Abstract
High risk human papillomavirus (HPV) has transformed head and neck oncology in the past several decades. Now that we have recognized that HPV-positive oropharyngeal squamous cell carcinoma (OPSCC) is a unique cancer type with distinct clinicopathologic features and favorable prognosis, it has become essential to test patients in routine practice. We have progressed greatly in our knowledge of this disease and gone, over the past two to three decades, from doing testing in highly variable amounts and methods to, now, with the help of national and international guidelines and patient staging requirements, to a situation where almost all patients with OPSCC are getting accurate classification through at least p16 immunohistochemistry. However, we are still struggling with how to accurately test specimens from cervical lymph nodes, and, in particular, on fine needle aspiration. In addition, many patients with non-oropharyngeal SCC are getting clinically unnecessary p16 and/or HPV-specific testing. The trends suggest progressive improvement in practices, but many practical questions still remain. On the horizon are myriad non-tissue-based tests, such as HPV serology and plasma DNA, DNA-based testing of fine needle aspirate fluid, computerized analysis of digitized pathology and radiology images, and machine learning from clinical and pathologic features, that may render pathologists largely obsolete for establishing HPV status for our patients’ tumors. This review takes a brief look back in time to where we have been, then characterizes current practices in 2020 and lingering questions, and, finally, looks ahead into the possible future of HPV testing in patients with head and neck SCC.
Keywords: Squamous cell carcinoma, Human papillomavirus, Testing, Guidelines, In situ hybridization, Classification, p16
Introduction
The changes in head and neck oncology by human papillomavirus (HPV) in the past 30 years has been remarkable. It is nothing short of a transformation, a “sea change”, where a distinct type of squamous cell carcinoma (SCC) has emerged, now with its own diagnostic features, clinical workup, staging, and treatment [1]. We searched for the last 100 or more years for a robust and clinically applicable biomarker for head and neck SCC and HPV (with its surrogate marker p16) emerged. Patients with p16 (HPV) positive oropharyngeal (OP) SCC have an almost four fold lower likelihood of dying from their disease than patients with p16 (HPV) negative OPSCC or with non-oropharyngeal SCC. The question of testing has shifted from “if” to “how”. It is now imperative that pathologists test patients with OPSCC, possible OPSCC, and metastatic SCC of unknown primary for p16 and sometimes for HPV specifically. This also needs to be done consistently and reported clearly [2, 3]. Many pitfalls and challenges exist, and we pathologists must adjust to the ever changing landscape, with the emergence of different types of tissue-based HPV tests and even many alternatives to tissue-based p16/HPV testing that threaten to take testing completely out of pathologists’ hands. How did this all come about and what does it mean for us now? This article will provide a brief history of p16/HPV positive OPSCC, where we are at the current time, and where we might be going in the future, with a specific focus on diagnosis and testing in clinical practice.
A Brief History
High risk HPV as a cause of cancer has been common knowledge for over 30 years. zur Hausen first speculated on this associated in the medical literature in 1976 [4], and he and his team went on to prove this association in the early 1980′s. After the association was extended to other genitourinary cancers, fast forward to 2000 when Gillison and colleagues published their seminal work on evidence for a causal association between HPV and OPSCC [5]. They described what would later be termed by many as a head and neck cancer “epidemic” among primarily Caucasian men. While cervical cancer still dominates as the most common HPV-related cancer worldwide, in the United States, OPSCC has now surpassed cervical cancer as the most common HPV-related cancer [6]. It constitutes approximately 80% of all OPSCC in the United States and parts of Europe. This has taken time to develop and appears to be related to sexual behavior changes and to underlying genetic and environmental factors that we are just beginning to understand. It became clear that HPV16 accounts for the majority of these tumors (90 to 95%), that patients have higher socioeconomic status, lower smoking exposure with a higher fraction of lifetime nonsmokers, and a different biology whereby the vast majority have cervical nodal metastases at presentation [7]. The prognostic implications also became much better recognized through work such as Ang et al., who published in 2011 in The New England Journal of Medicine [8] that patients in a prospective clinical trial who had p16 or HPV DNA positive tumors had three to fivefold lower risk of death from disease after treatment. Another story slowly began to emerge whereby the presence of HPV DNA alone (from classic DNA PCR-based studies), despite being associated with OPSCC, was not considered sufficient evidence to define these tumors as HPV-associated. Rather, the tumors needed to demonstrate transcriptionally-active high risk HPV [9]. And a surrogate marker of transcriptionally-active high risk HPV, p16 protein, emerged [8, 10, 11]. Starting out more as an interesting research test for tumor cells with active HPV, it was extensively studied in retrospective and prospective studies and even randomized controlled trials and shown to be strongly and independently prognostic in OPSCC patients. It showed a very high (but certainly not perfect) correlation with high risk HPV mRNA status, being incredibly sensitive (close to 100%) and relatively specific (~ 90%) [11].
The next “card to fall” was cancer staging. Many studies showed that the 7th edition AJCC staging of OPSCC did not predict outcomes effectively, with most “high stage” disease patients showing superior survival to Stage I and II patients, largely due to the strong emphasis on positive nodal status in this older staging system. In what was memorably worded, Sturgis et al. in the title of their work on this subject ended with “a Staging System in Need of Repair” [12]. The 8th edition of the manual truly codified HPV-related OPSCC as a distinct entity, with staging now completely dependent on testing for p16 (as a surrogate of HPV) status [13]. In fact, even metastatic SCC of unknown primary in cervical lymph nodes, if in upper jugular nodes (level II and III) and p16 positive, is staged using the p16 positive OPSCC system, on the assumption that the primary is in the oropharynx [13]. What a dramatic change to how we think of head and neck cancer!
HPV testing has been evolving all throughout this time. The very association of HPV and OPSCC had been on the basis of high risk HPV DNA PCR testing. And from knowledge in cervical cancer, we knew that p16 overexpression was a strong surrogate marker of the virus. It is a tumor suppressor protein that normally inhibits cell division, but is aberrantly overexpressed in tumor cells with transcriptionally-active high risk HPV because viral proteins cause Rb degradation and remove a normal physiologic repression of p16 expression. Early adopters of HPV testing, mainly at large academic centers, were testing for p16 and/or HPV DNA by PCR. Once DNA in situ hybridization and RNA-specific tests such as in situ hybridization and RTPCR came along, it became very clear that DNA PCR was a poor test [9]. DNA in situ hybridization emerged and could be visualized on the actual slides. Over this period, many studies examined p16 overexpression with high cutoffs (50, 70, or 75% nuclear and cytoplasmic staining) as a terrific prognostic marker with high correlation with HPV-specific testing. Due to its wide availability and easy application, it emerged as a practical test in routine practice [11]. Then, in the past 5 to 10 years, as it has become clear that transcriptionally-active high risk HPV is key to the entire disease, RNA-based tests have improved and now RNA in situ hybridization, which works incredibly well on formalin fixed paraffin embedded tissue and even on cytology cell blocks, has shown superior patient survival stratification, slightly better than p16 immunohistochemistry alone and better than DNA in situ hybridization and DNA PCR [9, 14–16]. It is now available as a large cocktail of 18 high risk types and on automated platforms that can be performed in routine clinical laboratories. It is still a somewhat expensive and limited availability test, but appears to finally be capable of uniting the features of direct testing for HPV and the ability to be applied across all modern pathology practices.
The Current Status of p16/HPV Testing
For many years, it was essentially the “wild west” when it came to HPV testing in clinical practice. People were doing anything from nothing at all to across the board HPV-specific testing on all head and neck cancers, including non-oropharyngeal and precursor lesions. Many had called for consistency and for formal guidelines. The answer to this was the College of American Pathologists (CAP) evidence-based guidelines that convened in 2014 [3]. With a formal evidence-based approach, organizational support, and a panel of experts across the field of head and neck oncology, after four years of discussion, literature reviews, data gathering, and a consensus conference, the guidelines were published in May of 2018. There were 14 recommendations and/or expert opinions, a written discussion of each, and an algorithm (decision tree) that deals with every possible patient with head and neck SCC, including cervical nodal metastasis of unknown primary. Within a year, an independent American Society of Clinical Oncology (ASCO) had reviewed the guidelines from a clinical perspective and had endorsed almost all of the guidelines [2], recommending only minor changes to testing approaches. The CAP guidelines publication is admittedly long and somewhat complicated, but the individual guidelines/recommendations/expert opinions are very clear [3]. Some of the most important and impactful are: (1) Guidelines #1 and 2: Across the board testing of all new OPSCC patients for p16 with a 70% nuclear and cytoplasmic cutoff (2) Guideline #4: No routine testing for non-oropharyngeal SCC patients or for patients with non-squamous oropharyngeal carcinomas (3) Guideline #7: HPV or p16 testing on fine needle aspiration specimens from the neck in patients with a known oropharyngeal mass lesion or, if in level II/III and no known primary (no specific testing modality recommended, but rather the type of testing to be validated by each institution on its own) and (4) Guideline #9: No routine testing for low risk HPV of any head and neck carcinoma.
Yet at this point, many important questions remain. We will discuss each of these in turn and look at the evidence behind them (Table 1).
Table 1.
Major questions in HPV testing in head and neck carcinomas
| Major questions |
| Can pathologists consistently apply (at least) p16 testing in oropharyngeal primary tumor specimens? |
| What is the current role for HPV-specific testing in primary tumor specimens? |
| What is the most appropriate approach to tissue specimens from neck metastases (needle core and excisional biopsies; neck dissections)? |
| What is the best approach to p16/HPV testing in neck cytology specimens? |
| Can pathologists avoid p16/HPV testing in non-oropharyngeal carcinomas (other than for diagnostic/definitional purposes)? |
Question 1: Can Pathologists Consistently Apply (at Least) p16 Testing in Oropharyngeal Primary Tumor Specimens?
One of the major problems before the guidelines were published and before staging made p16 (± HPV specific testing) essentially mandatory for all patients with known or suspected OPSCC was that practices were “all over the map”. Some pathologists were doing no testing at all, others suboptimal HPV-specific testing alone such as just HPV DNA PCR, some doing p16 without stringent cutoffs, and many testing all manner of head and neck carcinomas beyond just the oropharynx, beyond just high risk HPV, and even beyond just SCC. The guidelines should increase consistency in appropriate testing and decrease inappropriate testing. Anecdotally, this appears to be happening yet, even now, this author hears of academic practices where p16 immunohistochemistry is performed on all new head and neck SCC patients (regardless of primary site) and referral cases still come across my desk from primary OPSCC specimens where they are not tested at all. But how effectively has practice changed in the past few years? What instruments might help us assess the field? Academic practice reviews and large survey instruments should help us to assess practice. Two data sources, to date, can provide before and after data on HPV testing in practice. None has been reported since the CAP guidelines were published. We performed a study looking at all of our referral cases at Vanderbilt University from one year before the CAP guidelines were published and one year after and judged the practice of the outside pathologists based strictly on the CAP guidelines (unpublished data). This was quite revealing. For primary OPSCC specimens, 66.9% of patients from before publication were correctly tested and 90.7% afterwards, a substantial improvement. Most of the “off guidelines” testing was either no testing at all or ordering HPV-specific testing only. Since the criteria for p16 immunohistochemistry interpretation that were used were usually not stated in the outside reports, this study could not assess compliance with the 70% nuclear and cytoplasmic reactivity CAP guideline.
The CAP in situ hybridization survey which has been applied for years was modified to add questions that help to assess compliance with primary OPSCC HPV testing as well. The data has yet to be formally analyzed and published, but some generalities are expected. In a survey of laboratories from 2017, most performed high risk HPV testing of some form and almost all of the rest did so selectively based on the clinical scenario or upon request. Less than 5% did not perform testing at all. Based on 2019 data, most performed a single test, usually p16 immunohistochemistry. For p16 immunohistochemistry, most used a > 70% nuclear and cytoplasmic staining cutoff, while the fraction of staining used otherwise varied significantly, with significant minorities using anywhere from only a 10% cutoff all the way to 100%. This data, when finally published, will be invaluable to help us better understand what pathologists are actually doing in daily practice.
In summary, it seems that, at least in the United States, we pathologists can relatively consistently apply p16 immunohistochemistry to primary tumor OPSCC specimens. Based on this unpublished data, the majority of pathologists are following the guidelines Still, though, a surprisingly large minority are not.
Question 2: What is the Current Role for HPV-Specific Testing in Primary Tumor Specimens?
Since p16 immunohistochemistry is just a prognostic marker and surrogate marker of high risk HPV, it leaves “something to be desired” as a test for OPSCC patients. There are so many HPV-specific testing modalities, and they have various pros and cons. It is clear that DNA detection is not sufficient as a standalone modality [17], although in certain situations such as neck FNAs (see below) it may be a compromise test. For tissue, the ideal test is for high risk HPV E6/E7 mRNA, often called the “gold standard” [9, 18]. RT-PCR approaches [19] and even RNAseq are great at detecting high risk HPV E6/E7 mRNA but just are not practical for clinical application. For many years, RNA detection in formalin-fixed, paraffin-embedded (FFPE) tissue was difficult (nay impossible) due to degeneration and fragmentation. This has slowly changed over time to the point that RNA in situ hybridization methods that amplify signals have the ability to detect highly fragmented RNA in these specimens.
High risk HPV RNA in situ hybridization is increasingly available as a clinical test on automated stainers. This author has worked with it for four years for select applications in OPSCC and CUP patients, and the CAP in situ hybridization survey data mentioned here had a significant minority utilizing it either alone or in combination with p16 as their HPV-specific test. Of the laboratories who use HPV-specific testing in combination with other tests, HPV RNA in situ hybridization was more commonly used than DNA in situ hybridization or DNA PCR. All of this suggests that HPV RNA in situ hybridization is steadily emerging into routine clinical practice.
How does it perform as a standalone test for patient survival prediction compared against p16 immunohistochemistry? The data suggests that it is marginally better [16]. For example, in a well performed study of 79 OPSCC patients in Europe, Schache et al. [9], showed that hazard ratios for death of disease were 11.9 for high risk HPV RNA in situ hybridization versus 4.4 for p16 positive patients. Both were strongly and independently predictive of survival, but HPV RNA in situ hybridization was clearly better. Both had a sensitivity of 97% for high risk HPV compared to RT-PCR (the standard in this study), but specificity of RNA in situ hybridization was 93% versus just 82% for p16 alone [9]. High quality data from well performed studies using HPV-specific RNA testing like this is uncommon in the literature, and this type of data (survival data specifically) is lacking in the higher HPV attributable fraction U.S. population. Other indirect sources support this concept, however.
So is it time to switch to HPV RNA in situ hybridization? Can everyone start doing it across all of clinical practice [11, 20]? Maybe not, but we are inching closer, and the feeling is that the modest improvement HPV RNA in situ hybridization provides over p16 immunohistochemistry alone in categorizing patients as “favorable” or “unfavorable” prognosis is worth it because in each instant, we are dealing with an individual patient. When guidelines are revisited in coming years, this will be a major question to be addressed.
Question 3: What is the Most Appropriate Approach to Tissue Specimens from Neck Metastases (Needle core Biopsies, Excisional Biopsies, and Neck Dissections)?
Some specimens we receive in surgical pathology for primary diagnosis in OPSCC patients are tissue from cervical nodal metastases. Needle cores are sometimes taken at centers where FNA service is not present or reliable, when the patient has a history of another malignancy that may be in the differential diagnosis for their neck mass, or particularly when lymphoma is a consideration for the adenopathy. Excisional biopsies or limited neck dissections may be obtained as smaller centers, when a cystic neck mass is mistaken to be a branchial cleft cyst, or when FNA of the neck mass is non-diagnostic. CAP guidelines are that pathologists should only routinely test such patients for high risk HPV when there is an oropharyngeal mass on imaging or a suspected oropharyngeal tumor on clinical examination, when the specimen is from level II or III in the neck without a known or suspected primary lesion, or when the level of the neck is unknown [3]. Current guidelines allow that p16 immunohistochemistry is sufficient alone only if the tumor is from level II/III and has nonkeratinizing morphology. This latter allowance is the most controversial, but it was one that is backed by the requirement of three surrogates of high risk HPV status in order to forego HPV-specific testing, namely neck level, morphology, and positive p16 testing (Fig. 1). It obviates HPV-specific testing in at least 70% of these patients since the majority of patients’ tumors have all of these features. One of the most significant recommended modifications by the ASCO panel, however, which reviewed the CAP guidelines in 2019, was to simplify the neck testing to say that all p16 positive SCCs in the neck should then undergo HPV-specific testing [2].
Fig. 1.
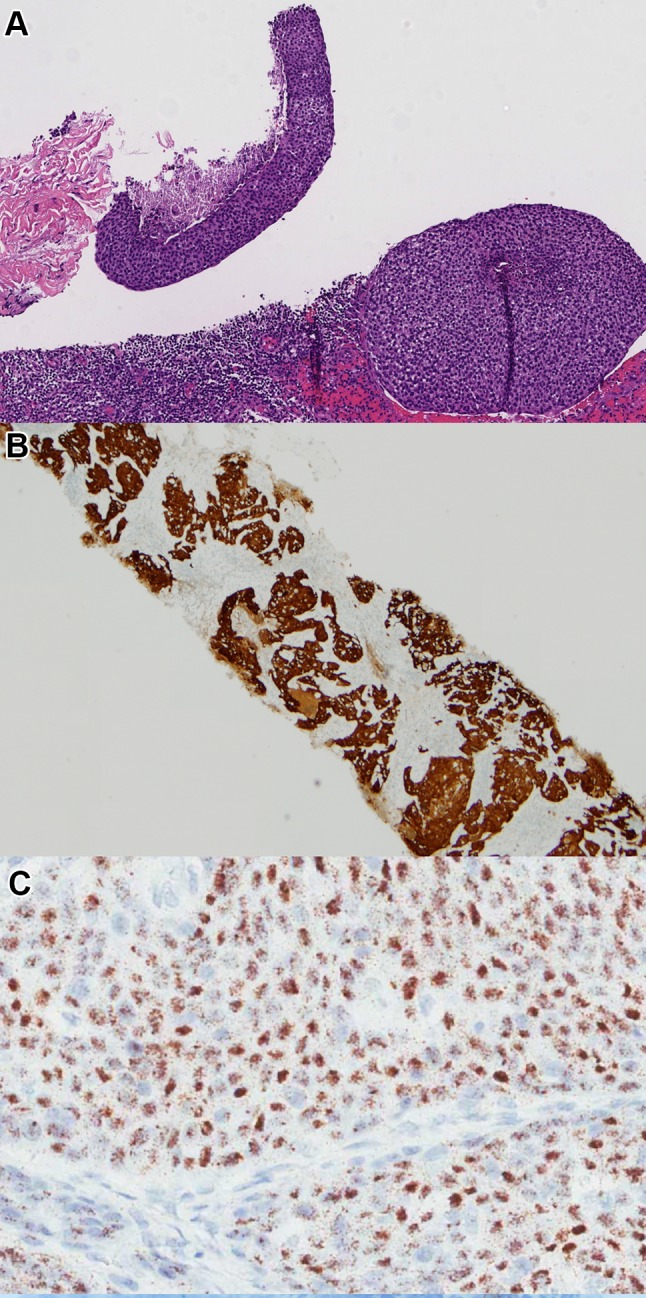
Core biopsy of a neck mass in a patient with no obvious primary lesion on clinical examination or computed tomography (CT) scanning. Level in the neck not clearly stated in clinical notes. a H&E morphology showing a nonkeratinizing squamous cell carcinoma (10X magnification). b p16 immunohistochemistry showing strong, diffuse positive nuclear and cytoplasmic staining (4X magnification) c High risk HPV E6/E7 mRNA in situ hybridization testing, performed according to CAP guidelines, showing punctate, granular, brown staining in the tumor cell cytoplasm (30X magnification). (Methods: p16 immunohistochemistry performed using E6H4 clone, prediluted, from Ventana Medical Systems, Inc. and HPV in situ hybridization performed using a probe cocktail covering HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82 from Advanced Cell Diagnostics, Inc.)
The major concern in the neck with p16 immunohistochemistry alone is that ~ 20% of metastatic skin and lung SCCs are extensively positive for p16, with > 70% nuclear and cytoplasmic staining yet without associated high risk HPV [21]. Further, a small fraction of non-oropharyngeal SCCs are also p16 diffusely positive without HPV association. There is no actual data in the literature on the effectiveness of the algorithmic CAP approach to HPV testing in neck tissue specimens versus an across the board p16 and HPV-specific testing on positive patients approach, but hopefully these studies will be performed in the future in order to guide practice more effectively.
Question 4: What is the Best Approach to p16/HPV Testing in Neck Cytology Specimens?
If tissue-based testing in primary OPSCC specimens is still “messy”, and on neck tissue cores and excisional biopsies still messier, then testing on cytology specimens from the neck is the messiest of all! Cervical nodal FNA specimens afford great convenience in OPSCC patients because so many patients have nodal disease at presentation (80–85%) and it is usually bulky. In clinic FNA without need for anesthesia is a quick and easy way to get a diagnosis. Technical challenges do occur, though, most importantly problems with aspirating cystic and necrotic nodes and getting representative, viable material. Recognizing the importance of HPV status in OPSCC and CUP patients, CAP guideline #7 calls for HPV or p16 testing on fine needle aspiration specimens from the neck in patients with a known oropharyngeal mass lesion or if the mass is in level II/III and no known primary is clinically apparent [3]. What test to do is the problem, so a specific testing modality was not recommended by the panel. Instead, the type of testing is to be validated by each institution on its own.
p16 immunohistochemistry can be performed on cytology material, best applied to cell block. However, it clearly doesn’t work in the same way as it does in tissue depending on how you fix the cell block. Several well performed studies showed that fraction of cells staining and intensity are less with various cytologic fixatives. Thus, what cutoff to use was unclear with some studies such as Holmes et al. saying “any confluent cell staining” [22] and other saying optimal cutoffs are 10 to 25% [23]. Then other studies came along showing that if you formalin fix the cell block, the situation is much better, almost comparable to tissue. Buonacore et al. [24] performed a side by side comparison of 25 patients with cervical nodal FNAs for OPSCC and showed that the formalin fixed cell blocks stained diffusely while the Cytolyte fixed ones averaged only 38% of the cells positive, effectively demonstrating side by side that the reactivity issue can be “fixed”/corrected with formalin fixation. HPV RNA in situ hybridization works well on cell blocks, independent of fixation method [23], so some laboratories are doing this with (or instead of) p16 immunohistochemistry.
An alternative that many laboratories are adopting is liquid-based HPV DNA testing on FNA fluid. This is highly convenient as many laboratories are already performing this testing on uterine cervical Papanicolau specimens. Strong data supports its use in the head and neck, including many studies in the literature using several different platforms for DNA detection [25]. Performance is essentially comparable to p16 immunohistochemistry on cell blocks, with > 90% concordance with corresponding tissue specimens. Most often, this requires an additional pass of the needle with the liquid set aside for the test. So, both liquid-based HPV testing and p16 both usually require dedicated needle passes, and often the cell blocks don’t have adequate viable tumor to either be diagnostic or to have reliable sampling for the staining. They are more desirable than having to go and obtain actual tumor tissue biopsies from the patient for p16/HPV testing, though, to be sure.
Question 5: Can Pathologists Avoid p16/HPV Testing in Non-Oropharyngeal Carcinomas (Other Than for Diagnostic/Definitional Purposes)?
In the complicated flow of life, human beings naturally use shortcuts and heuristics. It seems clear that pathologists have been hearing of data on high risk HPV in subsets of non-oropharyngeal SCCs [26] and extrapolating from OPSCC to say “well, probably having p16/high risk HPV expression in a non-oropharyngeal SCC must be a good thing, too. I think I’ll do a p16 stain.” From our unpublished Vanderbilt study from 2017, community pathologists ordered p16 or HPV specific testing on 45.5% of non-oropharyngeal SCCs including 45.2% of oral cavity SCCs and with significant fractions at all subsites, including larynx, hypopharynx, sinonasal tract and nasopharynx. Within the first year after the CAP guidelines, the numbers had actually increased to 54.7% and 57.1%, respectively, although there seemed to be a trend towards lower rates of inappropriate testing in the last few months of the study, suggesting a slow uptake in the guidelines or perhaps just return to baseline.
Coming back to the impending CAP in situ hybridization survey data, of the several hundred respondents, a surprisingly large minority still routinely perform high risk HPV testing of some form on non-oropharyngeal primary SCC patients, most of them at all anatomic subsites. Anecdotally, this author still hears of large academic institutions where p16 immunohistochemistry is performed on every new primary head and neck SCC patient.
While the data from some large studies that transcriptionally-active high risk HPV, and even p16 overexpression in the absence of HPV, in non-oropharyngeal SCCs is prognostic is compelling [26], until a clinical use for the information is established, there is no indication for all of this testing. Better communication of the guidelines including re-issues and social media posts may all improve pathology practice over time.
Where Are We Going?
With technology and innovation, change is inevitable. There is the specter on the horizon that surgical pathologists will no longer be doing any HPV testing on tissue or cytology specimens. Why is this? Because many promising alternatives are emerging, such as liquid-based HPV testing of the supernatant which is generated in every single cervical lymph node FNA, high risk HPV serology and circulating tumor cell and/or HPV DNA, computerized analysis of medical images, including cross sectional radiology and digitized H&E pathology slides, and machine learning from clinical and pathologic features in the electronic medical record (Table 2).
Table 2.
Emerging, potentially disruptive alternatives to tissue-based HPV testing
| Liquid-based HPV-specific testing on nodal cytology aspirates and effluent |
| Blood-based HPV-specific E6/E7 serology |
| Blood-based HPV DNA detection |
| Computerized image analysis of cross-sectional radiographic imaging such as CT scans and MRI |
| Computerized image analysis of digitized pathology slides, both H&E and cytology |
As previously mentioned, liquid-based HPV testing has been common place in gynecology practice for many years as part of work up for cervical dysplasia and carcinoma. Extensive literature now shows concordance rates with tissue based p16/HPV testing of > 90% when testing is applied according to the CAP recommendations (cervical metastatic SCC with known or suspected oropharyngeal primary, and metastatic SCC of unknown primary in a level II or III—or unknown level—lymph node) [25]. Some institutions have been performing this testing in routine clinical practice for the past several years [25]. Having a dedicated FNA pass put into liquid based medium is helpful, but now new data from Vanderbilt University (unpublished) is emerging that one can just use the supernatant from typical cell preparations for liquid based HPV testing suggesting that a dedicated pass is no longer needed. For those still using cell blocks, there may be a shift away from p16 immunohistochemistry. Since RNA in situ hybridization can be effectively applied for this purpose, some have started use this directly in lieu of p16 immunohistochemistry.
What is more jolting to the surgical pathologist is the number of biospecimen alternatives to tissue for HPV status. Data has shown that most patients with HPV16-related OPSCC develop robust, HPV specific IgG antibody responses. These can be detected by serologic testing. In some fascinating, long term, time course studies of healthy patients, high titer HPV16 specific IgG antibodies can be detected in patients up 20 to 30 years before they present with clinical disease [27]! And further, if one uses HPV serology as a diagnostic test just when the patient presents with clinical disease, it has a correlation of > 90% with high risk HPV-specific testing on the patient’s tissue, putting it close to on par with p16 immunohistochemistry. One limitation of serologic testing, currently, is that it is focused on HPV16 and is still suboptimal for other high risk HPV types such as 33, 35, etc., which account for ~ 5 to 10% of all HPV in OPSCC. But cross reactivity between HPV16 serology and these other high risk types helps to some extent. It feels like just a matter of time until HPV serology is improved and ready for “prime time”. This could become the diagnostic gold standard in the near future. HPV DNA in the circulating blood has also shown promising performance for diagnosis of HPV in OPSCC patients. In a recent study of 103 patients with OPSCC and 115 controls who underwent plasma high risk HPV detection, at presentation, performance was estimated to be 97% sensitive and 89% specific for tissue HPV RNA status [28]. This is a performance comparable to HPV serology. These tests are promising, but will have to be improved. Once they are “locked down”, they will need to be thoroughly validated according to FDA guidelines in order to be used as clinical tests.
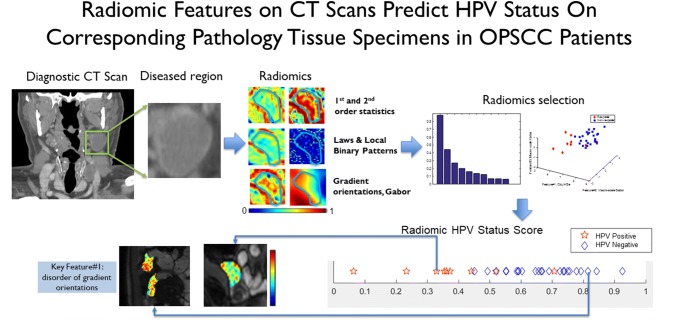
An even more fascinating and non-invasive testing alternative is image analysis of patient tissue images and/or radiology images (Fig. 2). In a very recent example, Leijenaar et al. [29], using a cohort of almost 1000 OPSCC patient CT scans for modeling and testing, showed a performance for patient survival stratification almost identical to p16 immunohistochemistry on tissue. In an open challenge on an MD Anderson OPSCC patient database of CT scan images and clinicopathologic features, the winner for predicting p16/HPV status (based on primary tumor tissue testing) achieved an area under the curve of 0.915 [30]. Needless to say, this type of modeling and testing with machine learning is extremely promising, suggesting that a great deal of information about p16/HPV status of a patient’s OPSCC is contained in radiology images and patient information in the EMR alone.
Fig. 2.
Computerized image analysis of digitized CT scan images. The images are analyzed for abnormal (tumor) regions, segmented and then normalized, with features such as texture, heterogeneity, shape, and many other possible features. Integration of imaging features across patients drawn a “classifier” with high likelihood of HPV positivity or negativity. (image courtesy of Anant Madabhushi, PhD and Cheng Lu, PhD
Image analysis and machine learning on digitized H&E tissue images is also promising. In unpublished data on BioRxIV, a preprint server for biology from Cold Spring Harbor, Kather et al. were able to obtain an AUC for p16 status prediction in head and neck SCC by image analysis of digitized H&E sections from The Cancer Genome Atlas of 0.70 [0.66; 0.74]. This included non-oropharyngeal SCC patients as well, so a somewhat “noisy” dataset. However, it speaks to the promise of morphologic features as a possible predictive feature for p16/HPV status in OPSCC patients. Imagine a future where a patient comes in with a neck mass or dysphagia, gets a CT scan showing a mass in the oropharynx, then gets an FNA for diagnosis of SCC, and a blood test or computerized analysis of the radiologic images, clinical features, and/or histologic slides that says it is an HPV-related tumor. Tissue based p16 or HPV specific testing may no longer be needed at all.
Summary
There has been a massive shift in head and neck pathology over the past several decades so that HPV status in OPSCC patients and patients with metastatic SCC of unknown primary is required information. Pathology-based testing has evolved from very inconsistent to more consistent, at least for OPSCC, where p16 immunohistochemistry is being performed and properly interpreted for the majority of patients in routine clinical practice. Practices will continue to change, likely with the adoption of high risk HPV RNA ISH on p16 positive patients, or perhaps instead of p16 immunohistochemistry. Cervical lymph node testing in routine clinical practice, both on tissue and cytology, needs much improvement and standardization still, however. While we continue to work on improving tissue-based HPV testing, surgical pathologists may soon be replaced by a number of highly promising tests, such as liquid-based HPV testing on FNA specimens, blood-based serology and HPV DNA testing, and even, more disruptively, on artificial intelligence-based analysis of standard digitized radiographic and pathologic images, and clinical data. When it comes to interesting developments, the testing to diagnose HPV-related cancers does not disappoint. Stay tuned, as the future promises to be even more interesting.
Acknowledgements
The author would like to thank William Faquin, MD, PhD, co-chair of the CAP evidence-based guidelines for HPV testing in head and neck carcinomas expert panel, for his generosity, collegiality, and support in this area. The author would also like to thank Joel T. Moncur MS, MD, PhD for collaborative research, collaboration on the CAP expert panel, and for conversations on the coming CAP surveys which he supervises.
Funding
No external funding was obtained for this work.
Compliance with Ethical Standards
Conflict of interest
All authors declare that he/she has not conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board, which did not require informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parvathaneni U, Lavertu P, Gibson MK, et al. Advances in diagnosis and multidisciplinary management of oropharyngeal squamous cell carcinoma: state of the art. Radiographics. 2019;39:2055–2068. doi: 10.1148/rg.2019190007. [DOI] [PubMed] [Google Scholar]
- 2.Fakhry C, Lacchetti C, Rooper LM, et al. Human papillomavirus testing in head and neck carcinomas: ASCO Clinical Practice Guideline Endorsement Of The College of American Pathologists Guideline. J Clin Oncol. 2018;36:3152–3161. doi: 10.1200/JCO.18.00684. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JS, Jr, Beadle B, Bishop JA, et al. Human papillomavirus testing in head and neck carcinomas: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2018;142:559–597. doi: 10.5858/arpa.2017-0286-CP. [DOI] [PubMed] [Google Scholar]
- 4.Zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1976;36:794. [PubMed] [Google Scholar]
- 5.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wurdemann N, Wagner S, Sharma SJ, et al. Prognostic impact of AJCC/UICC 8th edition new staging rules in oropharyngeal squamous cell carcinoma. Front Oncol. 2017;7:129. doi: 10.3389/fonc.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schache AG, Liloglou T, Risk JM, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–1339. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedghizadeh PP, Billington WD, Paxton D, et al. Is p16-positive oropharyngeal squamous cell carcinoma associated with favorable prognosis? A systematic review and meta-analysis. Oral Oncol. 2016;54:15–27. doi: 10.1016/j.oraloncology.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JS., Jr p16 Immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2012;6(Suppl 1):S75–82. doi: 10.1007/s12105-012-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119:81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lydiatt WM, Patel SG, O'Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 14.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooper LM, Gandhi M, Bishop JA, et al. RNA in-situ hybridization is a practical and effective method for determining HPV status of oropharyngeal squamous cell carcinoma including discordant cases that are p16 positive by immunohistochemistry but HPV negative by DNA in-situ hybridization. Oral Oncol. 2016;55:11–16. doi: 10.1016/j.oraloncology.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 17.Augustin J, Outh-Gauer S, Mandavit M, et al. Evaluation of the efficacy of the 4 tests (p16 immunochemistry, polymerase chain reaction, DNA, and RNA in situ hybridization) to evaluate a human papillomavirus infection in head and neck cancers: a cohort of 348 French squamous cell carcinomas. Hum Pathol. 2018;78:63–71. doi: 10.1016/j.humpath.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao G, Chernock RD, Gay HA, et al. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013;132:882–890. doi: 10.1002/ijc.27739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson M, Schache A, Sloan P, et al. HPV specific testing: a requirement for oropharyngeal squamous cell carcinoma patients. Head Neck Pathol. 2012;6(Suppl 1):S83–90. doi: 10.1007/s12105-012-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimoto M, Matsuzaki I, Takahashi Y, et al. High-risk human papillomavirus E6/E7 mRNA is rarely detected in nonanogenital cutaneous squamous cell carcinoma: an RNA in situ hybridization-based tissue microarray study. Am J Dermatopathol. 2019;41:205–210. doi: 10.1097/DAD.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 22.Holmes BJ, Maleki Z, Westra WH. The fidelity of p16 staining as a surrogate marker of human papillomavirus status in fine-needle aspirates and core biopsies of neck node metastases: implications for HPV testing protocols. Acta Cytol. 2015;59:97–103. doi: 10.1159/000375148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalaly JB, Lewis JS, Jr, Collins BT, et al. Correlation of p16 immunohistochemistry in FNA biopsies with corresponding tissue specimens in HPV-related squamous cell carcinomas of the oropharynx. Cancer Cytopathol. 2015;123:723–731. doi: 10.1002/cncy.21600. [DOI] [PubMed] [Google Scholar]
- 24.Buonocore DJ, Fowle E, Lin O, et al. Cytologic evaluation of p16 staining in head and neck squamous cell carcinoma in CytoLyt versus formalin-fixed material. Cancer Cytopathol. 2019;127:750–756. doi: 10.1002/cncy.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr DA, Pitman MB, Sweeney B, et al. Performance of the Roche cobas 4800 high-risk human papillomavirus test in cytologic preparations of squamous cell carcinoma of the head and neck. Cancer Cytopathol. 2014;122:167–174. doi: 10.1002/cncy.21372. [DOI] [PubMed] [Google Scholar]
- 26.Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic role of p16 in nonoropharyngeal head and neck cancer. J Natl Cancer Inst. 2018;110:1393–1399. doi: 10.1093/jnci/djy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: a pooled analysis. Cancer Epidemiol Biomarkers Prev. 2015;24:683–689. doi: 10.1158/1055-9965.EPI-14-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chera BS, Kumar S, Beaty BT, et al. Rapid clearance profile of plasma circulating tumor HPV type 16 dna during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin Cancer Res. 2019;25:4682–4690. doi: 10.1158/1078-0432.CCR-19-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leijenaar RT, Bogowicz M, Jochems A, et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol. 2018;91:20170498. doi: 10.1259/bjr.20170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Head MMDACC, Neck Quantitative Imaging Working G. Matched computed tomography segmentation and demographic data for oropharyngeal cancer radiomics challenges. Sci Data. 2017;4:170077. doi: 10.1038/sdata.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]