Abstract
Warthin tumor is the second most common benign parotid neoplasm. Its association with non-salivary gland neoplasms has been sporadically reported. We reviewed clinical records of Warthin tumor diagnosed on aspiration cytology and surgical pathology to determine if there is any association with other extra-salivary gland malignant neoplasms. Computer search was made for all cases of Warthin tumor diagnosed in the parotid gland by aspiration cytology and surgical pathology at our institution between January 2007 and August 2016. Clinical records of all cases were reviewed for any associated malignant neoplasms and any surgical follow up. All available cytology and histologic material was reviewed. Seventy-three patients (mean 66.9, M:F 1.1:1, age range 43 to 87 years) with Warthin tumor were identified. 45 (62%) were diagnosed on aspiration cytology only, 19 (26%) had cytologic diagnosis as well as concordant surgical follow up, and 9 (12%) were diagnosed based on surgical pathology only. Average age for patients with and without secondary malignancy was 70.5-years, and 63.4-years, respectively (p < 0.05). Average pack years for patients with and without secondary malignancy was 45.4, and 39.8, respectively (p > 0.05). Twenty-seven (37.0%) patients harbored a malignant neoplasm. Association of extra salivary gland malignant neoplasms in 37.0% of our cases suggest that the prevalence of secondary non-salivary neoplasms in patients harboring Warthin tumor might have been underestimated. Squamous cell carcinoma was the most commonly associated non-salivary malignant neoplasm. The association of Warthin tumor with smoking plays an important role in this increased rate of malignancy, and this is supported by the fact that smoking is highly associated with head and neck and lung cancers.
Keywords: Warthin tumor, Incidence, Smoking, Salivary glands
Introduction
Warthin tumor (papillary cystadenoma lymphomatosum or cystadenolymphoma) is a benign neoplasm, and is the second most common benign neoplasm of the parotid gland after pleomorphic adenoma. It most commonly occurs during the fifth to seventh decades, and is quite uncommon before the third decade [1]. Most cases occur in the parotid gland, however rare cases have been reported in the lip, tonsil, maxillary sinus, cervical lymph nodes, larynx, palate, and submandibular gland [2–5]. Warthin tumor is commonly seen in smokers, and presentation as a bilateral/multifocal tumor is an additional characteristic feature [6]. A recent association with obesity has been reported; showing a significant higher body mass index (BMI) in patients with Warthin tumor compared to other benign parotid gland tumors [7].
The association of Warthin tumor with other salivary gland neoplasms has been previously reported, and include benign neoplasms such as pleomorphic adenoma, and malignant salivary glands lesions such as epithelial-myoepithelial carcinoma [8–11]. Extra-salivary gland neoplasms such as non-Hodgkin lymphoma, Hodgkin lymphoma, squamous cell carcinoma, urothelial cell carcinoma, clear cell renal carcinoma, invasive ductal carcinoma of the breast, and basal cell carcinoma have been reported in this patient population as well [8–11]. In the present study we investigate the association of Warthin tumor cases at our institution with other non-salivary gland malignant neoplasms.
Materials and Methods
Computer search was made for all cases of Warthin tumor diagnosed in the parotid gland by aspiration cytology and surgical pathology at our institution between January 2007 and August 2016. Clinical records of all cases were reviewed for any associated malignant neoplasms and any surgical follow up. All available cytology and histologic material was reviewed. We performed statistical analysis using student t test with Social Science Statistics website (http://www.socscistatistics.com). A p value of < 0.05 was considered a significant difference between compared sets of data. The study was approved for IRB exemption from our institutional review board.
Results
Of the 73 patients with Warthin tumor, 45 (62%) were diagnosed on aspiration cytology only, 19 (26%) had cytologic diagnosis as well as concordant surgical follow up, and 9 (12%) were diagnosed based on surgical pathology only. Based on cyto-histologic correlation, accuracy of aspiration cytology diagnosis of Warthin tumor was 100% (n = 19). Aspiration cytology in all cases revealed oncocytes and lymphocytes.
The age of the patients ranged from 43 to 87-years (mean 66.9, M:F 1.1:1). Average age for patients with and without secondary malignant neoplasm was 70.5-years, and 63.4-years, respectively (p < 0.05) (Table 1). Smoking data was available in 31 patients with 30 of 31 (96.8%) patients reporting smoking. Average pack years for patients with and without secondary malignancy was 45.4, and 39.8, respectively (p > 0.05). Fifty-four (74.0%) cases presented with palpable lesions, 10 (13.7%) were discovered on positron emission tomography (PET) scan (Standardized uptake value (SUV) > 4.2), 8 (11%) were detected by computed tomography (CT), and 1 (1.3%) by magnetic resonance imaging (MRI). 11 of the 19 (57.9%) cases of Warthin tumor diagnosed after malignancy were discovered on imaging, and 8 out of 19 (42.1%) cases were palpable. In contrast, all 4 cases of Warthin tumors diagnosed before malignancy were found by palpation. In four of 27 (14.8%) cases the information was not available for the date of the malignant diagnosis. Among the Warthin tumor patients without an associated malignancy only seven of 46 (15.2%) were picked up by imaging. Most of the Warthin tumor patients without an associated malignancy were found by palpation (84.8% (39/46)).
Table 1.
Summary of demographics and how Warthin tumor was discovered
| All patients | Patients with an associated malignancy | Patients without an associated malignancy | |
|---|---|---|---|
| Total patients | 73 | 27 (37%) | 46 (63%) |
| Female | 34 (46.6%) | 12 (35.3%) | 22 (643%) |
| Male | 39 (514%) | 15 (38.5%) | 24 (61.5%) |
| Age (years, mean/range) | 66.9 | 70.5 (55 to 86) | 63.4 (43 to 87) |
| Pack years (mean/range) | 42.6 | 45.4 (20 to 70.5) | 39.8 (1.8 to 180) |
| Warthin tumor discovered by imaging | 19 (26%) | 12 (612%) | 7 (36.8%) |
| Warthin tumor discovered by palpation | 54 (74%) | 15 (27.8%) | 39 (72.2%) |
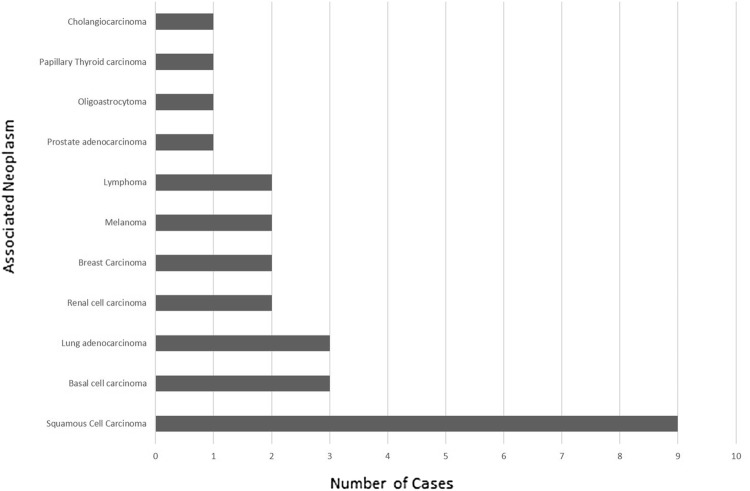
Twenty-seven (37.0%) patients harbored an associated malignant neoplasm [squamous cell carcinoma (9), basal cell carcinoma (3), lung adenocarcinoma (3), renal cell carcinoma (2), breast carcinoma (2), melanoma (2), lymphoma (2), prostate adenocarcinoma (1), oligoastrocytoma (1), papillary thyroid carcinoma (1), and cholangiocarcinoma (1)] (Fig. 1). Sites of involvement for squamous cell carcinoma, the most common malignant neoplasm detected, included lung (4), tongue (2), nostril (1), ear (1), and unknown primary (1). The unknown primary was diagnosed on a biopsy of a neck mass, and a primary was not subsequently discovered according to the records.
Fig. 1.
Non salivary gland malignant neoplasms seen in patients diagnosed as with Warthin tumor
70.4% (19/27) of the associated malignant neoplasms were diagnosed prior to the Warthin tumor diagnosis (average: 4.2 years; range: 6 days to 36 years). 14.8% (4/27) of cases (peripheral T cell lymphoma/leukemia, lung adenocarcinoma (2), breast carcinoma) were diagnosed after initial diagnosis of Warthin tumor (average: 5.4 years; range: 3 months to 10 years). For the 46 cases of Warthin tumor that were not associated with a malignancy, the average follow up after diagnosis was 4.9 years (range: 1.1 to 10.2 years).
Discussion
The association of extra-salivary gland malignant neoplasms in 37% of our cases suggest that the prevalence of secondary extra-salivary gland malignant neoplasms in patients harboring Warthin tumor is quite common. In our literature review, the association of Warthin tumor with extra-salivary gland malignant neoplasms has ranged from 1.1 to 27% in other studies, and has been reported with a wide range of different malignant neoplasms (Table 2) [8, 9, 11–51]. As previously reported in the literature, malignancies found in the current study include squamous cell carcinoma of the head and neck, lung carcinoma, basal cell carcinoma, renal cell carcinoma, breast carcinoma, melanoma, non-Hodgkin lymphoma, prostate adenocarcinoma, and thyroid carcinoma. In addition, we also found oligoastrocytoma and peripheral T-cell lymphoma; which are not reported in the literature. Additional reported neoplasms in the literature include urothelial cell carcinoma, Merkel cell carcinoma, diffuse large B cell lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, T-cell lymphoblastic lymphoma. Including data from the current study, 66 cases of squamous cell carcinoma (44 cases of squamous cell carcinoma of the head and neck), 43 cases of lung carcinoma (including 7 squamous cell carcinomas), 17 non-Hodgkin lymphomas, 11 basal cell carcinomas, and 6 thyroid carcinomas have been reported in the literature in patients with a diagnosis of Warthin tumor.
Table 2.
Literature review of the number of reported extra-salivary malignant neoplasms seen in patients with Warthin tumor [8, 9, 11–51]
| References | Percent (%) of associated extra-salivary malignant neoplasms | Number of associated extra-salivary malignant neoplasms | Cases of Warthin tumor | Head and neck SCC | Lung | Other | Unknown origin |
|---|---|---|---|---|---|---|---|
| Zaccarini et al. current study | 37.0 | 27 | 73 | SCC (nostril) (1), SCC (tongue) (2), SCC (ear) (1) | SCC (4), adenocarcinoma (3) | Basal cell carcinoma (3), renal cell carcinoma (2), Breast carcinoma (2), melanoma (2), Non-Hodgkin lymphoma (1), peripheral T-cell lymphoma/leukemia (1) prostate adenocarcinoma (1), oligoastrocytoma (1), thyroid carcinoma (1), cholangiocarcinoma (1) | SCC (1) |
| Naumaan et al. [10] | 27.0 | 17 | 63 | SCC (5) | Adenocarcinoma (4), small cell carcinoma (2) | Non-Hodgkin lymphoma (2), Hodgkin lymphoma (1), myxoinflammatory sarcoma (1), breast carcinoma (1), renal carcinoma (1) | |
| Cardoso et al. [51] | 25.0 | 19 | 76 | SCC (larynx) (4), SCC (oral cavity or oropharynx) (4) | Basal cell carcinoma (5), thyroid carcinoma (2), prostate adenocarcinoma (1) breast carcinoma (1), uterine cervical carcinoma (1) | ||
| Maiorano et al. [8] | 11.5 | 9 | 78 | SCC of larynx (2) | SCC (2) | Non-Hodgkin lymphoma (1), urothelial cell carcinoma (bladder) (1), clear cell renal cell carcinoma (1), invasive ductal carcinoma (breast) (1), basal cell carcinoma (1) | |
| Seifert et al. [9] | 2.9 | 8 | 275 | SCC (auricle) (1), SCC (lip) (1), SCC (larynx) (1) | SCC (1) | Non-Hodgkin lymphoma (1), basal cell carcinoma (2) | SCC (parotid) (1) |
| Xu et al. [11] | 1.1 | 11 | 994 | SCC (tongue) (5), SCC (buccal) (2), SCC (floor of mouth) (2), SCC (oropharynx) (1) | Non-Hodgkin lymphoma (1) | ||
| Other studies/case reports | SCC (floor of mouth) (1), SCC (larynx) (4), SCC (hypopbarynx) (2), SCC (ear) (1), SCC (tongue) (1), SCC (oral cavity) (1), SCC (retromolar trigone) (1), SCC (frontal and ethmoid sinus) (1) | Non-small cell lung carcinoma (20), Small cell carcinoma (7) | Non-Hodgkin lymphoma (11), Hodgkin lymphoma (4), thyroid carcinoma (3), breast carcinoma (2), merkel cell carcinoma (2), T-cell lymphoblastic lymphoma (1), melanoma (1) | SCC (parotid) (13) |
Squamous cell carcinoma was the most commonly associated secondary malignant neoplasm in patients with Warthin tumor; comprising 33.3% (9/27) of all associated malignancies in our study. Similar to our findings, squamous cell carcinoma has been reported as the most common associated secondary malignant neoplasm in patients with Warthin tumor in other studies. Maiorano et al. in their study of 78 cases demonstrated that 44.4% (4/9) of non-salivary gland malignancies were squamous cell carcinoma [8]. Recently Naumaan et al. revealed that 27% (17/63) of patients with Warthin tumor had secondary extra-salivary malignant neoplasms, and squamous cell carcinoma comprised 29.4% (5/17) of all malignancies [10]. Seifert et al. demonstrated 2.9% (8/275) with non-salivary malignant neoplasms, and 62.5% (5/8) of all malignancies were squamous cell carcinoma. Xu et al. showed 1.1% (11/994) with associated extra-salivary malignant neoplasms, and with 90.9% (10/11) of all malignancies diagnosed as squamous cell carcinomas [11]. Cardoso et al. demonstrated a rate of 25% (19/76) extra-salivary malignancies, and 42.1% (8/19) of all the malignancies were squamous cell carcinomas [51]. In the aforementioned studies, squamous cell carcinoma accounted for 45.1% (41/91) of the extra-salivary gland malignancies.
The close association of smoking with Warthin tumor likely explains the risk of secondary non-salivary gland malignancy in patients with Warthin tumor. This association is understandable considering its increased association with head and neck, lung, kidney, bladder, and many other cancers [52]. The risk of cancer in smokers is quite high, showing a 31.7% lifetime risk of lung cancer, and an odds ratio of 2.13 for head and neck squamous cell carcinomas [53, 54]. This is comparable to our rate of malignancy (37%) in this study, and supports that smoking is a clear risk factor for this patient population. 96.8% of patients with Warthin tumor in this study smoked, and this elevated rate of smoking has been reported before with 88% of men and 89% of women smoking in one study [3].
In our study imaging played a role in detection of 11 of 19 cases of Warthin tumor diagnosed after a malignant diagnosis. As opposed to this, all four cases of Warthin tumor diagnosed before a malignancy diagnosis were discovered by palpation. Imaging is commonly used in the workup for metastatic disease in patients with cancer. Caution is warranted in interpretation of PET scans commonly used to assess for metastatic disease. The prevalence of parotid gland incidentalomas (PGIs) is very high, and is often seen in smokers [55]. PGIs can be detected when screening for metastatic disease, and the decision to evaluate these lesions remains controversial [56]. Warthin tumor is known to cause false-positive 18F-fluorodeoxyglucose (FDG) PET/CT uptake [57]. In patients with asymmetric increased SUV, benign neoplasms such as Warthin tumor may be clinically mistaken as evidence for metastatic disease [57, 58]. In our series 6/9 (66.7%) squamous cell carcinoma were discovered by imaging, including five from increased PET/CT uptake. Imaging discernably played a role in the discovery of Warthin tumor in patients that already harbored a malignancy in this study.
There are two main theories regarding the development of Warthin tumor; with the chief concept supporting that it develops from salivary ductal inclusions within peri or intraparotid lymph nodes [59, 60]. The second theory is that it is a reactive growth arising within the parotid, while the lymphoid component is a response to this aforementioned growth [60]. In embryogenesis the parotid gland becomes encapsulated comparatively later, and this allows for salivary gland remnants to become entrapped in lymph nodes [59]. One study, supporting the first theory, demonstrated that salivary ductal inclusions were more frequent in parotid lymph nodes received from patients with Warthin tumor compared to pleomorphic adenoma [60].
Additionally, there is debate whether Warthin tumor is truly neoplastic or a reactive phenomenon. The epithelial component of Warthin tumor has been shown to be polyclonal, however one study displayed deletions of mitochondrial DNA in these tumors [61, 62]. Translocations involving 6p have been identified in a few cases, and the lymphoid component is usually polyclonal [63, 64]. Although the translocation t(11;19)(CRTC;MAML2) has been reported in a small number of Warthin tumors, it is possible these cases may have represented misdiagnosed mucoepidermoid carcinoma [65]. Nonetheless, a molecular underpinning for Warthin tumor has not been consistently elucidated. Surgical removal is usually curative with negative margins, however recurrence may rarely occur [6].
Although Warthin tumor has been traditionally known to have a male predominance; more recent studies show the ratio decreasing due to increased incidence in women, and possibly increased smoking in this population [3, 66]. The ratio of males to females in our study also does not show such a skewed ratio (1.1:1.0). The follow up period for patients diagnosed with a Warthin tumor, and subsequent malignancy diagnosis was 5.4 years (n = 4). For those without a subsequent malignancy diagnosis, our follow up time averaged 4.9 years (n = 46) after the Warthin tumor diagnosis. The length of follow up in this study may be one limitation, and a study looking at a longer follow up may be helpful to see if additional neoplasms are subsequently discovered. Most previous studies have not addressed follow up time [8–11]. However, a study looking at patients with lung cancer and Warthin tumor showed that three patients were diagnosed with lung carcinoma between 1 and 6 years after the diagnosis of Warthin tumor [47].
In conclusion, we report increased association of extra-salivary gland malignant neoplasms in patients with Warthin tumor. Presence of salivary gland swelling in patients with a malignant neoplasm may raise a false alarm in clinical and radiologic follow up of these cases. Fine needle aspiration cytology provides a simple tool to diagnose Warthin tumor and helps allay concerns regarding metastasis to the parotid gland in patients with malignant neoplasms, and associated parotid gland swelling.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel J. Zaccarini, Email: zaccarid@upstate.edu
Kamal K. Khurana, Email: khuranak@upstate.edu
References
- 1.Wenig BM. Atlas of head and neck pathology. Amsterdam: Elsevier Health Sciences; 2015. [Google Scholar]
- 2.Nisa L, Landis BN, Salmina C, Ailianou A, Karamitopoulou E, Giger R. Warthin’s tumor of the larynx: a very rare case and systematic review of the literature. J Otolaryngol Head Neck Surg. 2015;44(1):16. doi: 10.1186/s40463-015-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo GH, Eisele DW, Driben JS, Johns ME, Askin FB. Warthin’s tumor: a 40-year experience at the Johns Hopkins hospital. Laryngoscope. 1994;104(7):799–803. doi: 10.1288/00005537-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Struthers MA, William HL, Parkhill EM. Papillary cystadenoma of the maxillary paranasal sinus (atypical Warthin tumor) AMA Archiv Otolaryngol. 1954;59(2):241–244. doi: 10.1001/archotol.1954.00710050253016. [DOI] [PubMed] [Google Scholar]
- 5.Fahmy S. Adenolymphoma of the tonsillar fossa. J Laryngol Otol. 1973;87(7):675–679. doi: 10.1017/s002221510007746x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LD, Bishop JA. Head and neck pathology E-book: a volume in the series: foundations in diagnostic pathology. Amsterdam: Elsevier Health Sciences; 2017. [Google Scholar]
- 7.Kadletz L, Grasl S, Perisanidis C, Grasl MC, Erovic BM. Rising incidences of Warthin’s tumors may be linked to obesity: a single-institutional experience. Eur Archiv Oto-Rhino-Laryngol 2019;276:1–6. [DOI] [PubMed]
- 8.Maiorano E, Muzio LL, Favia G, Piattelli A. Warthin’s tumour: a study of 78 cases with emphasis on bilaterality, multifocality and association with other malignancies. Oral Oncol. 2002;38(1):35–40. doi: 10.1016/s1368-8375(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 9.Seifert G, Bull H, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Virchows Archiv A. 1980;388(1):13–38. doi: 10.1007/BF00430674. [DOI] [PubMed] [Google Scholar]
- 10.Naumaan A, Ghai R, Gattuso P. Warthin’s tumor of the parotid gland: a simple lesion with complex clinical implications; a clinical pathologic review. Lab Investig. 2017;97:330A. [Google Scholar]
- 11.Xu W, Lu H, Zhu Y, Ruan M, Zhang C, Yang W, et al. Warthin’s tumour in oral and maxillofacial regions: an 18-year retrospective study of 1084 cases in an eastern-Chinese population. Int J Oral Maxillofac Surg. 2018;47(7):913–917. doi: 10.1016/j.ijom.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Badve S, Evans G, Mady S, Coppen M, Sloane J. A case of Warthin’s tumour with coexistent Hodgkin’s disease. Histopathology. 1993;22(3):280–281. doi: 10.1111/j.1365-2559.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 13.Bunker ML, Locker J. Warthin’s tumor with malignant lymphoma DNA analysis of paraffin-embedded tissue. Am J Clin Pathol. 1989;91(3):341–344. doi: 10.1093/ajcp/91.3.341. [DOI] [PubMed] [Google Scholar]
- 14.Park C, Manning JT, Jr, Battifora H, Medeiros LJ. Follicle center lymphoma and Warthin tumor involving the same anatomic site: report of two cases and review of the literature. Am J Clin Pathol. 2000;113(1):113–119. doi: 10.1309/MJH0-RQGX-U128-VFC6. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros LJ, Rizzi R, Lardelli P, Jaffe ES. Malignant lymphoma involving a Warthin’s tumor: a case with immunophenotypic and gene rearrangement analysis. Hum Pathol. 1990;21(9):974–977. doi: 10.1016/0046-8177(90)90182-5. [DOI] [PubMed] [Google Scholar]
- 16.Banik S, Howell J, Wright D. Non-Hodgkin’s lymphoma arising in adenolymphoma—a report of two cases. J. Pathol. 1985;146(3):167–177. doi: 10.1002/path.1711460303. [DOI] [PubMed] [Google Scholar]
- 17.Damjanov I, Sneff E, Delerme A. Squamous cell carcinoma arising in Warthin’s tumor of the parotid gland: a light, electron microscopic, and immunohistochemical study. Oral Surg Oral Med Oral Pathol. 1983;55(3):286–290. doi: 10.1016/0030-4220(83)90329-8. [DOI] [PubMed] [Google Scholar]
- 18.Gunduz M, Yamanaka N, Hotomi M, Kuki K, Yokoyama M, Nakamine H. Squamous cell carcinoma arising in a Warthin’s tumor. Auris Nasus Larynx. 1999;26(3):355–360. doi: 10.1016/s0385-8146(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 19.Skálová A, Michal M, Nathanský Z. Epidermoid carcinoma arising in Warthin’s tumour: a case study. J Oral Pathol Med. 1994;23(7):330–333. doi: 10.1111/j.1600-0714.1994.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 20.Dreyer T, Battmann A, Silberzahn J, Glanz H, Schulz A. Unusual differentiation of a combination tumor of the parotid gland: a case report. Pathol Res Pract. 1993;189(5):577–581. doi: 10.1016/S0344-0338(11)80369-9. [DOI] [PubMed] [Google Scholar]
- 21.Gorai S, Numata T, Kawada S, Nakano M, Tamaru J, Kobayashi T. Malignant lymphoma arising from heterotopic Warthin’s tumor in the neck: case report and review of the literature. Tohoku J Exp Med. 2007;212(2):199–205. doi: 10.1620/tjem.212.199. [DOI] [PubMed] [Google Scholar]
- 22.Hall G, Tesluk H, Baron S. Lymphoma arising in an adenolymphoma. Hum Pathol. 1985;16(4):424–427. doi: 10.1016/s0046-8177(85)80238-0. [DOI] [PubMed] [Google Scholar]
- 23.Saxena A, Memauri B, Hasegawa W. Initial diagnosis of small lymphocytic lymphoma in parotidectomy for Warthin tumour, a rare collision tumour. J Clin Pathol. 2005;58(3):331–333. doi: 10.1136/jcp.2004.019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giaslakiotis K, Androulaki A, Panagoulias G, Kyrtsonis M, Lazaris AC, Kanakis DN, et al. T cell lymphoblastic lymphoma in parotidectomy for Warthin’s tumor: case report and review of the literature. Int J Hematol. 2009;89(3):359–364. doi: 10.1007/s12185-009-0271-z. [DOI] [PubMed] [Google Scholar]
- 25.Arcega RS, Feinstein AJ, Bhuta S, Blackwell KE, Rao NP, Pullarkat ST. An unusual initial presentation of mantle cell lymphoma arising from the lymphoid stroma of warthin tumor. Diagn Pathol. 2015;10(1):209. doi: 10.1186/s13000-015-0444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agaimy A, Wild V, Märkl B, Wachter DL, Hartmann A, Rosenwald A, et al. Intraparotid classical and nodular lymphocyte-predominant hodgkin lymphoma. Am J Surg Pathol. 2015;39(9):1206–1212. doi: 10.1097/PAS.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 27.Fornelli A, Eusebi V, Pasquinelli G, Quattrone P, Rosai J. Merkel cell carcinoma of the parotid gland associated with Warthin tumour: report of two cases. Histopathology. 2001;39(4):342–346. doi: 10.1046/j.1365-2559.2001.01240.x. [DOI] [PubMed] [Google Scholar]
- 28.Di Napoli A, Mallel G, Bartolazzi A, Cavalieri E, Becelli R, Cippitelli C, et al. Nodular lymphocyte-predominant Hodgkin lymphoma in a Warthin tumor of the parotid gland: a case report and literature review. Int J Surg Pathol. 2015;23(5):419–423. doi: 10.1177/1066896915582263. [DOI] [PubMed] [Google Scholar]
- 29.Iizuka T, Kohgo T, Amemiya A, Notani K, Totsuka Y, Fukuda H, et al. A case of adenolymphoma of parotid gland associated with squamous cell carcinoma of the floor of the mouth. Jpn J Oral Maxillofac Surg. 1986;32(8):1498–1504. [Google Scholar]
- 30.Allevi F, Biglioli F. Squamous carcinoma arising in a parotid Warthin’s tumour. BMJ Case Rep. 2014 doi: 10.1136/bcr2014-207870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cozzolino I, Zeppa P, Cuccuru A, Picardi M, Vetrani A, Palombini L. Collision Hodgkin lymphoma and Warthin tumour. Report of a case and review of the literature. Oral Surg. 2009;2(4):188–192. [Google Scholar]
- 32.Meikle D, Yarington CT. Synchronous parotid tumors of different histological types in association with metastasizing hypopharyngeal carcinoma. J Laryngol Otol. 1985;99(12):1261–1267. doi: 10.1017/s0022215100098509. [DOI] [PubMed] [Google Scholar]
- 33.Iannaccone PR. Multiple primary tumors. Four distinct head and neck tumors. Arch Pathol. 1975;99(5):270–272. [PubMed] [Google Scholar]
- 34.Volmer J. Multiple unilateral tumors of the salivary parotid gland. Zentralbl Allg Pathol. 1982;126(3–4):327–334. [PubMed] [Google Scholar]
- 35.Hall G, Tesluk H, Baron S. Lymphoma arising in an adenolymphoma. Hum Pathol. 1985;16(4):424–427. doi: 10.1016/s0046-8177(85)80238-0. [DOI] [PubMed] [Google Scholar]
- 36.Lederman M. Adenolymphoma of the parotid salivary gland. Br J Radiol. 1943;16(192):383–385. [Google Scholar]
- 37.de la Pava S, Knutson GH, Mukhtar F, Pickren JW. Squamous cell carcinoma arising in Warthin’s tumor of the parotid gland. First case report. Cancer. 1965;18(6):790–794. doi: 10.1002/1097-0142(196506)18:6<790::aid-cncr2820180617>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Little JW, Rickles NH. Malignant papillary cystadenoma lymphomatosum. Report of a case, with a review of the literature. Cancer. 1965;18(7):851–856. doi: 10.1002/1097-0142(196507)18:7<851::aid-cncr2820180712>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Baker M, Yuzon D, Baker BH. Squamous cell carcinoma arising in benign adenolymphoma (Warthin’s tumor) of the parotid gland. J Surg Oncol. 1980;15(1):7–10. doi: 10.1002/jso.2930150103. [DOI] [PubMed] [Google Scholar]
- 40.Mcclatchey KD, Appelblantt NH, Langin JL. Carcinoma in papillary cystadenoma lymphomatosum (Warthin’s tumor) Laryngoscope. 1982;92(1):98–99. doi: 10.1288/00005537-198201000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Bolat F, Kayaselcuk F, Erkan AN, Cagici CA, Bal N, Tuncer I. Epidermoid carcinoma arising in Warthin’s tumor. Pathol Oncol Res. 2004;10(4):240–242. doi: 10.1007/BF03033769. [DOI] [PubMed] [Google Scholar]
- 42.Sharma M, Saxena S, Agrawal U. Squamous cell carcinoma arising in unilateral Warthin’s tumor of parotid gland. J Oral Maxillof Pathol. 2008;12(2):82. [Google Scholar]
- 43.Sheahan P, Hafidh M, Toner M, Timon C. Unexpected findings in neck dissection for squamous cell carcinoma: incidence and implications. Head Neck. 2005;27(1):28–35. doi: 10.1002/hed.20110. [DOI] [PubMed] [Google Scholar]
- 44.Lesser RW, Spector JG. Facial nerve palsy associated with Warthin’s tumor. Archiv Otolaryngol. 1985;111(8):548–549. doi: 10.1001/archotol.1985.00800100096016. [DOI] [PubMed] [Google Scholar]
- 45.Miller R, Yanagihara ET, Dubrow AA, Lukes RJ. Malignant lymphoma in a warthin’s tumor report of a case. Cancer. 1982;50(12):2948–2950. doi: 10.1002/1097-0142(19821215)50:12<2948::aid-cncr2820501240>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.Melato M, Falconieri G, Fanin R, Baccarani M. Hodgkin’s disease occurring in a Warthin’s tumor: first case report. Pathol Res Pract. 1986;181(5):615–618. doi: 10.1016/S0344-0338(86)80158-3. [DOI] [PubMed] [Google Scholar]
- 47.White CK, Williams KA, Rodriguez-Figueroa J, Langer CJ. Warthin’s tumors and their relationship to lung cancer. Cancer Invest. 2015;33(1):1–5. doi: 10.3109/07357907.2014.979365. [DOI] [PubMed] [Google Scholar]
- 48.Zorlu E, Atasoy P, Günal N, Dural K, Özpolat B. Current thoracic surgery. 2018.
- 49.Haberal MA, Akar E, Dikis OS. Metastatic lung cancer associated with Warthin’s tumour. Niger J Clin Pract. 2019;22(4):585–587. doi: 10.4103/njcp.njcp_424_18. [DOI] [PubMed] [Google Scholar]
- 50.Snyderman C, Johnson JT, Barnes EL. Extraparotid Warthin’s tumor. Otolaryngol Head Neck Surg. 1986;94(2):169–175. doi: 10.1177/019459988609400207. [DOI] [PubMed] [Google Scholar]
- 51.Cardoso SV, do Nascimento Souza KC, de Faria PR, Lima RA, Nascimento MF, Eisenberg AL. Warthin’s tumor at the Brazilian National Cancer Institute: additional evidence of homogeneous sex prevalence and association with other neoplasms. ORL J Otorhinolaryngol Relat Spec. 2008;70(6):339–343. doi: 10.1159/000163028. [DOI] [PubMed] [Google Scholar]
- 52.The American Cancer Society medical and editorial content team. Health Risks of Smoking Tobacco. https://www.cancer.org/cancer/cancer-causes/tobacco-and-cancer/health-risks-of-smoking-tobacco.html. Accessed 20 May 2019.
- 53.Samet JM, Wiggins CL, Humble CG, Pathak DR. Cigarette Smoking and Lung Cancer in New Mexico1-3. Am Rev Respir Dis. 1988;137:1110–1113. doi: 10.1164/ajrccm/137.5.1110. [DOI] [PubMed] [Google Scholar]
- 54.Jethwa AR, Khariwala SS. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017;36(3):411–423. doi: 10.1007/s10555-017-9689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bothe C, Fernandez A, Garcia J, Lopez M, León X, Quer M, et al. Parotid incidentaloma identified by positron emission/computed tomography: when to consider diagnoses other than warthin tumor. Int Archiv Otorhinolaryngol. 2015;19(02):112–115. doi: 10.1055/s-0034-1397334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas R, Sharma N, Burke C, Maxwell D, Howlett DC. Parotid incidentaloma detected during thoracic PET imaging: how should these lesions be managed? Br J Hosp Med (2005). 2010;71(5):292–293. doi: 10.12968/hmed.2010.71.5.47915. [DOI] [PubMed] [Google Scholar]
- 57.Klijanienko J, Petras S, De Bosschere L, Paulmier B, Le Tourneau C, Rodriguez J. False-positive FDG PET/CT uptake in Warthin tumor in head and neck oncological patients confirmed by a fine needle aspiration. Diagn Cytopathol. 2012;40(3):282–284. doi: 10.1002/dc.21640. [DOI] [PubMed] [Google Scholar]
- 58.Basu S, Houseni M, Alavi A. Significance of incidental fluorodeoxyglucose uptake in the parotid glands and its impact on patient management. Nucl Med Commun. 2008;29(4):367–373. doi: 10.1097/MNM.0b013e3282f8147a. [DOI] [PubMed] [Google Scholar]
- 59.Teymoortash A, Werner J. Tissue that has lost its track: Warthin’s tumour. Virchows Arch. 2005;446(6):585–588. doi: 10.1007/s00428-005-1276-5. [DOI] [PubMed] [Google Scholar]
- 60.Cope W, Naugler C, Taylor S, Trites J, Hart R, Bullock M. The association of warthin tumor with salivary ductal inclusions in intra and periparotid lymph nodes. Head Neck Pathol. 2014;8(1):73–76. doi: 10.1007/s12105-013-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honda K, Kashima K, Daa T, Yokoyama S, Nakayama I. Clonal analysis of the epithelial component of Warthin’s tumor. Hum Pathol. 2000;31(11):1377–1380. [PubMed] [Google Scholar]
- 62.Lewis PD, Baxter P, Paul Griffiths A, Parry JM, Skibinski DO. Detection of damage to the mitochondrial genome in the oncocytic cells of Warthin’s tumour. J. Pathol. 2000;191(3):274–281. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH634>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 63.Martins C, Fonseca I, Roque L, Soares J. Cytogenetic characterisation of Warthin’s tumour. Oral Oncol. 1997;33(5):344–347. doi: 10.1016/s1368-8375(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 64.Takezawa K, Jackson C, Gnepp DR, King TC. Molecular characterization of Warthin tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 1998;85(5):569–575. doi: 10.1016/s1079-2104(98)90293-1. [DOI] [PubMed] [Google Scholar]
- 65.Fehr A, Röser K, Belge G, Löning T, Bullerdiek J. A closer look at Warthin tumors and the t (11; 19) Cancer Genet Cytogenet. 2008;180(2):135–139. doi: 10.1016/j.cancergencyto.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Franzen AM, Franzen CK, Guenzel T, Lieder A. Increased incidence of Warthin tumours of the parotid gland: a 42-year evaluation. Eur Arch Otorhinolaryngol. 2018;275(10):2593–2598. doi: 10.1007/s00405-018-5092-3. [DOI] [PubMed] [Google Scholar]