Abstract
Solitary fibrous tumors (SFT) arising in the head and neck region are uncommon yet well-recognized entities. Their biologic behavior and management still need to be elucidated. Systematically reviewing all published cases of SFT involving the head and neck region since 1991, a pooled meta-analysis was conducted to evaluate various demographic and tumor characteristics. 587 SFT in the head and neck have been reported; 343 met pooled analysis inclusion criteria. 61% of cases presented as a new mass; 89% were painless. Median onset of symptoms prior to evaluation was 8 months. Pre-operative local invasion and malignant histological features (hemorrhage, necrosis, mitoses > 4/10 hpf) were not statistically associated with decreased recurrence-free survival. Positive surgical margins was the only factor associated with shorter recurrence-free survival (p < 0.001). The evidence presented herein reveals novel associations between clinical presentation and tumor characteristics that provide otolaryngologists with new insight into SFT tumor behavior, thus prompting further investigations.
Electronic supplementary material
The online version of this article (10.1007/s12105-019-01058-6) contains supplementary material, which is available to authorized users.
Keywords: Solitary fibrous tumor, SFT, Head, Neck, Soft tissue tumor
Introduction
Solitary fibrous tumors (SFT) are rare spindle-cell neoplasms of mesenchymal origin, historically believed to arise from the pleura. As the contemporary knowledge of soft tissue tumors evolved, SFT have been identified in many extrathoracic sites. In 1991, Witkin and Rosai reported the first recognized case within the head and neck region [1]. Currently, it is estimated that 6–18% of all SFT arise within the head and neck region, representing approximately one quarter of all extrathoracic SFT [2–5].
Although SFT are well acknowledged in the discipline of head and neck surgery, the information regarding their behavior and management are still restricted to discussions and reports of institutional experiences and the complexity of establishing the diagnosis. Moreover, conclusions derived from intrathoracic SFT data have been applied to their extrathoracic counterparts, and propagated throughout the literature without proper validation. As a result of these suppositions and variations in management related to individual institutional practice, the clinical nuances and biologic behavior of these neoplasms within the head and neck remain poorly understood.
In an effort to better understand the actual and quantifiable behavior of these tumors, we systematically compiled and reviewed all reported cases of SFT in the head and neck region. Through the retrospective analysis detailed herein, epidemiological, histopathological, and malignant features of these tumors were more clearly defined and elucidated. Concurrently, we also illustrate a new case of a solitary fibrous tumor in the anterior neck to demonstrate the application and practicality of the conclusions drawn in this article. In the most comprehensive analysis to date, the following report is aimed to assist the practicing otolaryngology-head and neck surgeon, when faced with these challenging entities.
Case Presentation
A 79-year old female presented to our practice for the sole complaint of a right-sided neck mass that slowly progressed in size over the past 18 months. The patient denied associated fevers, pain, difficulty with mastication, dysphagia, or dysphonia. Her medical history was significant for non-Hodgkin lymphoma in the right neck, which was diagnosed 6 years’ prior and treated with a course of Rituximab.
Office-based physical examination revealed a 7-cm firm, mobile, non-fluctuant right-sided neck mass. There was no evidence of mucosal abnormality, mass or obvious tumor involving the upper aerodigestive tract. Computed tomography (CT) imaging of the neck showed a solid, lobulated soft tissue mass located immediately inferior to the anterior belly of the right digastric muscle (Fig. 1). Subsequent fine needle aspiration (FNA) yielded equivocal findings demonstrating atypical cells admixed within muscle and adipose tissue components. As a result of this non-diagnostic biopsy, a decision was made to proceed with mass excision for diagnostic and potentially curative purposes. Pre-operative positron emission tomography-computed tomography (PET-CT) was obtained for the presumed cervical malignancy, and only demonstrated minimal uptake within the mass (Fig. 2). The patient underwent an uneventful excision with grossly negative surgical margins and no post-operative complications. Frozen section of the mass was not taken.
Fig. 1.
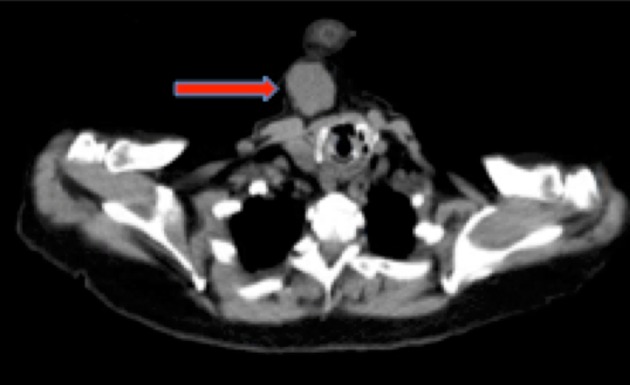
Axial computed tomography of the neck without contrast demonstrating a solid, lobulated soft tissue mass in the right neck immediately inferior to the anterior belly of the right digastric muscle, as indicated by the red arrow
Fig. 2.
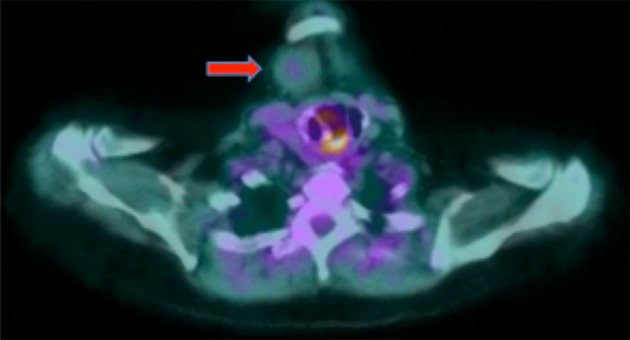
Axial positron emission tomography–computed tomography of the neck demonstrating minimal central FDG-avidity within the soft tissue lesion, as indicated by the red arrow
Histologic examination of the excised specimen demonstrated a well circumscribed mass composed of spindle cells with fine-to-vesicular chromatin and inconspicuous nucleoli, and arranged without a discernable pattern (Fig. 3). Prominent vessels with hyalinization and collagenous stroma were present. Mitotic figures numbered < 1 per 10 HPFs, and there was no necrosis, increased cellularity, or severe nuclear pleomorphism (Fig. 4). Immunohistochemical staining showed the tumor cells were positive for CD34 and CD99. Immunostains for CAM5.2, CK5/6, AE1/3, p63, EMA, CD23, CD21, CD35, SMA, desmin, and S100 were all negative. Ancillary immunohistochemistry on stored tissue specimens demonstrated diffuse, nuclear STAT6 expression. Based on the histopathologic findings, a final diagnosis of a solitary fibrous tumor was confirmed.
Fig. 3.
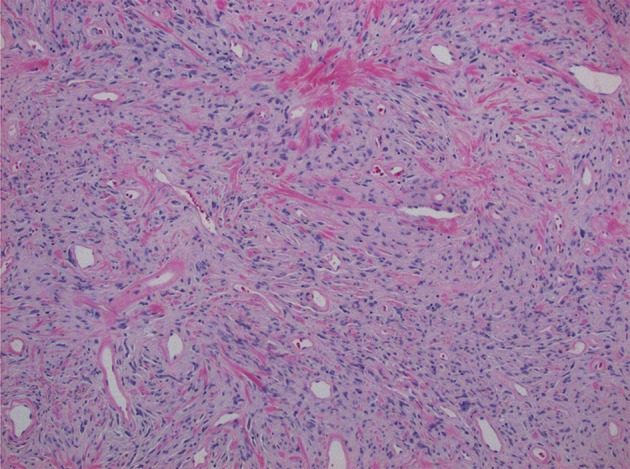
× 100 magnification photomicrograph stained with hematoxylin and eosin demonstrating histomorphological features of the tumor, composed of spindled cells without a discernible architecture, set within a collagenous stroma. Characteristic rounded vessels with hyalinized walls are prominent
Fig. 4.
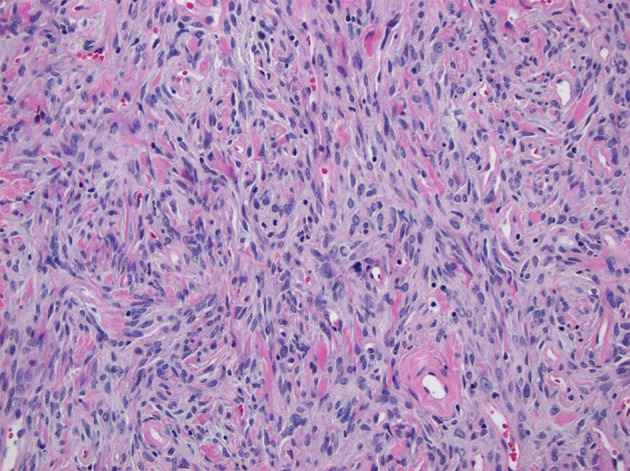
× 200 magnification photomicrograph stained with hematoxylin and eosin demonstrating tumor cells with vesicular chromatin and inconspicuous nucleoli without histological evidence of malignancy
The patient has continued to follow our practice in the post-operative period, with no evidence of recurrence at 18 months.
Methods
A systematic review and pooled meta-analysis was conducted of the published literature on Solitary Fibrous Tumors involving the head and neck region. Identification of studies was carried out through a literature search of the PubMed, Google Scholar, and ISI Web of Science databases by two independent researchers (LS, KL). Appropriately modified for each database, combinations of the following search terms were used to retrieve potentially eligible studies: “solitary fibrous tumor,” “buccal,” “extrathoracic,” “extrapleural,” “ear,” “external auditory canal,” “gingiva,” “head,” “hypopharynx,” “infratemporal fossa,” “larynx,” “lip,” “mouth,” “nasal,” “nasopharynx,” “neck,” “oral,” “oral cavity,” “oropharynx,” “paranasal,” “parotid,” “pharynx,” “pterygopalatine fossa,” “salivary gland” “scalp,” “sinonasal,” “thyroid,” and “upper aerodigestive tract.” An example of the search query used in the PubMed Database can be found in Supplementary Material, Fig. 1. The search included case reports and case series, but was not restricted to study type in order to ensure a thorough evaluation of the literature. All non-English and non-peer reviewed evidence were excluded, and only full-text articles were considered. The bibliographies of the potentially eligible studies were then hand-searched for additional studies. The systematic review process followed the guidelines set forth by the PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [6].
The inclusion criterion for our analysis was unambiguous evidence of a solitary fibrous tumor in the form of a histopathologic and immunohistochemical diagnostic confirmation. This review only included cases after 1991, as this was the year the first SFT was described in the head and neck. We included individual cases within the head and neck from case series describing extrapleural tumors from all anatomic regions, provided there was sufficient clinical data describing the individual cases. Consideration was taken by the principal investigators for inclusion or exclusion of the cases with incomplete data. Likewise, differences in data extraction between the two independent researchers was addressed via principal investigator consideration. In the case of duplicate publications describing the same institutional experience, the latest and most complete study was used. Cases were excluded based on the absence of either contributable clinical or immunohistochemical features. For example, cases that did not report immunohistochemical confirmation of the diagnosis were excluded. Similarly, cases without sufficient detail describing clinical presentation, management, or follow-up were excluded. Cases retrospectively re-classified as SFT from an alternative initial diagnosis were excluded. SFT arising in the orbit were excluded, as they exceeded the scope of our otolaryngology-targeted focus.
Associated demographic, clinical, pathologic, immunohistochemical, and follow-up data were extracted and compiled into a single contingent statistical package. Key items for analysis were defined as gender, age, presenting symptoms, duration of symptoms, notable history, anatomic location of tumor, size of tumor, presence of bone erosion, presence of vascular involvement, presence of neural involvement, modality of treatment, adjunctive therapy, complications of treatment, presence of negative surgical margins (when applicable), outcome (recurrence vs. disease-free survival vs. last known disease-free follow up vs. death), histological findings (mitotic activity per high-powered field, presence of cellular atypia, hemorrhage, or necrosis), and immunohistochemical markers (CD34, CD99, Vimentin, Bcl-2, S-100, Cytokeratins, Smooth Muscle Actin, Factor XIIIa, p53, STAT6). For the cases in which the duration of presenting symptoms was reported, estimated tumor growth rates (centimeters per month) were calculated. Cumulative incidences of recurrence, metastases, and disease-specific death were calculated.
Descriptive statistics including mean, median, standard deviation, interquartile range, and 95% confidence intervals (CI) were calculated for the collected variables. Normality was tested and log transformation was performed for skewed distributions. Differences in baseline characteristics were tested by student t tests (continuous variables), Chi square (categorical variables), or ANOVA with post hoc Bonferroni adjustment for multiple comparisons. Wilcoxon rank sum test and Fisher’s exact test were used to assess association between continuous or categorical variables and binary outcomes. In the cohort that had details of follow-up available, recurrence-free survival was estimated Kaplan–Meier analysis was utilized to estimate recurrence-free survival, and Log-rank test was used to assess differences by groups. Patients were censored accordingly at the date of last assessment, the end of the reported observation time, loss to follow up, or death from a cause other than SFT. Cox proportional hazards regression methodology was used to analyze the effect on recurrence-free survival by various demographic, histologic, and treatment factors. P < 0.05 using 95% confidence intervals was considered statistically significant. All statistical analysis was conducted using SPSS (IBM SPSS Statistics Software, version 24.0).
Results
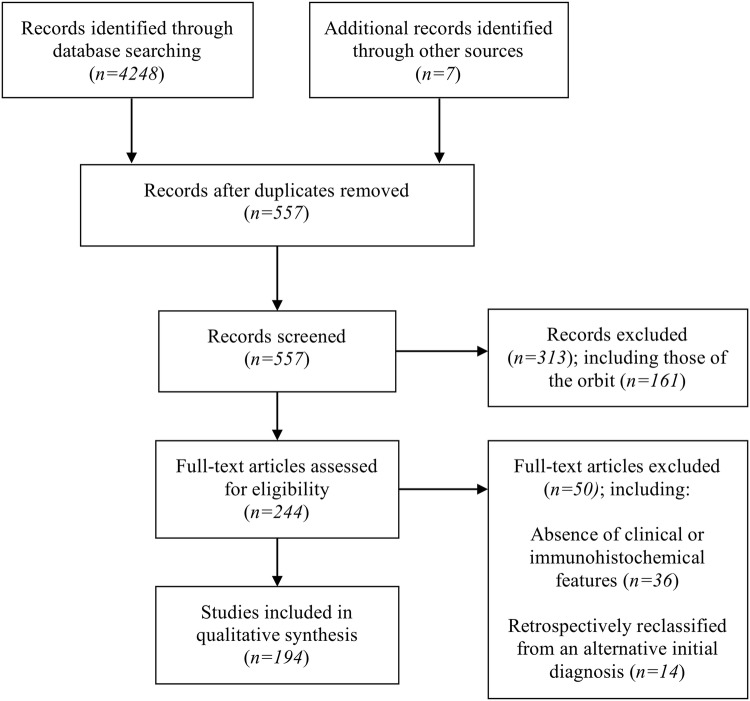
Following removal of duplicates, the search identified 557 citations, which were screened for possible inclusion. 244 articles were retained after title and abstract review, totaling 587 cases of solitary fibrous tumors in the head and neck regions. Reporting standards were unfortunately met with varying stringency and heterogeneity existed among the reported key items for analysis. For final pooled analysis purposes, we included data from 194 articles (Fig. 5), comprising 343 cases of SFT occurring in the head and neck from 1991 to 2019, including our own.
Fig. 5.
PRISMA flow diagram depicting the phases of the conducted systematic review
Solitary fibrous tumors in the head and neck demonstrate no predilection for gender; 50% of cases were reported in males (n = 173) and 50% in females (n = 170). The median age at presentation was 51 years old (range 8 months–94 years). No significant associations were observed with the presence of disease and notable patient histories including tobacco use or prior local injury/trauma. The oral cavity (31%) was the most commonly reported location, followed by the sinonasal (16%) and neck (12%) regions. The median size of the tumors was 4.0 cm (range 0.4–18.0 cm). After adjusting for multiple comparisons, significant differences were observed in the median tumor size between the oral cavity (2.0 cm) and sinonasal region (5.0 cm) (p < 0.001), oral cavity and neck (5.0 cm) (p < 0.001), and oral cavity and thyroid (4.7 cm) (p < 0.001).
Information regarding presenting symptoms was reported for 240 cases. Nearly 62% of valid cases reported a chief complaint of a new mass or localized swelling. Other symptoms prompting evaluation were linked to the anatomical location of the tumors: 87% of all laryngeal tumors presented with dysphonia and 63% of all sinonasal tumors with nasal obstruction. 25% of all sinonasal tumors presented with epistaxis with and without nasal obstruction. Six SFT were found incidentally on imaging performed for alternative purposes. Pain was only described in 38 cases (11%). The average tumor size among those who described pain (5.6 cm) was significantly larger compared to those who did not complain of pain (4.1 cm) (p = 0.005).
The median onset of symptoms prior to evaluation was 8 months (range 1–180 months). Specifically, new masses were described to be present for a median of 9 months prior to presentation, nasal obstruction for 8 months, and dysphonia for 11 months. No significant differences were observed among the duration of the symptoms and either the location of the tumors (p = 0.377) or the type of symptoms (p = 0.456) (i.e. those with a mass did not present sooner than those with nasal obstruction or dysphagia). There was no difference in the duration of symptoms in those experiencing and not experiencing pain (p = 0.071).
Definite details of local invasion on pre-operative imaging or gross examination during surgical intervention were available for 285 of the cases. Local invasion into bone, vasculature, and nerve was noted in 32 cases (13%). Bony erosion was noted 29 cases (10%), vascular invasion was noted in 5 cases (2%), and peri-neural involvement was reported in 3 cases (1%). Tumors with invasion were found to be larger (5.4 cm) compared to those without invasion (4.0 cm) (p = 0.001), and were associated with shorter duration of presenting symptoms (p = 0.038). There was no difference in the proportion experiencing pain (p = 0.597) when comparing cases with invasion to cases without invasion.
Surgery was used as the main modality of treatment in all but 2 cases, in which percutaneous cryoablation and Intensity Modulated Radiation Therapy (IMRT) were employed individually. Adjuvant therapy was utilized in 24 cases (7%); 14 (4%) included a course of post-operative radiation therapy, 5 (1.5%) described pre-operative embolization treatment. Status of surgical margins was reported in 284 cases, 18 (6%) of which were histologically positive. 33% of cases with positive margins received adjuvant therapy compared to only 4% of those with negative margins (p < 0.001). Likewise, there was a trend for those with invasion to receive adjunctive therapy compared to those without invasion (p = 0.005). Note, positive margins were reported in 23% of cases with pre-operative invasion compared to 4% of those without (p = 0.001).
Immunohistochemical staining was positive for CD34 in 98% of all cases. When tested, 98% of the tumors stained positive for vimentin (n = 173), 91% for bcl-2 (n = 175), and 75% for CD99 (n = 122). S-100 stained negatively in 98% of the tumors tested (n = 242), and smooth muscle actin was negative in 94% (n = 185). There were no cases reported in which cytokeratins stained positively (n = 192). Positive nuclear expression of STAT6 was noted in all cases when tested (n = 73).
Among those with reported counts (n = 209), the median number of mitoses per 10 high power fields (hpf) was 1.0 (range 0–85). 18% of these cases described a mitotic rate greater than or equal to 4 per 10 hpf. There was a significant difference in the mitotic rates of tumors with pre-operative invasion (2.75 per 10 hpf) compared to cases without invasion (1.6 per 10 hpf) (p = 0.039). No significant associations were observed between mitotic rate and location of the tumors (p = 0.086). Additionally, no significant correlations were observed when testing mitotic rate (continuous) and tumor size (p = 0.212).
Aberrant histological features including prevalent cellular atypia, nuclear pleomorphism, focal areas of necrosis, or hemorrhage were noted in 39 cases (11%), and each was described as malignant in their respective reports. There was a significant difference in median tumor size in those with malignant histological findings (4.5 cm) compared to those with benign findings (3.5 cm) (p = 0.003). Symptoms were present for a median of 6 months in malignant cases compared to 10 months in benign (p = 0.023). The median rate of growth in these tumors was 1.0 (mean = 1.44 cm/month, SD±1.49) versus 0.42 cm per month in the benign neoplasms (mean = 0.89 cm/month, SD±1.3) (p = 0.045). There was no difference in patient age at diagnosis (p = 0.426) or the proportion of those who reported pain (p = 0.054) comparing malignant versus benign cases. Of the 39 malignant cases, 13 received adjunctive therapy, compared to just 8 of 229 benign cases (p < 0.001). The aforementioned characteristics are summarized in Table 1.
Table 1.
Summary of patient, tumor, and treatment characteristics
| Characteristic | n | Number |
|---|---|---|
| Sex | 343 | |
| Male (%) | 173 (50) | |
| Female (%) | 170 (50) | |
| Age, median (range) | 51 (8 months–94 years) | |
| Size (cm), median (range) | 4.0 (0.4–18.0) | |
| Anatomic site | ||
| Oral cavity (%) | 106 (31) | |
| Sinonasal (%) | 56 (16) | |
| Neck (%) | 41 (12) | |
| Thyroid (%) | 39 (11) | |
| Parotid (%) | 29 (8) | |
| Scalp (%) | 23 (7) | |
| Larynx (%) | 15 (4) | |
| Symptoms | 237 | |
| Mass/swelling | 147 (62) | |
| Nasal obstruction | 40 (17) | |
| Dysphonia | 12 (5) | |
| Incidental | 6 (3) | |
| Symptom duration (months), median (range) | 8.0 (1–180) | |
| Pain | 38 (16) | |
| Invasion | 285 | 37 (13) |
| Bone (%) | 29 (10) | |
| Vasculature (%) | 5 (2) | |
| Perineural (%) | 3 (1) | |
| Adjuvant therapy (%) | 343 | 24 (7) |
| Radiation (%) | 14 (4) | |
| Pre-operative embolization (%) | 5 (1.5) | |
| Surgical margins | 284 | |
| Positive (%) | 18 (6) | |
| CD34 positive (%) | 343 | 335 (98) |
| Bcl-2 positive (%) | 175 | 160 (91) |
| Vimentin positive (%) | 173 | 169 (98) |
| CD99 positive (%) | 122 | 92 (75) |
| S-100 positive (%) | 242 | 5 (2) |
| SMA positive (%) | 185 | 12 (6) |
| CK positive (%) | 192 | 0 (0) |
| Mitoses (hpf), median (range) | 209 | 1 (0–85) |
| ≥ 4 hpf (%) | 38 (18) | |
| Aberrant histological features (%) | 343 | 39 (11) |
| Follow up (months), median (range) | 267 | 24 (1–160) |
| Recurrence (%) | 267 | 15 (6) |
hpf high powered field
Details of follow-up were reported in 267 of the cases. The last known disease-free interval for the cohort was a median of 24 months (range 1–160 months). Recurrence was reported in 15 cases (5%). Kaplan Meir analysis estimated that median recurrence-free interval was 36.5 months. Log-ranks testing demonstrated that the presence of positive margins was associated with shorter recurrence-free survival (p < 0.001). Both pre-operative invasion and the presence of malignant histological features (nuclear pleomorphism, hemorrhage, necrosis) were associated with decreased recurrence-free survival, although both of these failed to reach statistical significance (p = 0.064 and p = 0.069, respectively). Only two cases in the cohort described metastatic disease, in which bilateral pulmonary lesions were found 4 months and 5 months after initial resections. There were 2 cases in which the cause of patient death was attributable to the primary SFT.
Univariate cox proportional hazards regression analysis demonstrated that the presence of positive margins was the only factor predictive of time to recurrence (HR 0.069, CI 0.02–0.27, p < 0.001). No significant associations were noted between recurrence intervals and male gender, age (tested continuously and categorically using ≥ 55 years), estimated rate of growth, duration of symptoms, pain, tumor size (tested continuously and categorically using < 4.9 cm, 5.0–9.9 cm, ≥ 10 cm), mitotic activity (tested continuously and categorically using ≥ 4/10 hpf), presence nuclear atypia or foci of hemorrhage or necrosis, or local aggressive behavior (bone, vascular, and perineural invasion) (Table 2). As only one variable was statistically significant and outcome counts were limited, a multivariate analysis was not performed.
Table 2.
Respective critical values (P) and hazard ratios (HR) calculated via univariate cox proportional hazards regression analysis presented with presented with 95% Confidence Intervals (CI)
| Factor | P | HR | 95% CI |
|---|---|---|---|
| Male | 0.428 | 1.59 | 0.50–5.02 |
| Age (cont.) | 0.924 | 1.01 | 0.96–1.04 |
| Age ≥ 55 | 0.966 | 1.04 | 0.33–3.19 |
| Rate of growth | 0.904 | 0.94 | 0.33–2.57 |
| Duration of symptoms | 0.534 | 1.01 | 0.98–1.05 |
| Pain | 0.151 | 0.41 | 0.08–2.03 |
| Tumor size (cont.) | 0.151 | 1.21 | 0.94–1.54 |
| Size < 4.9 cm | 0.39 | 0.38 | 0.11–1.04 |
| Size 5.0–9.9 cm | 0.967 | 0.76 | 0.41–1.89 |
| Size ≥ 10 cm | 0.955 | 1.34 | 0.86–1.98 |
| Mitotic activity (cont.) | 0.354 | 1.04 | 0.96–1.12 |
| Mitotic activity ≥ 4/10 hpf | 0.291 | 0.40 | 0.07–2.19 |
| Positive margins | < 0.001 | 0.069 | 0.02–0.27 |
| Atypia, hemorrhage, or necrosis | 0.088 | 0.36 | 0.07–1.26 |
| Local invasion | 0.079 | 0.34 | 0.08–1.15 |
Bold values indicate statistical significance
cont. continuously tested variable, hpf high powered field
Discussion
Over 587 solitary fibrous tumors have been reported in the head and neck region since 1991. To date, this analysis serves as the most robust review describing the demographic, immunohistochemical, and clinical outcomes associated with SFT in this anatomic region. Consistent with prior case series and reviews, the descriptive findings from our pooled cohort showed a wide range in age distribution with no propensity for gender [7–9]. The oral cavity was the most frequent site of occurrence. Tumors of the oral cavity were significantly smaller than tumors in other sites of the head and neck. The mainstay of successful treatment remains surgical intervention with complete resection and attainment of clear margins. The addition of adjuvant treatment was significantly associated with pre-operative local invasion and post-operative positive surgical margins.
This is the first extensive report in which the symptoms of initial presentation and their relation to a variety of tumor characteristics and outcomes were examined. While our analysis did not identify any risk factors or patient populations related to the development of these neoplasms, the observations made regarding symptomatic presentation may offer a new level of insight into SFT tumor behavior. Nearly two-thirds of all cases presented with a complaint of a new mass and only 11% of all tumors were painful. Generally, appreciable symptoms were reflective of mass-related compression of local anatomic structures. Interestingly, the tumors causing pain were found to be larger than the painless ones. However, pain was not associated with local invasion or malignant histological findings, thus likely has minimal prognostic value at the time of presentation.
Both atypical histological makeup and local invasion were associated with shorter durations of symptom presentation. While factors such as symptom severity were not controlled for, this data helps demonstrate that intrinsically aggressive SFT grow more rapidly than benign lesions. By extrapolating approximate growth rates from this data, a new understanding on the natural history of SFT in the head and neck may be gained. In fact, the estimated rates of growth in the tumors post-operatively classified as malignant based on histologic criteria was nearly double compared to those that were classified as benign (1.44 cm/month vs. 0.88 cm/month) and their median size was significantly larger.
The conclusions from this data are derived from the onset of symptoms and should be interpreted with a generous level of caution. This data may not be reflective of the true growth rate of these neoplasms. Equally, these rates may be influenced by recall and reporting bias. The median duration of the development of a new mass was 9 months, contrary to the historical belief that these lesions are very slow growing. There are no current investigations examining the natural progression of these neoplasms nor evidence elucidating an interval from tumor origin to the development of symptoms. Unlike their intraplueral counterparts, we hypothesize that SFT in the head and neck are more likely to be identified at earlier stages and at smaller sizes secondary to their intimate proximity of anatomic structures and one’s ability to more easily visualize and examine these regions. The results from this analysis provide additional support that SFT in the head and neck are typically smaller than intrathoracic SFT at the time of diagnosis.
Various generalizations originally stemming from data on intrathoracic SFT have been unjustly propagated throughout the head and neck literature. The analyzes in this review directly challenge the validity of these conclusions. Although no universal definition for defining a malignant SFT is accepted, several histopathological criteria have been proposed. These customary markers of malignancy include histological foci of hemorrhage or necrosis, the presence of immature or pleomorphic tumor cells, and increased mitotic activity (≥ four mitoses per 10 hpf). These were first proposed by England et al. based on a single-institutional experience associating pleural fibrous tumors of multiple etiologies (fibrosarcomas, leiomyosarcomas, hemangiopericytomas, etc.) with both recurrence and metastasis [10]. Refining these criteria for extrathoracic SFT, Gold et al. and Demicco et al. demonstrated that age ≥ 55 years old, tumors ≥ 10 cm, positive surgical margins, the presence of histological necrosis, and mitoses ≥ 4/10 hpf predicted for local recurrence and metastasis [11, 12]. However, only 5 and 12 cases out of the 79 and 110 in these studies, respectively, were SFT of the head and neck. Despite a widespread impact on the current body of literature, additional external validation of such criteria has yet to be satisfied.
When retrospectively analyzing the model proposed by Gold and Demicco against the cases in our database, the only factor that significantly predicted for recurrence was the presence of positive margins. These results slightly diverge from recent SFT series which exhibited that the risk of local recurrence and metastasis correlates with tumor size and the aforementioned histological findings [13–15]. Most notably Smith et al. recently exhibited that size, mitotic rate, epithelioid morphology, and necrosis were adverse prognostic factors when modeled for local recurrence [15]. However, the rate of recurrence (36%) in their series was much greater than previously reported, potentially suggestive of referral center bias [8, 9, 16]. In the context of this conflicting information, we believe that nuclear atypia, greater than 4 mitoses/10 HPFs, and necrosis are likely associated with aggressive clinical behavior, but there is not enough current evidence to indicate they are predictive of recurrence themselves. Further, the current schemata of classifying tumors as malignant based upon histological characteristics alone should be reconsidered.
Contemporary studies have investigated the role of genetic markers in SFT behavior. In cases of uncertain malignant potential, Yokoi et al. suggested that expression of p53 protein may be associated with aggressive tumor behaviors [17]. Testing for p53 expression was only reported in 12 cases in our analysis cohort. In the 3 that were positive, only 1 demonstrated atypical histological characteristics and this case did not recur at a last known follow up of 12 months. The frequency of proliferating cells that express Ki-67 has been proposed as a prognostic factor for aggressive behavior. Sun et al. reported a higher Ki-67 labeling index in malignant SFT compared to benign SFT; however, Hasegawa et al. reported low Ki-67 index in three cases with recurrence [3, 18]. This inconsistent evidence indicates that Ki-67 is an unreliable prognostic factor at this time.
Detectable via a variety of molecular diagnostics, STAT6 expression is a worthwhile adjunct for head and neck SFT [19]. Diffuse nuclear expression of STAT6 protein, likely representing the presence of the pathognomonic NAB2-STAT6 gene fusion, carries a diagnostic sensitivity estimated above 95% [13, 20–23]. While other mesenchymal neoplasms frequently exhibit both nuclear and cytoplasmic positivity, SFT demonstrate exclusive nuclear expression. When tested, all cases including our own were positive for STAT6 nuclear expression. Although established as a reliable diagnostic marker, its clinical and biological relevance to disease behavior remains to be elucidated.
Our analysis possesses limitations inherent to all retrospective analyses. Reporting bias is certainly present in this assessment and must be acknowledged appropriately. There is a general tendency to under-report benign cases in the form of a publishing bias. Secondly, the decision to include and exclude data from various case series based on reporting stringency may have influenced the distribution of various clinical variables. In the most robust review of sinonasal SFT to date, Thompson and Lau identified 86 cases within our current literature [24]. Yet, clinical, tumor, and immunohistochemical details were limited in nearly 30 of these cases. As a result, they were individually excluded from our pooled analysis. Such exclusions were made with the intent to protect analysis fidelity; however, this concurrently narrows our investigation. Similarly, heterogeneity amongst the reported information restricts the factors for analysis. For example, information regarding epithelioid change—including round cell features which may occur in more aggressive tumors—was scarce amongst in included cases.
Nonetheless, the evidence from this cohort supports the rhetoric that head and neck SFT do not require any additional treatment provided that negative surgical margins are achieved. Although majority of cases do not recur, there are several case reports reported that had recurrence greater than a 10-year interval. In our pooled dataset, the median time to recurrence was greater than the overall median follow-up, which may potentially suggest that cases of recurrence should have been included in this dataset.
Conclusions
Solitary fibrous tumors are rare mesenchymal-based neoplasms that arise within many extrathoracic sites, including the head and neck region. In the most comprehensive review to date, this analysis provides evidence to support demographic and clinical conclusions derived from prior institutional series and targeted literature reviews. As the presence of positive margins was the only factor predictive of local disease recurrence, these results prompt the future investigation between traditional histological markers of malignancy and tumor behavior. Additionally, this review revealed novel associations between initial disease presentation and variety of tumor characteristics, providing clinicians with new insight into SFT presentation. By better understanding the extent of the current evidence in our specialty, practitioners are better equipped to provide superior care when faced with these challenging entities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 58971 kb)
Funding
The authors have no funding, financial relationships to disclose.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Witkin GB, Rosai J. Solitary fibrous tumor of the upper respiratory tract. A report of six cases. Am J Surg Pathol. 1991;15(9):842–848. doi: 10.1097/00000478-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kao YC, Lin PC, Yen SL, et al. Clinicopathological and genetic heterogeneity of the head and neck solitary fibrous tumours: a comparative histological, immunohistochemical and molecular study of 36 cases. Histopathology. 2016;68(4):492–501. doi: 10.1111/his.12772. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa T, Matsuno Y, Shimoda T, et al. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30(12):1464–1473. doi: 10.1016/S0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 4.Morimitsu Y, Nakajima M, Hisaoka M, et al. Extrapleural solitary fibrous tumor: clinicopathologic study of 17 cases and molecular analysis of the p53 pathway. APMIS. 2000;108(9):617–625. doi: 10.1034/j.1600-0463.2000.d01-105.x. [DOI] [PubMed] [Google Scholar]
- 5.Brunnemann RB, Ro JY, Ordonez NG, et al. Extrapleural solitary fibrous tumor: a clinicopathologic study of 24 cases. Mod Pathol. 1999;12(11):1034–1042. [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox DP, Daniels T, Jordan RC. Solitary fibrous tumor of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(1):79–84. doi: 10.1016/j.tripleo.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Bowe SN, Wakely PE, Jr, Ozer E. Head and neck solitary fibrous tumors: diagnostic and therapeutic challenges. Laryngoscope. 2012;122(8):1748–1755. doi: 10.1002/lary.23350. [DOI] [PubMed] [Google Scholar]
- 9.Ganly I, Patel SG, Stambuk HE, et al. Solitary fibrous tumors of the head and neck, a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg. 2006;132(5):517–525. doi: 10.1001/archotol.132.5.517. [DOI] [PubMed] [Google Scholar]
- 10.England DM, Hochholzer L, McCarthy MJ, et al. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13(8):640–658. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94(9):1057–1068. doi: 10.1002/cncr.10328. [DOI] [PubMed] [Google Scholar]
- 12.Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27(3):390–395. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]
- 14.Daigeler A, Lehnhardt M, Langer S, et al. Clinicopathological findings in a case series of extrathoracic solitary fibrous tumors of soft tissues. BMC Surg. 2006;6(1):10. doi: 10.1186/1471-2482-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SC, Gooding WE, Elkins M, et al. Solitary fibrous tumors of the head and neck: a multi-institutional clinicopathologic study. Am J Surg Pathol. 2017;41(12):1642–1656. doi: 10.1097/PAS.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunzel J, Hainz M, Ziebart T, et al. Head and neck solitary fibrous tumors: a rare and challenging entity. Eur Arch Otorhinolaryngol. 2016;273(6):1589–1598. doi: 10.1007/s00405-015-3670-1. [DOI] [PubMed] [Google Scholar]
- 17.Yokoi T, Tsuzuki T, Yatabe Y, et al. Solitary fibrous tumour: significance of p53 and CD34 immunoreactivity in its malignant transformation. Histopathology. 1998;32(5):423–432. doi: 10.1046/j.1365-2559.1998.00412.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Naito Z, Ishiwata T, et al. Basic FGF and Ki-67 proteins useful for immunohistological diagnostic evaluations in malignant solitary fibrous tumor. Pathol Int. 2003;53(5):284–290. doi: 10.1046/j.1440-1827.2003.01474.x. [DOI] [PubMed] [Google Scholar]
- 19.Chuang IC, Liao KC, Huang HU, et al. NAB2-STAT6 gene fusion and STAT6 immunoexpression in extrathoracic solitary fibrous tumors: the association between fusion variants and locations. Pathol Int. 2016;66(5):288–296. doi: 10.1111/pin.12408. [DOI] [PubMed] [Google Scholar]
- 20.Koelsche C, Schweizer L, Renner M, et al. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology. 2014;65(5):613–622. doi: 10.1111/his.12431. [DOI] [PubMed] [Google Scholar]
- 21.Demicco EG, Harms PW, Patel RM, Smith SC, Ingram D, Torres K, et al. Extensive survey of STAT6 expression in a large series of mesenchymal tumors. Am J Clin Pathol. 2015;143(5):672–682. doi: 10.1309/AJCPN25NJTOUNPNF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38(4):552–559. doi: 10.1097/PAS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar A, Sakthivel P, Rajeshwari M, et al. Recurrent sinonasal CD34-negative malignant solitary fibrous tumor diagnosed on STAT6 immunohistochemistry and NAB2-STAT6 fusion. Head Neck Pathol. 2019 doi: 10.1007/s12105-018-00999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson LDR, Lau SK. Sinonasal tract solitary fibrous tumor: a clinicopathologic study of six cases with a comprehensive review of the literature. Head Neck Pathol. 2018;12(4):471–480. doi: 10.1007/s12105-017-0878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 58971 kb)