Abstract
Inflammatory myofibroblastic tumor (IMT) is an uncommon neoplasm most frequently seen in the abdomino-pelvic region and lungs of children and young adults. Laryngeal tumors are rare. We present a case of a 23-year-old patient with a 5 month history of laryngitis and aphonia unresolved by corticotherapy. Laryngoscopy revealed a small, non-ulcerated, subepithelial, polypoid mass arising from the right vocal cord. The diagnosis of IMT with ALK expression was supported by histopathologic and molecular analysis. The THBS1 fusion partner was identified by RNA-sequencing analysis for the first time in a laryngeal IMT. This fusion partner has only been identified in six uterine IMTs thus far. Conservative excision of the lesion yielded excellent functional results for the patient. The voice was preserved and no recurrences were seen after 6 months of follow-up.
Keywords: Inflammatory myofibroblastic tumor, Laryngeal tumor, Anaplastic lymphoma kinase, Immunohistochemistry, ALK–THBS1 fusion
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare neoplasm comprising myofibroblastic and fibroblastic spindle cells with an accompanying inflammatory infiltrate of plasma cells, lymphocytes, and/or eosinophils [1]. It most commonly affects the abdominal cavity and lung but may occur at any anatomic site [1]. Wenig et al. first published a case series of IMTs affecting the larynx in 1995, and, to date, less than 50 laryngeal tumors with treatment and outcome data are described in the English literature [2–21]. The neoplastic vs reactive nature of IMTs has been long debated; however, discovery of ALK rearrangements in 50% to 70% of tumors helped to establish its clonal nature [22]. This paper aims to describe a rare case of laryngeal IMT confirmed by ALK overexpression and identification of an ALK–THBS1 fusion by RNA-sequencing analysis. Review of the literature emphases the particular clinical presentation and indolent course of IMTs of the larynx.
Observation
A 23-year-old male smoker was admitted to hospital for a progressive aphonia of 4 months duration and chronic laryngitis unresolved by corticosteroid treatment. There was no history of fever or weight loss and no cervical lymphadenopathy. Laryngoscopic examination revealed a small, non-ulcerated, red, subepithelial, polypoid mass arising from the right vocal cord and extending to the anterior commissure. The computed tomography (CT) imaging demonstrated heterogeneous enhancement of a nodular mass within the glottic and subglottic plane. It was lateralized to the right and measured 14 × 13 mm in size (Fig. 1a, b).
Fig. 1.
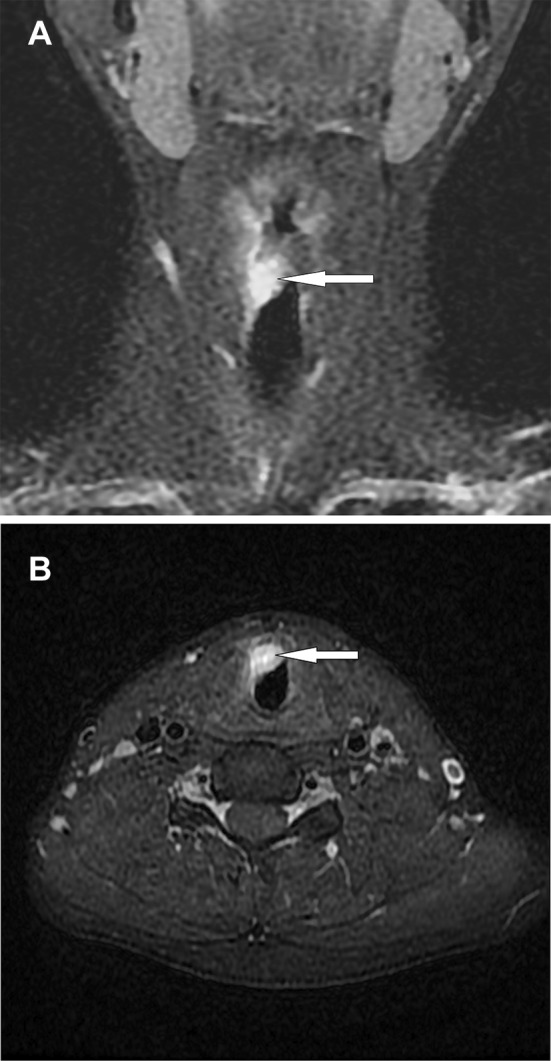
a Coronal fat saturation T1-weighted post contrast MRI shows thickening of the right true vocal fold (arrow). b MRI axial scan shows a soft tissue mass arising from the right vocal cord (arrow)
Histologic examination of the resulting biopsy specimens showed a proliferation of spindle cells in a background of mixed inflammatory cells including lymphocytes, histiocytes, plasma cells, and eosinophils. The spindle cells had eosinophilic cytoplasm and oval to elongated nuclei (Fig. 2a, b). They were positive for vimentin, smooth muscle actin (SMA), and anaplastic lymphoma kinase (ALK1) and negative for S-100 protein and desmin (Fig. 3a, b). The lymphoid cells were mixture of CD20-positive B cells and CD3-positive T cells. The diagnosis of IMT was rendered. A gene rearrangement was detected by NanoString technology and subsequent RNA sequencing revealed a THBS1-ALK fusion. The patient underwent conservative surgical treatment. Voice improvement was noted at 2.5 months post-surgery, and the patient remained free of disease at 6 month follow-up.
Fig. 2.
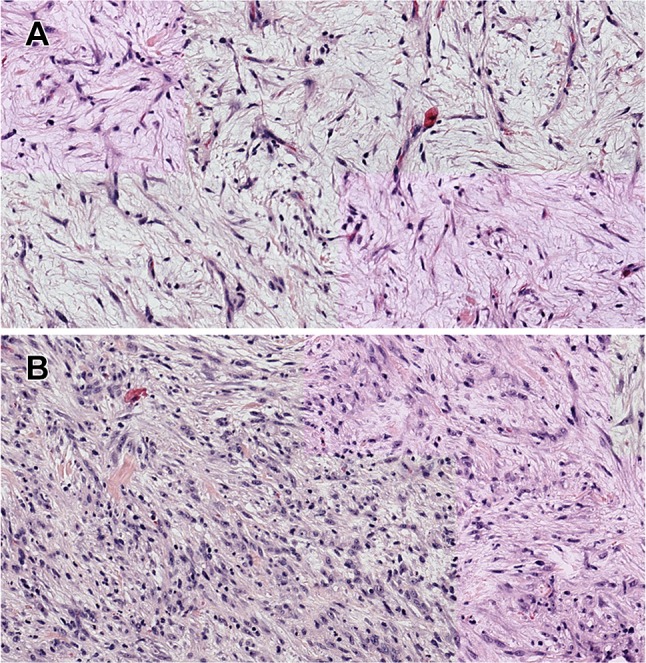
a Photomicrograph of the tumor specimen showing myxoid tumour composed of spindle-shaped myofibroblasts with scattered inflammatory cells (haematoxylin and eosin stain, magnification ×400). b Microscopic sections show a dense spindle cell proliferation associated with a lymphoplasmacytic infiltrate (haematoxylin and eosin stain ×400)
Fig. 3.
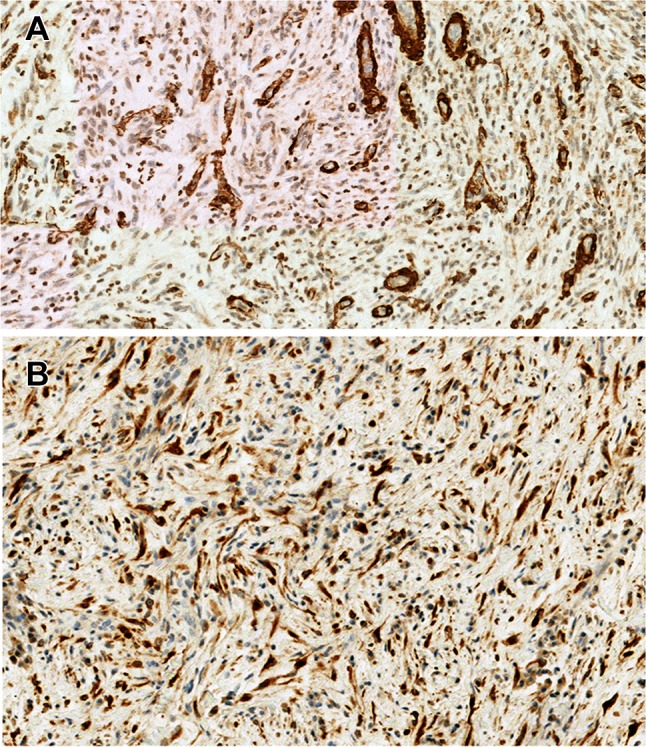
Immunohistochemical findings. The spindle cells were positive for a α-smooth muscle actin (SMA) and b anaplastic lymphoma kinase-1 (ALK-1) (immunohistochemistry staining ×200)
Discussion
IMT is defined by the World Health Organization as a neoplasm composed of myofibroblastic and fibroblastic spindle cells with a background of mixed inflammatory cells [1]. They tend to occur in children and young adults, with a mean age at diagnosis of 10 years, and a slight female predominance is observed [1, 23]. The most frequent locations are the abdominal and thoracic cavities, but IMTs are described across many anatomic sites.
Less than 50 IMTs affecting the larynx are reported in the English literature [2–21]. In a series of 84 extrapulmonary IMTs, Coffin et al. reported three laryngeal IMTs among nine IMTs of the upper respiratory tract [23]. In contrast to pulmonary tumors, IMTs of the larynx are mostly found in adults (median age of 48 years) with a male predominance (2.1:1 male to female ratio). Only three cases are reported in children. It is most often localized to the vocal folds (19/35, 54%). As in our case, laryngeal tumors often present as a polypoid lesion mimicking reactive polyps of the vocal cords; however, exophytic lesions suggesting papillomatosis or possible malignancies are also described [2, 10–12]. Imaging studies such as CT scans and magnetic resonance imaging (MRI) are usually performed to determine lesional extent.
Based on the histologic findings, Coffin et al. proposed a classification system distinguishing three main categories of IMTs according to the cell density [23]:
Hypocellular, myxoid: In this pattern, the myofibroblasts are organized loosely within a background of inflammatory cells such as plasma cells, lymphocytes, and eosinophils.
Hypercellular: Characterized by densely aggregated, elongated, short spindle cells or stellate cells arranged within a collagenized background.
Collagen pattern: The cellular component is loosely arranged, resembling scar tissue.
All three histologic patterns may be present in a single tumor, and the combinations create a diverse spectrum of tumor morphologies.
Immunohistochemical findings may also differ between tumors. SMA and desmin expression is variable, and 30 to 80% of IMTs express cytokeratin. In a tumor with nuclear atypia and cytokeratin reactivity, an IMT may be mistaken for the more common spindle cell squamous carcinoma [24]. Immunostaining for ALK is helpful to achieve the correct diagnosis. The expression of ALK is explained by clonal cytogenetic rearrangements involving chromosome band 2p23 that fuses the 3′ kinase region of ALK with partner genes. These include EML4, TPM3, TPM4, CLTC, RANBP2, ATIC, CARS, SEC31L1, and more as the list continues to expand [25]. EML4, TMP3, TMP4 and CLTC are the most frequently reported fusion partners. Recently, rearrangements involving ROS1, PDGFRB, RET, and NTRK1 have been identified in a subset of ALK-negative tumors [26]. In laryngeal IMTs, ALK immunohistochemistry was performed in 19 cases, 14 of which were positive (Table 1). Patients with ALK-positive IMTs were slightly younger than those with ALK-negative tumors (median age: 38 vs 46). For the first time, the THBS1 fusion partner was identified in a laryngeal IMT. This partner was only identified in six uterine IMTs prior to this case [27, 28].
Table 1.
Laryngeal inflammatory myofibroblastic tumors reported in the literature with determination of ALK protein expression
| Authors | Age (years) | Sex | Location | Size (cm) | Recurrence | Treatment | Follow-up | Status | ALK IHC |
|---|---|---|---|---|---|---|---|---|---|
| Guilemany et al. [6] | 62 | m | vc | 2 | 0 | Conservative surgery | 24 | ned | 0/1 |
| Belezza et al. [8] | 23 | m | vc | ni | 0 | Conservative surgery | 21 | ned | 1/1 |
| Idrees et al. [10] | 56 | m | Anterior commissure | 1.1 | 0 | Conservative surgery | 27 | ned | 0/1 |
| 28 | m | vc | 3 | 0 | Conservative surgery | 18 | ned | 1/1 | |
| Volker et al. [11] | 56 | m | Supraglottic | ni | stable | Laser debulking | 24 | stable | 0/1 |
| 34 | f | vc | 0.8 | 0 | Conservative surgery | 31 | ned | 1/1 | |
| Ni et al. [12] | 46 | f | Arytenoid fold | 3 | 0 | Conservative surgery | 12 | ned | 0/1 |
| He et al. [16] | 10 | m | 2 vc and AC | ni | 3 (incomplete resection) | Conservative surgery | 24 | ned | 1/1 |
| Kieu et al. [17] | 5 | f | Subglottic mucosa | ni | 0 | Conservative surgery | 12 | ned | 1/1 |
| Pierry et al. [18] | 54 | f | Right ventricular band | < 1 | 0 | Conservative surgery | 33 | ned | 1/1 |
| 40 | m | vc | < 1 | 0 | Conservative surgery | 5 | ned | 1/1 | |
| 42 | f | vc | < 1 | 0 | Conservative surgery | 28 | ned | 1/1 | |
| 25 | m | vc | < 1 | 0 | Conservative surgery | 26 | ned | 1/1 | |
| 86 | m | Ventricular band | < 1 | 0 | Conservative surgery | 21 | ned | 1/1 | |
| 48 | m | vc | < 1 | 0 | Conservative surgery | 12 | ned | 1/1 | |
| Yan et al. [19] | 73 | m | vc | ni | 0 | Conservative surgery | 12 | ned | 1/1 |
| Tay et al. [20] | 12 | f | vc | ni | 0 | Conservative surgery | 12 | ned | 0/1 |
| Izadi et al. [21] | 46 | f | Aryepiglottic fold and vallecula | 3 | 3 (incomplete resection) | Supraglottic laryngectomy | 42 | ned | 1/1 |
| Our case | 23 | m | vc | < 1 | 0 | Conservative surgery | 8 | ned | 1/1 |
m male, f female, vc vocal cord, ni no information, ned no evidence of disease, ALK anaplastic lymphoma kinase, IHC immunohistochemistry
IMTs are considered intermediate malignancies due to a local recurrence rate of about 25% and distant metastasis has been described [1]. All patients included in our literature review were initially treated with conservative surgery. The five recurrences observed were attributed to incomplete resection [2, 3, 13, 15, 16]. Two patients showed stable disease at follow-up but none developed metastases (follow-up ranging between 12 and 47 months). Recurrence was associated with incomplete resection and large tumor size with extent beyond the vocal cord.
Conclusion
Laryngeal IMTs often present as vocal cord polyps. It is important to consider this entity when evaluating a spindle cell proliferation with some nuclear atypia. ALK immunostaining is important to differentiate an IMT from a reactive polyp or spindle cell carcinoma. If negative, a next generation sequencing (NGS) study may be considered to detect genetic abnormalities. IMTs generally have a good prognosis and do not require aggressive therapy. Regular follow-ups are necessary to monitor for recurrence.
Acknowledgment
The authors would like to thank Dr. Marie Karanian for the RNA-sequencing analysis (Department of Pathology, Centre Leon Berard, Lyon).
Compliance with Ethical Standards
Conflicts of Interests
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Consent
Consent was obtained from the patient for the publication of materials related to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coffin CM, Flechter JA. World Health Organization classification of tumors of soft tissue and bone. 4. Lyon: IARC; 2013. pp. 83–84. [Google Scholar]
- 2.Wenig B, Devaney K, Bisceglia M. Inflammatory myofibroblastic tumor of the larynx. A clinicopathological study of eight cases simulating a malignant spindle cell neoplasm. Cancer. 1995;76:2217–2229. doi: 10.1002/1097-0142(19951201)76:11<2217::AID-CNCR2820761107>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Corsi A, Ciofalo A, Leonardi M, Zambetti G, Bosman C. Recurrent inflammatory myofibrobastic tumor of the glottis mimicking malignancy. Am J Otolayngol. 1997;18:121–126. doi: 10.1016/S0196-0709(97)90100-9. [DOI] [PubMed] [Google Scholar]
- 4.Martínez S, Bosch R, Pardo J, Salvadó MT, Alvaro TJ. Laryngeal inflammatory myofibroblastic tumour of larynx. J Laryngol Otol. 2001;115:140–142. doi: 10.1258/0022215011907541. [DOI] [PubMed] [Google Scholar]
- 5.Ereño C, López JI, Grande J, Santaolalla F, Bilbao FJ. Inflammatory myofibroblastic tumour of the larynx. J Laryngol Otol. 2001;115:856–858. doi: 10.1258/0022215011909189. [DOI] [PubMed] [Google Scholar]
- 6.Guilemany JM, Alós L, Alobid I, Bernal-Sprekelsen M, Cardesa A. Inflammatory myofibroblastic tumor in the larynx: clinicopathologic features and histogenesis. Acta Otolaryngol. 2005;125:215–219. doi: 10.1080/00016480410022796. [DOI] [PubMed] [Google Scholar]
- 7.Hanna SJ, Blenke E, Sharma R, Knight LC. Laryngeal inflammatory pseudotumour: an unusual cause of airway obstruction. Int J Pediatr Otorhinolaryngol. 2005;69:1253–1255. doi: 10.1016/j.ijporl.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Belezza G, Cavaliere A, Del Sordo R, Sidoni A. Inflammatory myofibroblastic tumor of the larynx with anaplastic lymphoma kinase protein overexpression. A case report. Tumori. 2006;92:449–451. doi: 10.1177/030089160609200516. [DOI] [PubMed] [Google Scholar]
- 9.Suh SI, Seol HY, Lee JH, Lee YH, Kim TK, Lee NJ, Woo JS, Kim IS. Inflammatory myofibroblastic tumor of the larynx. Head Neck. 2006;28:369–372. doi: 10.1002/hed.20413. [DOI] [PubMed] [Google Scholar]
- 10.Idrees MT, Huan Y, Woo P, Wang BY. Inflammatory myofibroblastic tumor of larynx: a benign lesion with variable morphological spectrum. Ann Diagn Pathol. 2007;11:433–439. doi: 10.1016/j.anndiagpath.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Völker HU, Scheich M, Zettl A, Hagen R, Müller-Hermelink HK, Gattenlöhner S. Laryngeal inflammatory myofibroblastic tumors: different clinical appearance and histomorphologic presentation of one entity. Head Neck. 2010;32:1573–1578. doi: 10.1002/hed.21232. [DOI] [PubMed] [Google Scholar]
- 12.Ni C, Xu YY, Zhou SH, Wang SQ. Differential diagnosis of inflammatory myofibroblastic tumour and low-grade myofibroblastic sarcoma: two case reports with a literature review. J Int Med Res. 2011;39:311–320. doi: 10.1177/147323001103900134. [DOI] [PubMed] [Google Scholar]
- 13.Alhumaid H, Bukhari M, Rikabi A, Farahat M, Mesallam TA, Malki KH, Aldkhyyal A. Laryngeal myofibroblastic tumor: case series and literature review. Int J Health Sci (Qassim). 2011;5:187–195. [PMC free article] [PubMed] [Google Scholar]
- 14.Dava CJ, Hajiioannou JK, Terzis A, Bizakis J. An inflammatory pseudotumour of the larynx: a case report and literature review of an unusual tumour. Ecancermedicalscience. 2012;6:273. doi: 10.3332/ecancer.2012.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do BA, Varshney R, Zawawi F, Levental M, Caglar D, Young J. Inflammatory myofibroblastic tumor of the larynx-a case report. J Voice. 2014;28:258–261. doi: 10.1016/j.jvoice.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 16.He CY, Dong GH, Liu HG. Recurrent laryngeal inflammatory myofibroblastic tumor with positive anaplastic lymphoma kinase mimicking recurrent respiratory papillomatosis: a case report. World J Surg Oncol. 2014;6(12):54. doi: 10.1186/1477-7819-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieu MQ, Thottam PJ, DaCosta V, Gonzalez-Krellwitz L, Poulik JM, Madgy DN. Treatment of inflammatory myofibroblastic tumor of the subglottis with KTP laser: a case report. J Voice. 2014;28(841):e1–e4. doi: 10.1016/j.jvoice.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Pierry C, Pérot G, Karanian-Philippe M, Neuville A, Gomez-Brouchet A, Crestani S, Coindre JM. Polypoid laryngeal inflammatory myofibroblastic tumors: misleading lesions: description of six cases showing ALK overexpression. Am J Clin Pathol. 2015;144:511–516. doi: 10.1309/AJCPCG8D6JAQBVLG. [DOI] [PubMed] [Google Scholar]
- 19.Yan Q, Hu XL. Inflammatory myofibroblastic tumor of the larynx: report of a case and review of the literature. Int J Clin Exp Pathol. 2015;8:13557–13560. [PMC free article] [PubMed] [Google Scholar]
- 20.Tay SY, Balakrishnan A. Laryngeal inflammatory myofibroblastic tumor (IMT): a case report and review of the literature. J Med Case Rep. 2016;23(10):180. doi: 10.1186/s13256-016-0967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izadi F, Ghanbari H, Azizi MR, Gasembaglou S, Manteghi MJ, Ghanbari A. Inflammatory myofibroblastic tumor of the larynx: a case report. Iran J Otorhinolaryngol. 2016;28:79–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14:569–576. doi: 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 23.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumour (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Thompson LD, Wieneke JA, Miettinen M, Heffner DK. Spindle cell carcinomas of the larynx: a clinicopathological study of 187 cases. Am J Surg Pathol. 2002;26:153–170. doi: 10.1097/00000478-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Lovly CM, Gupta A, Lipson D, Otto G, Brennan T, Chung CT, Borinstein SC, Ross JS, Stephens PJ, Miller VA, Coffin CM. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4:889–895. doi: 10.1158/2159-8290.CD-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonescu CR, Suurmeijer AJ, Zhang L, Sung YS, Jungbluth AA, Travis WD, Al-Ahmadie H, Fletcher CD, Alaggio R. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39:957–967. doi: 10.1097/PAS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett JA, Nardi V, Rouzbahman M, Morales-Oyarvide V, Nielsen GP, Oliva E. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod Pathol. 2017;30:1489–1503. doi: 10.1038/modpathol.2017.69. [DOI] [PubMed] [Google Scholar]
- 28.Haimes JD, Stewart CJR, Kudlow BA, Culver BP, Meng B, Koay E, Whitehouse A, Cope N, Lee JC, Ng T, McCluggage WG, Lee CH. uterine inflammatory myofibroblastic tumors frequently harbor ALK fusions with IGFBP5 and THBS1. Am J Surg Pathol. 2017;41:773–780. doi: 10.1097/PAS.0000000000000801. [DOI] [PubMed] [Google Scholar]


