Abstract
Surgical removal with negative margins is the preferred management of oral squamous cell carcinomas. This review summarizes statements by professional organizations and data supporting the specimen-driven approach to margin assessment. Practical aspects of the intraoperative margin assessment, as guided by gross examination, are presented. The most cost- and time-efficient method of intraoperative margin assessment depends on desired margin clearance and likelihood of other adverse histologic factors, such as extranodal extension, perineural invasion, which are likelier in advanced carcinomas. Intraoperative surgeon-pathologist communication can be improved by reporting to surgical team gross distances to all or selected closest margins, before choosing margins for microscopic frozen examination. Case specific mitigation strategies to minimize the negative impact of tumor-bed driven margin assessment or of suboptimal margin revision are proposed. Based on size, shape, histology, size of carcinoma at the margin, and orientation of the additional tissue, margin revision may be judged as adequate (conversion of a positive margin into a negative one), inadequate (positive margin remains positive), or indeterminate. The significance of anatomic subsite based labeling, radial margin sampling from the main resection specimen, and the relationship between the distance to closest margin and local control are highlighted. The modern definition of safe margin would account for other parameters, such as perineural invasion. An updated approach to resolution of frozen versus permanent sampling issues is outlined. Future studies are needed to design and validate risk models that would help to determine for individual patient what represents a safe margin and how to judge the quality of margin revision.
Keywords: Margin, Squamous cell carcinoma, Oral cavity, Frozen, Gross examination
Introduction
The principal aim of oncologic surgery is to remove the entire tumor with the minimal rim of normal tissue required to achieve local control while minimizing patient morbidity and preserving functionality. The challenges of achieving this are readily evident when approaching head and neck cancers given the limited “real estate” and specialized anatomic considerations. As such, surgeons’ and pathologists’ input are required to determine margin status. For the purposes of this review, we will mostly focus on early squamous cell carcinomas (SCC) of the oral cavity, particularly of oral tongue, one of the most anatomically accessible areas of head and neck.
A common practice regarding margin status assessment is intraoperative consultation. While routinely requested by surgeons, there is no evidence that intraoperative margin assessment as performed currently improves clinical outcome. Intriguingly, post hoc analysis of local control in 494 patients with early oral tongue carcinoma (< 4 cm in largest dimension, pN0) suggests that intraoperative margin assessment may be associated with worse local control (Fig. 1) [1]. We will herein review several aspects of margin assessment that may contribute to the breakdown of this seemingly logical method of margin assessment. Sections below will detail practical reasons as to why intraoperative margin assessment as performed now is at best inefficient. Since daily requests for routine intraoperative margin assessment are ingrained in current surgical practice (and are unlikely to cease after this review!), we suggest mitigation strategies to minimize the negative impact of such requests. This work was meant to complement a prior extensively illustrated review [2].
Fig. 1.
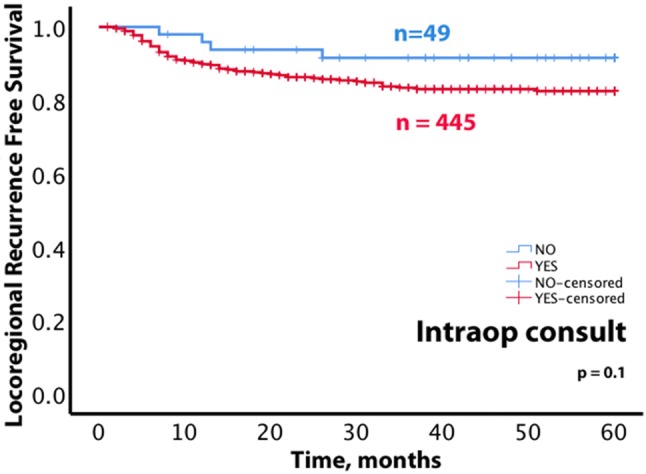
Preliminary review of locoregional recurrence free survival among patients with early oral tongue carcinoma. Of 494 patients [1], surgery for 49 patients did not include intraoperative margin assessment. The trend for better locoregional recurrence free survival among patients whose surgery did not include intraoperative margin assessment is not statistically significant (p = 0.1) and could not be explained by lower frequency of adverse histologic factors or higher rates of radiotherapy
What is a Safe Margin and What is an Acceptable Risk of Recurrence?
Adequate studies of margin clearance are rare, difficult to design, time-consuming, and exceedingly tedious [1–8]. Such a study, among other requirements, would use local recurrence as a primary endpoint, be specific to an anatomic sub-site, clearly distinguish true local recurrences from second primary SCC, and analyze the distance to closest margin as a continuous variable (without arbitrary grouping of cases into “close” and “negative” groups based on pre-conceived cut-offs, e.g., 5 mm). Also, adequate margin studies would specify whether margins were revised and exclude cases with positive margins when determining the best cut off between close and negative margins [7, 9, 10]. Given the lack of standardization in margin assessment, studies based entirely on review of surgical pathology reports or cancer registry databases are suboptimal, if not outright misleading [11]. Actual pathology glass slides have to be re-reviewed. When one attempts to critically review glass slides, it becomes clear that only specimens that were processed in a manner that allows measuring distance to margins can be adequately studied (i.e., type of margin sampling [perpendicular vs. shave] should be clearly documented along with inking key and summary of tissue sections). Most importantly, one has to unequivocally state what is the source of the tissue used to determine margin—main resection specimen or additional tissue obtained from the tumor bed?
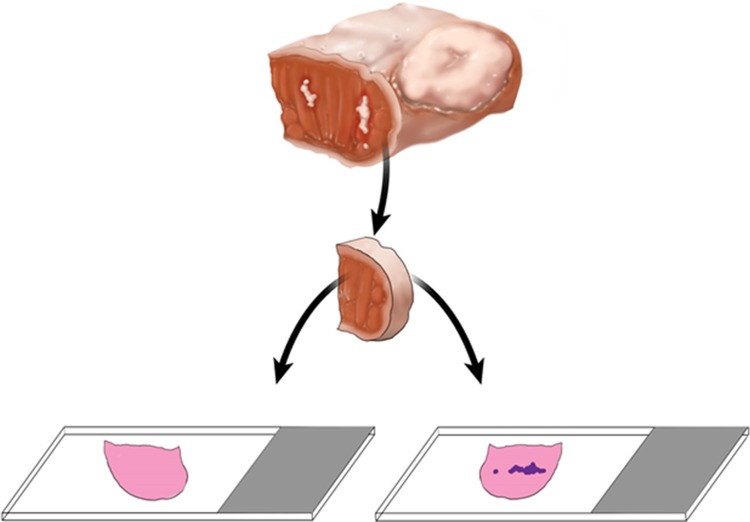
Although not explicitly presented in the literature, there seem to be a belief that one can add the width of the additional tissue obtained from the tumor bed to the margin clearance as measured from the main resection specimen. Except for rare cases when additional tissue is sutured to the main specimen (Fig. 2), reliably re-imagining the relationship between the main resection specimen and additional tumor bed tissue is very challenging, if at all possible (Fig. 3). In fact, a yet unknown number of tumor bed margins is sampled before tumor resection is even completed (see below)!
Fig. 2.
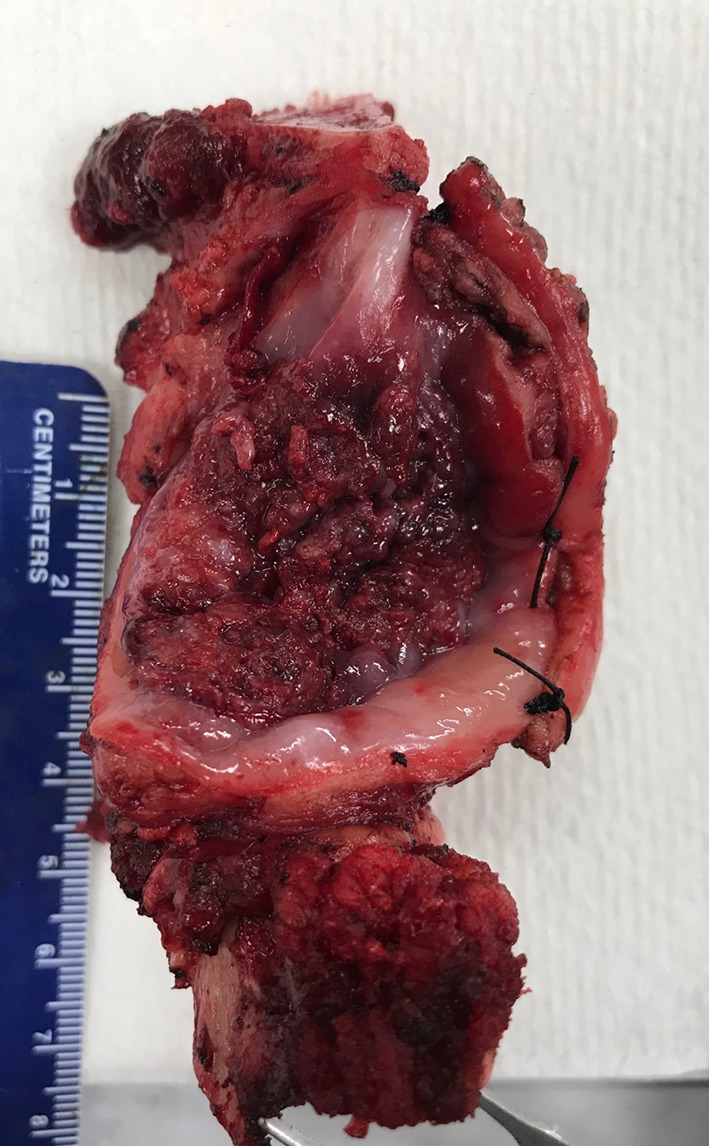
Right segmental mandibulectomy with revised buccal mucosal margin: additional rim of buccal mucosa sutured to the main resection specimen. For orientation, tips of the forceps are at the posterior (mandibular angle) aspect of the specimen. One of the hallmarks of likely adequate margin revision is additional tissue being sutured by surgeon to the main resection specimen: it eliminates the uncertainty over the spatial relationship between the main specimen and additional tissue/new margin. Coming up with the combined distance to the buccal margin, by adding distance to buccal margin on the main resection specimen and width of the additional tissue, would still be challenging. The additional buccal margin retracts, curls, and shrinks at its own rate. Measuring the width of additional buccal margin required two pairs of hands. The additional piece of buccal tissue had to be stretched out by a pathology assistant, while pathologist was applying the ruler. The distance to the closest buccal mucosal margin (on mandibulectomy) was 6 mm, while the closest soft tissue buccal margin was 4 mm (deeper/more submucosal than the depth of the additional buccal tissue). An attempt to submit initial and additional buccal margins as one tissue section in one cassette was made. However, microscopic measurement of the distance to buccal margin was complicated by the gap between the initial and additional buccal margins. Overall, reliable measurement of a distance to revised margin is possible only when residual carcinoma is identified in the additional tissue, new margin surface is clearly marked, and additional tissue is wide enough to be processed as a radial margin
Fig. 3.
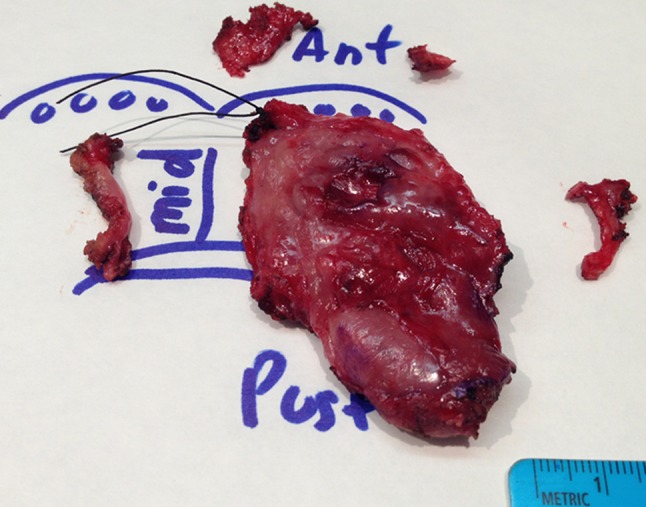
Attempting to re-imagine the spatial relationship between the main resection specimen and three mucosal tumor bed samples and to judge the quality of revision, an example of right base of tongue transoral robotically assisted resection. If the midline margin from the base of tongue resection specimen ends up being positive for carcinoma, then the additional midline tumor bed margin is likely to truly supersede it and the overall midline margin would be best considered negative. This scenario represents adequate revision, because the midline tumor bed sample is thick, about 5 mm, and spans the entire length and depth of the midline aspect of the base of tongue resection specimen. However, if the lateral (opposite to the midline, toward glossotonsillar sulcus and tonsil) margin of the main specimen is positive for carcinoma, the corresponding tumor bed margin (above the ruler) is too short and its exact position along the lateral aspect of the main specimen is too uncertain to supersede the positive specimen margin. Such revision is either inadequate or indeterminate and the overall lateral margin status is best considered still positive or indeterminate. Similarly, revision of the anterior (towards oral tongue) margin is of uncertain quality. For orientation, black suture designates anterior midline corner of the main specimen; handwritten in blue ink “Ant” stands for anterior, “mid” for midline, ‘post”, for posterior. Blue circles represent circumvallate papillae. Anterior tumor bed margin is represented by two fragments. True/new margin surface or any other orientation for tumor bed samples are unknown
Even the above-outlined strict criteria would not help to quantify the confounding effect of tumor bed samples, if any, on prognostic significance of margin clearance (as measured from the main resection specimen). However, it seems that tumor bed biopsies can be entirely ignored. In a risk model presented below, distance to closest margin was assessed from the main resection/glossectomy specimen only, while tumor bed samples were not accounted for (impossible to account for in a retrospective study, especially if gross images similar to the one in Fig. 3 are unavailable).
When above-outlined principles are followed, there appear to be a 33% decrease in risk of local recurrence for an increase of 1 mm of margin width (up to 4–5 mm of margin width for oral tongue, without adjusting for perineural invasion, PNI) [7, 10]. Apparently, on its own, distance to closest margin may be insufficient to accurately predict the risk of local recurrence in an individual patient. The analysis of 494 patients with early oral tongue SCC resulted in a risk model predicting both local recurrence and locoregional recurrence based on the distance to the closest margin and PNI (Fig. 4) [1]. In the design of the risk model numerous other clinicopathologic variables were considered, such as depth of invasion, vascular invasion, and were not included in the risk model due to the lack of predictive ability.
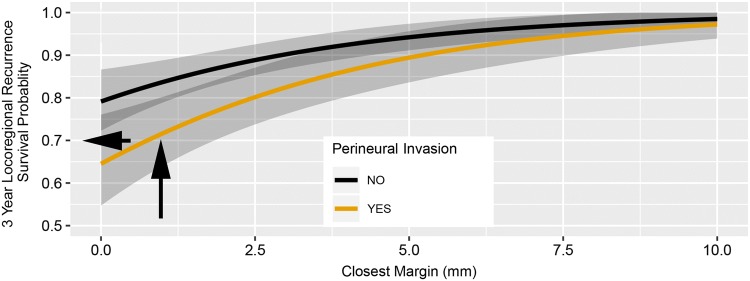
Fig. 4.
Risk model estimating probability of recurrence of early oral tongue squamous cell carcinoma based on distance to closest margin and perineural invasion. Curve for estimating locoregional recurrence are shown. The
source paper illustrates curves for local recurrence, too. Model-derived estimated probability of 3-year locoregional recurrence-free survival is plotted against the distance to closest margin and perineural invasion status. The presented curve functions like a nomogram. For instance, a patient with a closest margin of about 1 mm and positive for perineural invasion would have an estimated probability of 3-year locoregional recurrence free survival of about 0.7 (probability of recurrence at 30%). Arrows illustrate how referencing for individual patients can be done. A black vertical arrow is drawn from the point corresponding to the distance to closest margin on axis “x” until the line crosses the orange Kaplan–Meier curve (with PNI). From this point on Kaplan–Meier curve, horizontal black arrow towards the “y” axis will point out the estimated probability of 3-year locoregional recurrence free survival
A prognostic risk model for early oral tongue SCC may guide post-operative therapy choices following partial glossectomy for a specific patient. The risk model would predict probability of local recurrence and locoregional recurrence. If, for instance, the predicted local recurrence and locoregional recurrence probability at 3 years is < 5–10%, post-operative therapy would not be recommended.
However, once the relationship between the margin clearance, PNI, and risk of local recurrence is established, another question arises—what is the acceptable risk of recurrence? Determining the acceptable risk of local recurrence is a prerequisite step for defining safe margin. The acceptable risk of recurrence will likely depend on patient’s preferences and tolerance to (chemo)radiotherapy adverse effects, and expected 8% rate of second primary SCC. At 90% of recurrence free survival, the 10% risk of local recurrence at 4 years after surgery would be comparable to the risk of developing of second primary SCC [1, 7]. Perhaps for most head and neck SCCs, including early oral cavity SCC, a 90% cure rate following surgery alone would be accepted as a success and at that point one really should be concerned about the adverse effects of post-operative therapy. If 10% locoregional recurrence rate is deemed acceptable, then the safe margin clearance is 3 mm for a patient without PNI and 5.5 mm for a patient with PNI (Fig. 4). In addition, the indications for adjuvant radiotherapy in early stage oral SCC are unclear and largely based on retrospective data [1, 5, 12]. Overall, determining safe margin should be done in the context of other histologic findings, risk of developing second primary carcinoma, modern understanding of (chemo)radiotherapy efficacy, and patient’s tolerance to adverse effects.
Finally, to satisfy the need for instance for 5 mm margin clearance, one must account for a multi-factorial process of tissue shrinkage. First, the moment tissue is resected, it shrinks by 20–40% due to muscle contraction, perhaps more for anterior oral tongue than for floor of mouth [13, 14]. To a lesser extent (< 5%), tissue shrinkage appears to further depend on resection technique—cold steel, electrocautery, laser [15]. Formalin fixation would add 10% of shrinkage. So, to end up with a 5 mm margin clearance, surgeons may have to start with 8–10 mm of a cuff of normal tissue. To account for tissue curling and shrinkage, in our department, during intraoperative gross evaluation and measurement of distance to margins, we try to stretch the cuff of normal tissue to what it likely was when surgery started (see videos in [2]). Most importantly, tissue shrinkage happens in all cases and on its own does not explain margin status and certainly would not convert a close margin to a positive one [16, 17].
How does the Desired Margin Clearance Help with Selection of the Method of Intraoperative Margin Assessment?
The clinical value and method of intraoperative margin assessment (gross only versus routine microscopic examination of selected or all margins) are debated [18–20]. If one has to perform intraoperative margin assessment, the most cost- and time-efficient method depends on desired margin clearance and likelihood of other adverse histologic factors (i.e., extranodal extension [ENE], PNI), which are likelier in advanced carcinomas [21]. For instance, postoperative chemoradiotherapy is believed to be beneficial in patients with either ENE, or positive margin.
Knowing what is the practically achievable margin clearance, helps to determine what is an actionable intraoperative finding. In other words, what is the margin clearance which would prompt margin revision? Is it a microscopically positive margin (i.e., tumor at ink) or < 5 mm margin clearance?
If surgeon’s goal is > 5 mm clearance, than a gross only margin assessment would suffice in most cases [22]. Such precision can be achieved by examining mucosal surface for induration, leukoplakia, followed by cutting into the specimen (after inking), in preparation for sampling radial margins for permanent pathologic examination.
If a surgeon plans to revise a margin only knowing that there is an unequivocally positive margin or if additional histologic information is needed, such as diagnosis, depth of invasion, then one would have to extend gross examination and freeze the closest margin with tumor.
Every review on the topic of margins mentions the need for surgeon-pathologist collaboration and continuous communication. Specifically, both the surgeon and pathologist should agree on the main specimen orientation as well as what areas are concerning for a close or positive margin. Going further, we have found it helpful to report and demonstrate to surgical team the gross distance to all margins. This allows pathologists to learn what margins will be revised based on gross impression and what margins, even if positive, cannot be revised (e.g., deep margin against a carotid artery). This approach allows for prioritization of what margins, if any, have to be examined microscopically intraoperatively.
Who May Benefit the Most from Margin Assessment: Patients with Early or Advanced Carcinomas?
Privately, some surgeons accept that margin status, especially as assessed intraoperatively, may not affect overall management of many patients with oral SCC and many revisions do not convert a positive margin into a verifiably negative one. However, there is still no agreement on the subset of patients for whom intraoperative margin assessment is least relevant. One could argue for stopping routine intraoperative margin assessment for patients with advanced oral cavity SCC. However, in the absence of ENE, margin status may help to determine the need for chemotherapy or the field and dose of radiotherapy. The opposite approach would be to stop intraoperative margin assessment in patients with early oral cavity SCC, based on surgeons’ confidence in negative margins.
Even if Margin is Accepted as a Quality Measure, Does it have to be Frozen?
Most surgeons see value in margin assessment as a feedback on the quality of surgery. However, such feedback can be effective and useful even when it is not done intraoperatively. If real-time feedback is desired, it can be achieved by gross only examination. Even more importantly, if objective unimpeded pathologic examination of margins is truly valued, it must be done on the main resection specimen.
Why do some Surgeons Still Insist on Tumor Bed Sampling?
Aside from margins derived from the main resection specimen, tumor bed biopsies are still being sent for intraoperative assessment (although by fewer surgeons). Importantly, tumor bed biopsies are sent for intraoperative review without main resection specimen and likely prior to the resection of the main specimen. Outside of margin revision scenario, tumor bed sampling is not guided by the examination of the main resection specimen by pathologist. Reports attempting to justify tumor bed margins are exceedingly rare, inadequate, and were previously critiqued in detail [17].
Briefly, tumor bed biopsies do not reflect main specimen’s margin status [23], do not predict local control (over 90% of tumor bed biopsies are benign) [7], and prevent pathologists from reliably assessing margins. Tumor bed biopsies hinder pathologists’ ability to evaluate margin status in several ways. Objectively, any specimen fragmentation makes it impossible to establish relationship between tissue fragments (i.e., between one main specimen and several tumor bed samples). By convention, fragmentation represents one of the reasons to not provide margin assessment by pathologists. As a constraint on margin assessment, fragmentation cannot be reliably resolved by labeling small and commonly un-oriented tissue fragments as “margins” and submitting it to pathology as separate parts. Furthermore, to avoid interdisciplinary friction, up to one third of pathologists [24] do not sample margins from the main resection specimen when tumor bed biopsies were obtained. Some pathology reports resort to equivocal terminology (i.e., tissue edge) and misleading comments (e.g., see other parts for “true/final margins”).
Why then do some surgeons prefer tumor bed sampling? Personal communications revealed several perceived advantages of tumor bed-driven approach. Some reasoning behind upfront reliance on tumor bed margins identifies areas for improvement in pathologists’ skills and surgeon-pathologist communication.
Some surgeons prefer to control margin labeling (anatomically correct and detailed designation of margins). For instance, partial glossectomy specimens with SCC involving lateral edge of the oral tongue have superior/dorsal, deep/towards midline, and inferior/ventral/towards floor of mouth margins. When pathologists get partial glossectomy in the laboratory for intraoperative margin assessment it is apparently not uncommon to flatten the specimen and re-designate superior/dorsal margin as “medial” and inferior/ventral margin as “lateral”. Occasionally, an orienting stitch placed by surgeons on partial glossectomy and designated as “anterior”, would be assumed by pathologists to represent “12 o’clock” and all margins will be reported using a 12, 3, 6, and 9 o’clock orientation. Such confusions are better addressed by coming to a common understanding on preferred (anatomic sub-site based!) labeling terminology, rather than taking over margin labeling by upfront tumor bed sampling. Ideally, the margin labeling would be agreed upon intraoperatively with pathologist and surgeon and standardized for future use.
Surgeons know where tumor bed margins came from: Even if it is so, re-localizing this area after frozen section results are reported remains a problem: identifying the site of suboptimal margin is off target by about 1 cm in 1/3 of cases [25]. As it is now, up to 78% of the revised margins do not contain residual tumor, implying that the revised margin is taken from an area that does not correspond to positive margin in the main resection specimen [10]. Recent studies suggest to improve margin re-localization by placing tags [26].
Some surgeons rationalize upfront tumor bed sampling by work-flow and time-management advantages—“one can get the frozens (margins) off and going before the tumor is completely out”. However, is it really possible to assess margins before the tumor is out? It would be interesting to determine how often tumor bed biopsies are obtained before the main resection/tumor specimen was removed. Moreover, ablative surgeons may send off tumor bed before the entire tumor resection is completed to more quickly provide mucosa/skin dimensions for reconstructive surgeons harvesting free tissue for transfer. This technical detail (timing of tumor bed sampling) remains unexplored.
In most candid conversations, it is admitted that tumor bed biopsies represent a way to control pathology reports and margin status and to reliably get negative margins (even though in cases with positive margin on the main resection specimen, margin would commonly be negative on paper only). Such approach may backfire by setting unreasonable expectations and suggestions for 90% negative margin rate as a quality measure and/or as a condition for surgeon’s participation in clinical trials [11]. Most importantly, tumor bed biopsies result in multi-part pathology reports obfuscating overall margin status and complicating decisions by oncologists.
All involved, surgeons and pathologists, seem to have a good empiric understanding of what represents mucosal and deep margin. However, the band of tissue between the most superficial/immediately submucosal tissue and deep margin remains undesignated (Fig. 5) and is the most likely source of discrepant margins, i.e., positive main resection specimen margin and negative tumor bed biopsy.
One of the common concerns is submucosal spread of SCC that would presumably be difficult to detect on the main resection specimen. However, it was shown to be rare (1.2% of cases) and 7 mm margin clearance (from grossly identifiable tumor) was equivalent to negative frozen section [20, 22, 27].
Fig. 5.
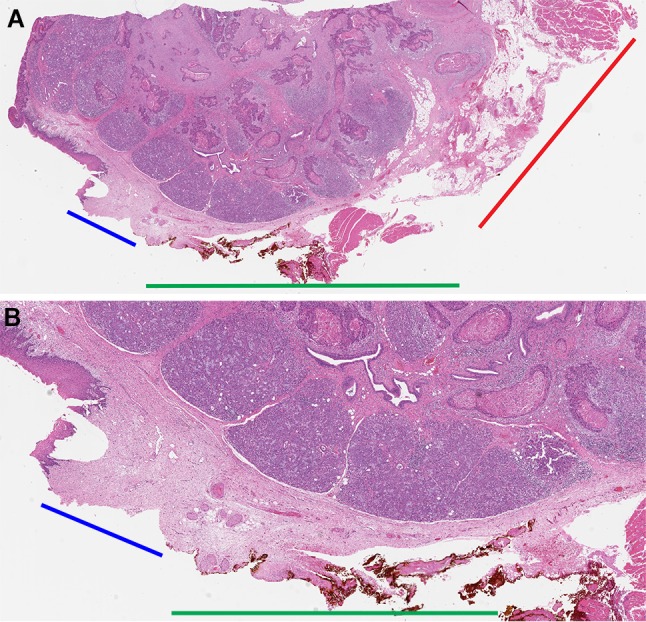
How to designate a margin between the mucosal and deep aspects of the resection specimen? An illustration of how superficial shaved mucosal margins can be. This right floor of mouth excision revealed squamous cell carcinoma extending into sublingual gland. The floor of mouth excision specimen was submitted for intraoperative margin assessment. Intraoperatively, all circumferential mucosal margins were inked and shaved off the main specimen. This is not an approach we would advocate for, but it helps to illustrate two points. The midline margin was inked orange. Shown at two different magnifications is radial tissue section of the closest midline margin obtained for permanent pathologic examination after mucosal margin was shaved off intraoperatively. a The blue bar outlines the depth of what was sampled as a mucosal margin. It is only 2–4 mm deep. These details can be deduced from the fact that the area corresponding to the blue bar lacks orange ink (the orange ink is on the shaved off mucosal margin). The red bar is against the deep margin. How to designate the margin against the green bar? The margin against the green bar seems to be too superficial for deep margin and too deep for mucosal margin (it certainly was not included in the intraoperatively shaved off mucosal margin). Such “nitpicking” is irrelevant when margins are sampled in radial fashion. However, the area against the green bar is the most likely explanation for under-sampled margins and discrepancies between mucosal tumor bed biopsies or mucosal margins shaved off the main specimen and radially sampled margins from the main resection specimen. Whole slide scanned image, original magnification of about x 1. b Higher magnification showing the absence of orange ink on tissue against the blue bar, illustrating the depth of the mucosal margin that was shaved off intraoperatively. Whole slide scanned image, original magnification of about x 3
Why Would Pathologists do not Oppose Tumor Bed Margins?
Depending on practice setting (i.e., general, rather than subspecialty driven intraoperative consultation service), pathologists may have little incentive to encourage the switch from tumor bed-driven to main resection specimen-driven margin assessment. Tumor bed margins are rarely oriented, require minimal-to-no understanding of anatomy, and, unlike resection specimens, require no handling by pathologists before microscopic sections are ready for review. Overall, tumor bed biopsies are quicker and easier to evaluate. Ironically, being submitted as distinct multiple parts of a case, tumor bed biopsies are also associated with higher Relative Value Units and reimbursement.
How Intraoperative Margin Assessment May Lead to Worse Local Control and Why Most Revisions, as Performed Currently, are Inadequate?
To understand why intraoperative margin assessment, as performed currently, is unlikely to improve local control and may result in worse local control, one has to again consider the two types of margins. Most reasons behind the inferiority of tumor bed samples were mentioned above.
Therefore, further discussion will focus on quality of margin revision—the second more understandable and justifiable scenario leading to targeted tumor bed sampling guided by the examination of the main resection specimen. In the literature, there seems to be a consensus that margin revision, as performed currently, is of little clinical value [10, 28–33]. There is no longer reason to assume or believe that every attempt to revise a margin results in a conversion of a positive margin into a negative one. As described by the College of American Pathologists Protocol for the Examination of Specimens from Patients with Cancers of the Lip and Oral Cavity and as illustrated in Figs. 3, 6, and previously [2], there are three possible outcomes of margin revision:
Conversion of a positive margin into a negative one,
Positive margin remains positive, and
Revision of indeterminate quality.
Fig. 6.
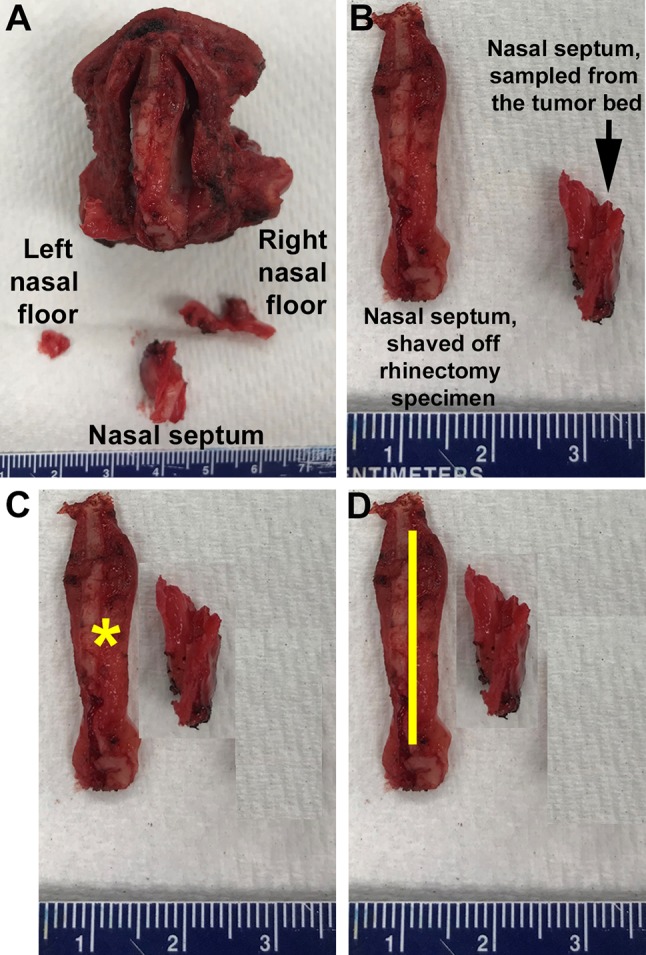
Total rhinectomy and additional tumor bed margins (left nasal floor, posterior nasal septum, and right nasal floor). Posterior view of the total rhinectomy (the specimen is placed on the nasal tip). Attempting to re-establish the spatial relationship between the rhinectomy specimen and three mucosal tumor bed margins may help to judge the adequacy of tumor bed biopsies and quality of margin revision. a The length of the tumor bed sample from the right nasal floor fits the right nasal floor aspect of the rhinectomy specimen. However, the left nasal floor tumor bed margin is about threefold smaller and is unlikely to be representative of the entire left nasal floor margin from rhinectomy specimen. b Comparing the superior-to-inferior (cranial-to-caudal) length of the posterior nasal septum margin shaved off the rhinectomy specimen (left, bigger) to nasal septum margin sampled by the surgeon from the tumor bed (right, smaller). c If the posterior nasal septum margin shaved off the rhinectomy is positive for a small focus of carcinoma (yellow asterisk), then the tumor bed nasal septum sample may supersede the carcinomatous focus at the rhinectomy nasal septum margin (if tumor bed sample originates from the exact part of the tumor bed where carcinoma was). In this scenario, the final/overall posterior nasal septum margin could be reported as positive, indeterminate, or negative. Discussing such scenarios with surgeons may help: pathologists could try to establish where the focus of carcinoma is, while surgeons may specify where from the tumor bed nasal septum sample came from (more caudal or more cranial?) d The overall/final margin should be reported as positive when the focus of carcinoma (yellow line) in the rhinectomy nasal septum margin is clearly larger than the tumor bed nasal septum fragment
In trying to assess the quality of margin revision pathologists would pay attention to spatial relationship between the main resection specimen and revised margin (Figs. 3 and 6), a challenging and frequently impossible task. Microanatomy/histologic features may be of help. For instance, if the tumor is cauterized and intermingled with the skeletal muscle at the deep inked margin, but a new separately submitted “deep margin” shows mucosa and minor salivary glands without skeletal muscle, then it is clear that the new “deep margin” sample comes from a wrong site and does not supersede positive deep margin from the main resection specimen.
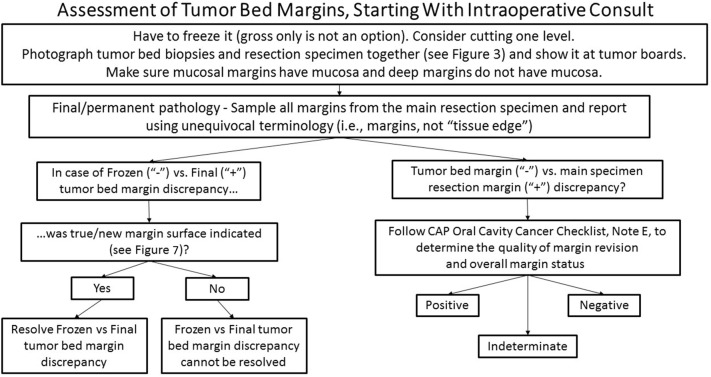
Even if additional tissue is taken from the correct site, the new true surface/margin may not always be designated and microscopic exam of the less relevant aspect of the additional tissue may be prioritized for microscopic examination intraoperatively or for final pathology (Figs. 7 and 8).
Fig. 7.
Orientation of the revised (or tumor bed) margin. Schematic representation of a left partial glossectomy with residual submucosal carcinoma at anterior margin, represented by white irregular areas in the anterior aspect of glossectomy specimen. After positive anterior margin was communicated to surgical team, additional issue was obtained; however, the new true margin surface of the revised anterior margin was not indicated. Without orientation, the surface to be examined intraoperatively or for final pathology is picked randomly. The dark blue irregular area on the right glass slide represents tumor. If the true new margin is indicated and examined intraoperatively and frozen section is negative for tumor, but permanent section of the frozen remnant reveals tumor, the overall margin status is “close, but negative”. However, if true new margin is not indicated, the determination as to what represents true margin (frozen or permanent) is impossible. Without orientation, assuming that frozen section represents the true margin is difficult to justify
Fig. 8.
Suggested framework for assessment and reporting of tumor bed biopsies
Finally, additional new margin is likely to be too thin (e.g., < 3 mm). Therefore, a positive margin would be revised to a close, rather than a negative margin.
Technical inadequacy of most revisions is not easily glimpsed from multi-part pathology reports. Revisions tend to obfuscate true margin status and may deprive patient of postoperative therapy (in the absence of other adverse factors that would warrant postoperative therapy, regardless of margins status). To summarize, variable, but predominantly suboptimal quality of margin revision, explains available data on the lack of benefit of margin revision as performed currently [10, 28–33]. Since tumor bed sampling and the need for margin revision correlates with higher rates of local recurrence, it seems safe to add such events to other known adverse features, such as PNI [4, 7, 10, 28–36].
How to Improve (Intraoperative) Margin Assessment?
The quickest and simplest way to improve overall margin evaluation would be to stop routine intraoperative margin assessment. This would shift focus to the quality of gross examination and result in more straightforward margin reporting on final pathology report. If revision is performed as a second procedure, the quality of the revision can still be judged using the criteria outlined above.
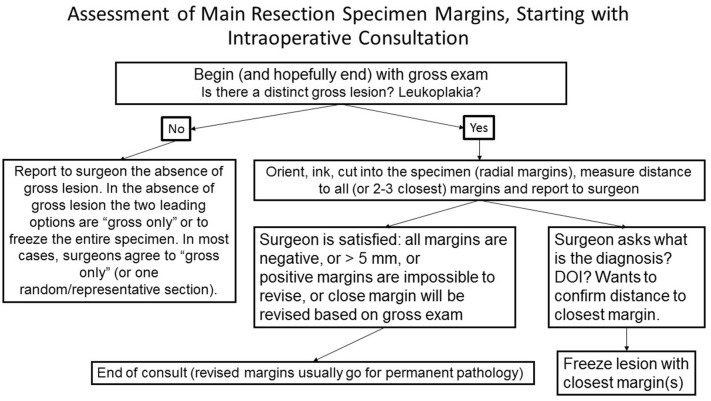
Since the cessation of routine requests for intraoperative margin assessment is unimaginable for now, the next best alternative is to convert to gross examination of the resection specimen as the mainstay of intraoperative margin assessment (Fig. 9). Gross examination of the main resection specimen should include its orientation, anatomy-based labeling of margins, inking of all margins, cutting into the specimen to allow for radial margin sampling and measurement of the distance to all margins. Detailed comparison of radial and shave margins was performed in a prior review [2]. Of note, the 8th edition of American Joint Committee on Cancer (AJCC) advocates for radial sampling of margins [37]. Shaved off circumferential margins tend to predominantly sample mucosal surface, do not consistently include the entire thickness/depth of the specimen (Fig. 5), and tend to underestimate margin clearance in irregular specimens [2].
Fig. 9.
Suggested framework for assessment and reporting of main resection specimen margins. DOI depth of invasion
Microscopic examination of selected margins after discussion of gross findings and distances to margins with surgical team seems to be most informative. Overall, it was repeatedly shown that gross only intraoperative examination, by surgeon and/or pathologist, is quicker and more cost-effective than microscopic examination [20, 22, 27].
The following approach to assessment of tumor bed biopsies may help to better elucidate their limitations (Fig. 8). When received for intraoperative consultations, pathologists have little choice but to freeze it. If the true margin surface is not indicated (Fig. 7), and in most cases it is not [23], then it is acceptable to cut and examine one technically adequate section: without knowing where the true margin is, by getting more sections one could get further away from the true margin. An adequate microscopic tissue section would have an overall contour, size, and number of fragments, resembling that of the submitted tissue, and would be otherwise technically acceptable (i.e., no tissue folds or gaps). This can be achieved to appropriately facing the frozen section block (i.e., if the strip of tissue was about 7 mm long before freezing, the length of the tissue on frozen section slide should be about 7 mm and still be in one piece/fragment). Without attention to such tissue characteristics one could examine 3–5 tissue sections, all of which may still be suboptimal.
For a mucosal margin to be potentially adequate, it should contain actual mucosal surface, while a deep margin should have no mucosa. With the exception of very small tumor bed biopsies, it is difficult to understand how a sample can be “exhausted” at the time of frozen (other than intentionally, with idea of avoiding dealing with the permanent section of the frozen section remnant). Each margin is best frozen in a separate block. Inking several tumor bed biopsies and freezing it all in one block interferes with obtaining best possible sections of each tissue fragment.
The following steps may help to demonstrate the inadequate nature of tumor bed biopsies in a given case.
First, it could be helpful to superimpose tumor bed biopsies next to the actual resection specimen and photograph it (Figs. 3 and 6). After such images are reviewed at tumor boards (multidisciplinary conferences), tumor bed biopsies tend to first get longer, wider, deeper, and better oriented. Later on, there is a good chance of surgeons converting from tumor bed-driven margin assessment to the resection specimen-drive approach: it is easier to remove more normal tissue with the main resection specimen, than to obtain 4–5 tumor bed samples that are sufficiently big and adequately labeled and oriented.
Second, the need to orient tumor bed samples as to the new margin surface is best highlighted when there is frozen versus permanent discrepancy. When tumor bed sample is reported as negative intraoperatively, but permanent section of the frozen section remnant reveals carcinoma, the final status of the tumor bed biopsy can only be deduced if the margin surface was indicated (Fig. 7). Without knowing where the new margin surface is, it is unclear whether the deeper permanent section is away from or closer to the new margin. In other words, when frozen section is benign but permanent tissue section is malignant, in the absence of orientation, there is no reason to assume that frozen section accurately reflects the status of the tumor bed biopsy.
Finally, when one of the main resection specimen margins is positive and the corresponding tumor bed sample is negative, one has to decide what the overall margin status is. It is best done by applying criteria used to judge the quality of margin revision (see above).
Summary and Challenges
The clinical value of routine intraoperative margin assessment as practiced currently is highly questionable. The absence of improvement in local control from intraoperative margin assessment is best explained by a combination of factors. The most common outcome of intraoperative margin assessment is the statement of all margins being negative. This is the result of intraoperative margin assessment in at least 70% of surgeries for early oral tongue SCC [7]. In this scenario, reassurance of the surgical team is the only contribution by the pathology team, frequently at the expense of 20–40 min of work by pathology assistant, pathology trainees, and pathologist and, perhaps increase in operating room time (especially when neck dissection is not performed and reconstruction is not planned).
Reassurance about negative margins would be an acceptable and worthy contribution, if identification of a positive margin were to lead to its revision into a truly negative one. However, research over the last three decades makes it increasingly clear that revisions, as performed currently, do not convert positive margins into negative ones and are therefore of little clinical benefit [2, 10, 28–32, 38]. Most recent meta-analysis confirmed that attempts for margin revision do not affect rates of local recurrence, which remain comparable to cases with positive margins without revision [33].
Perhaps the leading reason for intraoperative margin assessment being ineffective and potentially harmful, is the inadequacy of the tissue sent for intraoperative analysis—tumor bed biopsies. In short, tumor bed biopsies are so small, fragmented, unoriented, and not representative of the actual margin status (derived from the main resection specimen), that even based on empirical data and mostly retrospective studies, most professional organizations now advocate en bloc resection of primary tumor whenever possible (NCCN Guidelines Version 2.2019, Head and Neck Cancers [39], 8th edition of AJCC [37], 4th edition of World Health Organization Classification of Head and Neck Tumors) and many leading surgical centers move away from tumor bed based margin assessment [4, 7, 17, 23, 38–41].
Future studies are needed to design and validate risk models that would help to determine for each patient what represents a site-specific safe margin clearance, while accounting for histologic adverse factors. Such studies require multi-institutional cooperation to collect sufficient number of patients, with subgroup analysis of those patients whose early cancer did not require postoperative therapy and did not involve sampling of the tumor bed. There are currently no studies that would test how accurate are the above-outlined criteria for judging the quality of margin revision. Tumor bed-driven margin assessment should be actively discouraged by illustrating its inadequacy on a case-by-case basis.
Compliance with Ethical Standards
Conflict of Interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sridharan S, Thompson LDR, Purgina B, et al. Early squamous cell carcinoma of the oral tongue with histologically benign lymph nodes: A model predicting local control and vetting of the eighth edition of the American Joint Committee on Cancer pathologic T stage. Cancer. 2017;125(18):3198–3207. doi: 10.1002/cncr.32199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiosea SI. Intraoperative margin assessment in early oral squamous cell carcinoma. Surg Pathol Clin. 2017;10(1):1–14. doi: 10.1016/j.path.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CR, Sisson K, Moncrieff M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol. 2015;51(5):464–469. doi: 10.1016/j.oraloncology.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Buchakjian MR, Tasche KK, Robinson RA, Pagedar NA, Sperry SM. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142(12):1191–1198. doi: 10.1001/jamaoto.2016.2329. [DOI] [PubMed] [Google Scholar]
- 5.Liao CT, Chang JT, Wang HM, et al. Does adjuvant radiation therapy improve outcomes in pT1-3N0 oral cavity cancer with tumor-free margins and perineural invasion? Int J Radiat Oncol Biol Phys. 2008;71(2):371–376. doi: 10.1016/j.ijrobp.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Liao CT, Chang JT, Wang HM, et al. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann Surg Oncol. 2008;15(3):915–922. doi: 10.1245/s10434-007-9761-5. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell JH, Thompson LD, Brandwein-Gensler MS, et al. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1104–1110. doi: 10.1001/jamaoto.2015.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvvuri U, Seethala RR, Chiosea S. Margin assessment in oral squamous cell carcinoma. Cancer. 2014;120(3):452–453. doi: 10.1002/cncr.28432. [DOI] [PubMed] [Google Scholar]
- 9.Sperry SM, Varvares MA, Chiosea SI. Patients with revised surgical resection margins are best studied as a distinct group. Cancer. 2018;124(21):4262–4263. doi: 10.1002/cncr.31712. [DOI] [PubMed] [Google Scholar]
- 10.Chang AM, Kim SW, Duvvuri U, et al. Early squamous cell carcinoma of the oral tongue: comparing margins obtained from the glossectomy specimen to margins from the tumor bed. Oral Oncol. 2013;49(11):1077–1082. doi: 10.1016/j.oraloncology.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Duvvuri U, Johnson JT, Chiosea SI. Standardized Margin Assessment Is Needed Before Implementing Negative Margin as a Quality Measure. JAMA Otolaryngol Head Neck Surg. 2018;144(6):541–542. doi: 10.1001/jamaoto.2018.0074. [DOI] [PubMed] [Google Scholar]
- 12.Liao CT, Lin CY, Fan KH, et al. Identification of a high-risk group among patients with oral cavity squamous cell carcinoma and pT1-2N0 disease. Int J Radiat Oncol Biol Phys. 2012;82(1):284–290. doi: 10.1016/j.ijrobp.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Johnson RE, Sigman JD, Funk GF, Robinson RA, Hoffman HT. Quantification of surgical margin shrinkage in the oral cavity. Head Neck. 1997;19(4):281–286. doi: 10.1002/(sici)1097-0347(199707)19:4<281::aid-hed6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Mistry RC, Qureshi SS, Kumaran C. Post-resection mucosal margin shrinkage in oral cancer: quantification and significance. J Surg Oncol. 2005;91(2):131–133. doi: 10.1002/jso.20285. [DOI] [PubMed] [Google Scholar]
- 15.George KS, Hyde NC, Wilson P, Smith GI. Does the method of resection affect the margins of tumours in the oral cavity? Prospective controlled study in pigs. Br J Oral Maxillofac Surg. 2013;51(7):600–603. doi: 10.1016/j.bjoms.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Weinstock YE, Alava I, 3rd, Dierks EJ. Pitfalls in determining head and neck surgical margins. Oral Maxillofac Surg Clin North Am. 2014;26(2):151–162. doi: 10.1016/j.coms.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Chiosea S. On challenges of disproving inferiority of tumor bed margins. Oral Dis. 2019;25(8):2040–2041. doi: 10.1111/odi.13165. [DOI] [PubMed] [Google Scholar]
- 18.Gerber S, Gengler C, Gratz KW, Kruse AL. The impact of frozen sections on final surgical margins in squamous cell carcinoma of the oral cavity and lips: a retrospective analysis over an 11 years period. Head Neck oncology. 2011;3:56. doi: 10.1186/1758-3284-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gokavarapu S, Chandrasekhara Rao LM, Patnaik SC, Parvataneni N, Raju KV, Chander R. Prognostic value of frozen section in t1, t2 carcinoma of oral cavity. Indian J Otolaryngol Head Neck Surg. 2015;67(Suppl 1):86–90. doi: 10.1007/s12070-014-0783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaturvedi P, Datta S, Nair S, et al. Gross examination by the surgeon as an alternative to frozen section for assessment of adequacy of surgical margin in head and neck squamous cell carcinoma. Head Neck. 2014;36(4):557–563. doi: 10.1002/hed.23313. [DOI] [PubMed] [Google Scholar]
- 21.DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110(10 Pt 1):1773–1776. doi: 10.1097/00005537-200010000-00039. [DOI] [PubMed] [Google Scholar]
- 22.Datta S, Mishra A, Chaturvedi P, et al. Frozen section is not cost beneficial for the assessment of margins in oral cancer. Indian J Cancer. 2019;56(1):19–23. doi: 10.4103/ijc.IJC_41_18. [DOI] [PubMed] [Google Scholar]
- 23.Prabhu AV, Sturgis CD, Lai C, et al. Improving margin revision: Characterization of tumor bed margins in early oral tongue cancer. Oral Oncol. 2017;75:184–188. doi: 10.1016/j.oraloncology.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black C, Marotti J, Zarovnaya E, Paydarfar J. Critical evaluation of frozen section margins in head and neck cancer resections. Cancer. 2006;107(12):2792–2800. doi: 10.1002/cncr.22347. [DOI] [PubMed] [Google Scholar]
- 25.Kerawala CJ, Ong TK. Relocating the site of frozen sections–is there room for improvement? Head Neck. 2001;23(3):230–232. doi: 10.1002/1097-0347(200103)23:3<230::aid-hed1023>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.van Lanschot CG, Mast H, Hardillo JA, et al. Relocation of inadequate resection margins in the wound bed during oral cavity oncological surgery: a feasibility study. Head Neck. 2019;41(7):2159–2166. doi: 10.1002/hed.25690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mair M, Nair D, Nair S, et al. Intraoperative gross examination vs frozen section for achievement of adequate margin in oral cancer surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(5):544–549. doi: 10.1016/j.oooo.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Guillemaud JP, Patel RS, Goldstein DP, Higgins KM, Enepekides DJ. Prognostic impact of intraoperative microscopic cut-through on frozen section in oral cavity squamous cell carcinoma. J Otolaryngol 2010;39(4):370–7. [PubMed]
- 29.Jackel MC, Ambrosch P, Martin A, Steiner W. Impact of re-resection for inadequate margins on the prognosis of upper aerodigestive tract cancer treated by laser microsurgery. Laryngoscope. 2007;117(2):350–356. doi: 10.1097/01.mlg.0000251165.48830.89. [DOI] [PubMed] [Google Scholar]
- 30.Kwok P, Gleich O, Hubner G, Strutz J. Prognostic importance of "clear versus revised margins" in oral and pharyngeal cancer. Head Neck. 2010;32(11):1479–1484. doi: 10.1002/hed.21349. [DOI] [PubMed] [Google Scholar]
- 31.Patel RS, Goldstein DP, Guillemaud J, et al. Impact of positive frozen section microscopic tumor cut-through revised to negative on oral carcinoma control and survival rates. Head Neck. 2010;32(11):1444–1451. doi: 10.1002/hed.21334. [DOI] [PubMed] [Google Scholar]
- 32.Scholl P, Byers RM, Batsakis JG, Wolf P, Santini H. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue. Prognostic and therapeutic implications. Am J Surg 1986;152(4):354–60. [DOI] [PubMed]
- 33.Bulbul MG, Tarabichi O, Sethi RK, Parikh AS, Varvares MA. Does clearance of positive margins improve local control in oral cavity cancer? A meta-analysis. Otolaryngol Head Neck Surg 2019. 10.1177/0194599819839006 [DOI] [PubMed]
- 34.Buchakjian MR, Ginader T, Tasche KK, Pagedar NA, Smith BJ, Sperry SM. Independent predictors of prognosis based on oral cavity squamous cell carcinoma surgical margins. Otolaryngol Head Neck Surg 2018;154(4):675–82. [DOI] [PMC free article] [PubMed]
- 35.Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125(10):2298–2307. doi: 10.1002/lary.25397. [DOI] [PubMed] [Google Scholar]
- 36.Tassone P, Savard C, Topf MC, et al. Association of positive initial margins with survival among patients with squamous cell carcinoma treated with total laryngectomy. JAMA Otolaryngol Head Neck Surg. 2018;144(11):1030–6. [DOI] [PMC free article] [PubMed]
- 37.AJCC 8th Edition Updates and Corrections. In; 2019.
- 38.Giurintano JP, Ha PK. Should margin sampling be obtained from the specimen or from the resection bed in oral cavity cancer? In: Difficult decisions in head and neck oncologic surgery. Berliin: Springer; 2019. p. 31–39.
- 39.Network NCC. Head and Neck Cancers. In; 2019.
- 40.Amit M, Na'ara S, Leider-Trejo L, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: A prospective randomized controlled study. Head Neck. 2016;38(Suppl 1):E1803–E1809. doi: 10.1002/hed.24320. [DOI] [PubMed] [Google Scholar]
- 41.Ettl T, El-Gindi A, Hautmann M, et al. Positive frozen section margins predict local recurrence in R0-resected squamous cell carcinoma of the head and neck. Oral Oncol. 2016;55:17–23. doi: 10.1016/j.oraloncology.2016.02.012. [DOI] [PubMed] [Google Scholar]