Abstract
Based on evidence accumulated over the past three decades showing that noninvasive encapsulated follicular variant of papillary thyroid carcinoma has an indolent clinical behavior and a RAS-like molecular profile similar to follicular adenoma, the Endocrine Pathology Society working group in 2016 proposed to rename this entity as “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)” in order to eliminate the term “carcinoma” from the diagnosis. It is a major evidence-based attempt initiated by an international group of endocrine pathologists to tackle the epidemic of thyroid cancer overdiagnosis and overtreatment. However, its creation and continuous existence are not without controversies. NIFTP has sparked a wave of follow up studies aiming to decipher the exact nature of this new entity. In this review, we summarize the rationale, diagnostic criteria, controversies and subsequent changes to the NIFTP concept, and their impact on patient care and pathology practice.
Keywords: Thyroid, Follicular variant of papillary thyroid carcinoma, Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), BRAF, RAS
Introduction
The term FVPTC was coined by Stuart Lindsay in 1960 in a book entitled “Carcinoma of the thyroid gland” [1]. In his article, he described these tumors as having a follicular growth pattern and nuclear features similar to the one seen in papillary carcinoma. In 1977, Chen and Rosai reported the first detailed clinico-pathologic analysis on FVPTC and concluded that it is a tumor that “resembled papillary carcinoma in its biologic behavior and all morphologic features with the exception that papillae were not present” [2]. In this small series of six patients, where all tumors were infiltrative and lacked complete encapsulation, five individuals developed nodal metastasis and two died of or with disease.
Since that seminal article, FVPTC has gained in popularity among practicing pathologists, and the diagnosis of papillary thyroid carcinoma (PTC) became an exercise based largely on identifying the nuclear features of PTC regardless of architectural pattern, encapsulation and invasive status. Consequently, the diagnosis of papillary carcinoma follicular variant began to prevail, and FVPTC, including the encapsulated noninvasive form, became one of the most common subtype of PTC [3–5].
In 2000s, emerging molecular studies shed light on the nature of FVPTC. As a whole, FVPTC shows a molecular profile similar to follicular adenoma and follicular carcinoma, characterized with high frequency (approximately 43%) of RAS mutations [6–8]. The encapsulated form of FVPTC is characterized by the lack of BRAF mutations, a high prevalence of RAS mutations, and in some instances the presence of PAX8-PPARG rearrangement [9, 10]. In 2014, The Cancer Genome Atlas (TCGA) reported a comprehensive molecular profile of 496 PTCs, confirming that FVPTC has frequent RAS mutation and a RAS-like molecular signature, unlike classic and tall cell variants of PTC with a BRAFV600E-like profile [11].
Within the same period, several studies have investigated the clinical behavior of FVPTC. Liu et al. showed that the clinical behavior and outcome of encapsulated and infiltrative FVPTC were drastically different: while infiltrative FVPTC are akin to classic PTC with a propensity to spread to regional lymph nodes, encapsulated FVPTC, in particular the noninvasive form, behaved like follicular adenoma with negligible risk of nodal metastasis and recurrence [12]. Multiple additional studies have further confirmed that encapsulated FVPTC carries no risk of recurrence or death when capsular or vascular invasion is carefully excluded by adequate tumor capsule sampling [5, 13–18].
In 2016, a working group of 28 international experts, including 24 experienced thyroid pathologists, critically re-examined the entity using a cohort of 109 patients with noninvasive encapsulated FVPTC who had at least 10-year follow up and did not receive post-operative radioactive iodine (RAI) treatment. None (0%) developed recurrence [19] As a result of this endeavor, a consensus statement was published advocating for a nomenclature change from noninvasive encapsulated FVPTC to NIFTP with a fundamental aim to avoid the term “carcinoma” [19]. Soon after, NIFTP has been adopted by mainstream clinical management guidelines, including the American Thyroid Association (ATA) [20], the National Comprehensive Cancer Network (NCCN) [21] and the American Head and Neck Society (AHNS) guidelines [22].
The Original Diagnostic Criteria of NIFTP and Their Evolution
The original diagnostic criteria of NIFTP proposed by the consensus statement are summarized in Table 1. Although a diagnosis of NIFTP should be considered in any noninvasive encapsulated/well-demarcated follicular-patterned lesion with nuclear atypia, there are several exclusion criteria that need to be applied, namely psammoma bodies, > 30% solid growth, > 1% true papillae with fibrovascular cores lined by cells with PTC nuclear features, a mitotic rate of ≥ 3/10 high power fields, and/or features of other PTC variants, e.g. tall cell variant [19]. The nuclear score is determined by a 3-point system: (1) nuclear size and shape; (2) nuclear membrane irregularity; and (3) chromatin characteristics. A nuclear score of 2 or 3 (i.e. the presence of at least 2 of the above-mentioned nuclear features) is required for a diagnosis of NIFTP.
Table 1.
Consensus diagnostic criteria of noninvasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP),
adapted from Nikiforov et al. [19]
| Encapsulated or clear demarcation |
| Follicular growth pattern |
| Nuclear score 2–3 |
| No vascular or capsular invasion |
| Absence of all the following features |
| > 1% papillae |
| Psammoma bodies |
| > 30% solid/trabecular/insular growth patterns |
| Tumor necrosis |
| High mitotic activity (≥ 3 per 10 high power fields) |
| Cell/morphologic characteristics of other variants of PTC, e.g. tall cell and cribriform morular variants |
Since the consensus statement of 2016, multiple subsequent retrospective NIFTP studies have been published [5, 23–31]. In accordance with the findings from the NIFTP consensus cohort, most studies using the original NIFTP diagnostic criteria have reported negligible risk of nodal metastasis and/or recurrence as well as a molecular profile characterized by mutations in RAS and absence of BRAFV600E mutations [5, 23-28]. However, three studies, one by Parente et al. from Canada [30] and two by Kim et al. [29] and Cho et al. [31] from Korea, have found up to a 6% rate of metastasis (predominantly nodal) in NIFTP patients, particularly in lesions containing a small percentage (< 1%) of true papillae. Even in tumors without any papillae, the metastatic risk was reported at 2–5% in these three studies. However, the studies reported by Kim et al. and Cho et al. included tumors with synchronous papillary microcarcinoma in the specimens and none of the above three studies compared the molecular profile of NIFTP with the metastasis. Therefore, one cannot exclude with confidence the possibility that the nodal metastasis observed in these patients were associated with a separate papillary microcarcinoma, a well-reported and documented possibility [32–35]. Additionally, since metastatic nodal and even distant disease has been reported in total thyroidectomy specimen negative for carcinoma and entirely submitted for microscopic examination [36, 37], the metastatic deposits seen in these three articles may have even originated from separate infiltrative small carcinomas embedded in the paraffin block or which had undergone regression.
Nevertheless, because of the results of these studies, members of the NIFTP consensus group subsequently published two commentaries proposing to revise the diagnostic criteria from < 1% of papillae to no true papillae allowed [38, 39]. This revision practically means that the finding of a single true papillae in an otherwise noninvasive follicular-patterned lesion with nuclear score of 2 or 3 will lead to a diagnosis of cancer.
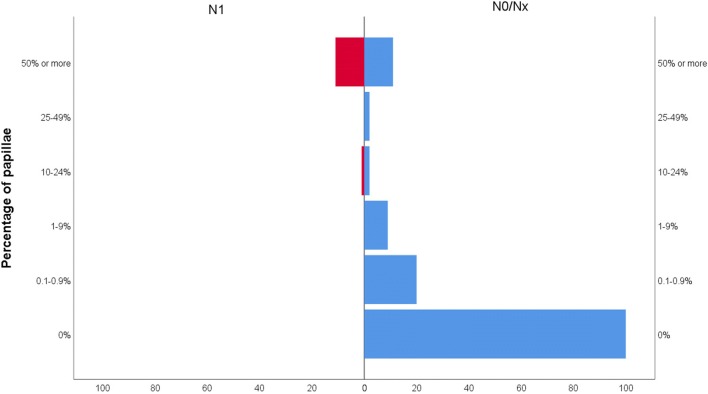
Exactly how many papillae are required for an encapsulated lesion to acquire the potential to develop lymph node metastasis? We have addressed this question in a large cohort of 235 unifocal encapsulated papillary thyroid carcinoma and NIFTP [40]. In noninvasive tumor, nodal metastasis was only observed in neoplasms containing at least 10% of papillae (Fig. 1), whereas in the entire cohort (invasive and non-invasive), nodal disease could be seen in tumors with 1% or more papillae. No nodal metastasis was observed in 127 patients without any true papillae and 31 patients with < 1% of true papillae [40]. The discrepancy between our study and most of the published literature on NIFTP on one side and those of Parente et al. [30], Kim et al. [29] and Cho et al. [31] on the other could be due to differences in defining or quantifying papillae. Whatever the reason for these discordant results, our findings indicate that the original criterion of 1% papillae may still be sound for the diagnosis of NIFTP. Future studies with larger number of encapsulated tumors with rare papillae (< 1%) will further help solve this controversy. Finally, in regard to the assessment of invasion, the original publication stated that it “requires adequate microscopic examination of the tumor capsule interface” [19]. There is now a general consensus that the whole tumor capsule should be submitted for histologic examination to exclude any invasion which is the most crucial defining feature of NIFTP [39].
Fig. 1.
Regional lymph node metastatic status according to percentage of papillae in 156 unifocal non-invasive encapsulated papillary thyroid carcinoma. Patients with < 10% papillae do not have any lymph node metastasis. Vertical axis represents percentage of papillae in the tumor while horizontal axis represents number of patients. Left red bars indicate patients with N1 (lymph node metastasis) and right blue bars patients with no nodal disease (N0/Nx). From reference [40]
Expansion of the Concept of NIFTP: Subcentimeter, Large (at Least 4 cm), Oncocytic Lesions and Lesions in Pediatric Patients
As the cohorts studied by the NIFTP consensus conference [19] and in previous reports [12, 17] did not include or specifically address subcentimeter lesions and oncocytic lesions, practice varies when such noninvasive lesions are encountered.
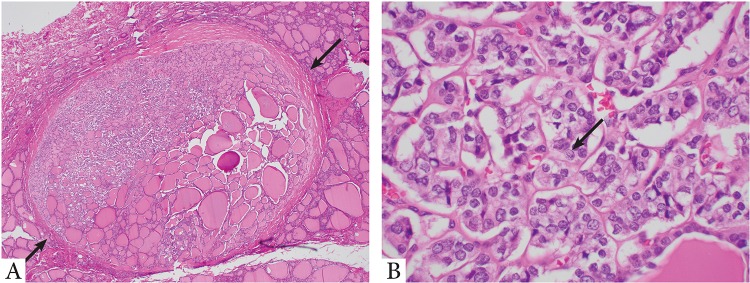
Although it is counter-intuitive to label a subcentimeter non-invasive encapsulated follicular patterned lesion with nuclear score of 2 to 3 as carcinoma while a similar histologic lesion that is > 1 cm in size is classified as NIFTP, some continuously label them as papillary microcarcinoma. Two recent studies have addressed this issue and independently demonstrated the highly indolent nature of papillary microcarcinoma, noninvasive encapsulated FVPTC: one included 52 cases with a median follow up of 6.3 years and the other reported 8 cases with a mean follow up of 12 years, all of which showed a negligible risk of nodal metastasis, distant metastasis, and recurrence [24, 28] (Fig. 2).
Fig. 2.
Forty-nine-year-old female with a 0.35 cm noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) who did not receive radioactive iodine (RAI) therapy. Patient is without nodal disease and no recurrence after 7 years. a Low power microscopic view of the tumor which is completely encapsulated (arrows) and non-invasive. b High power microscopic view shows enlarged, irregular, overlapping nuclei with chromatin clearing and nuclear grooves (arrow)
Large NIFTPs of at least 4 cm in size do exist. Prior to the introduction of NIFTP, these lesions would be staged as pT3a using the 8th edition American Joint Committee on Cancer (AJCC) staging manual [41]. According to the current American Thyroid Association (ATA) guidelines [20], post-operative radioactive iodine treatment would be offered as a management option to this cohort of patients. The implementation of NIFTP would alter the treatment from a total thyroidectomy with possible post-operative radioactive iodine treatment to lobectomy alone. In a recent international collaborative study involving four tertiary hospitals, we studied 79 patients with NIFTP of at least 4 cm in size [25]. All patients were disease free with a median follow up of 6.7 years, including 37 individuals that did not receive radioactive iodine therapy. Based on these results, it appears that large NIFTP can be safely and adequately managed by surgical treatment alone as long as the tumor capsule is entirely sampled to exclude invasion.
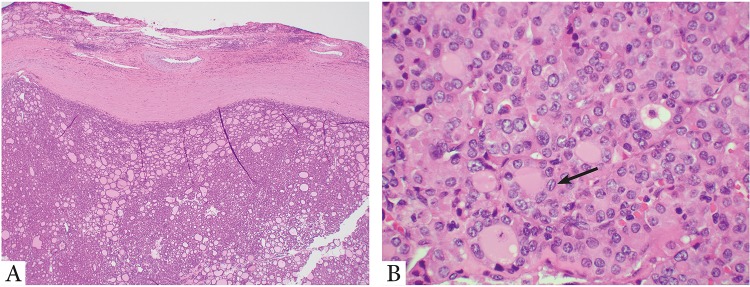
Similarly, we have recently demonstrated that noninvasive follicular patterned nodules with papillary carcinoma nuclear alterations and oncocytic change (i.e. noninvasive encapsulated oncocytic FVPTC, Fig. 3) follow an indolent clinical course without metastasis and recurrence with a median follow up of 10.2 years in an international multi-institutional cohort of 61 patients [23]. Additionally, noninvasive encapsulated oncocytic FVPTC has a high frequency of RAS mutations (being 33%) and lacks BRAFV600E mutation similar to what has been reported in NIFTP [23]. Mariani et al. have also shown that NIFTP may occur in pediatric patients and their NIFTP cases did not recur [26]. However, the pediatric patients in this report were very few in numbers. In regard to multifocal NIFTP, the number of reported cases is also too small to assess their behavior [42].
Fig. 3.
Forty-eight-year-old female with a 1.3 cm oncocytic noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) treated by lobectomy only. Patient is without nodal disease and no recurrence after 5 years. a Low power microscopic view of the tumor which is thickly encapsulated without invasion. b High power microscopic view shows enlarged, irregular, overlapping, clear nuclei with grooves (arrow). The tumor cells show oncocytic features with abundant eosinophilic granular cytoplasm
Taken together, this data provides support for the expansion of the concepts of NIFTP to specific patient populations, e.g. those with large, small or oncocytic tumors.
The Molecular Profile of NIFTP and Its Impacts on Commercially Available Molecular Platforms
Molecular testing has been adopted by the 2015 ATA guideline [20] as an alternative in managing thyroid nodules with an indeterminate diagnosis on FNA, in particular the atypia of undetermined significance (AUS) and the follicular neoplasm/suspicious for follicular neoplasms (FN/SFN) categories. A number of molecular classifiers have been studied, validated, and are available for clinical usage, with the most common used ones being Thyroseq [43] and Afirma assays [44–46].
As discussed in the previous section, studies have shown that NIFTP/non-invasive FVPTC is associated with a high frequency of RAS mutation [9-11] (Table 2). Similarly, Nikiforov et al. has reported the molecular profile of 27 cases from the consensus NIFTP cohort and detected a high frequency of RAS mutation (8/27, 30%) and PPARG fusion (6/27, 22%) (19). Other molecular events reported included: THADA (thyroid adenoma associated) fusion (6/27, 22%), EIF1AX (2/27, 7%, both coexisting with RAS mutation), and BRAFK601E (1/27, 4%) [19]. It is evident that all the common mutations and fusions detected in NIFTP can be captured by Thyroseq version 3, a next generation sequencing platform of 112 thyroid cancer-related genes [43]. Hence, it is reasonable to assume that the positive predictive value of Thyroseq in detecting malignancy would decrease, while the positive predictive value to predict neoplasm (i.e. the risk for a “surgically treatable condition”) remains unchanged if NIFTP is considered a non-malignant tumor. Indeed, in a large-scale prospective study investigating the performance of Thyroseq version 3 in indeterminate cytologic samples, NIFTP was detected in 11 of 257 cases (4%), all of which had a positive Thyroseq results and a RAS-like signature [43].
Table 2.
Molecular profile of noninvasive follicular thyroid neoplasms with papillary-like nuclear features (NIFTP)
| References | Detection method | RAS | BRAFV600E | PPARG fusion | THADA fusion | BRAF other than V600E | EIF1AX | PTEN | No mutations found |
|---|---|---|---|---|---|---|---|---|---|
| Nikiforov et al. [19] | NGS | 8/27 (30%)a | 0/27 | 6/27 (22%) | 6/27 (22%) | 1/27 (4%) | 2/27 (7%)a | 0/27 | 6/27 (22%) |
| Zhao et al. [51] | NGS | 27/48 (56%) | 1/48 (2%) | 2/48 (4%) | NA | NA | NA | NA | 18/48 (38%) |
| Brandler et al. [52] | NGS | 18/27 (67%)b | 0/27 | 3/27 (11%) | 3/27 (11%) | 1/27 (4%)c | 1/27 (4%)c | 1/27 (4%) | 1/27 (4%) |
| Total | 53/102 (52%) | 1/102 (1%) | 11/102 (11%) | 9/54 (17%) | 2/54 (4%) | 3/54 (6%) | 1/54 (2%) | 25/102 (25%) |
NGS next generation sequencing
aTwo cases had concomitant RAS and EIF1AX mutations
bThree of the 18 were associated with concurrent mutations (TP53, n = 1; PTEN, n = 2)
cOne case showed two mutations EIF1AX and BRAF T599_R603
Afirma gene expression classifier investigates the mRNA expression profile of 167 genes, and has been shown to be an excellent rule out test with a negative predictive value of 95%, 94%, and 85% for FNA diagnoses of AUS, FN/SFN, and suspicious categories [47]. Hang et al. have investigated 244 thyroid FNAs with a diagnosis of AUS and a suspicious or benign Afirma results. All their cases reclassified as NIFTP were suspicious by Afirma results [48]. In a study comparing Afirma and Thyroseq, all cases meeting the criteria for NIFTP demonstrated either high-risk mutations on Thyroseq or a "suspicious" result on Afirma GEC [49]. In the era of NIFTP, this clearly shows that a “positive" test result for either the Afirma or Thyroseq should not exclude conservative (i.e. lobectomy) surgical management [49].
Although almost all studies demonstrated that NIFTP has a RAS mutation profile and in general lacks BRAFV600E [50], one study showed a significant number of BRAFV600E mutation (10%) in NIFTP [31]. Other authors have found BRAFV600E mutation but at a much lower rate such as 1 in 50 cases in the article of Zhao et al. [51] In our most recent study, we were surprised to find one out of 50 (2%) NIFTPs positive for BRAFV600E by immunohistochemistry [40]. It was a 0.2 cm well-circumscribed tumor completely devoid of papillae on multiple H&E levels examined. We do not have a clear explanation for this unusual molecular finding. Whatever the reason, we are in agreement with the statement by some of the authors of the NIFTP working group that detection of BRAFV600E by immunohistochemistry or molecular studies should lead to an exhaustive search for papillae and invasion [39]. However, as stated by the same investigators, it cannot be used solely to exclude NIFTP which is a histologic diagnosis [39]. In summary, comprehensive genotypic studies in relatively large series (Table 2) and immunostaining on a large number of cases clearly show that NIFTP has mainly RAS mutations, some PPARG and THADA rearrangements and very rarely BRAFV600E mutation [52, 53].
Conclusions
Based on the clinical and molecular evidences accumulated over the past three decades, the term NIFTP was introduced into the field of thyroid pathology to spare patients afflicted with a very indolent tumor the side effects of completion thyroidectomy, RAI therapy and the psychosocial impact of a cancer diagnosis. Three years after the advent of NIFTP, multiple subsequent studies have been published further supporting the overall excellent prognosis of these lesions. Evidence based studies have suggested that NIFTP can include subcentimeter lesions, large size tumors, as well as nodules with oncocytic features. However, the concept of NIFTP was and continues to be controversial, as several studies have implied a small but non-zero risk of nodal metastasis. Additional studies, especially large-scale multi-center well-designed studies with long term follow up are needed to bring the controversies to an end.
Regardless, the introduction of NIFTP brings a shift in the practice of thyroid pathology. As it is now the invasive status that determines a cancer diagnosis, much needed attention has been switched from evaluation of nuclear features to capsular sampling and determination of invasion. Beyond thyroid pathology, the renaming of the non-invasive encapsulated FVPTC into NIFTP represents a road map that can be used to reduce the overdiagnosis and overtreatment of other indolent epithelial neoplasms through a change in nomenclature.
Acknowledgements
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflict of interest
No competing financial interests exist for all contributory authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lindsay S. Carcinoma of the thyroid gland: a clinical and pathologic study of 293 patients at the university of California hospital. Springfield Ill: Charles C Thomas; 1960. [Google Scholar]
- 2.Chen KT, Rosai J. Follicular variant of thyroid papillary carcinoma: a clinicopathologic study of six cases. Am J Surg Pathol. 1977;1(2):123–130. doi: 10.1097/00000478-197706000-00003. [DOI] [PubMed] [Google Scholar]
- 3.LiVolsi VA, Asa SL. The demise of follicular carcinoma of the thyroid gland. Thyroid. 1994;4(2):233–236. doi: 10.1089/thy.1994.4.233. [DOI] [PubMed] [Google Scholar]
- 4.Tallini G, Tuttle RM, Ghossein RA. The history of the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2016-2976. [DOI] [PubMed] [Google Scholar]
- 5.Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to noninvasive follicular thyroid neoplasm with papillary-like nuclear features would help prevent overtreatment. Mod Pathol. 2016;29(7):698–707. doi: 10.1038/modpathol.2016.65. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120(1):71–77. doi: 10.1309/ND8D-9LAJ-TRCT-G6QD. [DOI] [PubMed] [Google Scholar]
- 7.Wreesmann VB, Ghossein RA, Hezel M, Banerjee D, Shaha AR, Tuttle RM, Shah JP, Rao PH, Singh B. Follicular variant of papillary thyroid carcinoma: genome-wide appraisal of a controversial entity. Genes Chromosomes Cancer. 2004;40(4):355–364. doi: 10.1002/gcc.20049. [DOI] [PubMed] [Google Scholar]
- 8.Giordano TJ, Kuick R, Thomas DG, Misek DE, Vinco M, Sanders D, Zhu Z, Ciampi R, Roh M, Shedden K, et al. Molecular classification of papillary thyroid carcinoma: distinct BRAF, RAS, and RET/PTC mutation-specific gene expression profiles discovered by DNA microarray analysis. Oncogene. 2005;24(44):6646–6656. doi: 10.1038/sj.onc.1208822. [DOI] [PubMed] [Google Scholar]
- 9.Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, Ghossein RA. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23(9):1191–1200. doi: 10.1038/modpathol.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howitt BE, Jia Y, Sholl LM, Barletta JA. Molecular alterations in partially-encapsulated or well-circumscribed follicular variant of papillary thyroid carcinoma. Thyroid. 2013;23(10):1256–1262. doi: 10.1089/thy.2013.0018. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research N Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–1264. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 13.Widder S, Guggisberg K, Khalil M, Pasieka JL. A pathologic re-review of follicular thyroid neoplasms: the impact of changing the threshold for the diagnosis of the follicular variant of papillary thyroid carcinoma. Surgery. 2008;144(1):80–85. doi: 10.1016/j.surg.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Piana S, Frasoldati A, Di Felice E, Gardini G, Tallini G, Rosai J. Encapsulated well-differentiated follicular-patterned thyroid carcinomas do not play a significant role in the fatality rates from thyroid carcinoma. Am J Surg Pathol. 2010;34(6):868–872. doi: 10.1097/PAS.0b013e3181dbee07. [DOI] [PubMed] [Google Scholar]
- 15.Vivero M, Kraft S, Barletta JA. Risk stratification of follicular variant of papillary thyroid carcinoma. Thyroid. 2013;23(3):273–279. doi: 10.1089/thy.2012.0369. [DOI] [PubMed] [Google Scholar]
- 16.Ganly I, Wang L, Tuttle RM, Katabi N, Ceballos GA, Harach HR, Ghossein R. Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Hum Pathol. 2015;46(5):657–664. doi: 10.1016/j.humpath.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosario PW, Penna GC, Calsolari MR. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: is lobectomy sufficient for tumours %3e/=1 cm? Clin Endocrinol (Oxf) 2014;81(4):630–632. doi: 10.1111/cen.12387. [DOI] [PubMed] [Google Scholar]
- 18.Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA. Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern) Thyroid. 2009;19(2):119–127. doi: 10.1089/thy.2008.0303. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, Mandel SJ, Morris JC, Nassar A, Pacini F, et al. American thyroid association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27(4):481–483. doi: 10.1089/thy.2016.0628. [DOI] [PubMed] [Google Scholar]
- 21.NCCN clinical practive guidelines in oncology (NCCN guidelines): thyroid carcinoma. Version 1.2019 https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- 22.Ferris RL, Nikiforov Y, Terris D, Seethala RR, Ridge JA, Angelos P, Duh QY, Wong R, Sabra MM, Fagin JA, et al. AHNS Series: Do you know your guidelines? AHNS endocrine section consensus statement: state-of-the-art thyroid surgical recommendations in the era of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Head Neck. 2018;40(9):1881–1888. doi: 10.1002/hed.25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Reznik E, Tuttle RM, Knauf J, Fagin JA, Katabi N, Dogan S, Aleynick N, Seshan V, Middha S, et al. Outcome and molecular characteristics of non-invasive encapsulated follicular variant of papillary thyroid carcinoma with oncocytic features. Endocrine. 2019;64(1):97–108. doi: 10.1007/s12020-019-01848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Farhat N, Barletta JA, Hung YP, Biase D, Casadei GP, Onenerk AM, Tuttle RM, Roman BR, Katabi N, et al. Should subcentimeter non-invasive encapsulated, follicular variant of papillary thyroid carcinoma be included in the noninvasive follicular thyroid neoplasm with papillary-like nuclear features category? Endocrine. 2018;59(1):143–150. doi: 10.1007/s12020-017-1484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu B, Tallini G, Scognamiglio T, Roman BR, Tuttle RM, Ghossein RA. Outcome of large noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27(4):512–517. doi: 10.1089/thy.2016.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariani RA, Kadakia R, Arva NC. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: should it also be reclassified in children? Pediatr Blood Cancer. 2018;65(6):e26966. doi: 10.1002/pbc.26966. [DOI] [PubMed] [Google Scholar]
- 27.Johnson DN, Furtado LV, Long BC, Zhen CJ, Wurst M, Mujacic I, Kadri S, Segal JP, Antic T, Cipriani NA. Noninvasive follicular thyroid neoplasms with papillary-like nuclear features are genetically and biologically similar to adenomatous nodules and distinct from papillary thyroid carcinomas with extensive follicular growth. Arch Pathol Lab Med. 2018;142(7):838–850. doi: 10.5858/arpa.2017-0118-OA. [DOI] [PubMed] [Google Scholar]
- 28.Shafique K, LiVolsi VA, Montone K, Baloch ZW. Papillary thyroid microcarcinoma: reclassification to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a retrospective clinicopathologic study. Endocr Pathol. 2018;29(4):339–345. doi: 10.1007/s12022-018-9546-3. [DOI] [PubMed] [Google Scholar]
- 29.Kim MJ, Won JK, Jung KC, Kim JH, Cho SW, Park DJ, Park YJ. Clinical characteristics of subtypes of follicular variant papillary thyroid carcinoma. Thyroid. 2018;28(3):311–318. doi: 10.1089/thy.2016.0671. [DOI] [PubMed] [Google Scholar]
- 30.Parente DN, Kluijfhout WP, Bongers PJ, Verzijl R, Devon KM, Rotstein LE, Goldstein DP, Asa SL, Mete O, Pasternak JD. Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma: is NIFTP truly benign? World J Surg. 2018;42(2):321–326. doi: 10.1007/s00268-017-4182-5. [DOI] [PubMed] [Google Scholar]
- 31.Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017;30(6):810–825. doi: 10.1038/modpathol.2017.9. [DOI] [PubMed] [Google Scholar]
- 32.Gao X, Zhang X, Zhang Y, Hua W, Maimaiti Y, Gao Z. Is papillary thyroid microcarcinoma an indolent tumor?: a retrospective study on 280 cases treated with radioiodine. Medicine (Baltimore) 2016;95(40):e5067. doi: 10.1097/MD.0000000000005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, McCabe C, Boelaert K, Franklyn JA. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocr Metab. 2014;99(8):2834–2843. doi: 10.1210/jc.2013-2118. [DOI] [PubMed] [Google Scholar]
- 34.Piana S, Ragazzi M, Tallini G, de Biase D, Ciarrocchi A, Frasoldati A, Rosai J. Papillary thyroid microcarcinoma with fatal outcome: evidence of tumor progression in lymph node metastases: report of 3 cases, with morphological and molecular analysis. Hum Pathol. 2013;44(4):556–565. doi: 10.1016/j.humpath.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Tallini G, de Biase D, Durante C, Acquaviva G, Bisceglia M, Bruno R, Bacchi Reggiani ML, Casadei GP, Costante G, Cremonini N, et al. BRAF V600E and risk stratification of thyroid microcarcinoma: a multicenter pathological and clinical study. Mod Pathol. 2015;28(10):1343–1359. doi: 10.1038/modpathol.2015.92. [DOI] [PubMed] [Google Scholar]
- 36.Xu B, Scognamiglio T, Cohen PR, Prasad ML, Hasanovic A, Tuttle RM, Katabi N, Ghossein RA. Metastatic thyroid carcinoma without identifiable primary tumor within the thyroid gland: a retrospective study of a rare phenomenon. Hum Pathol. 2017;65:133–139. doi: 10.1016/j.humpath.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishikawa M, Toyoda N, Yonemoto T, Fujiyama A, Ogawa Y, Tokoro T, Sakaguchi N, Yoshimura M, Yoshikawa N, Tabata S, et al. Occult papillary thyroid carcinoma in Hashimoto's thyroiditis presenting as a metastatic bone tumor. Endocr J. 1998;45(1):111–116. doi: 10.1507/endocrj.45.111. [DOI] [PubMed] [Google Scholar]
- 38.Lloyd RV, Asa SL, LiVolsi VA, Sadow PM, Tischler AS, Ghossein RA, Tuttle RM, Nikiforov YE. The evolving diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) Hum Pathol. 2018;74:1–4. doi: 10.1016/j.humpath.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 39.Nikiforov YE, Baloch ZW, Hodak SP, Giordano TJ, Lloyd RV, Seethala RR, Wenig BM. Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol. 2018;4(8):1125–1126. doi: 10.1001/jamaoncol.2018.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu B, Serrette R, Tuttle RM, Alzumaili B, Ganly I, Katabi N, Tallini G, Ghossein R. How Many papillae in conventional papillary carcinoma? a clinical evidence-based pathology study of 235 unifocal encapsulated papillary thyroid carcinomas, with emphasis on the diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2019;29(12):1792. doi: 10.1089/thy.2019.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amin MB, Edge SB, Greene FL, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Madera M, et al. AJCC cancer staging manual. 8. New York: Springer Nature; 2017. [Google Scholar]
- 42.Canberk S, Montezuma D, Tastekin E, Grangeia D, Demirhas MP, Akbas M, Tokat F, Ince U, Soares P, Schmitt F. The other side of the coin": understanding noninvasive follicular tumor with papillary-like nuclear features in unifocal and multifocal settings. Hum Pathol. 2019;86:136–142. doi: 10.1016/j.humpath.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 43.Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, Figge JJ, Mandel S, Haugen BR, Burman KD, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2018.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Lin O. Molecular testing of thyroid nodules: a review of current available tests for fine-needle aspiration specimens. Arch Pathol Lab Med. 2016;140(12):1338–1344. doi: 10.5858/arpa.2016-0100-RA. [DOI] [PubMed] [Google Scholar]
- 45.Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ, 3rd, Ganly I, Hodak SP, Kebebew E, Patel KN, Shaha A, et al. American thyroid association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015;25(7):760–768. doi: 10.1089/thy.2014.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi ED, Larocca LM, Pantanowitz L. Ancillary molecular testing of indeterminate thyroid nodules. Cancer Cytopathol. 2018;126(Suppl 8):654–671. doi: 10.1002/cncy.22012. [DOI] [PubMed] [Google Scholar]
- 47.Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, Friedman L, Kloos RT, LiVolsi VA, Mandel SJ, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367(8):705–715. doi: 10.1056/NEJMoa1203208. [DOI] [PubMed] [Google Scholar]
- 48.Hang JF, Westra WH, Zhou AG, Cooper DS, Ali SZ. The impact of noninvasive follicular thyroid neoplasm with papillary-like nuclear features on the rate of malignancy for atypia of undetermined significance subcategories. Cancer Cytopathol. 2018;126(5):309–316. doi: 10.1002/cncy.21981. [DOI] [PubMed] [Google Scholar]
- 49.Jug RC, Datto MB, Jiang XS. Molecular testing for indeterminate thyroid nodules: performance of the Afirma gene expression classifier and ThyroSeq panel. Cancer Cytopathol. 2018;126(7):471–480. doi: 10.1002/cncy.21993. [DOI] [PubMed] [Google Scholar]
- 50.Johnson DN, Sadow PM. Exploration of BRAFV600E as a diagnostic adjuvant in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) Hum Pathol. 2018;82:32. doi: 10.1016/j.humpath.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 51.Zhao L, Dias-Santagata D, Sadow PM, Faquin WC. Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer. 2017;125(5):323. doi: 10.1002/cncy.21839. [DOI] [PubMed] [Google Scholar]
- 52.Brandler TC, Liu CZ, Cho M, Zhou F, Cangiarella J, Yee-Chang M, Shi Y, Simsir A, Sun W. Does noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) have a unique molecular profile? Am J Clin Pathol. 2018;150(5):451–460. doi: 10.1093/ajcp/aqy075. [DOI] [PubMed] [Google Scholar]
- 53.Basolo F, Macerola E, Ugolini C, Poller DN, Baloch Z. The molecular landscape of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a literature review. Adv Anat Pathol. 2017;24(5):252–258. doi: 10.1097/PAP.0000000000000163. [DOI] [PubMed] [Google Scholar]