Abstract
Depth of invasion (DOI) and tumour thickness (TT) are known prognostic indicators in oral squamous cell carcinoma (OSCC), but varying definitions have been used by pathologists for reporting. The American Joint Committee on Cancer (AJCC) has proposed adoption of a uniform definition of DOI and incorporated this measurement in the revised TNM staging (8th edition); however, unambiguous DOI determination can be a challenge in clinical practice. We reviewed archived slides of 95 cases of T1/T2N0 OSCC and listed the challenges in accurate DOI measurement with pictographical documentation. The impacts of DOI and TT on disease-free survival (DFS) were also assessed. The mean DOI and TT was 5.89 mm and 7.32 mm respectively. Challenge in horizon estimation for DOI measurement was experienced in 75/95 cases (78.9%). The most common challenges were lack of adjacent uninvolved mucosa in sections or presence only on one side, rounded/convoluted nature of the tumour surface for tongue and polypoidal tumours, and angulation of adjacent mucosa for alveolar or lip tumours. In cases with very thin epithelium, DOI was equal to TT. In spite of the challenges, Kaplan–Meier analysis showed DOI > 5 mm significantly predicted poorer DFS while TT did not. We recommend various guidelines to help improve consistency in measuring DOI and recording of TT in ambiguous cases for accurate staging of OSCC.
Keywords: Depth of invasion, Tumour thickness, Oral squamous cell carcinoma
Introduction
The American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) tumour staging for oral cavity squamous cell carcinoma (OSCC) has been unchanged since 1977. A major revision of the staging system was presented in 2017 with the release of the AJCC 8th edition staging manual [1]. One important change was the incorporation of depth of invasion (DOI) as a parameter for tumour staging of OSCC [1, 2].
DOI, as defined in the AJCC 8th edition and incorporated into the current College of American Pathologists (CAP) protocol, is measured from the horizon of the basement membrane of the adjacent squamous mucosa to the deepest point of the tumour in a perpendicular direction by dropping a plumbline. It is recorded in millimeters. The guidelines recommend use of transparent rulers printed on acetate paper to lay on the glass slide for measurement [2, 3].
Previously, DOI and tumour thickness (TT) for oral cancers were defined differently between various authors or used interchangeably in routine reporting and studies. This resulted in discrepant reports between centers, as lack of adherence to a uniform definition can significantly affect accurate staging. Pentenero et al. did a comprehensive review of all the different definitions used in literature for TT and DOI [4]. One of the earliest definitions was suggested in 1986 by Moore et al. who defined TT from the level of the surface of adjacent uninvolved epithelium to deepest point of tumour [5]. Woolgar et al. proposed a similar measurement but termed it DOI instead of TT. They recommended measuring depth from the adjacent non-ulcerated mucosal surface. This increased DOI to TT in ulcerated tumours and decreased DOI to TT in polypoidal tumours. The surface of the epithelium was the reference point rather than the basement membrane, unlike the current AJCC/CAP definition [6].
The CAP protocol used DOI and TT interchangeably in earlier editions. It defined TT as measured from the mucosal surface of the tumour to the deepest point of tissue invasion in a perpendicular direction, with exclusion of superficial parakeratotic layer in heavily keratinized tumours [3, 7]. To address possible discrepancies between centers, the AJCC 8th edition proposed the current consensus definition for DOI and recommended its universal adoption for staging of oral cavity tumours [2].
Based on the earlier (2013 and prior) editions of the CAP protocol, we measured TT for reporting resection specimens of OSCC until January 2018. After this time, we adopted the AJCC 8th edition definition of DOI and have since encountered many challenges in measuring this parameter. The difficulties lie in unambiguously drawing the horizon from the adjacent, uninvolved basement membrane.
The current retrospective review is aimed at reassessing archived cases of early stage OSCC (T1/T2N0) for DOI and pictographically documenting the challenges faced in measurement for uniform reporting and increased interobserver concordance. We also aimed to compare the difference between DOI and TT for each case, re-stage tumours based on DOI per the AJCC 8th edition, and assess the prognostic implications. As the likelihood of stage migration based on DOI is lower for high stage, larger tumours (i.e. T3/T4 tumours with more than 4 cm size or bone/masticator space muscle invasion), we concentrated this review on early stage cases to understand the true impact of DOI.
Materials and Methods
We reviewed archived hematoxylin and eosin (H&E) stained slides of pathologically proven early stage OSCC operated on at our center between May 2011 and November 2015. These included pT1N0 and pT2N0 (per AJCC 7th edition) cases of tongue and oral cavity (alveolus, buccal mucosa, lip) squamous cell carcinomas without evidence of metastasis. Recurrent cases or those with prior neoadjuvant treatment were rejected. All cases had elective nodal dissection, including the early stage cancers [8]. In all cases, the tumour was either submitted entirely or minimum of 4 sections from tumour was taken. All sections of tumour were reviewed by three pathologists (PR, PK and DP) to calculate the greatest DOI and TT for each case.
The CAP definitions of TT (mucosal surface of the tumour to the deepest point of invasion, measured perpendicularly) and DOI (from the level of the basement membrane of the closest adjacent normal mucosa to the deepest point of invasion) were adopted for measurement [1]. The line of horizon was drawn from the level of the adjacent closest basement membrane using a marker pen. DOI and TT were measured in millimeters by using a transparent scale on the slide and viewing under the 2.5× scanner objective. For cases with depth greater than 10 mm (> 1 low power field), corroboration was achieved by putting dots on the slide and then measuring with a scale. All sections of tumour were measured to ensure the section with the greatest DOI and TT was identified. With variations between these measurements, the two parameters were recorded on different slides (whichever had the greatest depth or thickness). In case of presence of worst pattern of invasion 5 (WPOI-5), the deeper focus was included in the depth calculation unless it was a single obvious focus of lymphovascular emboli or perineural invasion significantly distant from the main tumour. The challenges of recording an accurate DOI were listed and pictographically documented. Other histological parameters including tumour site, tumour size, and stage (per 7th and 8th AJCC editions) were also recorded.
Clinical follow-up to include local or distant failures were recorded from electronic medical records. The data was entered in REDcap data management software and de-identified for statistical analysis using SPSS version 20. Descriptive statistics were used to tabulate the various histologic parameters and Kaplan–Meier analysis was performed to evaluate disease free survival (DFS) of the various parameters. The interclass correlation coefficient (ICC) was evaluated to assess inter-rater concordance.
Results
The total number of cases studied was 95 and included 34 females and 61 males. The mean age was 55 years (range 25–81 years). Various clinicopathologic tumour characteristics are listed in Table 1. The most commonly affected site in our cohort was the tongue which comprised 68.4% cases (65/95).
Table 1.
Clinicopathological features
| Parameters | Category | No n = 95 | Percentage | p value |
|---|---|---|---|---|
| Tumour stage (AJCC 7) | T1N0 | 40 | 42 | |
| T2N0 | 55 | 57.9 | 0.822 | |
| Tumour site | Buccal mucosa | 20 | 21 | |
| Tongue | 65 | 68.4 | ||
| Lip | 3 | 3.15 | ||
| Alveolus | 5 | 5.2 | ||
| Retromolar trigone | 2 | 2.1 | ||
| Laterality | Right | 47 | 49.47 | |
| Left | 48 | 50.5 | ||
| Focality | Unifocal | 94 | 98.9 | |
| Multifocal | 1 | 1.1 | ||
| Margin status | Free (> 5 mm away) | 91 | 95.8 | |
| Close | 4 | 4.2 | ||
| Involved | 0 | 0 | ||
| Tumour type | Verrucous | 7 | 7.9 | |
| Hybrid | 3 | 3.4 | ||
| Early invasive | 2 | 2.2 | ||
| Not otherwise specified | 70 | 73.6 | ||
| Polypoidal | 13 | 14.6 | ||
| Grade | Well | 15 | 15.8 | |
| Moderate | 69 | 72.6 | ||
| Poor | 11 | 11.6 | 0.110 | |
| Lymphovascular emboli | Absent | 93 | 97.9 | |
| Present | 2 | 2.1 | 0.704 | |
| Perineural invasion | Absent | 63 | 66.3 | |
| Present | 32 | 33.7 | 0.124 | |
| Depth of invasion | ≤ 5 mm | 56 | 58.9 | |
| 6–10 mm | 25 | 26.3 | 0.019 | |
| > 10 mm | 14 | 14.7 | ||
| Tumour thickness | ≤ 5 mm | 41 | 43.15 | |
| 6–10 mm | 34 | 35.7 | 0.157 | |
| > 10 mm | 20 | 21.05 | ||
| Lymphocytic host response (LHR) | Type 1 and 2 (dense and moderate response) | 62 | 65.3 | |
| Type 3 (little or no response) | 33 | 34.7 | 0.019 | |
| Worst pattern of invasion (WPOI) | WPOI 1–3 (non-aggressive) | 47 | 49.5 | |
| WPOI 4–5 (aggressive) | 48 | 50.5 | 0.035 |
Tumour sizes ranged from 0.6 to 4.0 cm and DOI ranged from 0 to 20 mm (mean 5.65 mm, median 5 mm). TT ranged from 1 to 22 mm (mean 7.22 mm, median 6 mm).
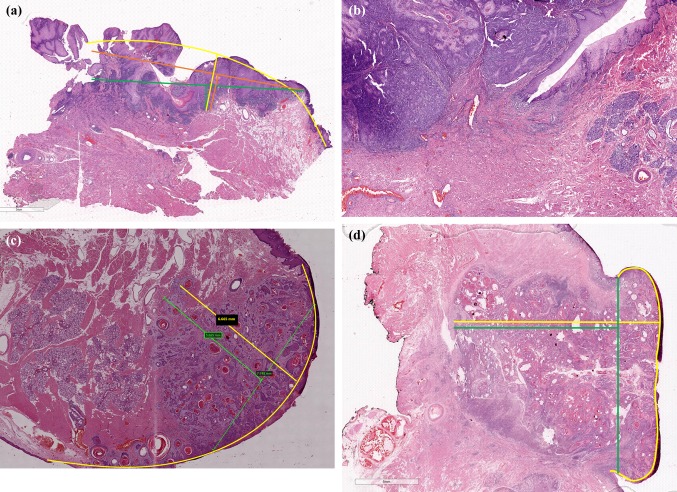
All the challenges encountered in horizon estimation (Fig. 1) and DOI calculation are listed in Table 2 and pictographically documented (Figs. 2, 3). Unambiguous measurement of DOI was possible in 20/95 cases (22.5%). The most common cause of difficulty, where DOI calculation was impossible, was complete lack of adjacent epithelium in the section (18/95; 20.2% cases). In 32/95 cases (44.4%), accurate horizon calculation was difficult due to presence of adjacent mucosa on one side only (Fig. 2a). In these cases, TT was recorded as the DOI for staging. In two cases, adjacent mucosa was present in some sections with tumour but absent in the section with the greatest thickness, limiting accurate assessment of depth of invasion.
Fig. 1.
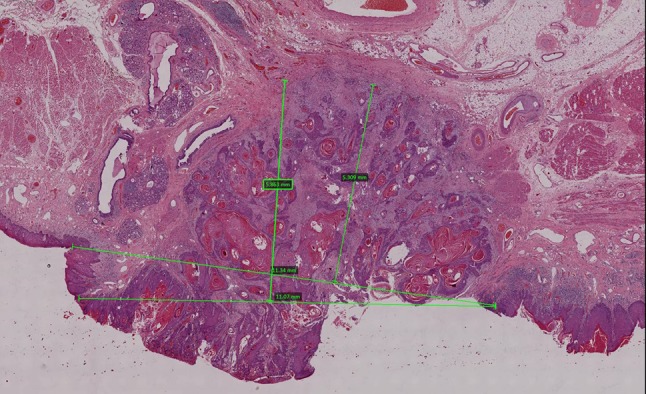
Difficulty in horizon estimation due to convolutions of adjacent mucosa which is not in a straight plane. The different lines show the various possibilities for drawing the horizon
Table 2.
Reasons for difficulty in depth of invasion measurement
| Reason | No. (percentage) | |
|---|---|---|
| 1 | Adjacent mucosa not present | 18/95 (20.2%) |
| 2 | Adjacent mucosa present in other sections, but absent in the section with the greatest thickness limiting accurate assessment of depth of invasion | 2/95 (2.8%) |
| 3 | Mucosa present on one side limiting assessment of horizon | 32/95 (44.4%) |
| 4 | Angulated adjacent mucosa (lip/retromolar trigone/alveolar tumour) or mucosa on both sides not in a straight line | 13/95 (13.9%) |
| 5 | Polypoidal tumour with uniform thickness | 15/95 (20.8%) |
| 6 | Irregular hyperplasia in adjacent mucosa with thick rete pegs leading to discrepancy in assessment of level of adjacent mucosa | 1/95 (1.4%) |
| 7 | Cut-off point of adjacent epithelium not clear (submucosal tumour) | 9/95 (12.5%) |
| 8 | Adjacent benign mucosa far away and cutoff point not clear due to zone of dysplasia | 15/95 (20.8%) |
| 10 | Adjacent mucosa is visible in a perpendicular direction to the surface of the tumour (in cases of lateral border of tongue tumours) | 26/95 (36.1%) |
| 11 | Adjacent mucosa very thin (DOI same as TT) | 31/95 (32.6%) |
| 12 | Rounded/arcuate horizon (based on the rounded natural contour of the tongue) | 26/95 (29.2%) |
Fig. 2.
a Difficulty in accurate horizon estimation due to adjacent uninvolved mucosa present on one side only. The arcuate yellow line represents the most likely natural contour and the most appropriate horizon. b Angulated adjacent mucosa limiting horizon estimation. c Arcuate line of horizon replicates the natural rounded contour of the lateral border of tongue rather than drawing a straight line from the edge of the adjacent uninvolved mucosa. d In a polypoidal non-verrucous/non-papillary tumour, the mucosa is heaped up by the tumour and thickness (calculated from yellow line) is a better measure of tumour bulk than depth of invasion
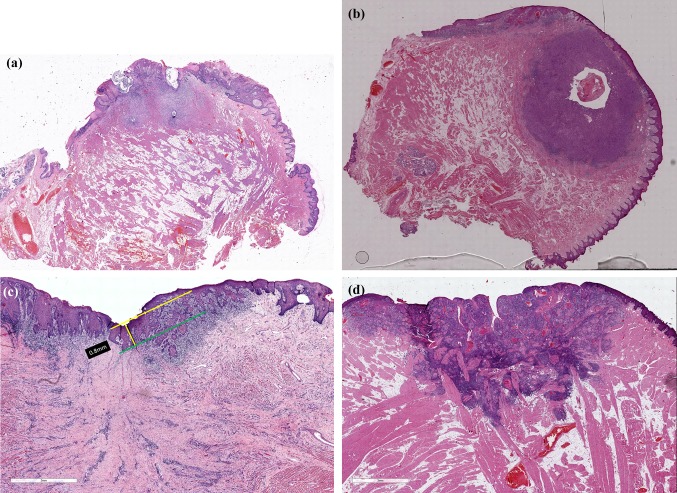
Fig. 3.
a Difficulty in horizon estimation due to extended zone of dysplasia adjacent to the tumour. The estimation of cut-off point of basement membrane of adjacent uninvolved mucosa is impaired. b Horizon estimation is not possible in small tumours post-biopsy which appear purely submucosal even after study of multiple deeper sections. c In cases with irregular hyperplasia of adjacent epithelium, the current AJCC guidelines do not specify whether measurement should be from the level of the tip of the submucosal papilla (yellow line) or the deepest point of the rete peg. This difference can sometimes vary by 1 mm or more. d When adjacent mucosa is very thin, depth of invasion measurements equal the tumour thickness
In 26/95 cases (29.2%), the reconstructed natural contour of the basement membrane was a rounded/arcuate horizon due to the rounded natural contour of the tongue, and this reference was used for DOI calculation rather than the straight line from the adjacent normal basement membrane recommended by AJCC (Fig. 2c). Reconstruction of the horizon of tumour and assessment of the hypothetical line for measurement of DOI was also difficult in cases with an angulated or convoluted tumour contour as in sections from lip, alveolus, or retromolar trigone. In these cases, TT was recorded as DOI (Fig. 2b). In 31 cases (32.6%), the adjacent mucosa was very thin (< 1 mm) (Fig. 3d), and the TT measurement was essentially the same as DOI. Some cases had multiple issues making DOI calculation difficult (Table 2).
Overall, in 52/95 cases (54.7%), DOI was measured as equal to TT due to tumour related difficulty in horizon estimation, thin adjacent mucosa, or rounded contour of the tongue.
DOI differed significantly from TT in polypoidal, papillary, and verrucous tumours. The difference varied by ≥ 5 mm in 11 cases (11.5%).
The ICC for TT and DOI measurement by three observers was 0.858 and 0.897 respectively (average measure), both of which indicate excellent inter-rater agreement/correlation [9].
The prognostic significance of the DOI and TT based on cut-offs of up to 5 and 10 mm are tabulated in Table 1. DOI > 5 mm significantly correlated with poorer prognosis (p = 0.019) (Fig. 4), while thickness > 5 mm did not show clinical significance in this cohort (p = 0.157).
Fig. 4.
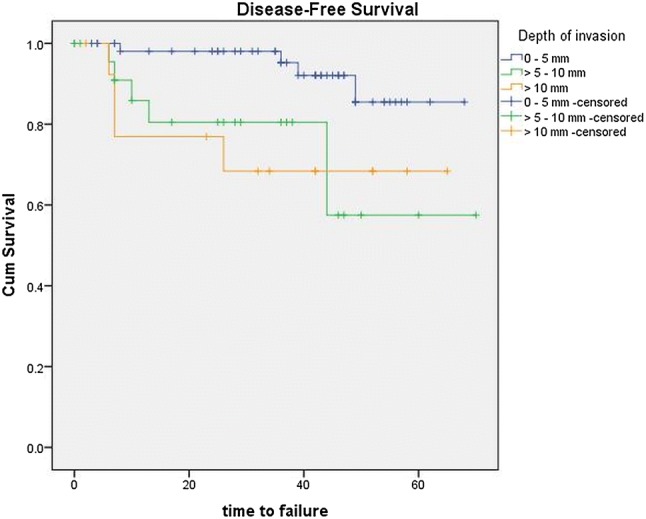
Kaplan–Meier curve showing disease-free survival stratified by depth of invasion
On restaging based on the AJCC TNM 8th edition, 25/95 (26.3%) cases had a T stage revision. Eleven (11.57%) T1 cases were upstaged to T2, one case was upstaged to T3, and 13 (13.6%) T2 cases changed to T3 based on DOI. If TT was used instead of DOI, 34/95 (35.7%) cases would have had stage revision (Fourteen T1 to T2 (14.7%), one T1 to T3 (1%) and nineteen T2 to T3 (20%)). Comparing outcomes by AJCC 7th edition pT stage (T1 versus T2; log rank, p = 0.822) and the AJCC 8th edition pT stage, (between T1, T2 and T3; log rank, p = 0.197) did not show a significant difference.
Discussion
Stage Revision Based on DOI and its Prognostic Significance
The recognition of DOI as a vital prognostic factor and its incorporation into the 8th edition AJCC TNM staging protocol necessitates accurate and unambiguous calculation of this parameter.
Several authors have shown the prognostic significance of both TT and DOI but used varying definitions [10–12]. Some have used the definition of DOI but labeled the measurement as TT and vice versa [13, 14]. Other studies have shown prognostic significance without clearly defining the criteria used for measurement as elaborated in the reviews by Pantenero et al. [4, 12, 15].
Ebrahimi et al. conducted a large multicenter retrospective analysis of 3149 patients with OSCC, treated surgically and reported in 11 different centers, to assess the impact of DOI on DFS and overall survival (OS) [16]. The different centers and pathologists, however, used differing definitions for TT and DOI. The authors accepted this heterogeneity in their cohort and aimed to assess whether there was inter-center heterogeneity in the clinical impact of DOI. They found DOI provided complementary information to tumour size based T staging and showed it to be an independent predictor of disease specific survival (p < 0.001). They demonstrated no inter-institutional prognostic heterogeneity by using a 2-staged random effects meta-analysis, proposed that DOI based staging was easy to apply worldwide, and recommended an AJCC 7th edition stage revision. They suggested cut off of 5 mm and 10 mm (as has been adopted by the AJCC 8th edition). Though there was no difference in clinical significance of DOI versus TT in their analysis, they highlighted the importance of adopting a worldwide consensus definition for consistent DOI measurement.
Kane et al. is the only group prior to us who compared the prognostic significance of DOI (p = 0.026) versus TT (p = 0.046) and shown the prior to be more significant [15]. In our series of early stage cases, DOI showed clinical significance with DFS while TT did not. Even after excluding the 14 cases of verrucous/polypoidal/papillary tumours with exophytic growth pattern, we saw no prognostic significance of TT (p = 0.129) while DOI was clearly prognostically significant in this cohort of non-verrucous tumours. The DOI in the verrucous and papillary carcinoma cases ranged from 0 to 2 mm while TT varied up to 22 mm. These tumours usually grow in a superficial and lateral manner rather than deeply, and staging mainly depends on the tumour size rather than the depth.
Dirven et al. also compared DOI with TT in their retrospective review of 456 patients and showed no difference in measurement in 57.7% [17]. The difference was less than 1 mm in 21.2% [17]. Similarly, 53% of cases in a study by Liu et al. had identical TT and DOI or a difference less than 1 mm [18]. Our review shows the difference between the mean DOI and TT to be 1.4 mm and median DOI and TT to be 1 mm. Similar to these previous studies, the measurement for both DOI and TT was identical in 52/95 (54.7%) cases in our series. Of these, in 20.2% of our cases, DOI could not be calculated at all due to complete absence of adjacent epithelium in the sections and TT had to be recorded.
Additionally, Dirven et al. showed a stage revision in 20.7% (T1–2), 6.7% (T1–3) and 39.9% (T2–3) cases when using DOI based on the AJCC 8th edition staging versus the prior AJCC 7th edition staging. An additional 3% changed from T1–2 and 2% changed from T2–3 when considering the TT measurement instead of DOI. In our study, the rates for stage change were 11.6% T1–2, 1.0% T1–3 and 13.6% T2–3 respectively when employing the DOI. Using the TT measurement, the changes were 14.7% T1–2, 1.0% T1–3 and 20.0% T2–3.
Difficulties in Horizon Calculation for DOI Assessment
We know that the intraoral mucosal surface is an irregular, convoluted lining, folding over the alveolus, retromolar trigone, and angle of mouth. The definition of DOI is difficult to apply in many cases due to complexities of the mucosal anatomy and tumour morphology. Few examples are provided in the AJCC 8th edition to explain the calculation of the ‘horizon’ from the level of adjacent basement membrane. The assumption is that the adjacent epithelium lies in a straight line (as demonstrated in the cartoon provided) [2, 4]. This is not always realistic and does not guide in many real-life, practical diagnostic situations and challenges (Figs. 1, 2a).
In this retrospective review, we have captured the difficulties in measurement to form a pictographic reference guide for accurate DOI calculation. We restricted our review to early stage cases with tumour size less than 4 cm for greater impact of DOI in accurate tumour staging.
We found the biggest challenge in assessing the level of horizon. This was because the adjacent basement membrane was completely absent in the sections with tumour or present only in sections with significantly lower depth/thickness rather than the section where the TT was maximum. In some cases, the uninvolved epithelium was only present on one side of the tumour section which led to an estimation of the angle of the horizon. This increased intra- and interobserver variability in measurement (Fig. 2a). Accurate assessment of the horizon was also difficult in tumours where the natural contour of the epithelium was not uniform or straight (lateral border of tongue, angle of mouth, lip, alveolus, retromolar trigone, or tumours extending over to the floor of mouth or gingivobuccal/gingivolabial sulci) (Fig. 2b, c). In all such cases, the TT was recorded as the DOI for stage assessment.
In OSCCs affecting the lateral border of the tongue, the majority of cases in our review, the natural contour of the uninvolved epithelium is convex. A straight line drawing from the junction of tumour with adjacent normal epithelium (Fig. 2c) is unlikely to represent the true bulk of tumour invading the underlying stroma. The reconstructed virtual line of original basement membrane should be convex, such as the natural contour, in these cases. Measurement of DOI from the tip of such a convex line is more likely to represent the true tumour bulk rather than DOI as defined by AJCC. This method has been adopted in our review. Berdugo et al. also recommended a similar approach of using an arcuate line for horizon estimation in tongue tumours [19].
A similar concept was used for polypoidal non-verrucous/non-papillary tumours where DOI calculation from the level of basement membrane of adjacent epithelium would be zero (Fig. 2d). Rather than being true exophytic tumours, these are cases where the polypoidal appearance is due to pushing up of the native tissue with the tumour actually invading into the stroma. So the TT measurement would capture the true potential of invasiveness of the tumour.
In cases with high grade dysplasia in the adjacent epithelium, extending for a large area beyond the confines of the invasive tumour, evaluation of the level of the adjacent normal basement membrane was not possible. In these situations, estimation of thickness (from the highest point of the surface to the deepest point of invasion in a perpendicular direction) is more practical and time efficient than drawing an imaginary horizon from the level of adjacent normal epithelium which is not visible (Fig. 3a). Horizon estimation and depth calculation is also not possible for submucosal tumours, and thickness can be measured instead (Fig. 3b).
The guidelines for measuring depth do not address the question of where to measure from in cases with thick irregular hyperplasia of the adjacent epithelium. The level of horizon calculation could be from the tips of the submucosal papillae or the deepest point of the epithelial rete ridges (Fig. 3c). In this study, we used the tip of the highest submucosal papilla for our reference for horizon estimation.
Methodology for DOI Measurement
Thickness equaled DOI in cases with very thin adjacent epithelium where measures of less than 1 mm could not be made without an ocular micrometer (Fig. 3d). Moreover, measuring in less than whole millimeter cut-offs is meaningless as rounding of the measurements to the closest millimeter is required for staging per the AJCC guidelines. The AJCC also clarifies that in case of ambiguity in recording depth, the lower millimeter value should be recorded for stage estimation [1, 3]. In cases where the depth was just beyond a particular millimeter measurement or almost reaching the next millimeter, we rounded to the closest lower or higher number accordingly.
We measured using a transparent scale on the glass slide and viewing through the 2.5× scanner objective. When we compared this method with putting dots with a marker and calculating with a scale outside the microscope, there was a discrepancy of 1 mm in some cases. However, if the depth was greater than 10 mm, the doting method was the easiest, quickest, and most accurate method of ensuring non-tangential (perpendicular) measurement. This method for recording DOI and TT also showed excellent inter-observer concordance with ICC values of > 0.8.
Using an ocular micrometer is recommended by AJCC and a good option, but availability of an ocular micrometer is not common for widespread adoption in reporting head and neck specimens. This is especially true in low-resource countries where OSCC is quite prevalent. Moreover, using an ocular micrometer can be tedious due to the conversion of micrometers to millimeters. In cases with > 4 mm depth (which is more than the length covered by a micrometer, usually 4 mm on 2.5×) the stage has to be moved multiple times in the correct perpendicular direction to capture the measurement of the entire depth with the ocular micrometer.
Recommendations for DOI Calculation
In conclusion, our study confirms the prognostic significance of DOI over TT and reestablishes that every attempt should be made to record this parameter accurately for uniform reporting, increased inter-center concordance, and appropriate tumour staging. We also suggest the following recommendations for unambiguous measurement of DOI in routine reporting of OSCC:
Tumours should be grossed in a gridded fashion so that level of adjacent mucosa can be assessed for horizon calculation.
In cases of mucosa on only one side, the plane of the surface of the tumour should be used as direction for horizon calculation.
Reconstructing the natural contour of the basement membrane at the region of tumour from the adjacent epithelium should be attempted for horizon assessment. In cases of lateral border of the tongue cancers or non-verrucous/non-papillary polypoidal tumours of the oral cavity, this is often a convex surface.
If accurate horizon calculation is not possible due to angulation of mucosal surface (lip, angle of mouth, alveolar tumours), it is appropriate and more consistent to record the tumour thickness from the surface of the tumour than trying to recapitulate an arbitrary adjacent basement membrane level.
Recording DOI by placing a transparent ruler on the slide and recording the measurement through the eye piece of the microscope, after marking the horizon and perpendicular plumbline with a marker, is the most accurate and time efficient method.
DOI should be rounded to the closest millimeter measurement, which can be the lower millimeter in ambiguous cases.
If a separate island of WPOI-5 is identified separate from the main bulk of tumour but deeper into the stroma, DOI should be recorded to that distance.
Acknowledgement
Mr. Arun Das and Ms. Rimi Bhowmik for technical assistance.
Funding
This study was funded by the departmental funds at Tata Medical Center.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study involves a retrospective review of archived paraffin blocks and an institutional review board waiver was available for this study.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
This was a technical review of reporting practices. Anonymized data was used for the study. Clinical trial was not involved hence informed consent was not taken.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 2.Ridge JA, Lydiatt WM, Patel SG. AJCC cancer staging manual. 8. New York: Springer; 2017. [Google Scholar]
- 3.Protocol for the examination of specimens from patients with carcinomas of the lip and oral cavity college of American pathologists. 2017. http://www.cap.org/. Accessed 1 Feb 2018.
- 4.Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005;27:1080–1091. doi: 10.1002/hed.20275. [DOI] [PubMed] [Google Scholar]
- 5.Moore C, Kuhns JG, Greenberg RA. Thickness as prognostic aid in upper aerodigestive tract cancer. Arch Surg. 1986;121:1410–1414. doi: 10.1001/archsurg.1986.01400120060009. [DOI] [PubMed] [Google Scholar]
- 6.Woolgar JA. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006;42:229–239. doi: 10.1016/j.oraloncology.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Protocol for the examination of specimens from patients with carcinomas of the lip and oral cavity college of American pathologists. 2013. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/liporalcaversion-13protocol.pdf. Accessed 28 July 2018.
- 8.D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373:521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 9.Cicchetti DV. Multiple comparison methods: establishing guidelines for their valid application in neuropsychological research. J Clin Exp Neuropsychol. 1994;16:155–161. doi: 10.1080/01688639408402625. [DOI] [PubMed] [Google Scholar]
- 10.Clark JR, Naranjo N, Franklin JH, et al. Established prognostic variables in N0 oral carcinoma. Otolaryngol Head Neck Surg. 2006;135:748–753. doi: 10.1016/j.otohns.2006.05.751. [DOI] [PubMed] [Google Scholar]
- 11.Ganly I, Goldstein D, Carlson DL, et al. Long-term regional control and survival in patients with “low-risk”, early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119:1168–1176. doi: 10.1002/cncr.27872. [DOI] [PubMed] [Google Scholar]
- 12.Huang SH, Hwang D, Lockwood G, et al. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. Cancer. 2009;115:1489–1497. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 13.Almangush A, Bello IO, Keski-Santti H, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36:811–818. doi: 10.1002/hed.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hori Y, Kubota A, Yokose T, et al. Predictive significance of tumor depth and budding for late lymph node metastases in patients with clinical N0 early oral tongue carcinoma. Head Neck Pathol. 2017;11:477–486. doi: 10.1007/s12105-017-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kane SV, Gupta M, Kakade AC, et al. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006;32:795–803. doi: 10.1016/j.ejso.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 16.International Consortium for Outcome Research in H. Neck C, Ebrahimi A, et al. Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140:1138–1148. doi: 10.1001/jamaoto.2014.1548. [DOI] [PubMed] [Google Scholar]
- 17.Dirven R, Ebrahimi A, Moeckelmann N, et al. Tumor thickness versus depth of invasion—analysis of the 8th edition American Joint Committee on cancer staging for oral cancer. Oral Oncol. 2017;74:30–33. doi: 10.1016/j.oraloncology.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Amaratunga R, Veness M, et al. Tumor depth of invasion vs tumor thickness in determining risk of nodal disease in early oral tongue squamous cell carcinoma. Red J. 2018;102:e331–e332. [Google Scholar]
- 19.Berdugo J, Thompson LDR, Purgina B, et al. Measuring depth of invasion in early squamous cell carcinoma of the oral tongue: positive deep margin, extratumoral perineural invasion, and other challenges. Head Neck Pathol. 2018;13(2):154–161. doi: 10.1007/s12105-018-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]