Abstract
To compare the risk of cancer development between patients with glaucoma and those without, we conducted a nationwide population-based cohort study using the Korean National Health Insurance Database. Individuals with diagnosis of glaucoma between 2007 and 2016 were identified, and controls were 1:1 matched based on age and sex. We calculated the incidence rates(IR) and hazard ratios(HR) before and after adjusting for age, gender, diabetes, smoking history, and body mass index. A total of 107,536 individuals with glaucoma and the same number of individuals without glaucoma were included. The IR of overall cancer were 12.23 and 11.62 per 1,000 individuals in the glaucoma and control groups, respectively. The HR of overall cancer was significantly higher in the glaucoma group before(HR: 1.053) and after adjusting for confounding factors(adjusted HR: 1.049) compared to that in the control group. The risk of overall cancer and specific cancers varied depending on gender and age groups, and the association was stronger in women and those under 65 years of age. Our study revealed that individuals with glaucoma showed higher risk of overall cancer and higher risk of specific cancers than those without glaucoma.
Subject terms: Optic nerve diseases, Epidemiology
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide, making it a major public health challenge1–3. It is a neurodegenerative disease characterized by progressive retinal ganglion cell death caused by multifactorial etiology including high intraocular pressure (IOP), neurotrophin insufficiency, local ischemia-hypoxia, inflammation, glutamate excitotoxicity, oxidative stress, and aberrant immunity4–8.
Among these risk factors, elevated IOP is the major risk factor1,4, however, patients with normal-tension glaucoma, the most predominant form of glaucoma in Korea9, have normal range IOP. This points to the importance of other mechanisms beyond IOP, such as inflammation in the pathogenesis of glaucoma7,8,10,11. Previous studies have shown that inflammatory mediators such as tumor necrosis factor alpha, interleukins, endothelin-1, and C-reactive protein are elevated in glaucoma12–15.
Inflammation plays decisive roles at all stages of cancer development16–18. Chronic inflammation increases the risk of carcinogenesis, from initiation, promotion, malignant conversion, to metastasis, and even subclinical, low grade inflammation may be as important in promoting cancer. Previous studies have shown strong and recurrent associations between inflammatory bowel diseases and cancer, not just limited to gut malignancies but also other sites including lung, kidney, endocrine, cervix, and prostate19,20. Increased risk of overall cancer and various focal malignancies has been reported in patients with rheumatoid arthritis and systemic lupus erythematosus (SLE)21,22.
In light with this, it is possible that glaucoma may contribute to increased risk of cancer, however, the association of the two disease entities has not yet been investigated. Identifying the risk of overall cancer and specific types of cancer in glaucoma patients may improve our understanding of both diseases and provide guidelines for appropriate diagnostic work-up for cancer screening in glaucoma patients.
Therefore, in this nationwide, population-based, age- and sex-matched cohort study, we aimed to investigate the risk of cancer development in patients with glaucoma using the National Health Insurance Database (NHID) provided by the Korean National Health Insurance Service (KNHIS), which covers almost the entire 50 million Korean population.
Results
In this study, 107,536 individuals with glaucoma and the same number of individuals without glaucoma matched for age and sex were included (Supplementary Fig. 1). Table 1 describes the baseline characteristics of the study population. The mean age of the study population was 62.33 ± 10.89 years, and 54,621 (50.79%) were men. The glaucoma group tended to live in the rural area (P < 0.001) and have more frequent diabetes (P < 0.001), hypertension (P < 0.001), and dyslipidemia (P < 0.001) than the control group. They also showed significantly lower cholesterol level (P < 0.001), higher glucose (P < 0.001), and lower systolic and diastolic blood pressure (P < 0.001, both) compared to controls. The mean follow-up time was 4.63 ± 2.50 years and 4.64 ± 2.51 years for individuals with and without glaucoma, respectively (P = 0.152).
Table 1.
Baseline characteristics of the study population.
| Parameters | Control | Glaucoma | P value |
|---|---|---|---|
| N = 107536 | N = 107536 | ||
| Age, years | 62.33 ± 10.89 | 62.33 ± 10.89 | 1 |
| Age groups | 1 | ||
| 40–49 | 15533(14.44) | 15533(14.44) | |
| 50–59 | 25944(24.13) | 25944(24.13) | |
| 60–69 | 33214(30.89) | 33214(30.89) | |
| 70–79 | 27870(25.92) | 27870(25.92) | |
| 80- | 4975(4.63) | 4975(4.63) | |
| Gender | 1 | ||
| Male | 54621(50.79) | 54621(50.79) | |
| Female | 52915(49.21) | 52915(49.21) | |
| Income | 0.2697 | ||
| Lowest quartile | 23140(21.52) | 22930(21.32) | |
| Remaining quartiles | 84396(78.48) | 84606(78.68) | |
| Residential area | <0.0001 | ||
| Urban | 46818(43.54) | 50478(46.94) | |
| Rural | 60718(56.46) | 57058(53.06) | |
| Diabetes mellitus | <0.0001 | ||
| No | 89959(83.65) | 82029(76.28) | |
| Yes | 17577(16.35) | 25507(23.72) | |
| Hypertension | <0.0001 | ||
| No | 58468(54.37) | 53146(49.42) | |
| Yes | 49068(45.63) | 54390(50.58) | |
| Dyslipidemia | <0.0001 | ||
| No | 77493(72.06) | 70228(65.31) | |
| Yes | 30043(27.94) | 37308(34.69) | |
| Smoking history | <0.0001 | ||
| No | 71102(66.12) | 72340(67.27) | |
| Ex-smoker | 17988(16.73) | 20330(18.91) | |
| Current smoker | 18446(17.15) | 14866(13.82) | |
| Body mass index | 24.07 ± 3.09 | 24.05 ± 3.07 | 0.0659 |
| Total cholesterol | 197.01 ± 38.18 | 194.97 ± 39.13 | <0.0001 |
| Systolic blood pressure | 126.62 ± 15.56 | 126.33 ± 15.63 | <0.0001 |
| Diastolic blood pressure | 77.47 ± 9.96 | 77.16 ± 9.96 | <0.0001 |
| Fasting glucose | 102.43 ± 25.82 | 106 ± 30.41 | <0.0001 |
Risk of cancer in glaucoma
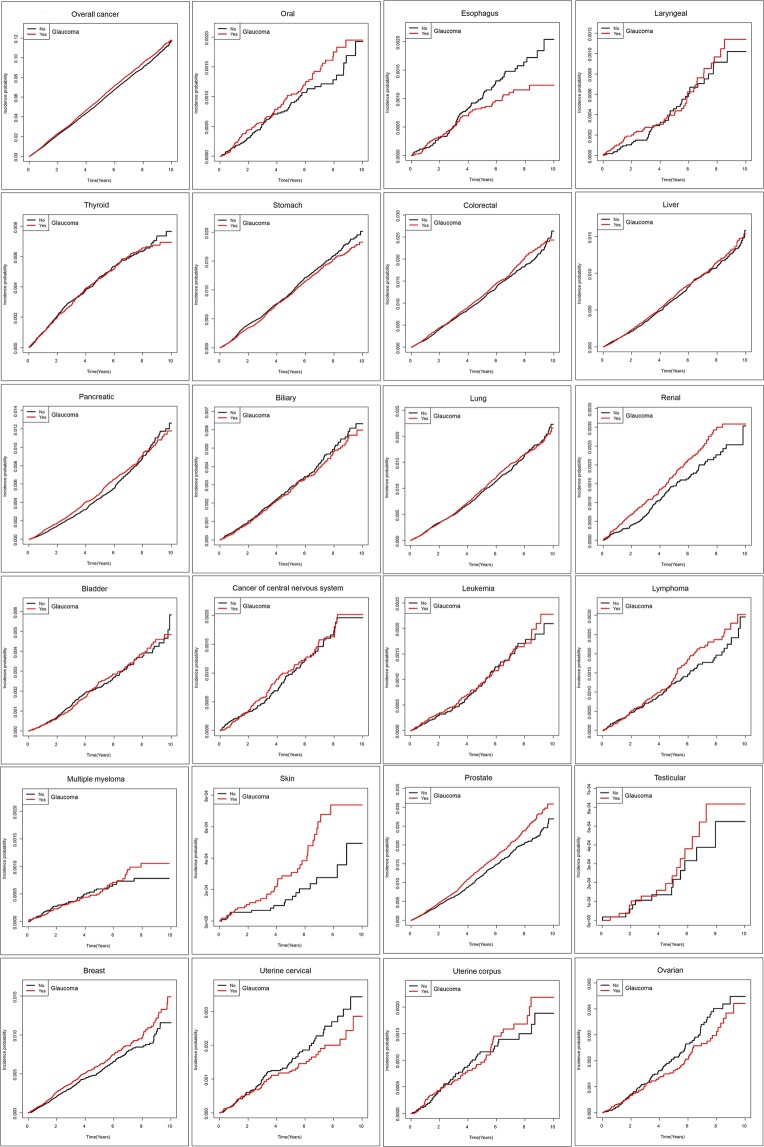
The number of newly diagnosed cancer and incidence rates are presented in Table 2. The cumulative incidence probability over time is presented in Fig. 1. The total cancer incidence rate was 12.23 per 1,000 persons in the glaucoma group, which was higher than 11.62 per 1,000 persons in the matched control group. The HR for overall cancer was significantly higher in the glaucoma group before (HR: 1.053, 95% CI: 1.015–1.091) and after adjusting for age, gender, diabetes, smoking history, and body mass index (adjusted HR: 1.049, 95% CI: 1.012–1.088) compared to that in the control group. Individuals with glaucoma exhibited increased risk of skin cancer before (HR: 2.003, 95% CI: 1.138–3.527) and after adjusting for confounding factors (adjusted HR: 1.944, 95% CI: 1.102–3.430); however, the confidence interval was large due to limited number of events. Risk of prostate cancer was also increased in the glaucoma group prior to (HR: 1.174, 95% CI: 1.055–1.307) and after adjustment (adjusted HR: 1.168, 95% CI: 1049–1.300).
Table 2.
Comparison of incidence rates and hazard ratios for overall and specific cancers between control and glaucoma groups.
| Control (N = 107536) | Glaucoma (N = 107536) | Hazard ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|
| N. of events | Incidence rate (per 1000) | N. of events | Incidence rate (per 1000) | Model 1 | Model 2 | Model 3 | |
| Overall cancer | 5802 | 11.617 | 6086 | 12.227 | 1.053 (1.015, 1.091)* | 1.051 (1.014, 1.09)* | 1.049 (1.012, 1.088)* |
| Oral | 89 | 0.174 | 106 | 0.207 | 1.192 (0.899, 1.58) | 1.191 (0.898, 1.578) | 1.217 (0.917, 1.616) |
| Esophagus | 103 | 0.201 | 81 | 0.158 | 0.787 (0.588, 1.053) | 0.784 (0.586, 1.049) | 0.805 (0.6, 1.079) |
| Laryngeal | 48 | 0.094 | 53 | 0.104 | 1.105 (0.748, 1.634) | 1.102 (0.746, 1.629) | 1.147 (0.773, 1.7) |
| Thyroid | 456 | 0.892 | 447 | 0.876 | 0.981 (0.861, 1.118) | 0.982 (0.862, 1.118) | 0.997 (0.874, 1.137) |
| Stomach | 1015 | 1.990 | 954 | 1.872 | 0.941 (0.861, 1.028) | 0.938 (0.859, 1.025) | 0.935 (0.856, 1.022) |
| Colorectal | 1162 | 2.279 | 1227 | 2.410 | 1.058 (0.976, 1.146) | 1.056 (0.974, 1.144) | 1.04 (0.959, 1.127) |
| Liver | 679 | 1.327 | 707 | 1.383 | 1.043 (0.938, 1.158) | 1.04 (0.936, 1.156) | 1.016 (0.914, 1.13) |
| Pancreatic | 505 | 0.986 | 545 | 1.066 | 1.081 (0.958, 1.22) | 1.078 (0.955, 1.217) | 1.055 (0.934, 1.191) |
| Biliary | 300 | 0.586 | 285 | 0.557 | 0.951 (0.809, 1.118) | 0.947 (0.806, 1.114) | 0.935 (0.795, 1.101) |
| Lung | 982 | 1.921 | 1007 | 1.972 | 1.027 (0.94, 1.121) | 1.023 (0.937, 1.117) | 1.059 (0.97, 1.157) |
| Renal | 139 | 0.271 | 178 | 0.348 | 1.282 (1.027, 1.601) | 1.281 (1.026, 1.599) | 1.239 (0.991, 1.549) |
| Bladder | 233 | 0.455 | 234 | 0.457 | 1.005 (0.839, 1.205) | 1 (0.834, 1.199) | 0.998 (0.831, 1.198) |
| Cancer of central nervous system | 102 | 0.199 | 108 | 0.211 | 1.06 (0.809, 1.39) | 1.059 (0.808, 1.388) | 1.074 (0.818, 1.409) |
| Leukemia | 98 | 0.191 | 102 | 0.199 | 1.042 (0.79, 1.375) | 1.04 (0.788, 1.373) | 1.03 (0.779, 1.361) |
| Lymphoma | 126 | 0.246 | 146 | 0.285 | 1.16 (0.914, 1.472) | 1.158 (0.912, 1.469) | 1.19 (0.937, 1.513) |
| Multiple myeloma | 57 | 0.111 | 60 | 0.117 | 1.054 (0.733, 1.514) | 1.051 (0.731, 1.51) | 1.033 (0.718, 1.488) |
| Skin | 18 | 0.035 | 36 | 0.070 | 2.003 (1.138, 3.527)* | 2 (1.136, 3.521)* | 1.944 (1.102, 3.43)* |
| Prostate | 625 | 2.427 | 732 | 2.849 | 1.174 (1.055, 1.307)* | 1.17 (1.052, 1.302)* | 1.168 (1.049, 1.3)* |
| Testicular | 12 | 0.046 | 15 | 0.058 | 1.252 (0.586, 2.676) | 1.25 (0.585, 2.67) | 1.289 (0.6, 2.768) |
| Breast | 278 | 1.099 | 324 | 1.284 | 1.167 (0.995, 1.37) | 1.169 (0.996, 1.372) | 1.168 (0.994, 1.372) |
| Uterine cervical | 82 | 0.324 | 67 | 0.265 | 0.818 (0.592, 1.13) | 0.818 (0.592, 1.129) | 0.814 (0.588, 1.126) |
| Uterine corpus | 53 | 0.209 | 56 | 0.221 | 1.058 (0.726, 1.54) | 1.058 (0.727, 1.54) | 1.051 (0.72, 1.535) |
| Ovarian | 111 | 0.438 | 93 | 0.367 | 0.839 (0.637, 1.105) | 0.839 (0.637, 1.105) | 0.845 (0.64, 1.115) |
Model 1 Unadjusted.
Model 2 Adjusted for age and gender.
Model 3 Adjusted for age, gender, diabetes, smoking history, and body mass index.
Female malignancies are analyzed in females(n = 52915).
Male malignancies are analyzed in males (n = 54621).
*P value < 0.05.
Figure 1.
Cumulative incidence probability of overall and specific cancers in control and glaucoma groups.
Risk of cancer according to gender
The number of cancer events and incidence rates in males and females are shown in Tables 3 and 4, respectively. In men, those with glaucoma were more likely to develop oral (adjusted HR: 1.491, 95% CI: 1.052–2.114), skin (adjusted HR: 4.42, 95% CI: 1.493–13.086), and prostate (adjusted HR: 1.168, 95% CI: 1.049–1.300) cancers, but less likely to develop biliary cancer (adjusted HR: 0.796, 95% CI: 0.639–0.993) than those without glaucoma.
Table 3.
Comparison of incidence rates and hazard ratios for overall and specific cancers between control and glaucoma groups in men.
| Control (N = 54621) | Glaucoma (N = 54621) | Hazard ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|
| N. of events | Incidence rate (per 1000) | N. of events | Incidence rate (per 1000) | Model 1 | Model 2 | Model 3 | |
| Overall cancer | 3582 | 14.256 | 3713 | 14.832 | 1.041(0.994, 1.09) | 1.038 (0.992, 1.087) | 1.039 (0.992, 1.088) |
| Oral | 54 | 0.209 | 79 | 0.305 | 1.465 (1.036, 2.07)* | 1.463 (1.035, 2.067)* | 1.491 (1.052, 2.114)* |
| Esophagus | 96 | 0.371 | 76 | 0.294 | 0.792 (0.586, 1.071) | 0.789 (0.584, 1.066) | 0.811 (0.599, 1.098) |
| Laryngeal | 44 | 0.170 | 50 | 0.193 | 1.138 (0.759, 1.706) | 1.135 (0.757, 1.701) | 1.178 (0.783, 1.773) |
| Thyroid | 104 | 0.402 | 115 | 0.445 | 1.107 (0.849, 1.443) | 1.108 (0.85, 1.444) | 1.091 (0.835, 1.425) |
| Stomach | 729 | 2.834 | 681 | 2.651 | 0.935(0.843, 1.038) | 0.933(0.84, 1.035) | 0.934(0.841, 1.038) |
| Colorectal | 721 | 2.802 | 710 | 2.762 | 0.986(0.889, 1.093) | 0.983(0.886, 1.09) | 0.967(0.871, 1.073) |
| Liver | 487 | 1.885 | 498 | 1.930 | 1.024(0.904, 1.16) | 1.022 (0.902, 1.158) | 0.993 (0.876, 1.126) |
| Pancreatic | 300 | 1.160 | 316 | 1.223 | 1.055 (0.901, 1.235) | 1.051 (0.898, 1.231) | 1.026 (0.875, 1.203) |
| Biliary | 180 | 0.695 | 144 | 0.557 | 0.801 (0.643, 0.997)* | 0.797 (0.64, 0.992)* | 0.796 (0.639, 0.993)* |
| Lung | 746 | 2.890 | 713 | 2.765 | 0.957 (0.864, 1.06) | 0.952 (0.859, 1.055) | 0.995 (0.898, 1.104) |
| Renal | 100 | 0.386 | 120 | 0.464 | 1.201 (0.921, 1.567) | 1.2 (0.921, 1.565) | 1.132 (0.866, 1.479) |
| Bladder | 191 | 0.739 | 198 | 0.766 | 1.038 (0.851, 1.266) | 1.032 (0.846, 1.259) | 1.029 (0.842, 1.257) |
| Cancer of central nervous system | 55 | 0.212 | 56 | 0.216 | 1.02 (0.703, 1.479) | 1.016 (0.701, 1.474) | 1.054 (0.725, 1.533) |
| Leukemia | 55 | 0.212 | 61 | 0.236 | 1.111 (0.771, 1.599) | 1.108 (0.77, 1.595) | 1.095 (0.759, 1.58) |
| Lymphoma | 87 | 0.336 | 85 | 0.329 | 0.978 (0.725, 1.318) | 0.975 (0.723, 1.314) | 1.013 (0.75, 1.369) |
| Multiple myeloma | 30 | 0.116 | 37 | 0.143 | 1.235 (0.763, 1.999) | 1.229 (0.76, 1.99) | 1.189 (0.733, 1.93) |
| Skin | 4 | 0.015 | 18 | 0.070 | 4.511 (1.527, 13.328)* | 4.5 (1.523, 13.296)* | 4.42 (1.493, 13.086)* |
| Prostate | 625 | 2.427 | 732 | 2.849 | 1.174 (1.055, 1.307)* | 1.17 (1.052, 1.302)* | 1.168 (1.049, 1.3)* |
| Testicular | 12 | 0.046 | 15 | 0.058 | 1.252 (0.586, 2.676) | 1.25 (0.585, 2.67) | 1.289 (0.6, 2.768) |
Model 1 Unadjusted.
Model 2 Adjusted for age.
Model 3 Adjusted for age, diabetes, smoking history, and body mass index.
*P value < 0.05.
Table 4.
Comparison of incidence rates and hazard ratios for overall and specific cancers between control and glaucoma groups in women.
| Control (N = 52915) | Glaucoma (N = 52915) | Hazard ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|
| N. of events | Incidence rate (per 1000) | N. of events | Incidence rate (per 1000) | Model 1 | Model 2 | Model 3 | |
| Overall cancer | 2220 | 8.946 | 2373 | 9.591 | 1.072 (1.012, 1.136)* | 1.072 (1.011, 1.135)* | 1.07 (1.009, 1.134)* |
| Oral | 35 | 0.138 | 27 | 0.107 | 0.772(0.467, 1.275) | 0.771(0.466, 1.273) | 0.791(0.478, 1.308) |
| Esophagus | 7 | 0.028 | 5 | 0.020 | 0.715(0.227, 2.253) | 0.711(0.226, 2.241) | 0.702(0.222, 2.217) |
| Laryngeal | 4 | 0.016 | 3 | 0.012 | 0.75 (0.168, 3.353) | 0.748 (0.167, 3.342) | 0.795 (0.177, 3.571) |
| Thyroid | 352 | 1.395 | 332 | 1.317 | 0.944 (0.812, 1.096) | 0.944 (0.813, 1.097) | 0.969 (0.833, 1.127) |
| Stomach | 286 | 1.131 | 273 | 1.080 | 0.955 (0.809, 1.127) | 0.953 (0.808, 1.125) | 0.94 (0.796, 1.111) |
| Colorectal | 441 | 1.746 | 517 | 2.051 | 1.175 (1.034, 1.334)* | 1.174 (1.034, 1.333)* | 1.162 (1.023, 1.32)* |
| Liver | 192 | 0.758 | 209 | 0.826 | 1.09 (0.896, 1.326) | 1.088 (0.894, 1.323) | 1.079 (0.886, 1.313) |
| Pancreatic | 205 | 0.809 | 229 | 0.905 | 1.119 (0.926, 1.351) | 1.117 (0.925, 1.349) | 1.099 (0.909, 1.328) |
| Biliary | 120 | 0.474 | 141 | 0.557 | 1.176 (0.922, 1.5) | 1.173 (0.92, 1.497) | 1.142 (0.894, 1.458) |
| Lung | 236 | 0.932 | 294 | 1.163 | 1.248 (1.051, 1.481)* | 1.246 (1.05, 1.479)* | 1.264 (1.064, 1.501)* |
| Renal | 39 | 0.154 | 58 | 0.229 | 1.489 (0.992, 2.235)* | 1.488 (0.992, 2.233)* | 1.519 (1.01, 2.283)* |
| Bladder | 42 | 0.166 | 36 | 0.142 | 0.858 (0.55, 1.34) | 0.856 (0.548, 1.335) | 0.856 (0.548, 1.339) |
| Cancer of central nervous system | 47 | 0.185 | 52 | 0.205 | 1.107 (0.746, 1.643) | 1.107 (0.746, 1.642) | 1.105 (0.743, 1.643) |
| Leukemia | 43 | 0.170 | 41 | 0.162 | 0.954 (0.622, 1.464) | 0.953 (0.621, 1.462) | 0.952 (0.619, 1.463) |
| Lymphoma | 39 | 0.154 | 61 | 0.241 | 1.566 (1.048, 2.341)* | 1.565 (1.047, 2.339)* | 1.582 (1.057, 2.368)* |
| Multiple myeloma | 27 | 0.107 | 23 | 0.091 | 0.853 (0.489, 1.488) | 0.852 (0.489, 1.486) | 0.863 (0.494, 1.51) |
| Skin | 14 | 0.055 | 18 | 0.071 | 1.287 (0.64, 2.588) | 1.285 (0.639, 2.583) | 1.231 (0.611, 2.483) |
| Breast | 278 | 1.099 | 324 | 1.284 | 1.167 (0.995,1.37) | 1.169 (0.996,1.372) | 1.168 (0.994,1.372) |
| Uterine cervical | 82 | 0.324 | 67 | 0.265 | 0.818 (0.592, 1.13) | 0.818 (0.592, 1.129) | 0.814 (0.588, 1.126) |
| Uterine corpus | 53 | 0.209 | 56 | 0.221 | 1.058 (0.726, 1.54) | 1.058 (0.727, 1.54) | 1.051 (0.72, 1.535) |
| Ovarian | 111 | 0.438 | 93 | 0.367 | 0.839 (0.637, 1.105) | 0.839 (0.637, 1.105) | 0.845 (0.64, 1.115) |
Model 1 Unadjusted.
Model 2 Adjusted for age.
Model 3 Adjusted for age, diabetes, smoking history, and body mass index.
*P value < 0.05.
Meanwhile, women with glaucoma had a greater risk for overall cancer (adjusted HR: 1.070, 95% CI: 1.009–1.134), colorectal (adjusted HR: 1.162, 95% CI:1.023–1.320), lung (adjusted HR: 1.264, 95% CI: 1.064–1.501), and renal (adjusted HR: 1.519, 95% CI: 1.010–2.283) cancers and lymphoma (adjusted HR: 1.582, 95% CI: 1.057–2.368) compared to those without glaucoma.
Risk of cancer according to age group
Younger patients (age <65 years) with glaucoma had a greater risk of overall cancer (adjusted HR: 1.086, 95% CI: 1.023–1.152) compared with those without glaucoma (Table 5). They showed increased risk of renal (adjusted HR: 1.462, 95% CI: 1.036–2.063), skin (adjusted HR: 3.437, 95% CI:1.125–10.498) and prostate (adjusted HR: 1.259, 95% CI: 1.022–1.552) cancers, but decreased risk of esophageal (adjusted HR: 0.564, 95% CI: 0.320–0.994) cancer.
Table 5.
Comparison of incidence rates and hazard ratios for overall and specific cancers between control and glaucoma groups in those <65 years.
| Control (N = 59567) | Glaucoma (N = 59567) | Hazard ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|
| N. of events | Incidence rate (per 1000) | N. of events | Incidence rate (per 1000) | Model 1 | Model 2 | Model 3 | |
| Overall cancer | 2140 | 7.496 | 2321 | 8.166 | 1.09 (1.027, 1.156)* | 1.09 (1.027, 1.156)* | 1.086 (1.023, 1.152)* |
| Oral | 36 | 0.124 | 39 | 0.134 | 1.086 (0.69, 1.708) | 1.086 (0.69, 1.708) | 1.089 (0.689, 1.723) |
| Esophagus | 35 | 0.120 | 19 | 0.065 | 0.544 (0.311, 0.951)* | 0.544 (0.311, 0.951)* | 0.564 (0.32, 0.994)* |
| Laryngeal | 15 | 0.052 | 24 | 0.083 | 1.605 (0.842, 3.059) | 1.604 (0.842, 3.058) | 1.705 (0.885, 3.285) |
| Thyroid | 326 | 1.124 | 334 | 1.155 | 1.027 (0.881, 1.196) | 1.027 (0.882, 1.196) | 1.05 (0.901, 1.225) |
| Stomach | 350 | 1.206 | 363 | 1.254 | 1.04 (0.898, 1.204) | 1.04 (0.898, 1.205) | 1.021 (0.88, 1.185) |
| Colorectal | 417 | 1.438 | 408 | 1.410 | 0.981 (0.856, 1.124) | 0.981 (0.856, 1.124) | 0.964 (0.84, 1.107) |
| Liver | 236 | 0.812 | 261 | 0.900 | 1.109 (0.93, 1.322) | 1.109 (0.93, 1.322) | 1.074 (0.898, 1.283) |
| Pancreatic | 163 | 0.560 | 176 | 0.606 | 1.083 (0.875, 1.34) | 1.083 (0.875, 1.341) | 1.035 (0.834, 1.284) |
| Biliary | 76 | 0.261 | 82 | 0.282 | 1.081 (0.792, 1.477) | 1.082 (0.792, 1.478) | 1.057 (0.771, 1.448) |
| Lung | 262 | 0.901 | 290 | 1.000 | 1.11 (0.939, 1.312) | 1.11 (0.939, 1.312) | 1.154 (0.975, 1.366) |
| Renal | 55 | 0.189 | 83 | 0.286 | 1.513 (1.076, 2.128)* | 1.514 (1.077, 2.128)* | 1.462 (1.036, 2.063)* |
| Bladder | 53 | 0.182 | 59 | 0.203 | 1.116 (0.77, 1.617) | 1.116 (0.77, 1.617) | 1.117 (0.767, 1.625) |
| Cancer of central nervous system | 43 | 0.148 | 39 | 0.134 | 0.909 (0.59, 1.403) | 0.91 (0.59, 1.403) | 0.919 (0.594, 1.424) |
| Leukemia | 35 | 0.120 | 36 | 0.124 | 1.032 (0.648, 1.643) | 1.032 (0.648, 1.643) | 1.018 (0.636, 1.629) |
| Lymphoma | 52 | 0.179 | 51 | 0.176 | 0.983 (0.668, 1.447) | 0.984 (0.668, 1.447) | 1.015 (0.687, 1.498) |
| Multiple myeloma | 16 | 0.055 | 24 | 0.083 | 1.503 (0.799, 2.83) | 1.504 (0.799, 2.831) | 1.489 (0.787, 2.818) |
| Skin | 4 | 0.014 | 14 | 0.048 | 3.509 (1.155, 10.661)* | 3.511 (1.156, 10.666)* | 3.437 (1.125, 10.498)* |
| Prostate† | 162 | 1.002 | 201 | 1.245 | 1.244 (1.011, 1.53)* | 1.245 (1.012, 1.531)* | 1.259 (1.022, 1.552)* |
| Testicular† | 3 | 0.018 | 8 | 0.049 | 2.674 (0.709, 10.079) | 2.675 (0.71, 10.083) | 2.903 (0.761, 11.072) |
| Breast‡ | 182 | 1.416 | 209 | 1.632 | 1.153 (0.945, 1.406) | 1.153 (0.945, 1.406) | 1.156 (0.946, 1.413) |
| Uterine cervical‡ | 32 | 0.248 | 30 | 0.233 | 0.94 (0.571, 1.547) | 0.94 (0.571, 1.548) | 0.936 (0.566, 1.548) |
| Uterine corpus‡ | 30 | 0.233 | 40 | 0.311 | 1.338 (0.833, 2.147) | 1.337 (0.833, 2.147) | 1.36 (0.843, 2.191) |
| Ovarian‡ | 58 | 0.450 | 53 | 0.412 | 0.917 (0.632, 1.331) | 0.917 (0.632, 1.331) | 0.914 (0.628, 1.332) |
Model 1 Unadjusted.
Model 2 Adjusted for age and gender.
Model 3 Adjusted for age, gender, diabetes, smoking history, and body mass index.
*P value < 0.05.
†Male malignancies are analyzed in males (n = 33247).
‡Female malignancies are analyzed in females (n = 26320).
Older patients aged 65 years or older with glaucoma exhibited increased hazard of developing prostate cancer (adjusted HR: 1.150, 95% CI: 1.015–1.304), but decreased hazard of stomach cancer (adjusted HR: 0.892, 95% CI: 0.798–0.997) incidence (Table 6).
Table 6.
Comparison of incidence rates and hazard ratios for overall and specific cancers between control and glaucoma groups in those ≥65 years.
| Control (N = 47969) | Glaucoma (N = 47969) | Hazard ratio (95% confidence interval) | |||||
|---|---|---|---|---|---|---|---|
| N. of events | Incidence rate (per 1000) | N. of events | Incidence rate (per 1000) | Model 1 | Model 2 | Model 3 | |
| Overall cancer | 3662 | 17.117 | 3765 | 17.632 | 1.03 (0.984, 1.078) | 1.03 (0.984, 1.078) | 1.032 (0.986, 1.08) |
| Oral | 53 | 0.239 | 67 | 0.303 | 1.264 (0.881, 1.812) | 1.263 (0.881, 1.811) | 1.298 (0.904, 1.865) |
| Esophagus | 68 | 0.307 | 62 | 0.280 | 0.911 (0.646, 1.285) | 0.91 (0.645, 1.284) | 0.933 (0.66, 1.319) |
| Laryngeal | 33 | 0.149 | 29 | 0.131 | 0.878 (0.533, 1.446) | 0.879 (0.533, 1.447) | 0.909 (0.55, 1.501) |
| Thyroid | 130 | 0.588 | 113 | 0.511 | 0.869 (0.675, 1.118) | 0.869 (0.675, 1.118) | 0.877 (0.681, 1.129) |
| Stomach | 665 | 3.025 | 591 | 2.685 | 0.888 (0.794, 0.992)* | 0.887 (0.794, 0.99)* | 0.892 (0.798, 0.997)* |
| Colorectal | 745 | 3.390 | 819 | 3.729 | 1.1 (0.996, 1.215) | 1.099 (0.995, 1.214) | 1.085 (0.982, 1.199) |
| Liver | 443 | 2.005 | 446 | 2.018 | 1.006 (0.882, 1.148) | 1.005 (0.881, 1.147) | 0.989 (0.866, 1.128) |
| Pancreatic | 342 | 1.547 | 369 | 1.669 | 1.079 (0.931, 1.25) | 1.078 (0.93, 1.249) | 1.064 (0.918, 1.234) |
| Biliary | 224 | 1.013 | 203 | 0.917 | 0.905 (0.749, 1.095) | 0.904 (0.747, 1.093) | 0.898 (0.742, 1.087) |
| Lung | 720 | 3.264 | 717 | 3.249 | 0.995 (0.898, 1.104) | 0.994 (0.896, 1.102) | 1.033 (0.931, 1.146) |
| Renal | 84 | 0.380 | 95 | 0.429 | 1.13 (0.843, 1.516) | 1.13 (0.843, 1.516) | 1.1 (0.819, 1.477) |
| Bladder | 180 | 0.814 | 175 | 0.791 | 0.971 (0.789, 1.196) | 0.968 (0.786, 1.192) | 0.969 (0.786, 1.195) |
| Cancer of central nervous system | 59 | 0.267 | 69 | 0.312 | 1.169 (0.826, 1.654) | 1.168 (0.825, 1.653) | 1.19 (0.839, 1.687) |
| Leukemia | 63 | 0.285 | 66 | 0.298 | 1.047 (0.741, 1.479) | 1.047 (0.741, 1.479) | 1.042 (0.737, 1.474) |
| Lymphoma | 74 | 0.334 | 95 | 0.429 | 1.283 (0.947, 1.739) | 1.281 (0.945, 1.736) | 1.319 (0.972, 1.79) |
| Multiple myeloma | 41 | 0.185 | 36 | 0.162 | 0.877 (0.561, 1.373) | 0.874 (0.559, 1.368) | 0.86 (0.549, 1.349) |
| Skin | 14 | 0.063 | 22 | 0.099 | 1.571 (0.804, 3.071) | 1.569 (0.803, 3.066) | 1.527 (0.78, 2.99) |
| Prostate† | 463 | 4.833 | 531 | 5.560 | 1.151 (1.016, 1.304)* | 1.149 (1.014, 1.301)* | 1.15 (1.015, 1.304)* |
| Testicular† | 9 | 0.093 | 7 | 0.072 | 0.778 (0.29, 2.09) | 0.779 (0.29, 2.092) | 0.788 (0.292, 2.125) |
| Breast‡ | 96 | 0.772 | 115 | 0.925 | 1.197 (0.913, 1.569) | 1.198 (0.914, 1.571) | 1.202 (0.915, 1.577) |
| Uterine cervical‡ | 50 | 0.402 | 37 | 0.297 | 0.739 (0.483, 1.131) | 0.739 (0.483, 1.131) | 0.739 (0.482, 1.132) |
| Uterine corpus‡ | 23 | 0.185 | 16 | 0.128 | 0.695 (0.367, 1.315) | 0.695 (0.367, 1.315) | 0.674 (0.355, 1.28) |
| Ovarian‡ | 53 | 0.426 | 40 | 0.321 | 0.754 (0.5, 1.136) | 0.754 (0.5, 1.136) | 0.762 (0.505, 1.15) |
Model 1 Unadjusted.
Model 2 Adjusted for age and gender.
Model 3 Adjusted for age, gender, diabetes, smoking history, and body mass index.
*P value < 0.05.
†Male malignancies are analyzed in males(n = 21374).
‡Female malignancies are analyzed in females(n = 26595).
Discussion
In this nationwide population-based study, we compared the incidence of overall cancer and various specific cancers between 107,536 glaucoma subjects and age- and sex- matched controls. We found that individuals with glaucoma showed significantly higher risk of developing cancer overall and specific cancers including prostate and skin than those without glaucoma before and after adjusting for confounding factors. To the best of our knowledge, this is the first study that has assessed the association between glaucoma and cancer incidence.
The risk of cancer in specific organs varied depending on gender and age groups, and the association was stronger in women and those under 65 years of age. In males, the risk of oral, skin, and prostate cancers increased and that of biliary cancer decreased compared to the age-matched control group. However, in females, individuals with glaucoma had higher risk of overall cancer as well as colorectal, lung, and renal cancers and lymphoma compared to the matched control group.
After stratifying by age, in those under the age of 65, individuals with glaucoma showed increased risk of overall, renal, skin, and prostate cancers and decreased risk of esophageal cancer. In individuals aged 65 years or older, those with glaucoma exhibited higher risk of prostate cancer. However, the risk of stomach cancer compared to matched controls was lower, which was unexpected, because significant association between H. Pylori infection, one of the major risk factors for stomach cancer, and glaucoma has been reported in previous studies23.
Although the reason for the association between glaucoma and various cancers is not well known, there are several potential mechanisms. First, the most important risk factor for glaucoma is elevated intraocular pressure, but there are other important factors including inflammation, which may link glaucoma with cancer. Inflammation plays a role in the pathogenesis of glaucoma, in which low grade chronic inflammation occurs in response to elevated intraocular pressure and vascular dysfunction24,25. In healthy eyes, the blood-ocular barrier prevents many molecular substances from crossing from the blood into the eye and vice versa, but in glaucomatous eyes, this barrier around the optic nerve head has been shown to leak26. Furthermore, inflammatory molecules including tumor necrosis factor alpha, interleukins, endothelin-1, C-reactive protein, vascular endothelial growth factor, and cyclooxygenase-2/prostaglandin E2 have been reported to be elevated in glaucoma12,15,27.
Inflammation predisposes to cancer development and plays critical roles at all stages of tumorigenesis from tumor initiation, growth, and progression, to metastasis18,28. Chronic systemic inflammation in glaucoma may contribute to enhanced carcinogenesis through inflammation-related mechanisms16,29,30. Other inflammatory diseases such as inflammatory bowel diseases19, rheumatoid arthritis31, systemic lupus arthritis21, and type 1 diabetes mellitus32 have been reported to increase the risk of cancer. In individuals with rheumatoid arthritis, the risk of overall cancer and the risk of specific cancers including lymphoma, larynx, oropharynx, esophagus, lung, kidney, bladder, prostate, liver, skin, and pancreas was increased33. In patients with SLE, the risk of overall cancer and also of focal malignancies such as lung, kidney, bladder, esophagus, stomach, colon, liver, and pancreas was shown to be increased. [21 Specific cancers related with these inflammatory diseases are similar to our results.
There are also environmental factors. Smoking may be another link between glaucoma and cancer. There are numerous studies reporting that smoking is associated with higher risk of glaucoma34,35. In our study, specific cancers shown to be increased in patients with glaucoma are mostly smoking-related cancers, which include lung, oral, renal, and colorectal36. However, the association between glaucoma and cancer was significant even after adjusting for smoking history, and other smoking-related cancers such as esophageal and gastric cancers showed decreased risk in patients with glaucoma, so the results of this study cannot be explained by smoking alone.
Furthermore, our findings could also be partly explained by the hypothesis that patients with glaucoma are expected to visit physicians more often and may show different health screening behavior compared to those without glaucoma. They may be more likely to receive screening for other diseases, leading to more frequent diagnosis of certain types of malignancies. On the other hand, this may also lead to lower incidence of certain tumors, for instance, they may receive endoscopy more often, enabling treatment of precancer lesions which may account for the lower risk of stomach cancer in the older group.
This study has several strengths. First, a cohort study using a large database such as the Korean NHID would be optimal in assessing the association between glaucoma and cancers. Randomized clinical trials would not be feasible, and case-cohort studies would be influenced by survival effects, indicating that if glaucoma-related mortalities occurred before the cancer diagnosis, the risk of cancer would be underestimated. Second, this nationwide population-based longitudinal database enables accurate follow up of medical records of almost the entire 50 million population in Korea, allowing evaluation of temporal association between glaucoma and various cancers. Furthermore, we also included results from the general health screening examinations, which enabled adjustments for various parameters such as body mass index.
There are still limitations of this study. First, glaucoma was defined based on medical records, indicating those who did not seek medical service were not included in the study underestimating the glaucoma population. However, it is likely that this would underestimate the HR. In addition, we could not perform further analysis on the risk of cancers in different types of glaucoma. Further studies are warranted to explore this relationship. Furthermore, although the cancer incidence that we report in this study is based on one of the largest cohorts of glaucoma, this study only included Koreans. As different ethnicity and environmental exposures affect disease pathogenesis, future studies are needed in different ethnic groups.
In conclusion, based on this nationwide longitudinal study investigating the association between glaucoma and cancer development, we found that glaucoma is a risk factor for overall cancer incidence. In addition, the risk of cancer was more prominent in female glaucoma patients and those younger than 65 years. Considering the globally growing number of individuals with glaucoma, healthcare providers should be aware of the increased risk of cancer in individuals with glaucoma.
Methods
National Health Insurance Database and national general health screening in Korea
In this nationwide, population-based cohort study, we used the NHID provided by the KNHIS, which mandates all nationals to enroll in the system. The KNHIS covers about 97% of the population and provides universal health coverage. The NHID includes information on sociodemographic characteristics, medical data based on medical claims, and the results of national general health screening for the whole population of South Korea37. Sociodemographic characteristics include anonymized code for each individual, age, gender, socioeconomic variables, residential area, and household income level. Medical data in NHID include inpatient and outpatient usage, diagnostic codes by the International Classification of Diseases 10th revision (ICD-10), treatment procedures, and prescription records. In addition, the national general health screening is provided free of charge for workplace subscribers and their dependents and all Koreans over the age of 40 at least biannually and the participation rate has been over 70% since 201137. It includes information on health behaviors and general laboratory tests. Furthermore, the KNHIS has a registration program which offers additional coverage for cancer-related medical fees once diagnosis of malignancy is confirmed, resulting in a reliable method for identifying cancer patients using the NHID.
This study was approved by the Institutional Review Board (IRB) of the Yeouido St. Mary’s Hospital, The Catholic University of Korea, and was conducted according to the ethical principles outlined in the Declaration of Helsinki. Informed consent was not obtained, because anonymized and de-identified information was used for analyses.
Study population and design
Subjects who were diagnosed with glaucoma between 2007 and 2016 who also received a national general health screening in the same year were assigned as the glaucoma group. Individuals under the age of 40 and those with less than 1 year follow up were excluded from the analysis. Glaucoma was defined based on ICD-10 code (H401). To enhance the validity of the diagnosis, those with at least 3 clinical records with glaucoma diagnosis during the index year were included in the study38.
Controls were 1:1 matched based on age and sex during the same period. We collected additional data on subjects’ household income (lowest quartile or the remaining quartiles), place of residence (urban or rural), smoking history (no, ex-smoker, or current smoker), comorbidities such as diabetes (E11-E14), hypertension (I10–13 and I15), and dyslipidemia (E78), using ICD-10 diagnostic codes, and the national general health screening results including body mass index, cholesterol level, systolic/diastolic blood pressure, and fasting glucose level. Presence of comorbidities was defined as diagnoses of the aforementioned codes within one year before the index date and prescription history of relevant medications.
To define individuals with various site-specific cancer, the ICD-10 diagnostic codes were used (Supplementary Table 1)39,40. To include only patients with newly-diagnosed cancer, we excluded those with prior diagnosis of cancer between 2002 and the index date. Each subject was followed up for development of cancer until 2017.
Statistical analysis
To compare the population characteristics between cohorts, Student’s t-test and χ2 test were used to compare continuous and categorical variables, respectively. Cancer incidence rates were calculated per 1,000 individuals.
To investigate the association between glaucoma and incident cancer, we calculated the hazard ratios (HRs) and 95% confidence intervals (CIs) using the multivariate Cox regression analysis with multiple adjustments; the fully-adjusted model included matched variables (age and gender) as well as unmatched variables (diabetes, smoking history, and BMI). We used the SAS ver 9.4 (SAS Institute, Cary, NC, USA) with P values < 0.05 considered as statistically significant.
Supplementary information
Acknowledgements
This work was funded by the Institute of Clinical Medicine Research in the Yeouido St. Mary’s hospital, Catholic University of Korea [no grant number applicable].
Author contributions
Y.J. analysed the data and wrote the main manuscript text; K.H. acquired and analysed the data; K.N. reviewed and revised the manuscript; G.K. prepared tables; M.H. acquired the data and revised manuscript; J.P. revised the manuscript; J.I.M. supervised the acquisition of the data, analysed the data, and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-65116-8.
References
- 1.Jonas JB, et al. Glaucoma. Lancet. 2017;390:2183–2193. doi: 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- 2.Tham Y-C, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Resnikoff S, et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 4.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 5.Von Thun Und Hohenstein-Blaul N, Bell K, Pfeiffer N, Grus FH. Autoimmune aspects in glaucoma. Eur. J. Pharmacol. 2016;787:105–118. doi: 10.1016/j.ejphar.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Gramlich OW, et al. Autoimmune biomarkers in glaucoma patients. Curr. Opin. Pharmacol. 2013;13:90–97. doi: 10.1016/j.coph.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Greco A, et al. Emerging Concepts in Glaucoma and Review of the Literature. Am. J. Med. 2016;129:1000.e1007–1000.e1013. doi: 10.1016/j.amjmed.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Williams PA, Marsh-Armstrong N, Howell GR. Neuroinflammation in glaucoma: A new opportunity. Exp. Eye Res. 2017;157:20–27. doi: 10.1016/j.exer.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C-S, Seong GJ, Lee N-H, Song K-C, Namil Study Group KGS. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011;118:1024–1030. doi: 10.1016/j.ophtha.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Vohra R, Tsai JC, Kolko M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013;58:311–320. doi: 10.1016/j.survophthal.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Williams PA, Marsh-Armstrong N, Howell GR, Astrocytes LIIO, Participants GN. Neuroinflammation in glaucoma: A new opportunity. Exp. Eye. Res. 2017;157:20–27. doi: 10.1016/j.exer.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leibovitch I, et al. C-reactive protein levels in normal tension glaucoma. J. Glaucoma. 2005;14:384–386. doi: 10.1097/01.ijg.0000176932.06606.6e. [DOI] [PubMed] [Google Scholar]
- 13.Li G, et al. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol. Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- 14.Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J. Neurosci. 2000;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawano T, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat. Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- 16.Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32:1119–1136. [PMC free article] [PubMed] [Google Scholar]
- 18.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldacre MJ, Wotton CJ, Yeates D, Seagroatt V, Jewell D. Cancer in patients with ulcerative colitis, Crohn’s disease and coeliac disease: record linkage study. Eur. J. Gastroenterol. Hepatol. 2008;20:297–304. doi: 10.1097/MEG.0b013e3282f2a5e2. [DOI] [PubMed] [Google Scholar]
- 20.Hemminki K, Li X, Sundquist J, Sundquist K. Cancer risks in ulcerative colitis patients. Int. J. Cancer. 2008;123:1417–1421. doi: 10.1002/ijc.23666. [DOI] [PubMed] [Google Scholar]
- 21.Bernatsky S, et al. An international cohort study of cancer in systemic lupus erythematosus. Arthritis Rheum. 2005;52:1481–1490. doi: 10.1002/art.21029. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y-J, Chang Y-T, Wang C-B, Wu C-Y. Malignancy in systemic lupus erythematosus: a nationwide cohort study in Taiwan. Am. J. Med. 2010;123:1150.e1151–1156. doi: 10.1016/j.amjmed.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Zeng J, Liu H, Liu X, Ding C. The Relationship Between Helicobacter pylori Infection and Open-Angle Glaucoma: A Meta-Analysis. Invest. Ophthalmol. Vis. Sci. 2015;56:5238–5245. doi: 10.1167/iovs.15-17059. [DOI] [PubMed] [Google Scholar]
- 24.Mac Nair CE, Nickells RW. Neuroinflammation in Glaucoma and Optic Nerve Damage. Prog. Mol. Biol. Transl. Sci. 2015;134:343–363. doi: 10.1016/bs.pmbts.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Bell K, Und Hohenstein-Blaul NVT, Teister J, Grus F. Modulation of the Immune System for the Treatment of Glaucoma. Curr. Neuropharmacol. 2018;16:942–958. doi: 10.2174/1570159X15666170720094529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arend O, Remky A, Plange N, Kaup M, Schwartz B. Fluorescein leakage of the optic disc in glaucomatous optic neuropathy. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243:659–664. doi: 10.1007/s00417-004-1092-7. [DOI] [PubMed] [Google Scholar]
- 27.Tezel G, Li LY, Patil RV, Wax MB. TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 2001;42:1787–1794. [PubMed] [Google Scholar]
- 28.Mantovani A. Molecular pathways linking inflammation and cancer. Curr. Mol. Med. 2010;10:369–373. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 29.Okada F. Inflammation-related carcinogenesis: current findings in epidemiological trends, causes and mechanisms. Yonago Acta. Med. 2014;57:65–72. [PMC free article] [PubMed] [Google Scholar]
- 30.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res. Ther. 2015;17:212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y-J, Chang Y-T, Wang C-B, Wu C-Y. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63:352–358. doi: 10.1002/art.30134. [DOI] [PubMed] [Google Scholar]
- 32.Shu X, et al. Cancer risk among patients hospitalized for Type 1 diabetes mellitus: a population-based cohort study in Sweden. Diabetic medicine: a journal of the British Diabetic Association. 2010;27:791–797. doi: 10.1111/j.1464-5491.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 33.Turesson C, Matteson EL. Malignancy as a comorbidity in rheumatic diseases. Rheumatology (Oxford) 2013;52:5–14. doi: 10.1093/rheumatology/kes189. [DOI] [PubMed] [Google Scholar]
- 34.Law SM, et al. Cigarette smoking and glaucoma in the United States population. Eye (Lond) 2018;32:716–725. doi: 10.1038/eye.2017.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain V, Jain M, Abdull MM, Bastawrous A. The association between cigarette smoking and primary open-angle glaucoma: a systematic review. Int. Ophthalmol. 2017;37:291–301. doi: 10.1007/s10792-016-0245-0. [DOI] [PubMed] [Google Scholar]
- 36.Islami F, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 37.Cheol Seong S, et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai SW, Lin CL, Liao KF. Glaucoma correlates with increased risk of Parkinson’s disease in the elderly: a national-based cohort study in Taiwan. Curr. Med. Res. Opin. 2017;33:1511–1516. doi: 10.1080/03007995.2017.1322570. [DOI] [PubMed] [Google Scholar]
- 39.Weng PH, et al. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin. J. Am. Soc. Nephrol. 2011;6:1121–1128. doi: 10.2215/CJN.09011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S, et al. Risk of cancer in pre-dialysis chronic kidney disease: A nationwide population-based study with a matched control group. Kidney Res. Clin. Pract. 2019;38:60–70. doi: 10.23876/j.krcp.18.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.