetic and pharmaceutical industries17,18. Industrial interest in fatty acyl glycosides of natural origin (biosurfactants) has recently increased due to their low toxicity, high biodegradability, foaming capacity, high selectivity and specificity at extreme temperatures, pH and salinity.
Fatty acyl glucosides have been characterized in numerous plant species belonging to Solanaceae19 and in a few members of the Caryophyllaceae20, Martyniaceae21 and Rubiaceae families22. Studies conducted under controlled and field growing conditions have demonstrated that they play an important role in plant-insect and plant-fungus interactions23–27.
Previous works in our laboratory have demonstrated the capacity of different strawberry extracts and metabolites to protect plants against pathogen infections through enhanced plant immunity and by direct antimicrobial activity28–31. In this study, we present a group of strawberry trisaccharide fatty acid esters, not previously described, which activate innate plant pathogen defense reactions, stimulate plant growth and exhibit important antimicrobial activity.
Results and discussion
Bioassay-guided purification and chemical structure determination of SAGs
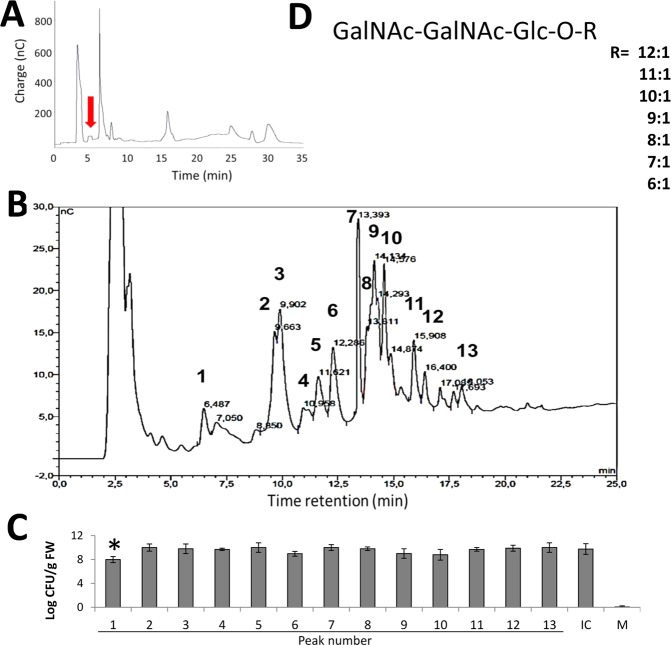
Aqueous extracts from strawberry leaves were fractionated by normal phase (Fig. 1A) and anionic exchange phase chromatography (Fig. 1B) and selected fractions were tested for protective effect against a pathogenic strain of Pseudomonas viridiflava on Arabidopsis plants (Fig. 1C). Leaves from pathogen-inoculated plants treated with the peak eluted at 6.487 minutes showed the greatest reduction of pathogenic bacteria, 1.75 logarithmic units, with respect to non-treated control plants, and was therefore selected for further studies and chemical identification as shown in Fig. 1D. The other fractions tested did not significantly reduce bacterial growth on inoculated plants. MALDI-TOF mass spectrum analysis of the selected fraction (Supplementary Figure S1) showed signals characteristic of an acylated trisaccharide with fatty acids of varying carbon chain length. Monosaccharides of this biologically active fraction were released by hydrolysis and identified as D-galactosamine and D-glucose in a 2:1 ratio (Supplementary Figure S2). In addition, fatty acids of the bioactive compound were derivatized and analyzed by gas-chromatography, which led to the identification of mono-unsaturated fatty acids, with a carbon chain ranging from 6 to 12 atoms. These analyses suggested a structure corresponding to a fatty acid glycoside composed of two molecules of N-acetylgalactosamine and a single glucose unit which in turn is bound by an ester linkage to the mono-unsaturated fatty acid (Fig. 1D). To the best of our knowledge this molecule has not been described previously, although similar fatty acid glycosides produced by microorganisms and plants have been reported16. Antibacterial and antifungal activities have been described for this group of compounds, and many acyl glycosides have been shown to act as emulsifier agents reducing both surface and interphase tension in organic/water liquid mixture. The latter property has been exploited for different applications in the oil, food, cosmetic, and pharmaceutical industries17,18. Although the chemical structure of SAGs indicates that these molecules may present amphipathic behavior, further studies have to be conducted to verify their possible surfactant characteristics.
Figure 1.
Purification and chemical structure of SAGs. (A) SAGs were purified from strawberry leaves by bioassay-guided normal phase (Zorbax-NH2) chromatography by collecting the unique fraction with plant defense inducing activity (arrow) which was later fractioned by (B) Carbopac PA-1 anionic exchange phase chromatography. (C) Bacterial count in leaves of Arabidopsis plants treated with each numbered peak and subsequently inoculated with the pathogen Pseudomonas viridiflava was determined and expressed as logarithm of colony forming units per leaf fresh weight (log CFU/g FW). Water-treated and thereafter inoculated plants are shown as infection control (IC) and non-inoculated plants as Mock treatment (M). (*) Denote statistically significant value using the DGC test (p < 0.05). Experiments were repeated four times with eight plants assayed for each peak. (D) Peak eluted at 6.487 minutes was chemically identified as the trisaccharide (GalNAc-GalNAc-Glc), esterified to a mono-unsaturated 6–12 carbons length fatty acid (R).
SAGs application activates innate plant defense systems and gives disease protection
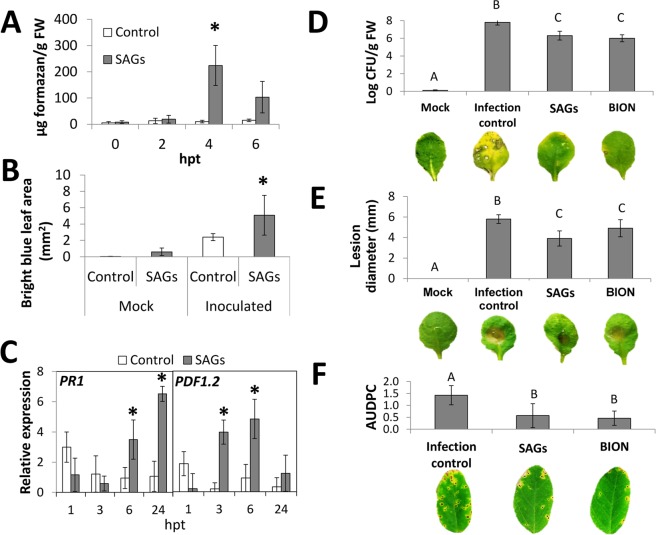
To study in more detail the protective effect of SAGs against plant pathogens, defense response mechanisms in SAGs-treated Arabidopsis plants were investigated. The activation of an innate immunity response in SAGs-treated plants was supported by an early and transient accumulation of reactive oxygen species (ROS) that reached a maximum 4 hours post treatment, as shown by spectrophotometrically quantifying purple formazan deposits in nitro blue tetrazolium-stained Arabidopsis leaves (Fig. 2A and Supplementary Figure S3). In addition, a minor callose deposition was observed in SAGs-treated plants after mock-treatment as a slight increase of brilliant areas in aniline blue-stained leaves (Fig. 2B and Supplementary Figure S4A). Callose deposition was enhanced in both SAGs-treated and water-treated plants after P. viridiflava infection (Supplementary Figure S4), although this increase was significant only in plants previously treated with SAGs (Fig. 2B). This result suggested that SAGs-treatment may have a priming effect on plant innate defense32.
Figure 2.
Pathogen defense-inducing activity of SAGs. (A) Superoxide radical production in leaves of plants after 0, 2, 4 and 6 hours post-treatment with SAGs (10 µg/ml) or water (control) measured by quantification of formazan produced after NBT histochemical staining. (B) Aniline blue staining to detect callose accumulation in leaves of SAGs- (10 µg/ml) or water-treated (control) plants later inoculated or not inoculated (Mock) with Pseudomonas viridiflava. Bright blue leaf areas (mm2) were measured from digital images of the stained leaves. Four leaves per plant and four plants per treatment were evaluated in three independent experiments for NBT and Aniline blue staining. (*) Denote values statistically different using the DGC test (p < 0.05). (C) Relative expression of PR1 and PDF1.2 genes calculated as 2∆CT in water-treated (control) and SAGs-treated (10 µg/ml) Arabidopsis plants at 1, 3, 6 and 24 hours post treatment (hpt). (*) Denote statistically significant differences in RNA expression between control and SAGs-treated plants using the ∆CT method. Three technical and three biological replicates were analyzed for each qPCR reaction. (D) Protection assay on Arabidopsis plants inoculated with P. viridiflava. Bacterial population in plants pretreated with SAGs (10 µg/ml), BION 500 or water (infection control) were expressed as the logarithm of colony forming units per leaf fresh weight (log CFU/g FW). (E) Protection assay on Arabidopsis plants inoculated with Botrytis cinerea. Disease severity was measured as average of leaf lesion diameters in plants pretreated with SAGs (10 µg/ml), BION 500, or water (infection control). Non-inoculated plants were denoted as Mock. (F) Protection assay on soybean plants inoculated with Corynespora cassiicola. The area under the disease progress curve (AUDPC) 4, 7 and 10 days post pathogen inoculation was determined in soybean plants pre-treated with SAGs (100 µg/ml), BION 500 or water (infection control). Eight and six plants per treatment were used in the protection assays on Arabidopsis and soybean, respectively. Both experiments were repeated four times. Different letters indicate significant difference among treatments using the DGC test (p < 0.05). Images of symptoms in leaves from plants corresponding to each treatment are shown under each graph bar.
Induction of defense-related genes in Arabidopsis was also associated with SAGs-treatment as expression levels of PR1 gene, a genetic marker for the salicylic acid defense pathway, significantly increased at 6 and 24 hours post treatment. Similarly, expression of PDF1.2 gene, a genetic marker for ethylene/jasmonate defense signaling, was up-regulated upon SAGs treatment (Fig. 2C). These results indicated that the SAGs-induced defense in Arabidopsis involves more than one defense signaling pathway and thereby may confer protection against different types of pathogens; an observation subsequently confirmed when SAGs-treated Arabidopsis plants showed increased disease protection against both a bacterial and a fungal pathogen. Deciphering the mechanism of action of a bioactive compound is very helpful in the development of a biostimulant or biocontroller, as such information will be of importance when optimizing use and time of application in any given agricultural system.
Arabidopsis plants spray-treated with SAGs 4 days before inoculation with a pathogenic strain of P. viridiflava resulted in an evident reduction of disease symptoms and 50-fold reduction of pathogenic bacterial population in infected leaves (Fig. 2D). This reduction is comparable to plants treated with the commercial biocontroller, BION 500 (Syngenta, Switzerland), based on the salicylic acid analog acibenzolar-S-methyl. A significant protective effect was also observed in Arabidopsis plants previously treated with SAGs when inoculated with the necrotrophic fungi Botrytis cinerea (Fig. 2E).
To evaluate the protective effect of SAGs in other plant species, soybean plants were sprayed with SAGs and three days later inoculated with the fungal pathogen Corynespora cassiicola, causal agent of the late season disease target spot. Soybean plants treated with SAGs at the V3 stage exhibited a 60% reduction in disease severity (Fig. 2F). These results indicated that SAGs may be used as biologically active ingredients in a biocontrol product designed for disease control in soybean.
Antimicrobial effect and mechanism of action of SAGs on phytopathogens
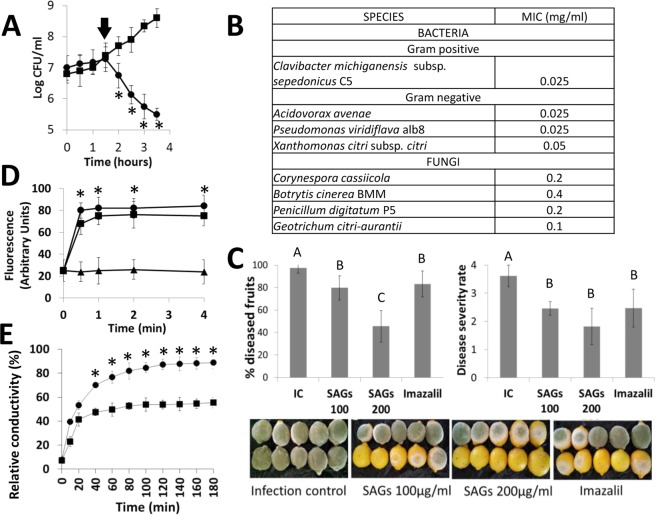
As antimicrobial activity has been reported for many different glycolipids including fatty acyl glucosides16,33,34, in vitro growth inhibition studies against various plant bacterial and fungal pathogens were carried out with the purified SAGs presented in this study. A six-fold higher concentration of SAGs (60 μg/ml) than what was used in the purification protocol (10 μg/ml), was found to inhibit both bacterial cell growth and significantly decrease the number of bacteria in an actively growing culture of the tomato pathogen Clavibacter michiganensis (Fig. 3A). Further studies confirmed an antimicrobial effect against a wide spectrum of both bacterial and fungal phytopathogens (Fig. 3B). SAGs were found to prevent bacterial growth at about a ten-fold lower concentration as compared to the concentration needed to inhibit the fungal pathogens tested. The broad-spectrum of antimicrobial activity at low concentrations exhibited by SAGs could be of interest for applications in the food, cosmetic and pharmaceutical industry.
Figure 3.
Antimicrobial activity of SAGs. (A) Effect of SAGs on Clavibacter michiganensis viability expressed as the logarithm of colony forming units per ml (log CFU/ml) at different time-points. SAGs application (arrow) at a final concentration of 60 μg/ml on fresh C. michiganensis liquid culture (circles) in comparison to non-treated bacteria (squares) was shown. (B) Antimicrobial activity of SAGs determined as Minimal Inhibitory Concentration (MIC) in vitro against different bacterial and fungal phytopathogens evaluated in at least three independent experiments. (C) Effect of SAGs-treatment on lemon fruits inoculated with Penicillium digitatum. Disease incidence (% diseased fruits) and disease severity (fruit area affected by fungus) were evaluated in inoculated fruits either treated with water (IC), SAGs at inhibitory (200 μg/ml) and sub-inhibitory (100 μg/ml) concentrations of pathogen growth, and 500 ppm of commercial fungicide Imazalil, 10 fruits were tested for each treatment in six independent assays. Different letters indicate significant difference among treatments using the DGC test (p < 0.05). Pictures in the lower panel show disease symptoms on lemon fruits for each treatment. (D) Effect on membrane permeability of bacterial cells treated with 60 μg/ml SAGs (squares), Valinomycin (circles) and water (triangles) at time-points indicated, using the intracellular fluorescent diSC35. (E) Effect of SAGs (200 μg/ml) on membrane permeability of fungal mycelia cells at time-points indicated (circles) compared to water-treated cells (squares). Membrane leakage was determined by measuring relative conductivity of C. cassiicola mycelia. Results represent the average ± standard deviation of three independent experiments. (*) Denote statistically significant differences with control treatment.
Antifungal activity of SAGs was also tested against the fungus P. digitatum, responsible for post-harvest decay in citrus fruits (Fig. 3C). Protection of P. digitatum infected lemon fruits with SAGs (100 μg/ml) was comparable to that obtained with Imazalil (Fungaflor 500EC, Janssen Lab., Belgium), a toxic fungicide commonly used in citrus production, whereas a higher concentration (200 μg/ml) clearly outperformed the commercial pesticide, significantly reducing the incidence of fungal disease symptoms in lemon fruits (Fig. 3C). SAGs and Imazalil were applied 6 hours after inoculation in order to imitate what occurs in the lemon packaging industry, where fruits suffer mechanical peel damages (primary infection sites) during harvest and transport to the packing plant where they later receive antifungal treatments. To study the possible underlying mechanism of the demonstrated antimicrobial activity of SAGs, taking into account the amphiphilic structure of these compounds, we applied the fluorescent dye diSC35, which is sensible to dissipation of membrane potential and a good indicator of cellular membrane damage, to C. michiganensis cells later treated with SAGs. Addition of 60 μg/ml SAGs to diSC35 containing bacterial cells induced a rapid increase of fluorescence very similar to that obtained with valinomycin, a well-known cell membrane potential dissipator (Fig. 3D). In addition, changes of the potential and/or permeability of the plasmatic membrane, seen as augmented fluorescence when increasing SAGs concentrations were successively tested, demonstrated a dose-dependent effect (Supplementary Figure S5). Similarly, action of SAGs on fungal membrane stability by measuring ion leakage, was studied. Relative ion-conductivity of C. cassiicola mycelia over time was significantly higher after SAGs treatment (200 μg/ml) as compared to mycelia treated with water (Fig. 3E). Cell membrane permeability of C. cassiicola mycelia treated with SAGs was noticeable higher (89%) when compared to water-treated control mycelia (55%) after 180 min of treatment. These results indicated that the antimicrobial effect observed for SAGs, at least partly, depends on membrane destabilization for both bacteria and fungus. A similar mechanism for antimicrobial activity has been suggested for closely related glycolipids including rhamnolipids35 and sophorolipids36. It is very probable that SAGs have amphiphilic properties that interact with both lipophilic and hydrophilic components, like many other saponin compounds, which often have antimicrobial activities and other biological effects due to their ability to complex with sterols in fungal membranes causing the loss of the membrane integrity with the formation of transmembrane pores8.
SAGs as plant growth stimulator
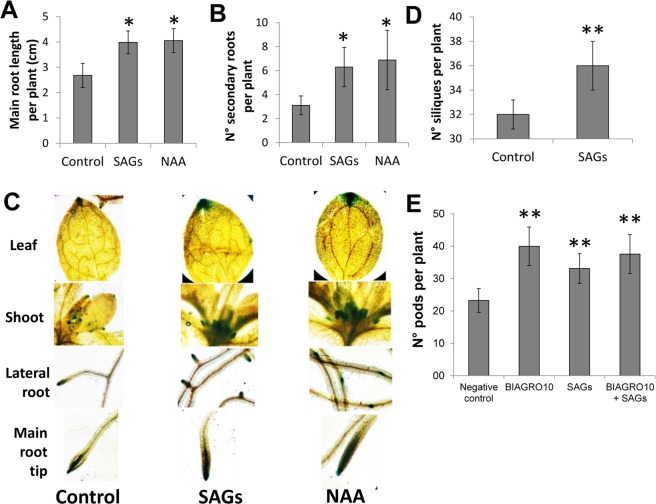
An interesting observation was made in strawberry plants treated with SAGs-containing extracts during field trials conditions, as a visible increase in plant biomass where registered, indicating a possible plant growth stimulating effect of SAGs (data not shown). Another growth effect was detected in soybean, where an increase in seed pods per plants were observed when pathogen defense experiments were performed under controlled growth conditions in the greenhouse. To further study a possible growth and development stimulating effect of SAGs in plants, in vitro growth assays using Arabidopsis seedlings were conducted and, as shown in Fig. 4A–C and Supplementary Figure S6, the bioactive compounds (0.16 μg/ml) promoted both elongation of the primary root as well as development of lateral roots. It is important to notice that the plant growth stimulation of SAGs in Arabidopsis was performed at very low concentrations 0.16 µg/ml for in vitro experiments and 1.6 µg/ml for greenhouse-grown plants. The reason for this was due to a phytotoxic effect seen in Arabidopsis when higher concentrations of SAGs were applied.
Figure 4.
Plant growth stimulation of SAGs. (A) Main root length and (B) number of secondary roots of Arabidopsis plants grown on MS medium supplemented with SAGs (0.16 µg/ml) or naphthalene acetic acid (NAA), and control plants grown on non-supplemented medium was shown. Ten individual plants were analyzed per treatment, and the experiment was carried out three times. (C) Auxin-dependent DR5::GUS gene expression visualized as blue staining in Arabidopsis seedlings grown on SAGs (0.16 µg/ml) - or NAA-supplemented medium or on non-supplemented medium (control). Three independent experiments were performed with 10 plants per treatment. (D) Number of siliques of Arabidopsis plants, 5 weeks post-foliar treatment with SAGs (1.6 µg/ml) or water (Control). Three independent experiments with four random blocks of 6 plants per block were evaluated. (E) Effect of SAGs (50 µg/ml), BIAGRO10, SAGs + BIAGRO10, or water treatment (negative control) on number of pods in greenhouse-grown soybean plants. BIAGRO10 is a commercial bioinoculant based on the nitrogen fixing nodule-forming strain E109 of Bradyrhizobium japonicum. Four random blocks with 5 plants in each block were used in two independent experiments. Asterisks indicate statistically significant differences using DGC test (*p < 0.001, **p < 0.05).
It is well known that auxins are involved in lateral root emergence and development in plants37–39 and a similar result, as seen for SAGs-treated plants, on radicular growth was observed in Arabidopsis plants treated with the auxin NAA (5 ng/ml), although lateral root formation was less pronounced. Furthermore, when the effect of SAGs were tested in the Arabidopsis transgenic line DR5::GUS, in which auxin-responsive tissues are blue-stained when treated with the substrate 5-bromo-4-chloro-3-indolyl glucuronide (XGluc), seedlings grown on medium supplemented with SAGs (0.16 μg/ml) or NAA (5 ng/ml) showed an almost identical staining pattern in leaf tips, shoots, in tips and primordia of lateral roots and in the main root tip (Fig. 4C). In contrast, in non-treated Arabidopsis seedlings a much less pronounced blue staining of the same tissues was observed (Fig. 4C). This minor blue-staining in non-treated plants is most probably due to endogenous auxin effects. These results suggested that the observed plant growth stimulation by SAGs could partly depend on the auxin response pathway by a direct activation of auxin signal transduction pathways or by an increase of endogenous auxins levels, but further studies are needed before a direct relationship with auxin signaling can be concluded. Additionally, foliar application of a higher concentration of SAGs (1.6 µg/ml) on mature Arabidopsis plants produced a significant increase (12%) of siliques per plant (Fig. 4D). Likewise, soybean plants sprayed with a SAGs solution (50 μg/ml) developed over 40% more seed pods per plant than water-treated control plants (Fig. 4E).
Conclusions
The different effects exerted by SAGs on plants and fruits showed a concentration dependent response. At higher concentrations (>100 µg/ml) SAGs exert a direct antimicrobial effect on phytopathogens, most likely by damaging the microbial plasma membrane. However, such high concentrations are phytotoxic for some plant species (i.e. Arabidopsis). Therefore application of these concentrations could be exploited to manage post-harvest fruit diseases before (preventive) or after (curative) infection occurs. At lower concentrations, (<50 µg/ml) where no antimicrobial or phytotoxic effect is observed, SAGs could be employed as elicitors of plant defense for disease management. At these concentrations a growth stimulating effect was observed in soybean and it is therefore plausible that application of concentrations in this range could have a dual effect, as activator of defense and stimulator of growth in this crop. However, before any final conclusions can be made field trials in different agroecosystems and crops will have to be performed.
Methods
Purification of SAGs
SAGs were obtained from freshly harvested Fragaria x ananassa leaves. Extraction was performed in distilled water acidified with 0.1% of trifluoroacetic acid (TFA) by homogenizing plant material (0.1 g fresh weight/ml of solvent) and shaking at 50 rpm (4 °C) for 24 hours. Clarified extract was recovered after centrifugation at 10.000 × g for 15 min and fractioned by preparative chromatography using a solid phase extraction (SPE) cartridge containing a C18-E matrix (Phenomenex, USA). Clarified extract was loaded on the column followed by washing with 0.1% TFA diluted in water. Initial loading volume together with washing flow-through was collected as a preliminary purification step as the SAGs molecules were not absorbed by the column matrix.
Recovered fraction was 40 times-concentrated in a vacuum concentrator (SpeedVac, Thermo Scientific), and further purified by HPLC using a reverse phase chromatography (SOURCE 5RPC, GE Healthcare Biosciences AB) column and a binary solvent system (distilled water and methanol, both acidified with 0.1% TFA). SAGs were recovered in the flow-through, which was 2 times vacuum-concentrated and purified using a normal-phase HPLC Zorbax-Amino (Waters, USA) column with an isocratic gradient of 80% acetonitrile (1 ml/min). The desired fraction was collected at around 5 minutes, vacuum-concentrated to dryness and resuspended in 1 ml of distilled water. In a final purification step a High Performance Anionic Exchange Chromatography with Pulse Amperometric Detector (HPAEC-PAD) system was used with a Carbopac PA-1 (Dionex, USA) column where bound SAGs were eluted by a gradient of a tripartite solvent (200 mM NaOH as solvent A, water as solvent B and 1 M AcONa as solvent C). An initial solvent gradient of 25% A, 75% B and 0% C was maintained for 10 min, followed by a linear gradient where concentrations of solvents B and C were changed until reaching a final solution after 30 min containing 25% A, 25% B and 50% C(1 ml/min) where SAGs were recovered at 6.49 minutes.
Determination of SAGs chemical structure
First matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometer analysis was performed. Pure SAGs were neutralized using acetic acid, vacuum-concentrated to dryness and analyzed by MALDI-TOF mass spectrometry using a 2,5-dihydroxybenzoic acid (DHB) matrix in reflectron positive mode. Monosaccharide composition was determined by dissolving SAGs in distilled water and later hydrolyzing in 2 N trifluoroacetic acid (TFA) during 4 hours at 100°C. The hydrolyzed sample was again vacuum-concentrated to dryness, dissolved in distilled water and analyzed by HPLC to identify released monosaccharides by comparison with standard samples of D-galactosamine, D-glucosamine, L-fucose, D-manose, D-galactose and D-glucose. Analysis was made by HPAEC-PAC using an anionic-exchange Carbopac P20 (Dionex, USA) column and a flow rate of 0.5 ml per minute. Neutral and amino monosaccharides were eluted by an isocratic program of 6% of solvent A (200 mM NaOH) and 94% of solvent B (distilled water). Acidic monosaccharides were eluted by a tripartite solvent (200 mM NaOH as solvent A, water as solvent B and 1 M AcONa as solvent C) using a isocratic program of 24% A, 62% B and 14% C.
Fatty acids composition was determined by gas chromatography of methyl ester derivatives. SAGs were hydrolyzed in 1.67% NaOH at room temperature with gentle shaking for 24 h and thereafter acidified with 0.5 M HCl and three times-extracted with an equal volume of dichloromethane. Organic phases were recovered, pooled and concentrated to dryness. The resulting residue was dissolved in 0.5 ml toluene and an equal volume of 20% boron trifluoride in methanol was added and incubated at 80 °C during 1 h under an atmosphere of nitrogen. After cooling to room temperature, the sample was washed 3 times by adding an equal volume of distilled water and recovering the organic phase, which was 3 times-extracted with toluene. Fatty acid analysis was performed by gas chromatography on a capillary column (Ultra 1,25 m × 0.20 mm) with an initial temperature of 80 °C for 2 min followed by a temperature-increase up to 290 °C (10 °C /min) during a total time of 30 min.
Disease resistance assay in Arabidopsis
Healthy 4-week-old Arabidopsis Col-0 plants sprayed with 10 μg/ml SAGs were 4 days later inoculated with the bacterial pathogen Pseudomonas viridiflava alb840 or the fungal pathogen Botrytis cinerea BMM41. Treatment with 0.4 mg/ml of the commercial biocontrol product BION 500 (Syngenta, Switzerland) was included as a plant defense-induction control whereas plants sprayed with sterile distilled water were used as negative controls and water-treated inoculated plants as infection controls. Pseudomonas viridiflava-inoculated plants were subsequently incubated in plant culture chambers under controlled growing conditions (26°C, 80% RH, 16 h photoperiod) and four days post bacterial inoculation leaf disease symptoms were recorded and colony forming units per grams of fresh weight determined by homogenization of leaves from infected plant and plating serial dilutions on LB-plates.
In addition, B. cinerea-inoculated plants were incubated in culture chambers at 22°C, 80% RH in darkness and three days later foliar lesion diameters were measured and recorded. Concentrations of SAGs used in the experiment were sub-inhibitory to both bacterial and fungal pathogen growth. Eight plants per treatment were assayed in four independent experiments.
Detection of reactive oxygen species (ROS) in Arabidopsis leaves
In situ detection of superoxide radical production in leaves of Arabidopsis was carried out by NBT histochemical staining42, with minor modifications. Plants were subjected to 10 μg/ml SAGs- or distilled water-treatments by foliar spraying. At 0, 2, 4 and 6 hours post treatment leaves were detached from treated plants, fresh weight determined and immersed in a 50 mM potassium phosphate buffer (pH 7.8) containing 0.1% NBT and 10 mM sodium azide. Stained leaves were vacuum-infiltrated by two vacuum shock-treatments for 1 min at 100 mm Hg, and thereafter incubated for 1 hour in darkness (without vacuum). After dark-incubation, leaves were first immersed in 96% (v/v) ethanol, to eliminate remaining chlorophyll, and then clarified and conserved in a solution of lactic acid/glycerol/water (3:3:4 v/v/v). Superoxide production was visualized as purple formazan deposits within leaflet tissues (Supplementary Figure S3), which were extracted and solubilized to spectrophotometrically quantify the superoxide accumulation in leaves, following the method of Grellet-Bournonville and Diaz-Ricci43. Briefly, NBT-stained and clarified leaves were homogenized in a mix of KOH (2 N):chloroform (1:1), the organic phase was recovered, vacuum-concentrated to dryness and the formazan extracted was dissolved in 350 µl dimethylsulfoxide and 300 µl KOH 2 N. Formazan was quantified in a spectrophotometer at 630 nm and expressed as µg of formazan per gram of leaf fresh weight using a calibration curve. Four leaves per plant were collected and four plants per treatment were evaluated in three independent experiments.
Callose accumulation in Arabidopsis leaves
Callose deposition was visualized using aniline blue dye44. Detached leaves of treated Arabidopsis Col-0 plants were discolored in 96% ethanol, and gradually rehydrated by being submerged sequentially in 50% ethanol, 25% ethanol and finally in 67 mM K2HPO4 (pH 12). After rehydration, leaves were stained for 1 hour with 0.05% aniline blue in darkness, and finally immersed in 30% glycerol before being analyzed under UV-light in a fluorescence microscope, to visualize callose accumulation as bright blue areas (Supplementary Figure 4 A). Before staining, Arabidopsis plants were sprayed with either 10 μg/ml SAGs or distilled water, and 6 days post-treatment detached leaves were stained as described above. In addition, a group of treated plants were inoculated with P. viridiflava alb8 4 days after SAGs- and water-treatment and stained with aniline 2 days later. Four leaves per plant were collected and four plants per treatment were evaluated in three independent experiments. Quantification of callose deposition was measured from digital images obtained from microscopic visualizations which correspond to leaf areas of 25 mm2, using the ImageJ (Image Processing and Analysis in Java) software (NIH, USA). Briefly, each image was converted to greyscale mode, a line with known length was drawn to define the real scale based on the eyepiece micrometer of the microscope. For all images threshold values for bright blue area was defined by only considering values ranging from 50–255 in the histograms showed. Selected areas were thereafter converted into red stained areas by the software (Supplementary Figure 4B) and measurement of the detected area was calculated using the analyze particle option, setting minimum particle size as 0.00002 cm2. Automatically, Image J software generated a new image with the detected areas (Supplementary Figure 4 C) and a results table obtained to each image (Supplementary Figure 4D). The calculation of the average Calculated Total Area value (cm2) for each microphotography was performed for each treatment and graphed as the bright blue area (mm2) per 25 mm2 of total leaf area.
Analysis of gene expression in Arabidopsis
Evaluation of gene expression in Arabidopsis plants was performed by real-time PCR. Total RNA was purified from leaves of Arabidopsis plants at 1, 3, 6 and 24 hours after foliar spraying with 10 μg/ml SAGs or distilled water. Detached leaves from treated plants were homogenized in liquid nitrogen using a mortar and pestle and total RNA was extracted by the Trizol method45. Briefly, 150 mg of leaves were homogenized in 1 ml of Trizol reagent, purified in a chloroform:isoamyl alcohol mix, and finally treated with DNAse I. Purity and quality of extracted RNA was determined by spectrophotometry and electrophoresis, respectively. Retrotranscription was performed with the reverse transcriptase M-MLV enzyme (Thermo Scientific) following the manufacturer´s instructions. Resulting cDNA was analyzed by real-time PCR using iQTM SYBR® Green Supermix (BioRad). Expression of PR1 (At2g14610; forward primer: GTCTCCGCCGTGAACATGT; reverse primer: CGTGTTCGCAGCGTAGTTGT) and PDF1.2 (At5g44420; forward primer: TTTGCTTCCATCATCACCCTTA; reverse primer: GCGTCGAAAGCAGCAAAGA) genes were studied. The housekeeping EF1 gene (At1g18070; forward primer: AGCACGCTCTTCTTGCTTTC; reverse primer: GGGTTGTATCCGACCTTCTTC) was used as reference gene whose expression did not change at each time point and treatment studied. Gene expression levels were measured using the ∆CT method calculated as 2∆CT to water-treated (control) or SAGs-treated plants46. ∆CT is the subtraction between reference gene CT value and target gene (PR1 or PDF1.2) CT value. The least number of cycles at which enough amplified product accumulates to yield a detectable fluorescent signal is called the threshold cycle, or CT. Three technical and three biological replicates were analyzed for each treatment.
Disease resistance assay in soybean
Healthy soybean (Glycine max) seeds of elite variety A8000 RG were sown in 4 l pots with soil, germinated in the greenhouse and grown to vegetative stage V3 (unifoliolate and first three trifoliolate leaves fully developed), when plants were sprayed with 100 μg/ml SAGs and three days later inoculated with virulent strain C4 of the pathogenic fungus C. cassiicola47, causal agent of soybean target spot. It should be noted that the concentration of SAGs used in the experiment were sub-inhibitory for growth of C. cassiicola. In addition, pre-treatment with sterile distilled water, or with 0.4 mg/ml of the commercial biocontrol product BION 500 were evaluated. Target spot-affected areas were evaluated on V3 and V4 plant segment leaves at 4, 7 and 10 days post-inoculation. Severity Index was calculated for each plant and each time point post-inoculation47 and the area under the disease progress curve (AUDPC) for each treatment was determined48. Four independent experiments were performed, and six soybean plants per treatment were used in each assay.
Liquid medium growth and membrane depolarization assays in Clavibacter michiganensis
The antimicrobial activity of SAGs was evaluated against the bacterial strain C. michiganensis subsp. sepedonicus C5 growing in liquid LB medium at 120 rpm and 26°C with an initial OD600 adjusted to 0.1 (7 log colony forming unit per ml) and OD600 measurements taken every half-hour. SAGs were added to growing bacteria after 1.5 hours of growth to a final concentration of 60 μg/ml. Membrane depolarization of C. michiganensis was monitored as changes in fluorescence emission intensity of the membrane-potential-sensitive dye diSC35,49. C. michiganensis was grown in LB liquid medium to mid log phase growth (OD600 = 0.5) where after cells were harvested by centrifugation and washed once (5 mM glucose and 20 mM HEPES pH 7.3) and resuspended to a concentration of OD600 of 0.05 in the same buffer. Aliquots of 100 µl were placed into quartz cuvettes containing 2.0 ml of 5 mM glucose, 100 mM KCl and 5 mM Na HEPES buffer pH 7.2. After addition of 0.4 µM diSC35, cuvettes containing bacterial cells were incubated at 26°C until a stable reduction of fluorescence (around 5 min), indicating incorporation of the dye into the bacterial membrane, was achieved. SAGs (60 μg/ml), Valinomycin (1 μM plus 100 mM KCl) or water were thereafter added and the dye fluorescence increase was recorded at 622 nm (excitation wavelength) and 670 nm (emission wavelength) with a RF-5301PC spectrofluorometer (Shimadzu, Kyoto, Japan) at 0, 0.5, 1, 2 and 4 min. The antibiotic Valinomycin, a well-known K+-selective ionophore that changes the bacterial membrane potential, was used as positive membrane-destabilizing control. A SAGs dose-response assay on membrane permeability of C. michiganensis was performed measuring the fluorescence after 1 minute at different concentrations (0, 7, 15, 30, 60 and 90 μg/ml) of SAGs. Experiments were repeated at least three times under identical experimental conditions.
Action on fungal cell membrane permeability
A method described by Elsherbiny and Taher50 was assayed. Briefly, C. cassiicola was cultured on Potato-Dextrose-Agar plates for 10 days and mycelial disks of 5 mm in diameter were taken from the margins of each plate to inoculate flasks with 100 ml of Sabouraud Maltose Broth. After 48 h of incubation with shaking at 150 rpm at 25°C, SAGs at a final concentration of 200 μg/ml was added. Flasks without application of SAGs were used as fungal control. After an additional 48 h of incubation under the same conditions, mycelia were collected from both treated and untreated flasks, washed twice with double distilled water and vacuum-filtrated. A measured amount of mycelia (0.5 g) was suspended in 20 ml of double distilled water and conductivity was measured after 0, 10, 20, 40, 60, 80, 100, 120, 140, 160 and 180 min with a pH/CON 510 series conductivity meter (Oakton, Illinois, USA). Finally, the mycelia was boiled for 5 min to measure total conductivity and the relative conductivity of mycelia was calculated (Relative conductivity (%) = conductivity/total conductivity × 100). The experiment was repeated three times.
Antimicrobial assay on agar plate
Antimicrobial activity of SAGs was evaluated against several bacterial plant pathogen species51. SAGs were diluted in sterile distilled water ranging from 6.25 to 100 μg/ml, and a drop of 10 μl of each was placed on LB agar plate until absorption. Molten sterile soft agar (0.7% in water) were cooled to 40 °C and inoculated with 107 cells/ml of an overnight culture of each bacterial phytopathogenic strain (C. michiganensis subsp. sepedonicus C5, Acidovorax avenae, Pseudomonas viridiflava alb8, Xanthomonas citri subsp. citri), poured on LB agar plates and incubated at 28°C. After 24 to 48 hours of incubation at 28°C, the zone of growth inhibition around each SAGs drop was observed. Minimal Inhibitory Concentration (MIC) was taken as the lowest concentration of SAGs that showed bacterial growth inhibition. LB medium was replaced with Cadmus medium to evaluate Xanthomonas citri subsp. citri.
Antifungal growth activity of SAGs was evaluated in a similar way as for bacteria. SAGs were diluted in sterile distilled water with a final concentration ranging from 5 to 1000 μg/ml, and a drop of 50 μl of each concentration was placed onto Potato-Dextrose-Agar (PDA) plates until absorption. Molten sterile soft agar (0.7% in water) was cooled to 40°C and inoculated with 104 conidia/ml with an overnight culture of each of the following fungal phytopathogenic strains (Corynespora cassiicola, Botrytis cinerea BMM, Penicillium digitatum P5 and Geotrichum citri-aurantii), poured on PDA plates containing drops of different concentrations of SAGs and incubated at 28°C for 48 to 72 hours. After incubation zones of growth inhibition around each SAGs dilution was observed and MIC was determined for each fungal strain. Each phytopathogen was evaluated in at least three independent experiments.
Assays of postharvest disease protection in lemon fruits
Lemon fruits previously disinfected by immersion in a 10% sodium hypochlorite solution and thereafter washed in distilled water, were inoculated by superficially wounding (1 mm in diameter and 2 mm in depth) the fruit surface by punching with a steel bar, and later apply a 20 μl aliquot of a 106 spores/ml suspension of the virulent strain P5 of P. digitatum. Infected fruits were sprayed with two concentrations of SAGs, 100 and 200 μg/ml, 6 hours after pathogen inoculation. As a positive control of fungal disease protection a commercial fungicide, 500 ppm Imazalil (Fungaflor 500EC, Janssen Lab., Belgium) was applied 6 hours after inoculation, while distilled water-treated lemons were used as infection controls. All treated fruits were incubated in a growth chamber at 25°C and 90% RH. Six days post pathogen-inoculation, fruit inspection was performed and the incidence (% of fruits with symptoms) and severity of the disease (fruit surface affected by mycelial development) were evaluated. Numerical visual rating with values ranging from 0 (without development of mycelium) to 4 (fruit completely covered with mycelium) was conducted. From the evaluation of the surface affected by mycelium for each fruit, a severity rate was calculated as the average frequency of affected fruits for each treatment. Evaluations were performed in 10 fruits for each treatment, and in six independent assays.
Growth promotion studies in Arabidopsis
Plants of Arabidopsis Col-0 and the DR5::GUS transgenic line were grown using a vertically oriented agar-plate culture system to evaluate aerial growth and root development52. SAGs (0.16ug/ml) were added to molten MS-medium (50°C) and poured onto agar plates. Phenotypes were generally observed at 7, 10 and 14 days after seed germination. For comparison, auxin effects were evaluated in plants grown in MS-medium supplemented with 5 ng/ml naphthalene acetic acid (NAA). Number of secondary roots per plant was determined at 10 days post germination using a light microscope. Main root length per plant was measured using the ImageJ software 1.48 v (NIH, USA) for digital imaging of roots. Experiments were repeated three times using 10 plants in each experiment. The DR5::GUS transgenic plants carries a strong synthetic auxin-responsive promoter (DR5) driving the β-glucuronidase reporter gene (gusA), which can be used to detect auxin distribution and responses in plant tissue by histochemical staining53. To visualize gusA expression in different plant tissues, seedlings grown as described previously were collected and submerged in the chromogenic substrate 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc) for 10 min at room temperature and then incubated at 37°C in the dark for 16 h. After dark-incubation seedlings were rinsed with 50 mM sodium phosphate buffer pH 7.0, cleared with 95% (v/v) ethanol and transferred to 70% (v/v) ethanol. Images of GUS-stained Arabidopsis tissue were recorded using a light microscope at 10X magnification equipped with a digital camera. Experiments were repeated three times using 10 plants for each experiment.
Plant growth promoting effects of SAGs on greenhouse-grown (25°C, 16 h photoperiod and 70% HR) Arabidopsis Col-0 plants were assayed by spraying SAGs (1.6 ug/ml) on aerial parts of healthy 3 weeks old plants in the beginning of flowering (onset of inflorescence). Total silique number per plant was evaluated 5 weeks after treatment. Plants sprayed with distilled water were used as control treatment. Experimental design consisted in 4 random blocks of 6 plants per block for each treatment; the experiment was repeated three times.
Pod setting in soybean
Glycine max plants (A8000 RG, maturity group VIII), sown in pots with a sterile low-fertility soil substrate and grown under controlled greenhouse conditions, were treated by spraying aerial parts with SAGs (50 μg/ml) and water (negative control) together with a siliconized adjuvant, nonylphenolethoxylate (0.3%), at plant growth stages V1, V7, R1 and R3. Vegetative V1 and V3 stages correspond to plants with a unifoliate leaf, and plants with the unifoliate leaf and first three trifoliate fully developed leaves, respectively. Reproductive R1 and R3 stages correspond to soybean plants at onset of flowering, and at beginning of pod formation, respectively. As positive growth promotion control, treatment of soybean seeds with the commercial bioinoculant BIAGRO10 (BIAGRO S.A., Argentina), based on nodule-inducing strain Bradyrhizobium japonicum 109 was carried out according to instructions from the manufacturer (77 g/L for 190 kg of seeds). Experiments were carried out in a greenhouse with temperatures ranging from 25 to 30°C and a controlled photoperiod of 18 hours light (12000–18000 lux). For each treatment, 4 random blocks with 5 plants in each were used in two independent assays. Once plants had reached stage R8, total number of pods per plant was determined for each treatment and the results were statistically analyzed by ANOVA using a random block experimental design.
Statistical analysis
Software InfoStat version 2018 (http://www.infostat.com.ar) was used for statistical analysis of all data and ANOVA by Di Rienzo, Guzmán and Casanoves (DGC) comparisons with p-value <0.05 was performed to detect statistically significant differences among treatments.
Supplementary information
Acknowledgements
We first would like to thank Dr. L. Toum and Dr. A. Vojnov for providing their experience and valuable suggestions in the work with Arabidopsis. We would also like to thank Dr. P. Carbonero and Dr. F. García Olmedo (UPM, Madrid, Spain) for their invaluable review and critical reading of the manuscript. This research was supported by funds from PICT 2013–3138 project (Agencia Nacional de Promoción Científica y Tecnológica Res. N°1055 214/14 and 539/13) and from EEAOC. CGB, MPF, ASC, JDR, BW and APC are researchers of CONICET. AMM, JDR and APC are professors at UNT. MFT and PAD are doctoral and postdoctoral students, respectively.
Author contributions
C.G.B., M.P.F., A.M.M., B.W. and A.P.C. designed the experiments. C.G.B. and A.S.C. designed and performed the SAGs purification method. A.S.C. determined the chemical structure of SAGs. C.G.B., P.A.D. and M.F.T. carried out the plant defense induction experiments. M.P.F., P.A.D. and A.M.M. studied and characterized antimicrobial activities. Plant growth promotion studies were carried out by C.G.B. and M.F.T. J.C.D.R., B.W. and A.P.C. interpreted the data. C.G.B. and B.W. wrote and edited the manuscript, A.P.C. and J.C.D.R. revised critically the article. All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Carlos Grellet Bournonville, María Paula Filippone and Pía de los Ángeles Di Peto.
Contributor Information
Björn Welin, Email: bwelin@gmail.com.
Atilio Pedro Castagnaro, Email: atiliocastagnaro@gmail.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-65125-7.
References
- 1.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418(6898):671. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 2.Baulcombe D. Reaping benefits of crop research. Science. 2010;327:761–761. doi: 10.1126/science.1186705. [DOI] [PubMed] [Google Scholar]
- 3.Dayan FE, Cantrell CL, Duke SO. Natural products in crop protection. Bioorganic & medicinal chemistry. 2009;17(12):4022–4034. doi: 10.1016/j.bmc.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH. Biostimulants in plant science: a global perspective. Frontiers in plant science. 2017;7:2049. doi: 10.3389/fpls.2016.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiesel L, et al. Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Frontiers in plant science. 2014;5:655. doi: 10.3389/fpls.2014.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocira S, et al. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Frontiers in plant science. 2018;9:388. doi: 10.3389/fpls.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.du Jardin P. Plant biostimulants: definition, concept, main categories and regulation. Scientia Horticulturae. 2015;196:3–14. doi: 10.1016/j.scienta.2015.09.021. [DOI] [Google Scholar]
- 8.Kim B, et al. Identification of novel compounds, oleanane-and ursane-type triterpene glycosides, from Trevesia palmata: Their biocontrol activity against phytopathogenic fungi. Scientific reports. 2018;8(1):14522. doi: 10.1038/s41598-018-32956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matyjaszczyk E. “Biorationals” in integrated pest management strategies. Journal of Plant Diseases and Protection. 2018;125(6):523–527. doi: 10.1007/s41348-018-0180-6. [DOI] [Google Scholar]
- 10.Grant, W. P. et al. Biopesticides: pest management and regulation. CABI, (2010).
- 11.Kuć, J. What’s old and what’s new in concepts of induced systemic resistance in plants, and its application. Multigenic and induced systemic resistance in plants, eds (Springer, Boston), pp. 9–20 (2006).
- 12.Reglinski, T., Dann, E. & Deverall, B. Implementation of induced resistance for crop protection. Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection, eds. Walters, D. R., Newton, A. C. & Lyon, G. D. (West Sussex, UK: John Wiley & Sons, Ltd), pp 249–299 (2014).
- 13.Lyon, G. D. Agents That Can Elicit Induced Resistance. Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection, eds Walters, D. R., Newton, A. C. & Lyon, G. D. (West Sussex, UK: John Wiley & Sons, Ltd), pp 11–31 (2014).
- 14.Heil, M. Trade-offs associated with induced resistance. Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection, eds Walters, D. R., Newton, A. C. & Lyon, G. D. (West Sussex, UK: John Wiley & Sons, Ltd), pp 171–185 (2014).
- 15.Walters D, Walsh D, Newton A, Lyon G. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology. 2005;95(12):1368–1373. doi: 10.1094/PHYTO-95-1368. [DOI] [PubMed] [Google Scholar]
- 16.Dembitsky VM. Astonishing diversity of natural surfactants: 1. Glycosides of fatty acids and alcohols. Lipids. 2004;39(10):933–953. doi: 10.1007/s11745-004-1316-1. [DOI] [PubMed] [Google Scholar]
- 17.Holmberg K. Natural Surfactants. Curr Opin Colloid Interface Sci. 2001;6:148–159. doi: 10.1016/S1359-0294(01)00074-7. [DOI] [Google Scholar]
- 18.Rosen, M. J. Surfactants and Interfacial Phenomena. 3rd eds John Wiley & Sons (New York), p 464 (2004).
- 19.Moghe GD, Leong BJ, Hurney SM, Jones AD, Last RL. Evolutionary routes to biochemical innovation revealed by integrative analysis of a plant-defense related specialized metabolic pathway. eLife. 2017;6:e28468. doi: 10.7554/eLife.28468.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asai T, Fujimoto Y. Cyclic fatty acyl glycosides in the glandular trichome exudate of Silene gallica. Phytochemistry. 2010;71:1410–1417. doi: 10.1016/j.phytochem.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Asai T, Hara N, Fujimoto Y. Fatty acid derivatives and dammarane triterpenes from the glandular trichome exudates of Ibicella lutea and Proboscidea louisiana. Phytochem. 2010;71:877–894. doi: 10.1016/j.phytochem.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Dalsgaard P, et al. Noniosides E-H, New Trisaccharide Fatty Acid Esters from the Fruit of Morinda citrifolia (Noni) Planta Medica. 2006;72(14):1322–1327. doi: 10.1055/s-2006-951706. [DOI] [PubMed] [Google Scholar]
- 23.Puterka GJ, Farone W, Palmer T, Barrington A. Structure-function relationships affecting the insecticidal and miticidal activity of sugar esters. Journal of Economic Entomology. 2003;96:636–644. doi: 10.1093/jee/96.3.636. [DOI] [PubMed] [Google Scholar]
- 24.Simmons AT, Gurr GM, McGrath D, Martin PM, Nicol HI. Entrapment of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) on glandular trichomes of Lycopersicon species. Australian Journal of Entomology. 2004;43:196–200. doi: 10.1111/j.1440-6055.2004.00414.x. [DOI] [Google Scholar]
- 25.Leckie BM, et al. Differential and synergistic functionality of acylsugars in suppressing oviposition by insect herbivores. PLoS One. 2016;11:e0153345. doi: 10.1371/journal.pone.0153345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luu VT, et al. O-acyl sugars protect a wild tobacco from both native fungal pathogens and a specialist herbivore. Plant Physiology. 2017;174:370–386. doi: 10.1104/pp.16.01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinhold A, Baldwin IT. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. PNAS. 2011;108:7855–7859. doi: 10.1073/pnas.1101306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippone MP, Diaz Ricci J, Mamaní de Marchese A, Farías RN, Castagnaro A. Isolation and purification of a 316 Da preformed compound from strawberry (Fragaria ananassa) leaves active against plant pathogens. FEBS letters. 1999;459(1):115–118. doi: 10.1016/S0014-5793(99)01231-4. [DOI] [PubMed] [Google Scholar]
- 29.Filippone MP, Diaz Ricci J. Mamaní de Marchese, A., Castagnaro, A. & Farías, R. N. Effect of fragarin on the cytoplasmic membrane of the phytopathogen Clavibacter michiganensis. Molecular plant-microbe interactions. 2001;14(7):925–928. doi: 10.1094/MPMI.2001.14.7.925. [DOI] [PubMed] [Google Scholar]
- 30.Mamaní A, et al. Pathogen-induced accumulation of an ellagitannin elicits plant defense response. Molecular plant-microbe interactions. 2012;25(11):1430–1439. doi: 10.1094/MPMI-12-11-0306. [DOI] [PubMed] [Google Scholar]
- 31.Martos GG, et al. Ellagitannin HeT obtained from strawberry leaves is oxidized by bacterial membranes and inhibits the respiratory chain. FEBS open bio. 2018;8(2):211–218. doi: 10.1002/2211-5463.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrath U, et al. Priming: getting ready for battle. Molecular plant-microbe interactions. 2006;19(10):1062–1071. doi: 10.1094/MPMI-19-1062. [DOI] [PubMed] [Google Scholar]
- 33.Cortes-Sanchez A, Hernandez-Sanchez H, Jaramillo-Flores ME. Biological activity of glycolipids produced by microorganisms: new trends and possible therapeutic alternatives. Microbiological Research. 2013;168(1):22–32. doi: 10.1016/j.micres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Cameotra SS, Makkar RS. Recent applications of biosurfactants as biological and immunological molecules. Current opinion in microbiology. 2004;7(3):262–266. doi: 10.1016/j.mib.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez M, et al. Modulation of the physical properties of dielaidoylphosphatidylethanolamine membranes by a dirhamnolipid biosurfactant produced by Pseudomonas aeruginosa. Chemistry and physics of lipids. 2006;142(1):118–127. doi: 10.1016/j.chemphyslip.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Kim KJ, Kim YB, Lee BS, Shin DH, Kim EK. Characteristics of sophorolipid as an antimicrobial agent. Journal of microbiology and biotechnology. 2002;12(2):235–241. [Google Scholar]
- 37.Casimiro I, et al. Auxin transport promotes Arabidopsis lateral root initiation. The Plant Cell. 2001;13(4):843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhalerao RP, et al. Shoot‐derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. The Plant Journal. 2002;29(3):325–332. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- 39.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115(5):591–602. doi: 10.1016/S0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 40.Jakob K, et al. Pseudomonas viridiflava and P. syringae—natural pathogens of Arabidopsis thaliana. Molecular plant-microbe interactions. 2002;15(12):1195–1203. doi: 10.1094/MPMI.2002.15.12.1195. [DOI] [PubMed] [Google Scholar]
- 41.L’Haridon F, et al. A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS pathogens. 2011;7(7):e1002148. doi: 10.1371/journal.ppat.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohlgemuth H, et al. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant, Cell &. Environment. 2002;25(6):717–726. [Google Scholar]
- 43.Grellet-Bournonville CF, Díaz‐Ricci JC. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochemical Analysis. 2011;22(3):268–271. doi: 10.1002/pca.1275. [DOI] [PubMed] [Google Scholar]
- 44.Currier HB, Strugger S. Aniline blue and fluorescence microscopy of callose in bulb scales of Allium cepa L. Protoplasma. 1956;45(4):552–559. doi: 10.1007/BF01252676. [DOI] [Google Scholar]
- 45.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical biochemistry. 1987;162(1):156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 46.Bio‐Rad, L. Real‐Time PCR Applications Guide. Bio-Rad Laboratories, Inc, 41 (2006)..
- 47.Chalfoun NR, et al. Development of PSP1, a biostimulant based on the elicitor AsES for disease management in monocot and dicot crops. Frontiers in Plant Science. 2018;9:844. doi: 10.3389/fpls.2018.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaner G, Finney RE. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology. 1977;67(8):1051–1056. doi: 10.1094/Phyto-67-1051. [DOI] [Google Scholar]
- 49.Wu M, Maier E, Benz R, Hancock RE. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38(22):7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 50.Elsherbiny EA, Taher MA. Silicon induces resistance to postharvest rot of carrot caused by Sclerotinia sclerotiorum and the possible of defense mechanisms. Postharvest Biology and Technology. 2018;140:11–17. doi: 10.1016/j.postharvbio.2018.02.004. [DOI] [Google Scholar]
- 51.De Caleya RF, Gonzalez-Pascual B, García-Olmedo F, Carbonero P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Applied microbiology. 1972;23(5):998–1000. doi: 10.1128/AEM.23.5.998-1000.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vellosillo T, et al. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. The Plant Cell. 2007;19(3):831–846. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avsian-Kretchmer O, Cheng JC, Chen L, Moctezuma E, Sung ZR. Indole acetic acid distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny. Plant Physiology. 2002;130(1):199–209. doi: 10.1104/pp.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.