Abstract
The domestic yak (Bos grunniens) from the Qinghai–Tibet Plateau is an important animal model in high-altitude adaptation studies. Here, we performed the genome-wide selective sweep analysis to identify the candidate copy number variation (CNV) for the high-altitude adaptation of yaks. A total of 531 autosomal CNVs were determined from 29 yak genome-wide resequencing data (15 high- and 14 low-altitude distributions) by using a CNV caller with a CNV identification interval > 5 kb, CNV silhouette score > 0.7, and minimum allele frequency > 0.05. Most high-frequency CNVs were located at the exonic (44.63%) and intergenic (46.52%) regions. In accordance with the results of the selective sweep analysis, 7 candidate CNVs were identified from the interaction of the top 20 CNVs with highest divergence from the FST and VST between the low (LA) and high (HA) altitudes. Five genes (i.e., GRIK4, IFNLR1, LOC102275985, GRHL3, and LOC102275713) were also annotated from the seven candidate CNVs and their upstream and downstream ranges at 300 kb. GRIK4, IFNLR1, and LOC102275985 were enriched in five known signal pathways, namely, glutamatergic synapse, JAK–STAT signaling pathway, cytokine–cytokine receptor interaction, neuroactive ligand–receptor interaction, and olfactory transduction. These pathways are involved in the environmental adaptability and various physiological functions of animals, especially the physiological regulation under a hypoxic environment. The results of this study advanced the understanding of CNV as an important genomic structure variant type that contributes to HA adaptation and helped further explain the molecular mechanisms underlying the altitude adaptability of yaks.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02254-w) contains supplementary material, which is available to authorized users.
Keywords: High-altitude adaptability, Yak, Copy number variation, Selection signal analysis
Introduction
Yaks, which are important herbivores in the Qinghai–Tibet Plateau, provide protein food to local herders and are integrated into the local culture as carriers of culture and religion (Ma et al. 2013; Yue et al. 2016). Numerous local domestic breeds with outstanding plateau adaptability and diverse human production expectations have been successfully bred due to the natural selection and the human domestication of yaks (Zhang et al. 2016; Lan et al. 2018a, b). Numerous studies have used yak as an animal model to study the genetic mechanism of high-altitude (HA) adaptiveness (Qiu et al. 2012). Particularly, the widespread application of whole-genome next-generation sequencing technology has led to the identification of a series of related candidate genes (Guang-Xin et al. 2019; Lan et al. 2018a, b; Goshu et al. 2019).
As an important member of the genomic structure variation family, the copy number variation (CNV) has been paid increasing attention in recent years. Numerous studies have confirmed that CNV participates in several human tissue development processes and diseases (Signore et al. 2019; Dasouki et al. 2019). Domestic animal studies have confirmed that abundant CNV mutations are involved in the economic traits and development of many animals, such as litter size and egg production (Huang et al. 2018; Zhang et al. 2019), milk production performance (Di Gerlando et al. 2019), and growth traits (Wang et al. 2019a). An increasing number of studies have reported on the population phylogeny and special economic traits of yak by using CNVs (Jia et al. 2019; Goshu et al. 2019; Ge et al. 2019).
In the present study, the selective sweep analysis of CNVs was performed to further identify the genetic divergence between yaks habituated under extreme HA and low altitude (LA). Our findings may help in further understanding the molecular genetic mechanism of the HA adaptation of yaks.
Materials and methods
The unpublished CNV analytical results from our previously published sequencing data (SRA: SRX4605921–SRX4605949; Guang-Xin et al. 2019) were presented to survey the divergence in the CNV distribution among 15 yaks at extreme HA (4800–6100 m) in Tibet Naqu and 14 yaks at LA (2450–2966 m) regions in the Gansu Zhaxixiulong grassland.
The adapter and low-quality raw paired reads were filtered initially. Then, the adapter and read with N ratio greater than 10% were removed. In addition, data with the number of bases with a quality value (Q) ≤ 20 exceeding 50% of the entire reading were deleted to obtain high-quality reads.
High-quality reads were mapped into the yak genome (BosGru_v2.0) through the BigBWA (Abuín et al. 2015) with ‘mem 4 -k 32 -M’, where -k is the minimum seed length. The -M option was used to mark shorter split alignment hits as auxiliary alignments. The SAM tools were used to convert the generated sequence alignment/graph format files into binary alignment/graph files. The Picard (V 1.129) (https://broadinstitute.github.io/picard/) was applied to sort, index, and delete duplicates.
The CNV was identified using the CNV caller (Wang et al. 2017) in accordance with CNV identification interval > 5 kb, CNV silhouette score > 0.7, and minimum allele frequency > 0.05. The selective sweep analysis was performed using the pairwise fixation indices, FST (Hudson et al. 1992) and VST (Sudmant et al. 2015). Here, VST was calculated using the equation: VST = (Vtotal–[Vpop1 × Npop1 + Vpop2 × Npop2]/Ntotal)/Vtotal, where Vtotal is the total variance, Npop is the CN variance for each respective population, Npop is the sample size for each respective population, and Ntotal is the total sample size. Statistical analysis and plot visualizations were achieved using the Perl and the R scripts. The gene-enriched signaling pathway was estimated using the KEGG database (https://www.genome.jp/kegg/pathway.html).
Results and discussion
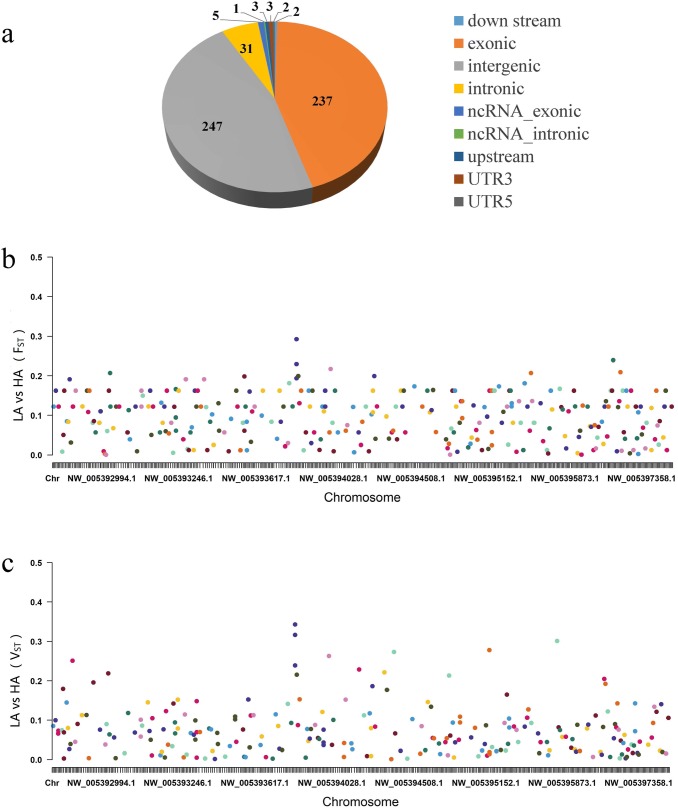
A total of 531 CNVs were identified from 430 scaffolds and classified into six types (Supporting Material I, Fig. 1a). The majority of the high-frequency CNVs belonged to the exonic (44.63%) and intergenic (46.52%) types. The lowest count of CNVs was found at the intron region of the noncoding RNA (ncRNA_intronic, 0.19%). The relative variant data were published and uploaded in the genome variation map (GVM000055, https://bigd.big.ac.cn/gvm/getProjectDetail?project=GVM000055).
Fig. 1.
Copy number variation (CNV) type description and genome-wide selection scan for CNV in high- and low-altitude yaks by using FST and VST. a CNV frequency and karyotypic location type. Manhattan plots show the selection signal of the CNV of the high- and low-altitude yaks by using bFST and cVST
The results of the selective sweep analysis (Fig. 1b, c) showed that the FST of each CNV ranged from -0.0331 (CNV_492) to 0.2926 (CNV_199), whereas the VST of each CNV ranged from -0.0387 (CNV_353) to 0.3431 (CNV_200). Seven CNVs (i.e., CNV_199, CNV_201, CNV_231, CNV_202, CNV_265, CNV_200, and CNV_430) were identified from the intersection of the top 20 CNVs from the FST and the VST (Supporting Material I). The genes annotated with the location and their upstream–downstream 300 kb ranges in these seven CNVs were displayed, and five genes were found. These genes were glutamate ionotropic receptor kainate type subunit 4 (GRIK4), interferon lambda receptor 1 (IFNLR1), olfactory receptor 1052 (LOC102275985), grainyhead-like transcription factor 3 (GRHL3), and olfactory receptor 8H3-like (LOC102275713). GRIK4, IFNLR1, and LOC102275985 were annotated in five known signaling pathways (i.e., glutamatergic synapse, JAK–STAT signaling pathway, cytokine–cytokine receptor interaction, neuroactive ligand–receptor interaction, and olfactory transduction; Supporting Material II).
Several studies have shown that the GRIK4 gene, which is annotated in the glutamatergic synapse and neuroactive ligand–receptor interaction pathway, is involved in human autism, neurodepression, and nervous system development (Minelli et al. 2017; Ren et al. 2017; Arora et al. 2018; Sun et al. 2019). A large number of other genes from these two signaling pathways also participate in neural signal transmission and sensory learning (Rao et al. 2019; Quinn et al. 2019). Accumulating evidence confirms that the re-establishment of the behavioral and the emotional neural responses of an animal under HA hypoxic environment is critical to improve the adaptive evolution of animals and humans (Ustinova et al. 1989; Livanova et al. 1993). Specifically, according to thr recently published proteomics studies, GRIK4 may be involved in the molecular mechanism of estrogen-mediated neuroprotection to reduce cerebral ischemic injury (He et al. 2018). Studies have confirmed that the cooperative expression pattern of genes directly or indirectly interacting with GRIK4 and NMDA receptors is involved in regulating the response of the retina to hypoxia (Crosson et al. 2009).
The present study suggested that the CNV_202 in the intron region of the GRIK4 gene may change the splicing and expression of the GRIK4 gene. This process assists the behavioral cognition and the nervous system of yak at different altitudes to adapt to the pressure of natural selection.
Furthermore, IFNLR1 belongs to the class II cytokine receptor family. IFNLR1 was annotated in the JAK–STAT signaling pathway and the cytokine–cytokine receptor interaction. An interferon lambda (IFN) is a cytokine induced by viral infection and has antiviral and antitumor effects (Peterson et al. 2019). IFN can activate the signal transduction pathway and exert antiviral and antitumor effects after binding to the receptor (Fragale et al. 2017; Hemann et al. 2019). Studies have shown that mutations in IFNLR1 are associated with autosomal dominant nonsyndrome hearing loss (Gao et al. 2018).
The signal pathway of JAK–STAT is divided into three parts, namely, cell surface receptors, a kinase (Janus kinase, JAK), and a signal transduction and transcription activation factor (signal transducer and activator of transcription [STAT]). This system transmits extracellular signals into the nucleus and activates the transcription of downstream target genes, including a series of genes related to immunity, proliferation, differentiation, apoptosis, and oncogenes (Morris et al. 2018; Hashimoto et al. 2020). Thus, the JAK–STAT pathway may be involved in the multiple adaptive evolutions of yak caused by differences in the habitat altitude.
Another outstanding highly selective CNV (CNV_199) from NW_005393834.1 (126,001–144,000 bp) was observed and located downstream of LOC102275985 (olfactory receptor [OR] 1052) at 10, 287 bp, which was enriched in the olfactory transduction signal pathway. The OR belongs to the G protein-coupled receptor family and identifies thousands of odor molecules in the olfactory sensory system (Antunes and Simoes de Souza 2016; Zhang et al. 2020; Krolewski et al. 2020). To date, OR genes have been found to belong to a multi-gene family distributed in various species, such as fish and mammals (Liu et al. 2019; Wakisaka et al. 2017). Several studies have reported the expression pattern and the genomic structure of OR genes under adaptive evolution with different ecological habitats (Madsen et al. 2019; C Silva et al. 2020). Thus, the OR genes of yak have evolved adaptively due to the diversity in the distribution of vegetation species at different altitudes. Specifically, yaks in LA habitats are more likely to benefit from the rich byproducts of agricultural areas than those in HA regions. As a result, the OR genes of local yaks have possibly adapted with the agricultural crops provided by humans.
The annual average temperature gradually decreases, whereas precipitation and wind speed increase with increasing altitude in the Qinghai–Tibet Plateau. These harsh ecological climatic conditions on the Tibetan Plateau limit the expansion of biological genetic diversity. However, animals that have undergone long-term natural selection and have adapted to HA climates have already exhibited a corresponding adaptive phenotype physiologically. Several studies have suggested that certain OR genes are involved in the growth and development of animal hair. For example, the OR2AT4 stimulates the proliferation of skin keratinocytes, and its silencing can inhibit hair growth, indicating that OR-dependent chemosensation is involved in human hair follicle growth (Chéret et al. 2018; Busse et al. 2014). The JAK–STAT pathway is also widely recognized as an important signal regulating pathway for determining skin and hair follicle development (Wang et al. 2019b; Samadi et al. 2017; Kim et al. 2016). Thus, results indicated that yak populations under different altitude distributions can undergo natural selection from specific ecological conditions in the neurosensing system and exhibit various types of growth.
Conclusion
HA adaptability is an important physiological characteristic of Tibetan plateau animals, such as yaks. In this study, the genome-wide selection signature analysis of CNV among 15 yaks at extreme HA and 14 yaks at LA were compared. Candidate CNV and genes (i.e., GRIK4, IFNLR1, LOC102275985, GRHL3, and LOC102275713) were identified.
Therefore, this study may contribute to the in-depth understanding of the molecular regulation of the HA adaptability of yaks. However, the authenticity and the positive rate of the identified CNVs confirmed by a large sample size and their molecular mechanism for HA adaptability still need further study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supporting material I. Complete information of the candidate CNV in 29 yaks (XLSX 189 kb)
Supporting material II. Pathway annotation of the candidate CNV (XLSX 12 kb)
Acknowledgements
This work was supported by Open Project Program of State Key Laboratory of Barley and Yak Gemplasm Resources and Genetics Improvement (Tibet Academy of Agricultural and Animal Husbandry Sciences (TAAAS)), Lhasa Tibet 850002, China.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
Footnotes
E. Guang-Xin, Bai-Gao Yang, and Yan-Bin Zhu contributed equally to this work.
References
- Abuín JM, Pichel JC, Pena TF, Amigo J. BigBWA: approaching the burrows-wheeler aligner to big data technologies. Bioinformatics. 2015;31(24):4003–4005. doi: 10.1093/bioinformatics/btv506. [DOI] [PubMed] [Google Scholar]
- Antunes G, Simoes de Souza FM. Olfactory receptor signaling. Methods Cell Biol. 2016;132:127–145. doi: 10.1016/bs.mcb.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Arora V, Pecoraro V, Aller MI, Román C, Paternain AV, Lerma J. Increased Grik4 gene dosage causes imbalanced circuit output and human disease-related behaviors. Cell Rep. 2018;23(13):3827–3838. doi: 10.1016/j.celrep.2018.05.086. [DOI] [PubMed] [Google Scholar]
- Busse D, Kudella P, Grüning NM, Gisselmann G, Ständer S, Luger T, Jacobsen F, Steinsträßer L, Paus R, Gkogkolou P, Böhm M, Hatt H, Benecke H. A synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor OR2AT4. J Invest Dermatol. 2014;134(11):2823–2832. doi: 10.1038/jid.2014.273. [DOI] [PubMed] [Google Scholar]
- Chéret J, Bertolini M, Ponce L, Lehmann J, Tsai T, Alam M, Hatt H, Paus R. Olfactory receptor OR2AT4 regulates human hair growth. Nat Commun. 2018;9(1):3624. doi: 10.1038/s41467-018-05973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson LA, Kroes RA, Moskal JR, Linsenmeier RA. Gene expression patterns in hypoxic and post-hypoxic adult rat retina with special reference to the NMDA receptor and its interactome. Mol Vis. 2009;15:296–311. [PMC free article] [PubMed] [Google Scholar]
- Dasouki MJ, Wakil SM, Al-Harazi O, Alkorashy M, Muiya NP, Andres E, Hagos S, Aldusery H, Dzimiri N, Colak D. New insights into the impact of genome-wide copy number variations on complex congenital heart disease in Saudi Arabia. OMICS. 2019 doi: 10.1089/omi.2019.0165. [DOI] [PubMed] [Google Scholar]
- Di Gerlando R, Sutera AM, Mastrangelo S, Tolone M, Portolano B, Sottile G, Bagnato A, Strillacci MG, Sardina MT. Genome-wide association study between CNVs and milk production traits in Valle del Belice sheep. PLoS ONE. 2019;14(4):e0215204. doi: 10.1371/journal.pone.0215204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale A, Romagnoli G, Licursi V, Buoncervello M, Del Vecchio G, Giuliani C, Parlato S, Leone C, De Angelis M, Canini I, Toschi E, Belardelli F, Negri R, Capone I, Presutti C, Gabriele L. Antitumor effects of epidrug/IFNα combination driven by modulated gene signatures in both colorectal cancer and dendritic cells. Cancer Immunol Res. 2017;5(7):604–616. doi: 10.1158/2326-6066.CIR-17-0080. [DOI] [PubMed] [Google Scholar]
- Gao X, Yuan YY, Lin QF, Xu JC, Wang WQ, Qiao YH, Kang DY, Bai D, Xin F, Huang SS, Qiu SW, Guan LP, Su Y, Wang GJ, Han MY, Jiang Y, Liu HK, Dai P. Mutation of IFNLR1, an interferon lambda receptor 1, is associated with autosomal-dominant non-syndromic hearing loss. J Med Genet. 2018;55(5):298–306. doi: 10.1136/jmedgenet-2017-104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F, Jia C, Chu M, Liang C, Yan P. Copy number variation of the CADM2 gene and its association with growth traits in yak. Animals (Basel) 2019;9(12):pii: E008. doi: 10.3390/ani9121008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshu HA, Chu M, Xiaoyun W, Pengjia B, Zhi DX, Yan P. Genomic copy number variation of the CHKB gene alters gene expression and affects growth traits of Chinese domestic yak (Bos grunniens) breeds. Mol Genet Genomics. 2019;294(3):549–561. doi: 10.1007/s00438-018-01530-y. [DOI] [PubMed] [Google Scholar]
- Guang-Xin E, Basang WD, Zhu YB. Whole-genome analysis identifying candidate genes of altitude adaptive ecological thresholds in yak populations. J Anim Breed Genet. 2019;136(5):371–377. doi: 10.1111/jbg.12403. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Kakigi R, Miyamoto Y, Nakamura K, Itoh S, Daida H, Okada T, Katoh Y. JAK-STAT-dependent regulation of scavenger receptors in LPS-activated murine macrophages. Eur J Pharmacol. 2020;871:172940. doi: 10.1016/j.ejphar.2020.172940. [DOI] [PubMed] [Google Scholar]
- He J, Gao Y, Wu G, Lei X, Zhang Y, Pan W, Yu H. Molecular mechanism of estrogen-mediated neuroprotection in the relief of brain ischemic injury. BMC Genet. 2018;19(1):46. doi: 10.1186/s12863-018-0630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann EA, Green R, Turnbull JB, Langlois RA, Savan R, Gale M., Jr Interferon-λ modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat Immunol. 2019;20(8):1035–1045. doi: 10.1038/s41590-019-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Cheng S, Feng Y, Sheng Z, Gong Y. A copy number variation generated by complicated organization of PCDHA gene cluster is associated with egg performance traits in Xinhua E-strain. Poult Sci. 2018;97(10):3435–3445. doi: 10.3382/ps/pey236. [DOI] [PubMed] [Google Scholar]
- Hudson RR, Slatkin M, Maddison WP. Estimation of levels of gene flow from DNA sequence data. Geneties. 1992;132(2):583–589. doi: 10.1093/genetics/132.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Wang H, Li C, Wu X, Zan L, Ding X, Guo X, Bao P, Pei J, Chu M, Liang C, Yan P. Genome-wide detection of copy number variations in polled yak using the Illumina BovineHD BeadChip. BMC Genomics. 2019;20(1):376. doi: 10.1186/s12864-019-5759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YE, Choi HC, Lee IC, Yuk DY, Lee H, Choi BY. 3-Deoxysappanchalcone promotes proliferation of human hair follicle dermal papilla cells and hair growth in C57BL/6 mice by modulating WNT/β-catenin and STAT signaling. Biomol Ther (Seoul) 2016;24(6):572–580. doi: 10.4062/biomolther.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski RC, Lin B, Stampfer S, Packard A, Schwob JE. A group of olfactory receptor alleles that encode full length proteins are down-regulated as olfactory sensory neurons mature. Sci Rep. 2020;10(1):1781. doi: 10.1038/s41598-020-58779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan D, Xiong X, Ji W, Li J, Mipam TD, Ai Y, Chai Z. Transcriptome profile and unique genetic evolution of positively selected genes in yak lungs. Genetica. 2018;146(2):151–160. doi: 10.1007/s10709-017-0005-8. [DOI] [PubMed] [Google Scholar]
- Lan D, Xiong X, Mipam TD, Fu C, Li Q, Ai Y, Hou D, Chai Z, Zhong J, Li J. Genetic diversity, molecular phylogeny, and selection evidence of Jinchuan yak revealed by whole-genome resequencing. G3 (Bethesda) 2018;8(3):945–952. doi: 10.1534/g3.118.300572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, He F, Shen L, Liu R, Wang Z, Zhou J. Convergent degeneration of olfactory receptor gene repertoires in marine mammals. BMC Genomics. 2019;20(1):977. doi: 10.1186/s12864-019-6290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livanova LM, Luk’ianova LD, Torshin VI. The effect of long-term adaptation to hypoxia on the open-field behavioral reactions in rats with different types of behavior. Zh Vyssh Nerv Deiat Im I P Pavlova. 1993;43(4):808–817. [PubMed] [Google Scholar]
- Ma ZJ, Zhong JC, Han JL, Xu JT, Liu ZN, Bai WL. Research progress on molecular genetic diversity of the yak (Bos grunniens) Yi Chuan. 2013;35(2):151–160. doi: 10.3724/SP.J.1005.2013.00151. [DOI] [PubMed] [Google Scholar]
- Madsen SS, Winther SST, Bollinger RJ, Steiner U, Larsen MH. Differential expression of olfactory genes in Atlantic salmon (Salmo salar) during the parr–smolt transformation. Ecol Evol. 2019;9(24):14085–14100. doi: 10.1002/ece3.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Congiu C, Ventriglia M, Bortolomasi M, Bonvicini C, Abate M, Sartori R, Gainelli G, Gennarelli M. Influence of GRIK4 genetic variants on the electroconvulsive therapy response. Neurosci Lett. 2016;626:94–98. doi: 10.1016/j.neulet.2016.05.030. [DOI] [PubMed] [Google Scholar]
- Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27(12):1984–2009. doi: 10.1002/pro.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ST, Kennedy EA, Brigleb PH, Taylor GM, Urbanek K, Bricker TL, Lee S, Shin H, Dermody TS, Boon ACM, Baldridge MT. Disruption of type III interferon (IFN) genes Ifnl2 and Ifnl3 recapitulates loss of the type III IFN receptor in the mucosal antiviral response. J Virol. 2019 doi: 10.1128/JVI.01073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q, Zhang G, Ma T, Qian W, Wang J, Ye Z, Cao C, Hu Q, Kim J, Larkin DM, Auvil L, Capitanu B, Ma J, Lewin HA, Qian X, Lang Y, Zhou R, Wang L, Wang K, Xia J, Liao S, Pan S, Lu X, Hou H, Wang Y, Zang X, Yin Y, Ma H, Zhang J, Wang Z, Zhang Y, Zhang D, Yonezawa T, Hasegawa M, Zhong Y, Liu W, Zhang Y, Huang Z, Zhang S, Long R, Yang H, Wang J, Lenstra JA, Cooper DN, Wu Y, Wang J, Shi P, Wang J, Liu J. The yak genome and adaptation to life at high altitude. Nat Genet. 2012;44(8):946–949. doi: 10.1038/ng.2343. [DOI] [PubMed] [Google Scholar]
- Quinn DP, Kolar A, Harris SA, Wigerius M, Fawcett JP, Krueger SR. The stability of Glutamatergic synapses is independent of activity level, but predicted by synapse size. Front Cell Neurosci. 2019;13:291. doi: 10.3389/fncel.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Kay Y, Herring BE. Tiam1 is critical for Glutamatergic synapse structure and function in the hippocampus. J Neurosci. 2019;39(47):9306–9315. doi: 10.1523/JNEUROSCI.1566-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Bi Y, Xu F, Niu W, Zhang R, Hu J, Guo Z, Wu X, Cao Y, Huang X, Yang F, Wang L, Li W, Xu Y, He L, Yu T, He G, Li X. Common variants in GRIK4 and major depressive disorder: an association study in the Chinese Han population. Neurosci Lett. 2017;653:239–243. doi: 10.1016/j.neulet.2017.05.071. [DOI] [PubMed] [Google Scholar]
- Samadi A, Ahmad Nasrollahi S, Hashemi A, Nassiri Kashani M, Firooz A. Janus kinase (JAK) inhibitors for the treatment of skin and hair disorders: a review of literature. J Dermatolog Treat. 2017;28(6):476–483. doi: 10.1080/09546634.2016.1277179. [DOI] [PubMed] [Google Scholar]
- Sebe JY, Cho S, Sheets L, Rutherford MA, von Gersdorff H, Raible DW. Ca2+-permeable AMPARs mediate Glutamatergic transmission and excitotoxic damage at the hair cell ribbon synapse. J Neurosci. 2017;37(25):6162–6175. doi: 10.1523/JNEUROSCI.3644-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signore F, Gulìa C, Votino R, De Leo V, Zaami S, Putignani L, Gigli S, Santini E, Bertacca L, Porrello A, Piergentili R. The role of number of copies, structure, behavior and copy number variations (CNV) of the Y chromosome in male infertility. Genes (Basel) 2019 doi: 10.3390/genes11010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CM, Chibucos M, Munro JB, Daugherty S, Coelho MM, Silva CJ. Signature of adaptive evolution in olfactory receptor genes in Cory's Shearwater supports molecular basis for smell in procellariiform seabirds. Sci Rep. 2020;10(1):543. doi: 10.1038/s41598-019-56950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudmant PH, Mallick S, Nelson BJ, Hormozdiari F, Krumm N, Huddleston J, Coe BP, Baker C. Global diversity, population stratification, and selection of human copy-number variation. Science. 2015;349(6253):aab3761. doi: 10.1126/science.aab3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yuan F, Yuan R, Ren D, Zhu Y, Bi Y, Hu J, Guo Z, Xu F, Niu W, Ma G, Wu X, Yang F, Wang L, Li X, Yu T, He L, He G. GRIK4 and GRM7 gene may be potential indicator of venlafaxine treatment reponses in Chinese of Han ethnicity. Medicine (Baltimore) 2019;98(19):e15456. doi: 10.1097/MD.0000000000015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova EE, Strekalova NV, Meerson FZ. The effect of adaptation to the periodic action of high-altitude hypoxia on the emotional behavior of rats. Zh Vyssh Nerv Deiat Im I P Pavlova. 1989;39(6):1112–1115. [PubMed] [Google Scholar]
- Wakisaka N, Miyasaka N, Koide T, Masuda M, Hiraki-Kajiyama T, Yoshihara Y. An adenosine receptor for olfaction in fish. Curr Biol. 2017;27(10):1437–1447. doi: 10.1016/j.cub.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Wang X, Zheng Z, Cai Y, Chen T, Li C, Fu W, Jiang Y. (2018) CNVcaller: highly efficient and widely applicable software for detecting copy number variations in large populations. Gigascience. 2017;6(12):1–12. doi: 10.1093/gigascience/gix115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ECE, Dai Z, Ferrante AW, Drake CG, Christiano AM. A Subset of TREM2+ dermal macrophages secretes Oncostatin M to maintain hair follicle stem cell quiescence and inhibit hair growth. Cell Stem Cell. 2019;24(4):654–669. doi: 10.1016/j.stem.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Wang X, Cao X, Wen Y, Ma Y, Elnour IE, Huang Y, Lan X, Chaogetu B, Hu L, Chen H. Associations of ORMDL1 gene copy number variations with growth traits in four Chinese sheep breeds. Arch Anim Breed. 2019;62(2):571–578. doi: 10.5194/aab-62-571-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Liang Y, Liang Y, Li F. Comprehensive investigation of nucleotide diversity in yaks. Anim Genet. 2016;47(6):752–755. doi: 10.1111/age.12467. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sun B, Yu Q, Ji Q, Xie P, Li H, Wang L, Zhou Y, Li Y, Huang C, Liu X. The breed and sex effect on the carcass size performance and meat quality of yak in different muscles. Korean J Food Sci Anim Resour. 2016;36(2):223–229. doi: 10.5851/kosfa.2016.36.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RQ, Wang JJ, Zhang T, Zhai HL, Shen W. Copy-number variation in goat genome sequence: a comparative analysis of the different litter size trait groups. Gene. 2019;696:40–46. doi: 10.1016/j.gene.2019.02.027. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang P, Yu S, Hansbro P, Wang H. Computerized screening of G-protein coupled receptors to identify and characterize olfactory receptors. J Toxicol Environ Health A. 2020;5:1–11. doi: 10.1080/15287394.2019.1709305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material I. Complete information of the candidate CNV in 29 yaks (XLSX 189 kb)
Supporting material II. Pathway annotation of the candidate CNV (XLSX 12 kb)