Abstract
Neonatal morbidities are associated with long term neurological deficits in life and have also been associated with dysbiosis. We tested whether optimizing the neonate’s microbiome through maternal probiotic supplementation can improve offspring’s neurodevelopmental outcomes. Maternal LB supplementation, carried out by giving Lactobacillus acidophilus and Bifidobacterium infantis (LB) to pregnant C57/BL6J mice daily from E16 to weaning, significantly suppressed postnatal peripheral proinflammatory insult-induced systemic inflammation and normalized compromised blood-brain barrier permeability and tight junction protein expression in the offspring at pre-weaned age. Maternal LB exposure also regulated markers associated with leukocyte transendothelial migration, extracellular matrix injury and neuroinflammation. The suppressed neuroinflammation by maternal LB supplementation was associated with reduced astrocyte/microglia activation and downregulation of the transcriptional regulators CEBPD and IκBα. Furthermore, maternal LB supplementation promoted neuronal and oligodendrocyte progenitor cell development. Our study demonstrates the efficacy of maternal LB supplementation in modulating systemic and central nervous system inflammation as well as promoting neural/oligodendrocyte progenitor development in the offspring. This evidence suggests that maternal probiotic supplementation may be a safe and effective strategy to improve neurological outcomes in the offspring.
Subject terms: Blood-brain barrier, Neuroimmunology
Introduction
The rate of preterm birth and the cost of neurodevelopmental diseases associated with preterm birth have been rising1,2. Thus, there is an increasing demand to develop interventions to reduce prematurity-associated neurological deficits. Preterm infants are at high risk for neurodevelopmental complications such as periventricular leukomalacia3, cerebral palsy4, and reduced cognitive performance later in life5. Microbiota is defined as the collection of microbial organisms that inhabits a certain environment6 and dysbiosis of the gut has been associated with the onset of neurodevelopmental disorders including attention-deficit/hyperactivity disorder5,7,8 and autism spectrum disorders9–11. Dysbiosis in preterm infants also contributes to neonatal sepsis12, necrotizing enterocolitis (NEC)13 and neuroinflammation14, which are also associated with long term neurological deficits15–17.
In animal models, sub-optimal microbial communities result in neuroinflammation and are associated with delayed brain development18–21. In particular, we have previously shown in a transfaunation of pregnant germ-free (GF) dam model that a microbial community associated with growth retardation in human preterm infants delayed neuronal development and resulted in both systemic and central nervous system (CNS) inflammation in the offspring20,22. Other studies have suggested that systemic inflammation is the driving factor to trigger CNS inflammation23,24 and several neurological conditions including sickness behaviors, depression and impaired learning and memory, due to the contribution of circulating cytokines to the communication between the immune system and the CNS23,25–29.
In recent years, targeting the microbiome for host benefits has gained significant attention30–32. The microbiome of neonates can be affected by postmenstrual age, mode of delivery, maternal and postnatal antibiotic usage, feeding patterns, and hospital and post-discharge environments33. Until recently, most of the current knowledge regarding targeting the microbiome to improve neonatal outcomes came from studies investigating direct administration of probiotics, most commonly Lactobacillus acidophilus and Bifidobacterium infantis (LB), to preterm infants to prevent NEC and/or associated mortality34–37. Probiotics are described as “live microorganisms which when administered in adequate amounts confer a benefit to the host”38. Studies have strongly documented the beneficial attributes of probiotics in host physiology including regulation of pathogenic bacterial colonization, mucosal barrier integrity, mucosal IgA responses, and anti-inflammatory cytokines. However, even with emerging evidence for a microbiome-brain communication pathway, few studies have explored optimization of the neonatal microbiome as a potential therapeutic intervention to improve neurological outcomes. This is potentially due to 1) the functional down-regulation of neonatal leukocytes (e.g., neutrophils, monocytes, and NK cells) and the complement system of the innate immune system in both term and preterm infants leading to suspected higher susceptibility of neonates to infections and other pathological conditions39 and 2) reported sepsis cases when probiotics were given prophylactically to reduce the incidence of NEC and mortality in preterm infants37,40,41. Therefore, one potential alternative yet to be explored is to change the maternal microbiome to improve neurological outcomes in the offspring.
Probiotic supplementation during pregnancy is generally regarded as safe since mothers do not have the same immune system immaturities as the neonates and has been found to confer benefit to the mother, protecting against preeclampsia42, gestational diabetes43, and vaginal infection44. In addition, maternal supplementation with probiotics during pregnancy and/or during lactation has been demonstrated to be an effective route to alter the infant microbiome45,46 as well as provide protection against diseases47–49. In a double-blinded placebo-controlled randomized clinical trial (RCT)45, antibiotics and birth mode (caesarean section) were associated with decreased Bifidobacterium abundance in infants. Maternal supplementation during pregnancy and breastfeeding of Bifidobacterium breve Bb99, Propionibacterium freundenreichii subsp. shermanii JS, Lactobacillus rhamnosus Lc705, and Lactobacillus rhamnosus GG normalized the Bifidobacterium abundance in the infants at three months of age. In another double-blinded placebo-controlled RCT study, both pre- and post-natal supplementation of a probiotic cocktail that included Bifidobacterium breve Bb99, Lactobacillus rhamnosus Lc705, and Lactobacillus rhamnosus GG reduced the risk of allergic disease among caesarean-born infants49. These limited but timely studies suggest that maternal probiotic supplementation can confer beneficial traits to the offspring.
In adults, probiotics have been shown to reduce circulating levels of systemic pro-inflammatory biomarkers in patients with a range of systemic inflammatory conditions including ulcerative colitis and psoriasis50, rheumatoid arthritis51,52, and liver disease53,54. Furthermore, a probiotic mixture (VSL#3, which contains four strains of Lactobacillus, three strains of Bifidobacterium and one Streptococcus salivarius subsp. thermophilus) has been shown to be able to reduce peripheral TNF-activated neuroinflammation marked by microglial activation and cerebral monocyte infiltration and altered sickness behaviors in the setting of peripheral organ inflammation55. These studies suggest that probiotics might exert effects on the CNS through an anti-inflammatory mechanism.
Therefore, we hypothesized that maternal probiotic supplementation confers protection on the CNS of offspring from inflammatory stimuli. Since IL-1β is a master regulator of neuroinflammation and elicits greater neuroinflammation when compared to other cytokines such as TNF or lipopolysaccharide (LPS, which represents exclusively gram-negative bacteria-induced inflammation)24, we chose to use IL-1β as the postnatal proinflammatory insult in this study. Prior to 21 days of life (weaning age) is a stage during which the rodent brain undergoes most of its neurogenesis, gliogenesis and myelination and is comparable to human infant neurodevelopment from birth to two to three years old56. Since studies have suggested that pre-wean rodents are more susceptible to inflammatory insults with adverse brain outcomes57, we investigated the effect of postnatal inflammatory insult on the offspring at pre-wean (two weeks) and post-wean (four weeks) age. The overall aim of this study was to investigate the effects of maternally administrated LB on inflammatory responses, neuroinflammation and neurodevelopment in the offspring. We demonstrate that maternally administrated LB from pregnancy to weaning protects the offspring brain from postnatal systemic proinflammatory insults and suppresses systemic inflammation-induced blood-brain barrier (BBB) dysfunction as well as immune cell activation and neuroinflammation. LB also actively promotes the development of neurons and oligodendrocyte progenitor cells in the brain.
Results
Maternal administration of Lactobacillus acidophilus and Bifidobacterium infantis (LB) significantly attenuated postnatal IL-1β-induced systemic inflammation in the offspring
We modeled systemic inflammation in C57BL/6J specific-pathogen free (SPF) mice at both two and four weeks of age with a peripheral immune challenge of intraperitoneal (i. p.) injection of IL-1β. As shown in Table 1, in two-week old mice four hours after injection, the serum levels of IL-6, KC, MCP-1, and IL-1β, but not IL-1α, evaluated by multiplex ELISA were significantly higher than those with saline injections (at least p < 0.01, n = 3). At four weeks of life there was not a significant inflammatory cytokine response to IL-1β injection (Table 2) (p > 0.05, n = 3) except for an increase in the serum MCP-1 level (p < 0.05, n = 3).
Table 1.
Systemic cytokine/chemokine levels after four hours i.p. IL-1β injection in the offspring at two-week old age.
| mean±S.E.M (pg/mL) Treatment | IL-6 | KC | MCP-1 | IL-1α | IL-1β |
|---|---|---|---|---|---|
| Saline | 24 ± 23 | 128.8.5 ± 94.51 | 36.32 ± 29.94 | 89.82 ± 9.45 | 6.49 ± 5.75 |
| IL-1β (i.p.) | 18747 ± 6037* | 20339 ± 386.5* | 13195 ± 1273* | 91.66 ± 12.25 | 1019 ± 215.3* |
Results are presented as: mean ± S.E.M, n = 3. *Comparison was performed with t-test, at least p < 0.05 was considered significantly different when compared with saline group.
Table 2.
Systemic cytokine/chemokine levels after four hours i.p. IL-1β injection in the offspring at four-week old age.
| mean±S.E.M (pg/mL) Treatment | IL-6 | KC | MCP-1 | IL-1α | IL-1β |
|---|---|---|---|---|---|
| Saline | 3.85 ± 2.56 | 59.37 ± 18.75 | 13.26 ± 7.34 | 116.8 ± 15.01 | 0.5 ± 0.02 |
| IL-1β (i.p.) | 364.5 ± 252.1 | 9355 ± 3796 | 2877 ± 719.4* | 129.9 ± 11.86 | 5.55 ± 2.69 |
Results are presented as: mean ± S.E.M, n = 3. *comparison was performed with t-test, at least p < 0.05 was considered significantly different when compared with saline group.
We then investigated whether maternal supplementation of LB reduces systemic responses to IL-1β insult in the offspring. The baseline circulating levels of IL-6, KC, MCP-1, IL-1α, and IL-1β were not statistically significantly different in the maternally LB supplemented vs unsupplemented offspring at either two or four weeks of age (Fig. 1a–j, p > 0.05, n = 3). However, maternal treatment with LB significantly downregulated the IL-1β-induced systemic levels of IL-6, KC, MCP-1, and IL-1β (Figs. 1a–c,e) at two weeks of age, by one-way ANOVA with Tukey’s post hoc analysis (at least p < 0.05, n = 3). Maternal LB supplementation did not affect the IL-1β-induced increased MCP-1 levels in the offspring at four weeks of age (Fig. 1h, p > 0.05, n = 3).
Figure 1.
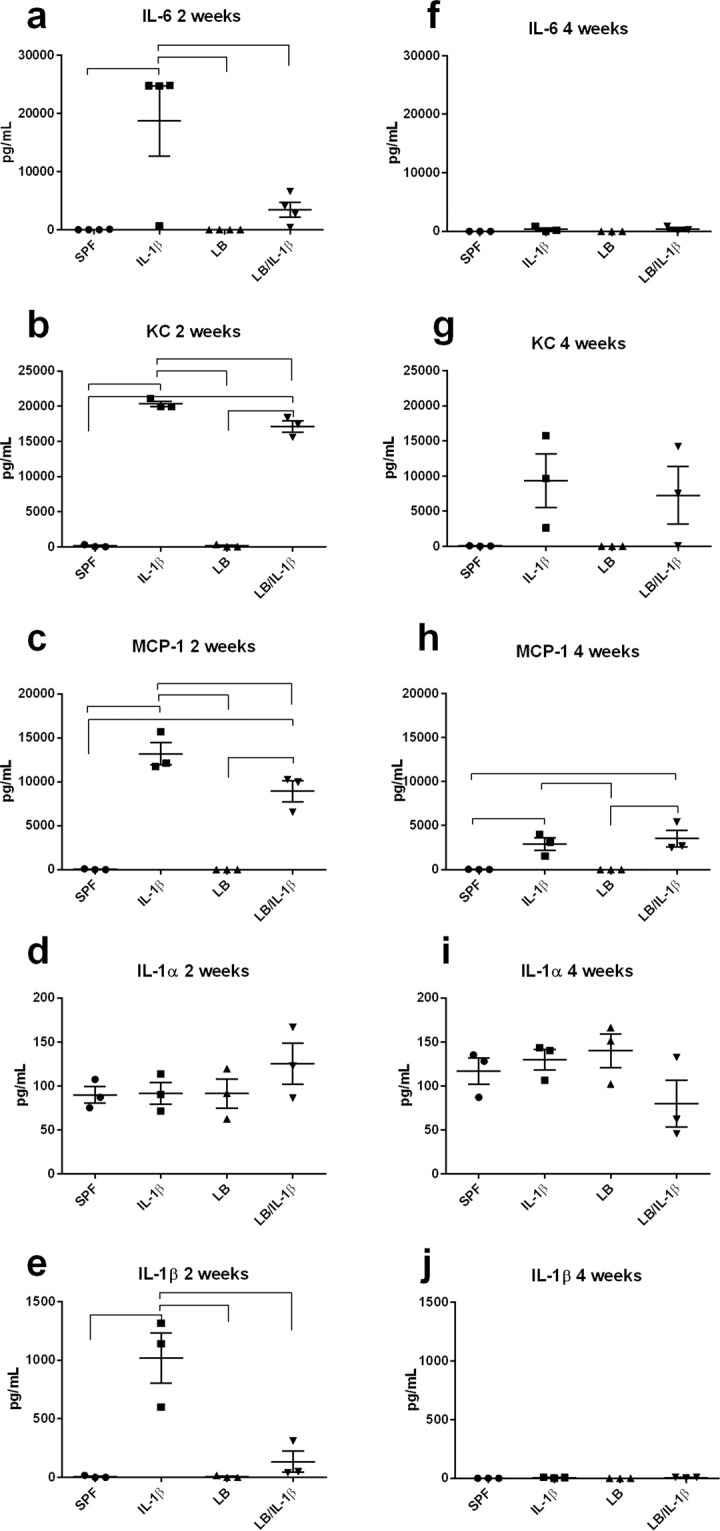
Maternal LB treatment significantly reduces systemic inflammation induced by postnatal IL-1β insult in the offspring. Systemic levels of IL-6 (a), KC (b), MCP-1 (c), IL-1α (d), and IL-1β (e) at two weeks of age (at least n = 3) and IL-6 (f), KC (g), MCP-1 (h), IL-1α (i), and IL-1β (j) at four weeks of age (n = 3) were measured by ELISA in serum four hours after saline or IL-1β (i.p.) injection. Data were presented as mean ± SEM. Bars with ⎴ denote significant difference between experimental groups (at least p < 0.05).
Maternal LB exposure rescued IL-1β -induced blood-brain barrier (BBB) dysfunction in two weeks old offspring
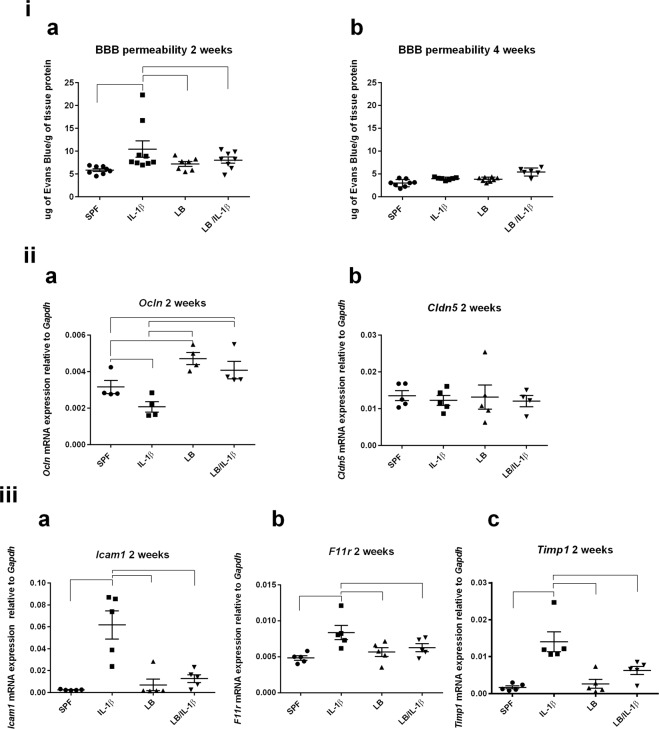
Studies have suggested that systemic inflammation can disrupt BBB integrity58. We evaluated BBB permeability of cerebral blood vessels, measured by extravasation of Evans blue dye. A two-way ANOVA analysis was first conducted to examine the effects of age (two weeks vs four weeks) and proinflammatory insult (without vs with) on BBB permeability in SPF mice (see Supplemental Fig. S1). There was not a statistically significant interaction between the effects of age and proinflammatory insult on BBB permeability, F (1, 28) = 2.891, p = 0.1002. Sidak’s multiple comparisons test revealed that IL-1β (i. p.) induced a significant increase in Evans blue leakage into the mouse brain of two (pre-wean), but not four (post-wean), weeks old mice (Fig. S1, p < 0.01, for 2 weeks n = 8–9 and p > 0.05, for four weeks n = 7–8). This result is consistent with previous reports that early postnatal rodents are more susceptible to systemic inflammation-induced BBB permeability (pre-wean in rodents, a stage of brain development equivalent to 22–42 weeks of gestation in humans59,60). Maternal exposure to LB normalized the IL-1β-induced increased BBB permeability at two weeks of age (Fig. 2i a, n = 7–8). Neither maternal supplementation with LB nor IL-1β (i. p.) had impact on BBB permeability at four weeks of age (Fig. 2i b, n = 6–8, p > 0.05).
Figure 2.
Maternal LB supplementation regulates several BBB characteristics in the offspring. (i). Intraperitoneally administered Evans blue dye (4 ml/kg, 2% [w/v]) in saline appeared significantly higher in the homogenized brains following the injection of IL-1β compared to the control group administered with saline only at two weeks of age (n = 8–9, p < 0.05) (a), but not at four weeks of age (n = 7–8) (b). Increased BBB permeability in two weeks old IL-1β-treated offspring was prevented by maternal LB treatment (n = 7–8, p < 0.05). (ii) Maternal LB supplementation promoted Occludin gene expression and prevented IL-1β-induced decreased Occludin expression in the two-week old offspring. Transcripts of Occludin (Ocln) (n = 4, (a) and Claudin-5 (Cldn5) (n = 4–5, (b) were evaluated by RT-PCR in the cerebral cortex of the two weeks old offspring in both the maternally un-supplemented and supplemented groups after four hours of saline or postnatal IL-1β injection. (iii) Maternal exposure of LB blocked IL-1β-induced expression of markers for vascular injury, leukocyte recruitment, and ECM integrity. Transcripts of Icam1 (n = 5, (a), F11r (n = 5, (b) and Timp1 (n = 5, (c) were evaluated by RT-PCR in the cerebral cortex of the two weeks old offspring in both the maternally un-supplemented and supplemented groups after four hours of saline or postnatal IL-1β injection. For (ii) and (iii), data were normalized to Gapdh gene expression and presented as mean ± SEM. For (i), (ii), and (iii), Bars with ⎴ denote significant difference between experimental groups (at least p < 0.05).
Since we observed most of the systemic inflammatory responses and increased BBB permeability in the two week old mice, the remainder of our investigations focused on this more vulnerable pre-wean population. The IL-1β-induced increased permeability in two-week old mice was associated with reduced cerebral gene expression of the tight junction (TJ) protein Occludin (Ocln) (Fig. 2ii a, p < 0.001, n = 4) but not Claudin‐5 (Cldn5, Fig. 2ii b, p > 0.05, n = 4–5), evaluated by RT-PCR. LB maternal supplementation significantly increased the expression of Ocln without postnatal inflammatory insult (Fig. 2ii a, p < 0.0001, n = 4) and the Ocln levels in the offspring remained significantly higher than unsupplemented pups even after IL-1β treatment (Fig. 2ii a, p < 0.001, n = 4).
Maternal LB exposure regulated IL-1β-induced leukocyte recruitment and extracellular matrix (ECM) damage in two-week old offspring
Leukocyte recruitment and extracellular matrix (ECM) damage are two other signature processes of compromised BBB integrity. Both intercellular adhesion molecule-1 (Icam-1) and junctional adhesion molecule-1 (F11r) serve as ligands for lymphocyte function-associated antigen-1 (LFA-1) to regulate leukocyte transendothelial migration known as diapedesis into the CNS61,62. The tissue inhibitors of metalloproteinases (TIMPs) are tissue specific, endogenous inhibitors of metalloproteinases, including the matrix metalloproteinases (MMPs), and regulate ECM proteolysis and turnover63. IL-1β (i.p.) increased the expression of brain Icam-1 (Fig. 2iii a, p < 0.0001, n = 5), Jam-1/F11r (Fig. 2iii b, p < 0.0001, n = 5), and Timp1 expression (Fig. 2iii c, p < 0.0001, n = 5), hallmarks for vascular injury, leukocyte recruitment and ECM injury. Maternal exposure to LB completely blocked the IL-1β-induced increased Icam-1 and F11r expression (Fig. 2iii a and 2iii b, p < 0.0001, n = 5; respectively). Offspring of the maternal supplemented group challenged by IL-1β had significantly lower Timp1 gene expression when compared to the offspring challenged by IL-1β without maternal exposure to probiotics (Fig. 2iii c, p < 0.05, n = 5). Taken together, these data demonstrate that maternal LB exposure protected against systemic inflammation-induced BBB dysfunction in the offspring by regulating tight junction disruption, vascular injury, leukocyte recruitment and ECM repair.
Maternal administration of LB attenuated postnatal IL-1β-induced astrocyte and microglia activation
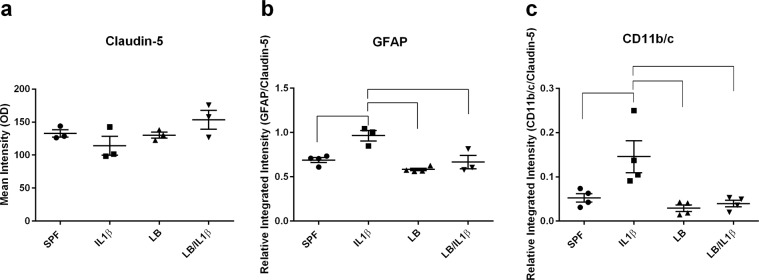
To determine whether changes in circulating cytokine levels and BBB integrity induce astrocyte activation and microglial recruitment to cerebral vasculature, we performed immunohistochemical staining for glial fibrillary acidic protein (GFAP)+ astrocytes (Fig. 3, green) and CD11b/c+ microglia (Fig. 4, green). Brain blood capillaries were defined by co-staining with a BBB-specific TJ protein claudin-5 (Figs. 3 and 4, red). Consistent with the gene expression data (Fig. 2ii b), we did not find any differences in claudin-5 (red, Figs. 3 and 4) protein levels in the cerebral cortex of the offspring from either the LB-supplemented or un-supplemented group with or without postnatal peripheral IL-1β insult. Integrated intensity levels of claudin-5 were obtained from analysis using ImageJ (NIH) and are presented in Fig. 5a (p > 0.05, at least n = 4). Systemic inflammation induced by IL-1β (i. p.) resulted in significantly increased GFAP staining (green) around the blood vessel (identified by claudin-5 staining, red), indicating a strong astrocyte activation in the BBB (Fig. 3), with quantification presented as GFAP intensity over claudin-5 measurement in Fig. 5b (p < 0.01, n = 4). Maternal LB exposure did not change the GFAP expression (Fig. 3) but attenuated IL-1β-induced increased astrocyte activation (Fig. 3). Systemic inflammation caused by IL-1β (i. p.) also induced microglia recruitment and activation around the blood capillaries (Fig. 4). Maternal LB exposure attenuated IL-1β-induced increased CD11b/c expression around the blood vessels, indicating reduced microglia recruitment to the vasculature (Fig. 4, p < 0.05, n = 4). Quantitative analysis CD11b/c is presented in Fig. 5c using ImageJ and expressed as integrated intensity levels of CD11b/c over claudin-5.
Figure 3.
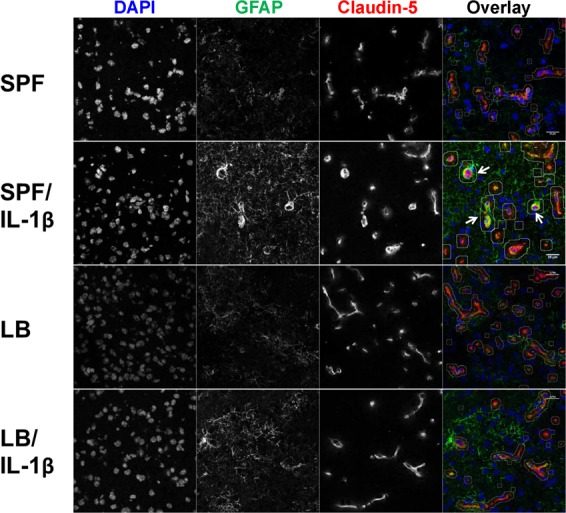
Increased astrocyte activation in IL-1β-stimulated two-week old offspring is reduced by maternal LB supplementation. Representative images of fluorescence microscopy of claudin-5+ (location of the brain capillaries, red), GFAP+ astrocyte (green), and DAPI (nuclei, blue). Three to five sections per mouse were examined and at least three mice were examined in each group. Stronger than control SPF GFAP staining was observed around the blood vessel (see arrow) after IL-1β insult. Maternal supplemented group (LB) with or without postnatal insult had GFAP levels similar to the control group indicating that LB supplementation prevented astrocyte activation around the BBB endothelium.
Figure 4.
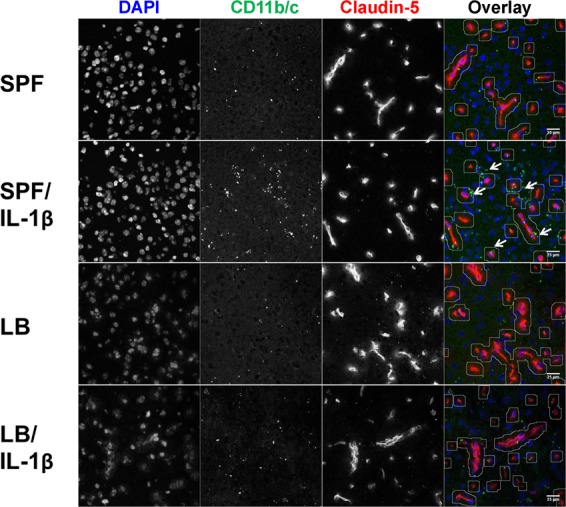
Increased microglia activation in IL-1β-stimulated two-week old offspring is reduced by maternal LB supplementation. Representative images of fluorescence microscopy of claudin-5+ (location of the brain capillaries, red), CD11b/c+ microglia (green), and DAPI (nuclei, blue). Three to four sections per mouse were examined and at least four mice were examined in each group. The CD11b/c+ staining of the maternally probiotic supplemental group remained similar to the un-supplemented group in term of both pattern (scattered) and intensity (see Fig. 5). However, IL-1β stimulated the recruitment of microglia to the BBB, demonstrated by increased CD11b/c+ staining around the BBB endothelium (see arrow). Maternal LB supplementation reduced the IL-1β-induced microglia translocation to the vicinity of BBB.
Figure 5.
Quantification of astrocyte and microglia activation using ImageJ (NIH). Overall expression of claudin-5 was not affected by LB supplementation or inflammatory insult (a). Thus, the GFAP and CD11b/c expression in the vicinity of the blood vessel is expressed as GFAP (n = 3–4, (b) or CD11b/c (n = 4, (c) levels over claudin-5 levels in the selected blood vessel area. Bars with ⎴ denote significant difference between experimental groups (at least p < 0.05).
Maternal administration of LB ameliorated postnatal IL-1β-induced neuroinflammation
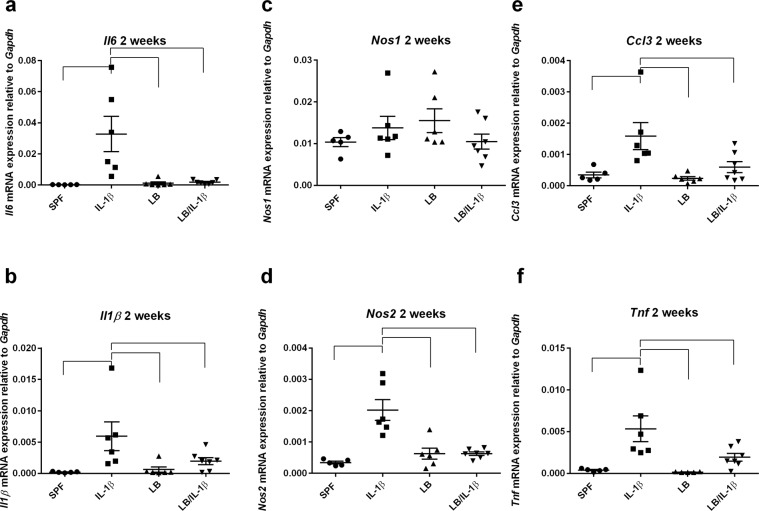
To further investigate the effects of maternal exposure to LB on offspring brain inflammatory status, we evaluated biomarkers of neuroinflammation in the cerebral cortex tissues at two weeks of age by RT-PCR. As depicted in Fig. 6, maternal LB did not change the steady-state inflammation status of the offspring brain. IL-1β injection (i. p.), when compared to saline, resulted in robust increases in cerebral cortex mRNA expression of Il6 (Fig. 6a), Il1β (Fig. 6b), Nos2 (Fig. 6d), Ccl3 (Fig. 6e), and Tnf (Fig. 6f), but not Nos1 (Fig. 6c) in the offspring at two weeks of age (at least p < 0.01, n = 5-6). Maternal exposure to LB completely mitigated the Il-1β-induced Il6, Il1β, Nos2, Ccl3, and Tnf gene expression increases (at least p < 0.05, n = 6-7; Tukey’s post hoc test after one-way ANOVA).
Figure 6.
Systemic inflammation-induced neuroinflammation is blocked in the two-week old offspring of maternal LB supplemented mice. Neuroinflammation markers Il6 (a), Il1β (b), Nos2 (d), Ccl3 (e) and Tnf (f), but not Nos1 (c), evaluated by RT-PCR, were upregulated by i.p. injection of IL-1β after four hours in two weeks old mice (n = 5–6). Maternal exposure of LB blocked the Il-1β induced increased expression of these markers (n = 6–7). Data were normalized to Gapdh gene expression and are presented as mean ± SEM. Bars with ⎴ denote significant difference between experimental groups (at least p < 0.05).
Maternal administration of LB regulated neuroinflammation through modulation of transcriptional factors
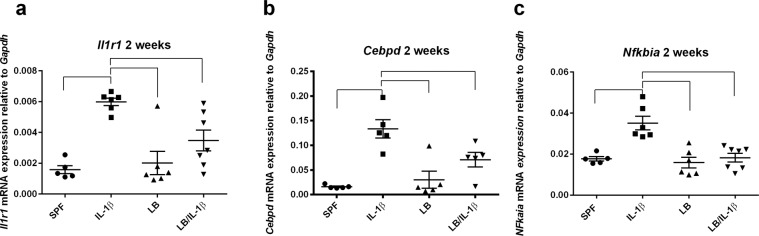
To delineate the potential mechanisms by which maternal LB supplementation modulates IL-1β-induced neuroinflammation in the offspring, we first investigated whether the reduced responses in the offspring brain were due to the downregulation of interleukin-1 receptor (IL1r). As demonstrated in Fig. 7a, there was no difference in Il1r1 gene expression between the control and the maternal supplemented groups. We then determined the transcriptional regulators in the brain responsible for the observed proinflammatory profile. Both CCAAT/enhancer-binding protein delta (CEBPD)64–71 and NF-κB/IκB72,73 pathways have been shown to be master transcriptional regulators of inflammatory responses. We found that postnatal insult with IL-1β (i. p.) significantly increased the mRNA expression of Cebpd and Nfkbia (which encodes IκBα) in the cortex of the mice at two weeks old of age (Fig. 7b,c, respectively, at least p < 0.01, at least n = 4). LB maternal supplementation did not change the baseline transcriptional regulators expression when compared to the unsupplemented controls and significantly ameliorated the IL-1β-induced Cebpd and Nfkbia gene expression levels (Fig. 7b,c, respectively, at least p < 0.01, at least n = 4). Additionally, Pearson correlation analysis revealed that there were strong statistically significant correlations between Cebpd (Fig. S2A), or Nfkbia (Fig. S2B), and neuroinflammation gene expression levels (see r values and p values under each plot), with the exception of two comparisons: Nfkbia or Cebpd versus brain levels of Nos1. Together, these data suggest that modulation of IL-1β-induced neuroinflammation in the offspring by maternal LB exposure could be due to regulation of the transcriptional regulators CEBPD and IκBα.
Figure 7.
Regulation of neuroinflammation by maternal LB supplementation in the two-week old offspring is mediated by transcriptional regulators. Transcripts of the IL-1β receptor Il1r1 (a) and the transcriptional regulators Cebpd (b) and Nfkbia (c) were evaluated by RT-PCR. Il1r1 gene expression was not affected by maternal LB supplementation (n = 5–6, (a). Cebpd (b) and Nfkbia (c) were upregulated by i.p. injection of IL-1β after four hours (n = 5-6). Maternal exposure of LB blocked the Il-1β-induced increased expression of these markers (n = 6–7). Data were normalized to Gapdh gene expression and are presented as mean ± SEM. Bars with ⎴ denote significant difference between experimental groups (at least p < 0.05).
Maternal administration of LB promoted the development of neurons and oligodendrocyte precursor cell (OPC) in the offspring
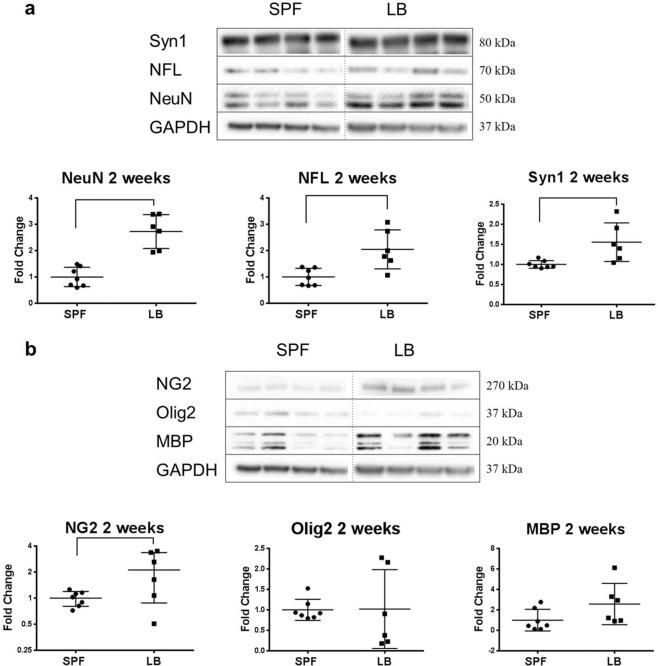
We have previously shown that a microbial community from a poor growth preterm infant was associated with delayed neuronal and oligodendrocyte development and myelination in the early (two weeks of age) postnatal brain20, thus we next investigated the effects of maternal LB supplementation on the expression levels of NeuN, a postmitotic mature neuron marker; neurofilament protein-L (NFL), marker for neuronal axon growth; Synapsin 1 (Syn1), marker for synapse formation; neuroglia antigen 2 (NG2), marker for OPCs; oligodendrocyte transcription factor (Olig2), marker for pre-myelinated oligodendrocytes; and myelin basic protein (MBP), marker for oligodendrocyte and white matter maturation; respectively. As demonstrated in Fig. 8a with representative western blots on the top panel and quantification on the bottom panel, LB maternal exposure significantly increased the expression of NeuN, NFL, and Syn1 (2.72, 2.05, 1.55 times of the control SPF; respectively) when compared to the control group (p < 0.05, n = 7 in SPF, n = 6 in LB group). LB maternal supplementation did not affect the expression of Olig2 and MBP; however, NG2 expression in the maternal LB group was found to be 2.12 times of the control group (Fig. 8b, p < 0.05, n = 7 in SPF, n = 6 in LB group). These data demonstrate that maternal LB promotes neuronal and oligodendrocyte precursor cell development in the brains of the offspring.
Figure 8.
Maternal LB exposure promotes neuronal and oligodendrocyte precursor cell development in the offspring. Proteins of two weeks old cortex tissues were separated on SDS-PAGE gels and probed with NeuN, NFL and Syn1 for neuronal development (a) and NG2, Olig2, MBP for oligodendrocyte development (b). For both (a) and (b), top: representative raw images of western blot analysis; bottom: scatter plots of densitometry results normalized to GAPDH displayed as a fold of control. Unpaired t test with Welch’s correction was used to detect the difference between SPF (n = 7) and LB (n-6) group. NeuN-Neuronal Nuclei; NFL-Neurofilament-L; Syn1-Synapsin; NG2-Neuroglia antigen 2; Olig2-Oligodendrocyte Transcription Factor 2; MBP-Myelin Basic Protein. Bars with ⎴ denote significant difference between experimental groups (at least p < 0.05). Blots were cropped and the original blots are presented in Supplementary Figure S3.
Discussion
The human microbiome has increasingly been shown to be an important regulator of CNS development and functions19,31,56,74. The effect of the initial establishment of the microbiome in neonates has been associated with basic processes such as neurogenesis, myelination, the establishment of the blood-brain barrier, and microglia maturation as well as with behavioral responses18,20,74–76. Preterm delivery disrupts important in utero brain maturation31,56. Dysbiosis, or altered gut bacterial composition, predisposes preterm infants to inflammatory diseases and infections such as NEC and sepsis that have long term adverse neurological outcomes14. Therefore, studies have explored means to modify the preterm microbiome to improve neonatal outcomes. With direct supplementation of probiotics to preterm infants, studies have demonstrated reduced NEC incidence, decreased length of the hospital stay, increased tolerance to feeding, and reduced mortality rate in the preterm population34. However, the Food and Drug Administration has not approved the usage of probiotics for the prevention and treatment of disease due to the lack of regulation of strains, dose, duration and quality of probiotics and reported cases of sepsis34,40,41. Maternal probiotic supplementation has been shown to be safe and effective to reduce NEC, atopic diseases and IgE-associated allergy in the offspring47–49,56, therefore in this study we investigated whether maternal probiotic supplementation can have neuroprotective effects from postnatal inflammatory insult or can have direct impacts on the brain development in the offspring. We first demonstrated in this study that maternal probiotic (LB) supplementation can change systemic immune responses in the offspring with suppression of circulating pro-inflammatory cytokines/chemokines, including IL-6, KC, MCP-1, and IL-1β, after postnatal peripheral inflammatory insult. This protection was then found not only in the blood but also extended to the brain. Postnatal peripheral inflammatory stimuli-induced BBB dysfunction, astrocyte and microglial activation, and neuroinflammation were normalized by maternal LB supplementation. Furthermore, we demonstrated that the regulation of neuroinflammation in the offspring by maternal LB supplementation was mediated through transcriptional regulators such as CEBPD and IκBα. Lastly, maternal LB supplementation promoted neuronal development evidenced by increased expression of NeuN, NFL, and Syn1 and oligodendrocyte progenitor cell development evidenced by increased NG2 expression in the offspring brain. These findings support the current paradigm to beneficially shift systemic immunity through probiotics administration, but this study is the first to demonstrate neuroprotective beneficial effects and the promotion of brain development in the offspring through maternal supplementation from pregnancy to weaning instead of direct administration to the pups.
Elevated circulating inflammatory cytokine levels are associated with a variety of medical conditions in infants. For example, postnatal episodes of inflammation with elevated proinflammatory IL-6 and TNF levels are associated with the development of severe retinopathy of prematurity77. Proinflammatory circulating cytokines IL-6 and IL-8 are strongly elevated and IL-4, an immune modulator that inhibits the release of proinflammatory cytokines, such as TNF and IL-1β, is suppressed in NEC patients compared to the healthy controls78. One of the proposed benefits of probiotics consumption is the ability to modulate the systemic immune function. In our study, maternal LB supplementation from pregnancy to weaning reduced postnatal inflammatory stimuli-induced systemic inflammation by significantly suppressing the circulating proinflammatory cytokines IL-6, KC, MCP-1, and IL-1β. The observed significantly increased circulating cytokines at two weeks of age is consistent with previous studies24,58 demonstrating that the acute systemic inflammatory response is associated with a fundamental proinflammatory cytokine profile consisting of IL-6 and IL-1β, and that IL-1β is a potent inducer of systemic inflammation. Our study agrees with the anti-inflammatory characteristics of probiotics but the novelty of our results lies in the fact the immunomodulatory effects of the LB were transferred from mothers to the offspring examined at the pre-weaned stage when the population is more vulnerable to inflammatory insult.
Emerging evidence has demonstrated communication between the immune system and CNS during peripheral inflammation23,25–28. Signaling at the BBB appears to be the prominent communication site at which the brain responds to the peripheral inflammation status27,79,80. The BBB is the vascular endothelium that tightly governs the interaction between the circulatory system and CNS to allow for proper brain function and provide protection to the CNS from toxins, pathogens, and inflammation81. Studies have discovered that systematic inflammation can increase BBB permeability modeled by i.p. injection of IL-1β24,57,79 and LPS82. There seems to be a specific window of susceptibility to systemic insults to the BBB since systemic inflammation-induced increased permeability of cerebral blood vessels was only observed in rats before postnatal age of 20 (P20, pre-wean)57, but not at P28 (post-wean). Our results are in agreement with these studies demonstrating that IL-1β (i.p.) induced increased BBB permeability at two weeks of age (P14), but not four weeks of age (P28), indicating a developmental maturation of BBB from pre-weaned to post-weaned age in mice.
Studies have further revealed that microbial communities can modulate BBB integrity75,83. Compared to SPF mice with normal gut flora, GF mice display increased BBB permeability and reduced expression of the TJ proteins occludin and claudin-5. Exposure of GF mice to a commensal microbiota or monocolonization with C. tyrobutyricum or B. thetaiotaomicron decreases BBB permeability and normalizes the expression of TJ proteins75. In this study, we have shown that maternal LB modulates BBB function in the offspring brain evidenced by normalized increased BBB permeability and decreased Ocln gene expression in response to postnatal inflammatory insult-induced systemic inflammation. Maternal LB supplementation resulted in a significant increase in Ocln gene expression compared to the unsupplemented group, suggesting that the protective effect of maternal LB supplementation on BBB is not only due to the suppression of systemic inflammation but also to upregulation of the TJ protein occludin itself.
In the presence of systemic inflammation signals, activation of the cerebral endothelium in the BBB by cytokines results in increased endothelial cell expression of adhesion factors such as ICAM1 and redistribution of JAM-1 (F11r) from the TJs62,84. The activated ICAM1 and JAM-1(F11r) both serve as ligands for LFA-1 and lead to adhesion, arrest and ultimately extravasation of leukocytes through the endothelium and into the brain84. In particular, the efficiency of this extravasation mediated by LFA-1 and ICAM-1 remained low unless the endothelium was stimulated by various inflammatory stimuli in mouse models of experimental autoimmune encephalomyelitis85 and liver disease25,86. In our study, we discovered that i.p. injection of IL-1β induced the expression of Icam1 and Jam-1 (F11r) expression in the offspring and maternal supplementation of LB significantly inhibited postnatal IL-1β-induced Icam1 and Jam-1 (F11r) expression. Furthermore, among the four tissue inhibitors of metalloproteases, TIMP-1 is the inducible form and is up-regulated by proinflammatory factors such as IL-1β and IL-687. It has been hypothesized that acute immune activation of astrocytes increases TIMP-1 in an attempt to repair and protect whereas chronic inflammation down-regulates TIMP-1 impairing the ability of TIMP-1 to preserve ECM88. In our study, an acute inflammatory response was modeled by i.p. injection of IL-1β and termination of the experiment four hours after injection. We observed that Timp1 gene expression was induced by postnatal IL-1β insult but that maternal LB supplementation maintained Timp1 at a steady-state level and prevented IL-1β-induced Timp1 increase in the acute inflammation state. Together, these data demonstrate that maternal LB supplementation can mediate the signaling of leukocyte extravasation and ECM homeostasis in the offspring in response to postnatal inflammatory insult.
Strong evidence suggests that peripheral inflammation can result in an inflammatory response within the brain, characterized by synthesis and action of cytokines in the CNS89–94. A single dose of a peripheral proinflammatory insult activated microglia and increased prolonged expression of MCP-1, IL-1β, and NF-κB in an adult model of neurodegenerative disease91. Additional astrocyte loss and structural changes95 and astrocyte gene transcription with a proinflammatory profile in response to peripheral inflammation were also reported96. The host microbiota’s role in shaping the brain’s innate immune system was recently reported by a study76 showing that GF mice displayed global defects in microglia with altered cell proportions and an immature phenotype, leading to impaired innate immune responses. Previous studies have further demonstrated that probiotics such as VSL#3 reduced systemic immune activation and cerebral monocyte infiltration and attenuated sickness behaviors associated with peripheral liver inflammation55. In our study, GFAP and CD11b/c immunostaining demonstrated that maternal LB exposure attenuates postnatal peripheral IL-1β-induced recruitment to the site adjacent to the BBB in the brain and activation of astrocytes and microglial cells. These results provide evidence that there is a signaling pathway between LB-mediated peripheral inflammation, BBB and the brain immune system.
We further demonstrated that postnatal IL-1β insult caused severe neuroinflammation marked by robustly increased Il6, Il1β, Nos2, Ccl3, and Tnf expression. Endothelial cells at the BBB, the recruited inflammatory cells from the blood, as well as activated microglia, pericytes, and astrocytes are all potential sources of IL-1β, IL-6, MIP-1α (CCL3), and TNF contributing to neuroinflammation94,97–103. Furthermore, non-immune cell-produced cytokines can in turn trigger further microglial activation and neuroinflammation104. Maternal LB supplementation significantly ameliorated the induced expression of neuroinflammation markers in our study. Other strains of probiotics have been shown to have an anti-inflammatory effect in the brain. Supplementation of L. helveticus downregulated inflammatory markers such as NOS2, prostaglandin E2, and IL-1β in the brain105. A multi-strain probiotics treatment consisting of L. helveticus R0052, L. plantarum R1012, and B. longum R0175 improved chronic mild stress (CMS)-induced anxiety- and depressive-like behaviors in a mouse model of CMS and ameliorated CMS-induced TNF increase in the brain106. Supplementation of LB has been widely used to reduce inflammatory bowel diseases especially in preterm infants with NEC35,36. Together with the systemic inflammation results, our study demonstrates that LB is anti-inflammatory and that these immunomodulation properties of LB: 1) can be transferred from the mother to the offspring 2) can be extended from the systemic system to the CNS of the offspring.
Since the observed peripheral inflammation-induced neuroinflammation is at least partially due to the locally produced inflammatory markers evidenced by increased transcriptional levels of these factors, we examined the transcriptional regulators potentially responsible for the up-regulation. As a member of the CCAAT/enhancer-binding protein (C/EBP) family, C/EBP delta (CEBPD) is expressed at relatively low levels under physiological conditions and is upregulated by a variety of extracellular stimuli, such as IL-664, LPS65, IL-1β66,67, TNF68 and interferon-γ107. CEBPD can reciprocally induce expressions of cytokines including IL-6, IL-1β, and TNF69–71,101. Moreover, enhanced expression of CEBPD plays a critical role in the pathogenesis of inflammatory diseases, such as AD and rheumatoid arthritis68,108,109. The expression level of the NF-κB inhibitor NFKBIA (which encodes IκBɑ) is a direct reflection of activation of NF-κB signaling by forming an autoregulatory loop with activated NF-κB transcription factors72,73. IκB ɑ protein is degraded upon proinflammatory stimulation, resulting in rapid translocation of NF-κB from the cytoplasm to the nucleus. In turn, IκB ɑ is quickly induced by the activated NF-κB transcriptional pathway72. In our study, the peripheral inflammation-induced neuroinflammation was mediated by upregulation of these prototypical proinflammatory transcriptional regulators and maternal LB modulated neuroinflammation in the offspring through downregulation of these factors. Interestingly, IL-1β peripheral insult resulted in the highest increase of Cebpd in astrocytes compared to microglia and endothelial cells. These increased astrocytic Cebpd expression contributed to the chemoattractant activity and migration and activation of microglia/macrophages in the brain in an animal model of Alzheimer’s disease101. Therefore, the observed reduced microglia migration in the offspring by maternal LB supplementation in this study could also be mediated by the Cebpd expression.
While mounting studies have demonstrated the impact of the intestinal microbiota on host behaviors such as anxiety and stress in adults and aging related-neurodegeneration19,23,110, few studies have investigated the early critical brain development window across prenatal and early postnatal stages. We have previously demonstrated that a microbial community associated with a poor postnatal growth resulted in delayed neuron development using NeuN and NFL expression as markers at the pre-weaned age of P14 and myelination using MBP as a marker at the post-weaned age of P2820. In this study, we provide novel observations that maternal supplementation of LB from late gestation to weaning promotes neuron development evidenced by increased NeuN, NFL, and Syn1 expression in the offspring brain. Furthermore, the OPC marker NG2 was also increased by this supplementation. These observations strongly suggest that maternal LB administration can actively modulate brain development in the offspring with therapeutic potential.
We acknowledge that we cannot determine whether the observed neuroinflammation was caused by cytokines crossing the compromised BBB or locally produced cytokines by endothelial cells or brain immune cells such as astrocyte and microglia. As previously discussed, multiple brain cell types including endothelial cells, astrocytes, microglial cells, pericytes, and neurons are all capable producing proinflammatory factors constituting neuroinflammation. We speculate that the deleterious effects in overall neuroinflammation are mediated by a combination of potential pathways: 1) systemic inflammation can directly induce BBB leakage allowing passage of proinflammatory cytokines such IL-1β and IL-6 which then stimulate immune cells and neurons in the brain to produce additional inflammatory cytokines/chemokines. These proinflammatory factors can in turn signal to recruit leukocyte infiltration of the blood vessels to amplify the inflammatory responses; 2) systemic inflammation can induce leukocyte infiltration and/or act directly on endothelial cells of the BBB vasculature. Additionally, a limitation of this study is that we only used CD11b/c to identify macrophages in the brain therefore, it is difficult to distinguish if the activated immune cells were local microglia, which can be identified by CD11b + /CD45low labeling, or infiltrating macrophages, which can be identified by CD11b/c + /CD45high labeling. We provide indirect evidence that leukocyte recruitment might be increased by induced Icam1 and F11r expression, however future studies can elucidate this process further by isolating microglia from the brain and sorting by CD11b/c and CD45 double-labelling using flow cytometry. Finally, investigation of the mechanisms by which maternal LB promotes neuron and OPC development in the offspring is warranted. Future studies will utilize metabolomics analysis to evaluate Sdifferences in the metabolites between the LB-treated group and untreated group to identify potential regulators signaling from the microbiome to the gut to the brain. Epigenetics can also be used to reveal whether maternal LB supplementation genetically programs the offspring to have decreased inflammatory responses and better neurodevelopmental outcomes.
In conclusion, the present study is novel in revealing the impact of the maternal probiotic route on brain development and immune (systemic and neuronal) responses in the offspring. Our data demonstrate that maternal LB supplementation inhibits peripheral inflammation-induced increases in circulating IL-6, KC, MCP-1, and IL-1β levels, BBB dysfunction, cerebral astrocytes and microglial activation, and neuroinflammation and promotes neuron and OPC development in the brain of the offspring. Therefore, targeting the maternal microbiome with probiotics may have a therapeutic role in conferring resilience against pro-inflammatory events and associated impacts on brain health/development.
Methods
Ethics approval
All animal procedures were approved by the Institutional Animal Care and Use Committee under the animal protocol No. 71703 and performed strictly in accordance with approved Animal Care and Use Protocols (ACUPs) by the U.S. National Institutes of Health at The University of Chicago.
Animals
Time pregnant C57/BL6J specific pathogen-free (SPF) mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and were kept on a 12-hour light/dark cycle and had access to food and water ad libitum. At embryonic days 16 (E16), dams were randomized to be fed daily with either a combination of 109 Lactobacillus acidophilus and 109 Bifidobacterium infantis (LB) at a ratio of 1:1 from ATCC (No. 53544 and 15697, respectively; Manassas, VA, USA) or vehicle until weaning at postnatal day 21 (P21). Pups delivered naturally and stayed with the mothers until P21. At P14 (two weeks of age) or P28 (four weeks of age), pups were weighed and challenged with intraperitoneal (i.p.) injection of IL-1β (50 ng/g body weight), modeling postnatal systematic inflammation. Groups included both male and female animals. Based on our previous studies20,74, sex difference was not prominent in C57BL/6 J mice at this age thus sex effect was not investigated separately. The offspring used in this study were from at least five different litters for SPF mice and six different litters for LB mice. This resulted in four study groups: SPF, IL-1β, LB, and LB/IL-1β. After four hours of IL-1β treatment, mice were sacrificed and serum was collected and stored at −80 °C for cytokine/chemokine multiplex analysis. Tissue harvesting was carried out with left cortex snap frozen for biochemical analysis and right cortex for immunohistochemical analysis.
Cytokine assay
Multiplex analysis was performed according to the manufacturer’s instructions using a kit for a panel of mouse cytokines/chemokines based on the Luminex xMAP technology with magnetic beads (EMD Millipore Corporation, Billerica, MA, USA). Experiments were performed in triplicate. The kit enables a simultaneous multiplex analysis of 12 cytokines, chemokines, and interleukins in a 25 µl (2x diluted) serum sample.
Blood-brain barrier (BBB) Permeability Evaluation
Evaluation of BBB permeability was carried out as previously described111,112. Briefly, mouse pups receive a 2% solution of Evans Blue (w/v) in normal saline (4 ml/kg of body weight, i.p.), followed four hours later by transcardiac perfusion with 40 ml of ice-cold PBS. Brain tissues were removed from the skull and divided into right and left hemispheres, snap-frozen in liquid nitrogen, and stored at −80 °C. Right hemisphere samples were homogenized in 1000 μl of PBS and centrifuged at 12, 000 × g for 30 mins at 4 °C. 500 μl of the supernatant was mixed with 500 μl 50% trichloroacetic acid, incubated at 4 °C overnight and then centrifuged at 12, 000 × g for 30 mins at 4 °C. Evans Blue stain was measured by ELx800 spectrophotometer (BioTek Instruments, Winooski, VT, USA) at 610 nm and quantified using a standard curve. The results are presented as (μg of Evans Blue stain)/(g of total protein).
RNA isolation and real-time PCR
Using the RNeasy Plus Mini Kit (QIAGEN GmbH, Hilden, Germany) total RNA from snap frozen brains was isolated. 500 ng of isolated total RNA was used to synthesize cDNA using RT2 First Strand Kit from QIAGEN. TaqMan Assays (Thermo Scientific Inc., Waltham, MA, USA) were used for genes of interest and the housekeeping gene Gapdh. Gene expression was normalized to the housekeeping gene.
Immunohistochemistry
Immunostaining protocol was as described previously20. Brains were obtained from mice at postnatal age of two weeks and embedded in OCT. Eight μm frozen sections were fixed in ice-cold methanol at −20 °C for 20 minutes. The samples were incubated with blocking solution (5% goat serum) in 0.2% Triton-X PBS (PBST) for one hour at room temperature (RT) after rinsing with 1x PBS three times and permeabilized with PBST for 15 mins. The tissue sections were then incubated with respective 50 μL of primary antibody solution overnight at 4 °C. After wash with PBST four times for 10 mins, the sections were incubated with respective fluorophore-conjugated secondary antibodies for one hour at RT. The sections were counterstained with DAPI-antifade mounting medium (Invitrogen Inc., Carlsbad, CA, USA). Imaging was performed at the University of Chicago Integrated Light Microscopy Facility. Images were captured with a Leica TCS SP8 laser scanning confocal microscope (Leica Microsystems, Inc., Buffalo Grove, IL, USA). Imaging processing and analysis was obtained using ImageJ (U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2012)113.
Western blotting
Immunoblotting protocol was as described previously20. Samples were lysed in 300 µL ice-cold 1x RIPA lysis buffer (50 mM Tris-Cl at pH=7.5, 150 mM NaCl, 1% NP-40 alternative (EMD Millipore Corporation), 0.5% (wt/vol) Sodium deoxycholate, 0.1% (wt/vol) SDS with protease inhibitors (Roche Diagnostics GmbH, Mannheim, Germany) and spun at 12,000 g for 10 minutes at 4 °C. Total protein concentration was measured via Pierce BCA Protein Assay (Thermo Scientific Inc.). Equal amounts of protein lysate were subjected to SDS-PAGE electrophoresis using Criterion XT 4–12% Bis-Tris precast gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and transferred to PVDF membranes using a semi-dry transfer system (Bio-Rad). The membranes were blocked with 5% nonfat milk (NFM) (Bio-Rad) in Tris-buffered saline (TBS, 0.02 mol/l Tris-HCl, 0.137 mol/l NaCl, pH=7.5) with 0.1% Tween-20 (TBST) for an hour on a shaker at RT. The membranes were cut into pieces depending on the molecular weights of the proteins of interest and then incubated with respective primary antibodies in 5% NFM in TBST overnight at 4 °C. The membranes were washed four times for 10 min with TBST and then incubated with secondary antibodies for one hour at RT. The chemiluminescent signal was developed using SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific) and captured using a Molecular Imager ChemiDoc XRS + imaging system (Bio-Rad). Densities were quantified using Fiji (NIH, Bethesda, MD, USA), normalized to that of GAPDH, and reported as a fold increase to SPF control.
Antibodies and reagents
Monoclonal Claudin-5 antibody (#35–2500) was purchased from Thermo Fisher Scientific (Rockford, IL, USA). Polyclonal GFAP antibody (#NB300–141) and CD11b/c antibody (#NB 110–70466) were purchased from Novus Biologicals, LLC (Centennial, CO, USA). Neuronal nuclei (NeuN #MAB377), synapsin (Syn1 #AB1543), oligodendrocyte transcription factor 2 (Olig2 #MABN50) and Neural/glial antigen 2 (NG2 #AB5320) antibodies were purchased from EMD Millipore (Billerica, MA, USA). Myelin basic protein (MBP #13344), neurofilament-Light chain (NFL #2837), and GAPDH (#5174) antibody were purchased from Cell Signaling Technology (Danvers, MA, USA). Secondary anti-mouse-HRP and anti-rabbit-HRP antibodies were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Secondary Alex Fluor goat anti-rabbit IgG-488 (H + L) (A11034) and Alex Fluor goat anti-mouse-594 (H + L) (A11032) were purchased from Invitrogen Inc. (Thermo Fisher Scientific). Recombinant murine IL-1β (Cat# 211-11B) was purchased from PeproTech Inc (Rocky Hill, NJ, USA).
Statistics
Data are presented as mean ± SEM. One-way ANOVA followed by Tukey’s post-hoc test for multiple comparison test among all experimental groups was performed when more than two groups were analyzed using GraphPad Prism version 6.07 for Windows, GraphPad Software, La Jolla, California, USA, www.graphpad.com. Unpaired t-test with Welch’s correction for unequal variances was used to detect the difference when only two groups were compared. P value less than 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
The authors would like to thank the Digestive Disease Research Core Center at The University of Chicago for its support on this study and Dr. Christine Labno at the Integrated Light Microscopy Core Facility at the University of Chicago for her technical support for imaging analysis. The current work is supported by NIH R01 HD083481 (E.C. Claud), NIDDK P30DK42086 (Digestive Disease Research Core Center at The University of Chicago), and the Duchossois Family Institute.
Author contributions
J.L. performed biochemical assays, Western blot assays, immunohistochemical staining, real time RT-PCR and data analysis. J.L., L.L. and Y.Y.Y. contributed to the animal experiments and sample collection. Y.Y.Y. and J.L. performed ELISA for serum cytokines. J.B. and J.L. performed Western blot assays. J.L. and E.C. designed the study and wrote the manuscript. All authors reviewed the manuscript.
Data availability
The data generated during the current study are available from the corresponding author on reasonable written request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-65180-0.
References
- 1.Boyle CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 2.Synnes A, Hicks M. Neurodevelopmental Outcomes of Preterm Children at School Age and Beyond. Clin. Perinatol. 2018;45:393–408. doi: 10.1016/j.clp.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Huang J, et al. Association between perinatal hypoxic-ischemia and periventricular leukomalacia in preterm infants: A systematic review and meta-analysis. PLoS One. 2017;12:e0184993. doi: 10.1371/journal.pone.0184993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirvonen M, et al. Cerebral palsy among children born moderately and late preterm. Pediatrics. 2014;134:e1584–1593. doi: 10.1542/peds.2014-0945. [DOI] [PubMed] [Google Scholar]
- 5.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 6.Kuczynski J, et al. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2011;13:47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sucksdorff M, et al. Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/ Hyperactivity Disorder. Pediatrics. 2015;136:e599–608. doi: 10.1542/peds.2015-1043. [DOI] [PubMed] [Google Scholar]
- 8.Aarts E, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One. 2017;12:e0183509. doi: 10.1371/journal.pone.0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front. Neurosci. 2017;11:490. doi: 10.3389/fnins.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams BL, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang DW, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wischmeyer PE, McDonald D, Knight R. Role of the microbiome, probiotics, and ‘dysbiosis therapy’ in critical illness. Curr. Opin. Crit. Care. 2016;22:347–353. doi: 10.1097/MCC.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claud EC, et al. Bacterial community structure and functional contributions to emergence of health or necrotizing enterocolitis in preterm infants. Microbiome. 2013;1:20. doi: 10.1186/2049-2618-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Underwood MA, Sohn K. The Microbiota of the Extremely Preterm Infant. Clin. Perinatol. 2017;44:407–427. doi: 10.1016/j.clp.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strunk T, et al. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect. Dis. 2014;14:751–762. doi: 10.1016/S1473-3099(14)70710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagberg H, et al. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin CR, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J. Pediatr. 2010;157:751–756 e751. doi: 10.1016/j.jpeds.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudo N, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, et al. Effects of Intestinal Microbiota on Brain Development in Humanized Gnotobiotic Mice. Sci. Rep. 2018;8:5443. doi: 10.1038/s41598-018-23692-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nino, D. F. et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci Transl Med10, 10.1126/scitranslmed.aan0237 (2018). [DOI] [PMC free article] [PubMed]
- 22.Lu L, et al. Transcriptional modulation of intestinal innate defense/inflammation genes by preterm infant microbiota in a humanized gnotobiotic mouse model. PLoS One. 2015;10:e0124504. doi: 10.1371/journal.pone.0124504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1beta, [corrected] TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8:e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front. Neuroendocrinol. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serrats J, et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holzer P, et al. Visceral Inflammation and Immune Activation Stress the Brain. Front. Immunol. 2017;8:1613. doi: 10.3389/fimmu.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugh CR, et al. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav. Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- 30.Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borre YE, et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol. Med. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Borre YE, Moloney RD, Clarke G, Dinan TG, Cryan JF. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Claud EC. Connection between gut microbiome and brain development in preterm infants. Dev. Psychobiol. 2019;61:739–751. doi: 10.1002/dev.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Underwood MA. Arguments for routine administration of probiotics for NEC prevention. Curr. Opin. Pediatr. 2019;31:188–194. doi: 10.1097/MOP.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet. 2007;369:1614–1620. doi: 10.1016/S0140-6736(07)60748-X. [DOI] [PubMed] [Google Scholar]
- 36.Martin CR, Walker WA. Probiotics: role in pathophysiology and prevention in necrotizing enterocolitis. Semin. Perinatol. 2008;32:127–137. doi: 10.1053/j.semperi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Hartel C, et al. Prophylactic use of Lactobacillus acidophilus/Bifidobacterium infantis probiotics and outcome in very low birth weight infants. J. Pediatr. 2014;165:285–289 e281. doi: 10.1016/j.jpeds.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 38.Hill C, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 39.Yu JC, et al. Innate Immunity of Neonates and Infants. Front. Immunol. 2018;9:1759. doi: 10.3389/fimmu.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dani C, et al. Lactobacillus Sepsis and Probiotic Therapy in Newborns: Two New Cases and Literature Review. AJP Rep. 2016;6:e25–29. doi: 10.1055/s-0035-1566312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esaiassen E, et al. Bifidobacterium longum Subspecies infantis Bacteremia in 3 Extremely Preterm Infants Receiving Probiotics. Emerg. Infect. Dis. 2016;22:1664–1666. doi: 10.3201/eid2209.160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brantsaeter AL, et al. Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011;174:807–815. doi: 10.1093/aje/kwr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolatkhah N, et al. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015;33:25. doi: 10.1186/s41043-015-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am. J. Obstet. Gynecol. 2010;203:120 e121–126. doi: 10.1016/j.ajog.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Korpela K, et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 2018;6:182. doi: 10.1186/s40168-018-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swartwout B, Luo XM. Implications of Probiotics on the Maternal-Neonatal Interface: Gut Microbiota, Immunomodulation, and Autoimmunity. Front. Immunol. 2018;9:2840. doi: 10.3389/fimmu.2018.02840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aceti A, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants: systematic review and meta-analysis. Ital. J. Pediatr. 2015;41:89. doi: 10.1186/s13052-015-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuccotti G, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 49.Kuitunen M, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 2009;123:335–341. doi: 10.1016/j.jaci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Groeger D, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325–339. doi: 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaghef-Mehrabany E, et al. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014;30:430–435. doi: 10.1016/j.nut.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Alipour B, et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014;17:519–527. doi: 10.1111/1756-185X.12333. [DOI] [PubMed] [Google Scholar]
- 53.Loguercio C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J. Clin. Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 54.Dhiman RK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–1337 e1323. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 55.D’Mello C, et al. Probiotics Improve Inflammation-Associated Sickness Behavior by Altering Communication between the Peripheral Immune System and the Brain. J. Neurosci. 2015;35:10821–10830. doi: 10.1523/JNEUROSCI.0575-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stolp HB, Dziegielewska KM, Ek CJ, Potter AM, Saunders NR. Long-term changes in blood-brain barrier permeability and white matter following prolonged systemic inflammation in early development in the rat. Eur. J. Neurosci. 2005;22:2805–2816. doi: 10.1111/j.1460-9568.2005.04483.x. [DOI] [PubMed] [Google Scholar]
- 58.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr. Pharm. Des. 2005;11:973–984. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 59.Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106-107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stolp HB, Dziegielewska KM. Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2009;35:132–146. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 61.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 62.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 63.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44-46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Sanford DC, DeWille JW. C/EBPdelta is a downstream mediator of IL-6 induced growth inhibition of prostate cancer cells. Prostate. 2005;63:143–154. doi: 10.1002/pros.20159. [DOI] [PubMed] [Google Scholar]
- 65.Liu YW, Tseng HP, Chen LC, Chen BK, Chang WC. Functional cooperation of simian virus 40 promoter factor 1 and CCAAT/enhancer-binding protein beta and delta in lipopolysaccharide-induced gene activation of IL-10 in mouse macrophages. J. Immunol. 2003;171:821–828. doi: 10.4049/jimmunol.171.2.821. [DOI] [PubMed] [Google Scholar]
- 66.Ko CY, et al. CCAAT/enhancer binding protein delta (CEBPD) elevating PTX3 expression inhibits macrophage-mediated phagocytosis of dying neuron cells. Neurobiol. Aging. 2012;33:422 e411–425. doi: 10.1016/j.neurobiolaging.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svotelis A, et al. IL-1 beta-dependent regulation of C/EBP delta transcriptional activity. Biochem. Biophys. Res. Commun. 2005;328:461–470. doi: 10.1016/j.bbrc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29:91–97. doi: 10.1002/(SICI)1098-1136(20000101)29:1<91::AID-GLIA9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 69.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. doi: 10.1096/fasebj.4.11.2199284. [DOI] [PubMed] [Google Scholar]
- 70.Kinoshita S, Akira S, Kishimoto T. A member of the C/EBP family, NF-IL6 beta, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc. Natl Acad. Sci. USA. 1992;89:1473–1476. doi: 10.1073/pnas.89.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 72.Brown K, Park S, Kanno T, Franzoso G, Siebenlist U. Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc. Natl Acad. Sci. USA. 1993;90:2532–2536. doi: 10.1073/pnas.90.6.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 74.Lu J, et al. Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PLoS One. 2018;13:e0201829. doi: 10.1371/journal.pone.0201829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braniste V, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hellgren G, et al. Increased postnatal concentrations of pro-inflammatory cytokines are associated with reduced IGF-I levels and retinopathy of prematurity. Growth Horm. IGF Res. 2018;39:19–24. doi: 10.1016/j.ghir.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benkoe T, et al. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PLoS One. 2013;8:e58720. doi: 10.1371/journal.pone.0058720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol. Psychiatry. 2008;13:480–497. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- 80.Saper CB. The dance of the perivascular and endothelial cells: mechanisms of brain response to immune signaling. Neuron. 2010;65:4–6. doi: 10.1016/j.neuron.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 81.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Frohlich EE, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 85.Piccio L, et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J. Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- 86.Kerfoot SM, et al. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology. 2006;43:154–162. doi: 10.1002/hep.21003. [DOI] [PubMed] [Google Scholar]
- 87.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 88.Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J. Neurosci. Res. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res. Mol. Brain Res. 1994;27:157–162. doi: 10.1016/0169-328X(94)90197-X. [DOI] [PubMed] [Google Scholar]
- 90.Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc. Natl Acad. Sci. USA. 2009;106:9069–9074. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qin L, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riazi K, et al. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl Acad. Sci. USA. 2008;105:17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Turrin NP, et al. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res. Bull. 2001;54:443–453. doi: 10.1016/S0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- 94.Van Dam AM, et al. Vagotomy does not inhibit high dose lipopolysaccharide-induced interleukin-1beta immunoreactivity in rat brain and pituitary gland. Neurosci. Lett. 2000;285:169–172. doi: 10.1016/S0304-3940(00)01031-4. [DOI] [PubMed] [Google Scholar]
- 95.Biesmans S, et al. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm. 2013;2013:271359. doi: 10.1155/2013/271359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zamanian JL, et al. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sebire G, et al. In vitro production of IL-6, IL-1 beta, and tumor necrosis factor-alpha by human embryonic microglial and neural cells. J. Immunol. 1993;150:1517–1523. [PubMed] [Google Scholar]
- 98.Buttini M, Boddeke H. Peripheral lipopolysaccharide stimulation induces interleukin-1 beta messenger RNA in rat brain microglial cells. Neuroscience. 1995;65:523–530. doi: 10.1016/0306-4522(94)00525-A. [DOI] [PubMed] [Google Scholar]
- 99.Fabry Z, et al. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J. Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 100.Wong ML, Bongiorno PB, Gold PW, Licinio J. Localization of interleukin-1 beta converting enzyme mRNA in rat brain vasculature: evidence that the genes encoding the interleukin-1 system are constitutively expressed in brain blood vessels. Pathophysiological implications. Neuroimmunomodulation. 1995;2:141–148. doi: 10.1159/000096884. [DOI] [PubMed] [Google Scholar]
- 101.Ko CY, et al. Glycogen synthase kinase-3beta-mediated CCAAT/enhancer-binding protein delta phosphorylation in astrocytes promotes migration and activation of microglia/macrophages. Neurobiol. Aging. 2014;35:24–34. doi: 10.1016/j.neurobiolaging.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 102.Matsumoto J, et al. Tumor necrosis factor-alpha-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation. Neurosci. Lett. 2014;578:133–138. doi: 10.1016/j.neulet.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 103.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsumoto J, et al. TNF-alpha-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IkappaB-NFkappaB and JAK-STAT3 pathways. Brain Res. 2018;1692:34–44. doi: 10.1016/j.brainres.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 105.Luo J, et al. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 2014;57:327–335. doi: 10.1007/s11427-014-4615-4. [DOI] [PubMed] [Google Scholar]
- 106.Li N, et al. Oral Probiotics Ameliorate the Behavioral Deficits Induced by Chronic Mild Stress in Mice via the Gut Microbiota-Inflammation Axis. Front. Behav. Neurosci. 2018;12:266. doi: 10.3389/fnbeh.2018.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tengku-Muhammad TS, Hughes TR, Ranki H, Cryer A, Ramji DP. Differential regulation of macrophage CCAAT-enhancer binding protein isoforms by lipopolysaccharide and cytokines. Cytokine. 2000;12:1430–1436. doi: 10.1006/cyto.2000.0711. [DOI] [PubMed] [Google Scholar]
- 108.Wang SM, et al. Increase of zinc finger protein 179 in response to CCAAT/enhancer binding protein delta conferring an antiapoptotic effect in astrocytes of Alzheimer’s disease. Mol. Neurobiol. 2015;51:370–382. doi: 10.1007/s12035-014-8714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li R, Strohmeyer R, Liang Z, Lue LF, Rogers J. CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer’s disease. Neurobiol. Aging. 2004;25:991–999. doi: 10.1016/j.neurobiolaging.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 110.Dantzer R, et al. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008;33:18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J. Neurosci. Methods. 2011;195:206–210. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaya M, Ahishali B. Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol. Biol. 2011;763:369–382. doi: 10.1007/978-1-61779-191-8_25. [DOI] [PubMed] [Google Scholar]
- 113.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during the current study are available from the corresponding author on reasonable written request.