Abstract
The identification of vascular invasion in follicular thyroid neoplasms is essential for categorizing lesions as benign (follicular adenomas) or malignant (follicular thyroid carcinomas). Among the histologic criteria diagnostic of true vascular invasion is tumor-cell associated thrombosis, including fibrin deposition and platelet clumping. This study aims to evaluate whether an immunohistochemical stain for the platelet-associated protein CD61 could assist in identifying tumor-associated thromboses and thereby confirm vascular invasion in follicular thyroid neoplasms. Histologic review and CD61 immunostaining of 19 atypical follicular adenomas, 13 non-metastatic follicular thyroid carcinomas, and 11 metastatic follicular thyroid carcinomas was performed. Linear arrays or clustered groups of CD61-expressing intravascular platelets were present in 51% of cases overall, including 54% of follicular thyroid carcinomas and 47% of follicular adenomas, mostly within intracapsular or peritumoral vessels. In three follicular thyroid carcinomas (all with distant metastases), CD61-expressing platelets were present in association with intravascular tumor cells. This finding was not present in adenomas. CD61 staining alone did not distinguish between atypical follicular adenomas, non-metastatic carcinomas, and metastatic carcinomas. When present in association with intravascular tumor cells, however, CD61-expressing platelets may serve as a marker for vascular invasion and aid in the diagnosis of follicular thyroid carcinoma.
Keywords: CD61, Follicular thyroid carcinoma, Atypical follicular adenoma, Vascular invasion, Angioinvasion, Platelets, Fibrin, Thrombi
Introduction
Follicular thyroid neoplasms are diagnostically challenging entities which necessitate a thorough histologic evaluation to distinguish benign follicular adenomas (FAs) from follicular thyroid carcinomas (FTCs). A follicular-patterned lesion must demonstrate full-thickness capsular and/or vascular invasion for a diagnosis of FTC [1]. Unencapsulated lesions may be diagnosed as FTC when tumor cells invade into the adjacent thyroid parenchyma (i.e. intrathyroidal invasion) [1].
Identifying definitive vascular invasion may be troublesome for pathologists and subject to interobserver variability [2]. Recent recommendations define true vascular invasion as tumor cells, with associated thrombosis, residing in the lumen of a large-caliber vessel that is located within the capsule or beyond the outer edge of the nodule. The tumor cells may or may not be covered by endothelium, and endothelialized tumor adherent to the vascular wall is also considered to represent true vascular invasion by many [2–4]. When tumor cells protrude through a vessel wall with associated thrombus formation, the frequency of metastasis is as high as 35% [2, 4]. This suggests these features represent clinically meaningful vascular invasion and their use as diagnostic criteria may help avoid an over diagnosis of carcinoma [2, 4]. Involvement of vessels within the tumor itself (i.e. intratumoral vascular invasion) has not conventionally been considered diagnostic of malignancy [5]. Histologic mimics of vascular invasion can include: (1) mechanical tumor displacement during tissue processing (detached intraluminal tumor cells without thrombosis); (2) tumor undermining of vascular endothelium without breach into the lumen, thrombosis, or adherence; and (3) an intravascular endothelialized tumor nodule without thrombosis or adherence [4].
Immunohistochemical (IHC) stains may assist in diagnosing vascular or lymphatic invasion by highlighting peritumoral endothelial cells via CD31, CD34, ERG, and FLI-1 or specifically highlighting lymphatic endothelium via D2-40 [2, 4, 6, 7]. Use of IHC in identification of tumor-associated thrombi has not been evaluated in follicular thyroid neoplasms. CD61 (integrin beta 3 chain) is a transmembrane protein expressed on the surface of platelets and megakaryocytes. It functions in thrombus formation by acting as a binding receptor for fibrinogen, fibronectin, plasminogen, prothrombin, von Willebrand factor, and other prothrombotic molecules [8]. CD61 may assist in marking tumor-associated thrombi in FTC in order to distinguish true vascular invasion from mimics. This study aims to evaluate whether an IHC stain for the platelet-associated protein CD61 could assist in identifying tumor-associated thromboses and thereby confirm vascular invasion in follicular thyroid neoplasms. These neoplasms include atypical FAs (aFA), non-metastatic FTCs (nmFTC), and FTCs with distant (hematogenous) metastases (mFTC). This study is the first to evaluate CD61 as a possible marker of vascular invasion in solid tumors.
Materials and Methods
Case Selection and Histologic Review
The internal pathology archive of the University of Chicago Medicine (2002–2018) was surveyed for patients with aFA, nmFTC, and mFTC. Nineteen aFAs, thirteen nmFTCs, and eleven mFTCs were selected for inclusion based on tissue block availability. Hematoxylin and eosin (H&E) slides from all cases were reviewed for the presence of capsular and vascular invasion as well as the number of foci of vascular invasion. The College of American Pathologists (CAP) guidelines for thyroid carcinoma evaluation were used to define the presence of capsular and vascular invasion [4]. Specifically, true capsular invasion required tumor cell growth into and beyond the outer edge of the capsular contour. True vascular invasion required tumor cells within the lumen of an intracapsular or peritumoral vessel with associated thrombus (with or without endothelium) or an endothelial-covered tumor nodule adherent to the vessel wall. If present, intraluminal tumor within intratumoral vessels was also noted. Atypical FAs were those that lacked true capsular, vascular, or intrathyroidal invasion by the above definitions but had one or more of the following features: intralesional fibrosis mimicking capsular invasion; endothelial undermining mimicking vascular invasion, or solid growth with increased mitoses. These lesions are uncommon but are thought to behave in an indolent fashion [9]. Non-metastatic FTCs demonstrated any number of foci of definitive capsular and/or vascular invasion but did not have distant metastasis at the time of the study. All mFTCs had pathologic documentation of distant (non-nodal) metastases. In order to avoid possible papillary carcinomas, cases with lymph node metastases only were excluded, and cases with papillary-like nuclei were excluded. Demographics and follow-up data were gathered from the electronic medical record.
For each case, one representative tumor block was selected specifically to include the tumor periphery/capsule. When available, blocks with vascular invasion or areas of greatest suspicion were selected. Whole tissue sections 3 μm in thickness were cut. One slide was stained with H&E and a second slide was stained for CD61 as follows: the slides were deparaffinized and rehydrated with xylene, serial rehydrants, and water. After antigen retrieval treatment (epitope retrieval solution II AR9640, Leica Biosystems) for 20 min, anti-CD61 antibody (Agilent, Cat#M0753, clone Y2/51, mouse monoclonal antibody) (1:40) was applied on tissue sections for overnight incubation at 4 °C. The antigen–antibody binding was detected with Bond Polymer Refine Detection (Leica Biosystems, DS9800) and DAB + chromogen (DAKO, K3468) system. Tissue sections were briefly immersed in hematoxylin for counterstaining and then covered with cover glasses.
CD61 immunostained slides and all available H&E stained slides for all included cases were reviewed by two pathologists (V.C. and N.A.C.). CD61 was considered positive if expressed in intravascular aggregated platelets. The location of the involved vessel was noted as being intratumoral (within the tumor), intracapsular (within the fibrous capsule), peritumoral (outside of but adjacent to the capsule or nodule periphery), or distant (> 1.0 cm from the nearest tumor edge). The morphologic pattern of CD61 expression was described. Chi squared testing for statistical significance was performed using online software (available at socscistatistics.com, 2019, © Stangroom).
Results
Clinical and Histologic Features of Thyroid Nodules
Nineteen aFAs were reviewed (8 males and 11 females). The average age at presentation was 53.9 years (range 16–81). The average tumor size was 3.8 cm (range 1.0–12.0 cm). All were confirmed to lack true capsular and vascular invasion. No patients had clinical or pathologic evidence of metastasis or local recurrence (average follow-up: 9.1 months, range 1–48 months).
Thirteen nmFTCs were reviewed (4 males and 9 females). The average age at presentation was 53.8 years (range 29–66). The average tumor size was 3.8 cm (1.9–5.0 cm). Unequivocal capsular and/or intrathyroidal invasion was present in 92% (12/13). Vascular invasion was identified in 38% (5/13) of cases: 2 cases had 1 focus, 1 case had 2 foci, 1 case had 3 foci, and 1 case had 4 foci. No patients had evidence of lymph node or distant metastasis (average follow-up: 25.7 months; range 1–82 months). One patient had a local recurrence in the thyroid bed 82 months after initial diagnosis.
Eleven mFTCs were reviewed (4 males and 7 females). The average age at presentation was 67.3 years (range 56–87). The average tumor size was 2.8 cm (range 0.4–4.8 cm). Unequivocal capsular and/or intrathyroidal invasion was identified in 81% (9/11) of cases. Vascular invasion was identified in 55% (6/11) of cases: 1 case had 1 focus, 1 case had 2 foci, 1 case had 3 foci, 1 case had 4 foci, 1 case had 6 foci, and 1 case had 7 foci. Two cases entirely submitted for histologic examination did not demonstrate definitive capsular or vascular invasion. Both of these patients presented with distant metastatic lesions, and subsequent thyroidectomies demonstrated extensive intratumoral fibrosis approximating regression, the features of which have been previously described [10]. All patients had histologically-confirmed distant (non-nodal) metastases (average length of follow-up: 34.6 months; range 0–96 months). Seven patients had isolated bone metastases, 3 patients had isolated lung metastases, and 1 patient had concurrent kidney, lung, and bone metastases.
CD61 Expression
In total, CD61-positive intravascular platelets were present in 51% of cases (22/43), including 47% (9/19) aFAs, 54% (7/13) nmFTCs, and 55% (6/11) mFTCs. Of the 5 and 8 nmFTCs with and without vascular invasion, respectively, 3 (60%) and 4 (50%) had CD61-positive intravascular platelets. Of the 6 and 5 mFTCs with and without vascular invasion, respectively, 4 (67%) and 2 (40%) had CD61-positive intravascular platelets. Rate of expression was not different by tumor type (p = 0.90; Table 1). Of the 22 positive cases, the location of vessels containing CD61-positive intravascular platelets was peritumoral in 60% (13), intracapsular in 23% (5), and distant in 9% (2). The location of vessels was not different by tumor type (p > 0.6; Table 1). Only mFTCs had CD61-positive platelets in intratumoral vessels (two cases). The incidence was too low to reach statistical significance compared to aFAs and nmFTCs (p = 0.44).
Table 1.
Pathologic features and intravascular CD61-expression in follicular thyroid neoplasms
| Feature | Follicular adenoma (n = 19) | Non-metastatic FTC (n = 13) | Metastatic FTC (n = 11) | Total (n = 43) |
|---|---|---|---|---|
| Capsular invasion | 0% (0/19) | 92% (12/13) | 82% (9/11) | 88% of carcinomas (21/24) |
| Angioinvasion | 0% (0/19) | 38% (5/13) | 55% (6/11) | 46% of carcinomas (11/24) |
| <4 foci | 80% (4/5) | 50% (3/6) | 29% of carcinomas (7/24) | |
| ≥4 foci | 20% (1/5) | 50% (3/6) | 17% of carcinomas (4/24) | |
|
CD61-positive intravascular platelets p = 0.90 |
47% (9/19) | 54% (7/13) | 55% (6/11) | 51% of all neoplasms (22/43) |
| CD61 location | ||||
| Peritumoral p = 0.7 | 55% (5/9) | 71% (5/7) | 50% (3/6) | 60% of positive cases (13/22) |
| Intracapsular p = 0.61 | 33% (3/9) | 14% (1/7) | 17% (1/6) | 23% of positive cases (5/22) |
|
Distant p = 0.97 |
11% (1/9) | 14% (1/7) | 0% (0/6) | 9% of positive cases (2/22) |
| Intratumoral p = 0.44 | 0% (0/9) | 0% (0/7) | 33% (2/6) | 9% of positive cases (2/22) |
| CD61 Pattern | ||||
|
Clustered p = 0.88 |
44% (4/9) | 57% (4/7) | 50% (3/6) | 50% of positive cases (11/22) |
|
Linear p = 0.97 |
33% (3/9) | 29% (2/7) | 33% (2/6) | 32% of positive cases (7/22) |
| Clustered and Linear p = 0.91 | 22% (2/9) | 14% (1/7) | 17% (1/6) | 18% of positive cases (4/22) |
|
Tumor Cell-Associated (all clustered) p = 0.12 |
0% (0/9) | 0% (0/7) | 50% (3/6) | 14% of positive cases (3/22) |
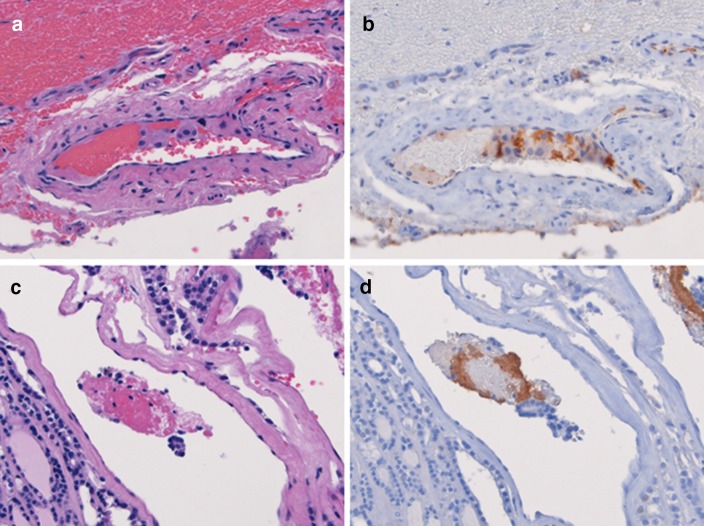
Two distinct patterns of CD61 expression were observed. The first was a linear pattern that manifested as positive staining in a single-file arrangement of platelets along the inner endothelial surface of a vessel (Fig. 1a, b). The second was a clustered pattern that showed positive staining in a group or cluster of platelets located within the luminal space or at inner endothelial edge of a vessel (Fig. 1c, d). Of the 22 positive cases, 50% (11) demonstrated pure clustered staining, 43% (7) demonstrated pure linear staining, and 18% (4) demonstrated mixed clustered and linear staining. The staining pattern was not different by tumor type (p > 0.8; Table 1).
Fig. 1.
Patterns of intravascular CD61-expression in follicular thyroid neoplasms. The linear pattern manifests as a linear array of CD61-positive platelets along the inner endothelium within the vascular lumen. The entire endothelium is not coated, as both positive and negative patches are present within a single vessel. There is some variability in strength of staining between cases (a, b). The clustered pattern demonstrates rounded clusters of CD61-positive platelets attached to a vessel wall (c) and/or present within the luminal space (d)
However, only FTCs with true angioinvasion demonstrated CD61-expressing platelets present in conjunction with intravascular tumor cells. Of the 11 FTCs with vascular invasion on H&E, 64% (7) demonstrated CD61-expressing platelets, 3 of which had clustered groups of platelets associated with intravascular tumor cells (Fig. 2). Platelet-tumor thrombi were identified in intratumoral vessels in 2 cases, and in 1 case they were in peritumoral vessels. All were in mFTCs. The incidence was too low to reach statistical significance compared to aFAs and nmFTCs (p = 0.12). Of the 4 FTCs with 4 or more foci of vascular invasion, 75% (3) had CD61-expressing platelets. Of the 7 FTCs with less than 4 foci of vascular invasion, 57% (4) had CD61-expressing platelets. Of the 32 aFAs and FTCs without vascular invasion on H&E, 47% (15) demonstrated CD61-expressing platelets.
Fig. 2.
CD61-expressing platelets associated with intravascular tumor cells. This case of follicular thyroid carcinoma shows tumor cells within a peritumoral vessel (a) as well as CD61-positive platelets associated with tumor cells (b). Only in rare cases were tumor cells present within intratumoral vessels (c). In this follicular thyroid carcinoma, tumor cells are associated with fibrin and with CD61-positive platelet clusters (d). Both cases (a, b and c, d) had multiple foci of angioinvasion on H&E as well as distant metastasis
Overall, the most common pattern was peritumoral clustered, followed by peritumoral linear, peritumoral linear and clustered, intracapsular clustered, and intracapsular linear and clustered (Table 2). Staining in vessels distant from the nodule was rare, as was staining in intratumoral vessels. The sensitivity (54%), specificity (53%), positive predictive value (60%), and negative predictive value (48%) were not sufficient to distinguish carcinomas from adenomas. Similarly, the sensitivity (64%), specificity (53%), and positive predictive value (32%) were not sufficient to distinguish angioinvasive tumors from non-angioinvasive tumors. The negative predictive value (81%) was somewhat higher.
Table 2.
Location and Staining Pattern of CD61-Positive Intravascular Platelets
| Type of Tumor | Type of staining | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PC | PL | PLC | IcC | IcL | IcLC | DC | DL | ItC | |
| FA (n = 9) | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 0 |
| nmFTC (n = 7) | 2 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| mFTC (n = 6) | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Total (n = 22) | 5 (23%) | 5 (23%) | 3 (14%) | 2 (9%) | 1 (5%) | 2 (9%) | 1 (5%) | 1 (5%) | 2 (9%) |
FA follicular adenoma, nmFTC non-metastatic follicular thyroid carcinoma, mFTC metastatic follicular thyroid carcinoma, P = peritumoral, Ic = intracapsular, D distant, It intratumoral, L linear, C clustered
Discussion
CD61-expressing intravascular platelets were present in aFAs (47%) and FTCs both with and without distant hematogenous metastases (54%). The platelets were present in either clusters or linear arrays and located predominantly in peritumoral and intracapsular vessels. They were rarely present in distant vessels (> 1 cm away from tumor edge) or intratumoral vessels. However, only in FTCs were CD61-expressing intravascular platelets present in association with intravascular tumor cells. This phenomenon was rare (3/24 FTCs) and only present in FTCs with distant metastasis.
There was no significant difference in the overall presence, pattern, or location of CD61-expressing intravascular platelets between aFAs, nmFTCs, and mFTCs (p = 0.9) (Table 1). The presence of CD61-positive intravascular platelet arrays or clusters did not correlate to the presence of angioinvasion on H&E or to a diagnosis of FTC regardless of the presence of angioinvasion. Relatively low sensitivities and specificities made CD61 a poor marker for vascular invasion in follicular thyroid neoplasms. CD61 immunostaining did not reveal occult vascular invasion in cases without vascular invasion on H&E. However, the presence of CD61-positive platelets associated with intravascular tumor cells was only present in FTCs and may help confirm true, clinically significant angioinvasion in equivocal or diagnostically challenging cases.
CD61-positive platelets were present in intratumoral vessels in 2/11 mFTCs, both of which demonstrated vascular invasion on H&E. This feature was not seen in aFAs nor nmFTCs. In both instances, the CD61-positive platelets were associated with tumor cells and fibrin. There was only one additional case of intravascular CD61-positive platelets associated with tumor in a peritumoral vessel, also in a mFTC. Conventionally, intratumoral vascular invasion has not been considered diagnostic of carcinoma in follicular thyroid neoplasms [4]. This exclusion may be related to the rich vascular network supplying thyroid neoplasms, fenestrated nature of thyroid endothelium, and relatively small amount of fibrous stroma separating follicular epithelial cells from endothelium [2, 11, 12]. Occasionally, follicular epithelial cells can be seen bulging into the intratumoral vessels of benign thyroid nodular diseases [2, 11, 12]. While rare in this study, the presence of true vascular invasion in intratumoral vessels may be supported by the association of platelet–fibrin thrombi with tumor. In this study, both cases had distant metastatic disease, suggesting that this finding may be of possible clinical significance. Additional studies may be warranted to assess the clinical relevance of intratumoral vascular invasion.
CD61 (integrin beta 3 chain) functions as a protein associated with thrombus formation by acting as a binding receptor for fibrinogen, fibronectin, plasminogen, prothrombin, von Willebrand factor, and other prothrombotic molecules [8]. CD61 is expressed in platelets, megakaryocytes, and in some myeloid lineage precursor cells. Given its strength in staining platelet–fibrin thrombi, CD61 may assist in marking tumor-associated thrombi in FTC and distinguishing true vascular invasion from mimics. In this study, CD61 did serve to highlight tumor-associated thrombi in a minority of cases. The significance and pathogenic mechanisms of platelet clustering in adenomas and in vessels not associated with tumor cells is unknown. This finding may be due to a local prothrombotic effect in the tumor milieu or procedure-related vascular microtrauma leading to thrombosis [13].
The presence of tumor-associated platelets may signify an increased likelihood of metastasis through a variety of biologic mechanisms. Recent evidence suggests that platelets may independently promote tumor cell metastasis through improved intravascular tumor cell survival, induction of an epithelial-to-mesenchymal phenotypic transformation, and modulation of niche environments to be more hospitable to metastases [14]. Though recognition of tumor-associated platelets is not an explicit component of identifying angioinvasion in the thyroid or elsewhere, the active role platelets play in facilitating metastasis is becoming increasingly apparent. Their presence may have prognostic or targeted therapeutic implications in the future.
Outside the bone marrow, use of CD61 IHC to detect thrombosis is relatively uncommon. Previous studies have used CD61 IHC to assist in differentiating gastric antral vascular ectasia from portal hypertensive gastropathy, mark cutaneous small vessel vasculitis, assess the putative etiology of thrombocytopenia in kaposiform hemangioendothelioma, and differentiate types of thrombi in thrombotic thrombocytopenia purpura and coronary artery thrombosis [15–19]. CD61 IHC has also been described in the identification of microthrombi in renal biopsies from patients with lupus nephritis and those receiving calcineurin inhibitor therapy [20, 21]. In addition to CD34, CD31, ERG, and FLI-1, Factor VIII and Ulex europaeus IHC have also been explored as possible agents to assist in identifying vascular invasion in thyroid neoplasms [22–24]. The current study is the first to explore the relationship of CD61-expression in platelets and vascular invasion in a solid organ neoplasm. CD61 may be a relevant additional marker to consider when employing IHC to assist in recognizing vascular invasion. If CD61-expressing platelets are present in association with intravascular tumor cells, true vascular invasion can be supported.
Reporting of the number of foci of vascular invasion in thyroid carcinomas is not required by CAP for accreditation; however, it is recommended as it may affect patient management. Although often requested by endocrinologists, endocrine surgeons, and oncologists, there is no minimum number of involved vessels or foci of capsular invasion needed to reach a malignant diagnosis [1, 25]. Nevertheless, some studies report an improved prognosis for patients with only capsular invasion or less than four foci of vascular invasion [2, 25, 26]. Of the nmFTCs in this study, two demonstrated more than four foci of capsular invasion, and one case demonstrated four or more foci of both capsular and vascular invasion. To date (46.6 months mean follow-up), none of these cases have metastasized.
Limitations of this study include the relatively focal nature of vascular invasion within the carcinomas. Vascular invasion was detected in 46% (11/24) of carcinomas, of which 64% (7/11) contained three or fewer foci of invasion. Some foci may have been eliminated from tissue blocks during sectioning for research staining thus minimizing opportunities for identification of tumor-associated platelet-thrombi. Also, the low incidence of tumor-associated CD61-expressing platelets may be due to the limitation of having performed CD61 immunostain on only a single representative section. This number may have increased if additional sections of tumor were stained.
In summary, linear arrays or clustered groups of CD61-expressing intravascular platelets were present in intracapsular and peritumoral vessels of aFAs, nmFTCs, and mFTCs. While the mechanism is unknown, the presence of intravascular aggregated platelets is not a good marker for vascular invasion in follicular thyroid neoplasms. Immunostaining for CD61 should not be used as a screening test for vascular invasion in follicular-patterned thyroid neoplasms, as it is not specific for angioinvasive tumors or metastatic behavior. However, CD61-expressing platelets associated with intravascular tumor cells were only present in follicular carcinomas and could support true vascular invasion in equivocal or diagnostically challenging cases.
Acknowledgement
The authors would like to acknowledge the Human Tissue Resource Center at the University of Chicago for the contribution to this project.
Compliance with Ethical Standards
Research Involving Human and Animal Participants
This article does not contain any studies with animals performed by any of the authors. This retrospective study is approved under the University of Chicago IRB #16-0948.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baloch ZW, Livolsi VA. Follicular-patterned afflictions of the thyroid gland: reappraisal of the most discussed entity in endocrine pathology. Endocr Pathol. 2014;25(1):12–20. doi: 10.1007/s12022-013-9293-4. [DOI] [PubMed] [Google Scholar]
- 2.Mete O, Asa SL. Pathologic definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol. 2011;24:1545–1552. doi: 10.1038/modpathol.2011.119. [DOI] [PubMed] [Google Scholar]
- 3.Suster S. Thyroid tumors with a follicular growth pattern: problems in differential diagnosis. Arch Pathol Lab Med. 2006;130(7):984–988. doi: 10.5858/2006-130-984-TTWAFG. [DOI] [PubMed] [Google Scholar]
- 4.Seethala RR, Asa SL, Bullock MJ, et al. Protocol for the examination of specimens from patients with carcinomas of the thyroid gland. College of American Pathologists Website. 2017. www.cap.org. Accessed March 2019.
- 5.Rosai J, Carcangiu ML, Delellis RA. Tumors of the thyroid gland, atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 1992. pp. 49–62. [Google Scholar]
- 6.Baloch Z, Mete O, Asa SL. Immunohistochemical biomarkers in thyroid pathology. Endocr Pathol. 2018;29(2):91–112. doi: 10.1007/s12022-018-9532-9. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, Zhu B, Liu Y, et al. Follicular thyroid carcinoma invades venous rather than lymphatic vessels. Diagn Pathol. 2010;5:8. doi: 10.1186/1746-1596-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett JS. Structure and function of the platelet integrin alpha 2B beta 3. J Clin Invest. 2005;115(12):3363–3369. doi: 10.1172/JCI26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera M, Ricarte-Filho J, Patel S, et al. Encapsulated thyroid tumors of follicular cell origin with high grade features (high mitotic rate/tumor necrosis): a clinicopathologic and molecular study. Hum Pathol. 2010;41(2):172–180. doi: 10.1016/j.humpath.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cracolici V, Kadri S, Ritterhouse LL, et al. Clinicopathologic and molecular features of metastatic follicular thyroid carcinoma in patients presenting with a thyroid nodule versus a distant metastasis. Am J Surg Pathol. 2018;43(4):514–522. doi: 10.1097/PAS.0000000000001208. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov YE, Ohori NP. Follicular carcinoma. In: Nikiforov YE, Biddinger PW, Thompson LDR, editors. Diagnostic pathology and molecular genetics of the thyroid. A comprehensive guide for practicing thyroid pathology. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 132–159. [Google Scholar]
- 12.Boerner SL, Asa SL. Biopsy interpretation of the thyroid. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 13.Ordookhani A, Motazedi A, Burman KD. Thrombosis in thyroid cancer. Int J Endocrinol Metab. 2018;16(1):e57897. doi: 10.5812/ijem.57897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leblanc R, Peyruchaud O. Metastasis: new functional implications of platelets and megakaryocytes. Blood. 2016;128(1):24–31. doi: 10.1182/blood-2016-01-636399. [DOI] [PubMed] [Google Scholar]
- 15.Westerhoff M, Tretiakova M, Hovan L, et al. CD61, CD 31, and CD34 improve diagnostic accuracy in gastric antral vascular ectasia and portal hypertensive gastropathy: an immunohistochemical and digital morphometric study. Am J Surg Pathol. 2010;34(4):494–501. doi: 10.1097/PAS.0b013e3181d38f0a. [DOI] [PubMed] [Google Scholar]
- 16.Meijer-Jorna LB, Mekkes JB, van der Wal AC. Platelet involvement in cutaneous small vessel vasculitis. J Cutan Pathol. 2002;29(3):176–180. doi: 10.1034/j.1600-0560.2002.290309.x. [DOI] [PubMed] [Google Scholar]
- 17.Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28(5):559–568. doi: 10.1097/00000478-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Burke AP, Mont E, Kolodgie F, et al. Thrombotic thrombocytopenic purpura causing rapid unexpected death: value of CD61 immunohistochemical staining in diagnosis. Cardiovasc Pathol. 2005;14(3):150–155. doi: 10.1016/j.carpath.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Arbustini E, Dal Bello B, Morbini P, et al. Immunohistochemical characterization of coronary thrombi in allograft vascular disease. Transplantation. 2000;69(6):1095–1101. doi: 10.1097/00007890-200003270-00013. [DOI] [PubMed] [Google Scholar]
- 20.Galindo M, Gonzalo E, Martinez-Vidal MP, et al. Immunohistochemical detection of intravascular platelet microthrombi in patients with lupus nephritis and anti-phospholipid antibodies. Rheumatology (Oxford) 2009;48(8):1003–1007. doi: 10.1093/rheumatology/kep152. [DOI] [PubMed] [Google Scholar]
- 21.Meehan S, Baliga R, Poduval R, et al. Platelet CD61 expression in vascular calcineurin inhibitor toxicity of renal allografts. Hum Pathol. 2008;39(4):550–556. doi: 10.1016/j.humpath.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Campora R, Montero C, Martin-Lacave I, et al. Demonstration of vascular endothelium in thyroid carcinomas using Ulex europaeus I agglutinin. Histopathology. 1986;10:261–266. doi: 10.1111/j.1365-2559.1986.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 23.Harach HR, Jasani B, Williams ED. Factor VIII as a marker of endothelial cells in follicular carcinoma of the thyroid. J Clin Pathol. 1983;36:1050–1054. doi: 10.1136/jcp.36.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephenson TJ, Griffiths DW, Mills PM. Comparison of Ulex europaeus I lectin binding and factor VIII-related antigen as markers of vascular endothelium in follicular carcinoma of the thyroid. Histopathology. 1986;10:251–260. doi: 10.1111/j.1365-2559.1986.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 25.Janovitz T, Barletta JA. Clinically relevant prognostic parameters in differentiated thyroid carcinoma. Endocr Pathol. 2018;29(4):357–364. doi: 10.1007/s12022-018-9548-1. [DOI] [PubMed] [Google Scholar]
- 26.Thompson LD, Wieneke JA, Paal E, et al. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with review of the English literature. Cancer. 2001;91(3):505–524. doi: 10.1002/1097-0142(20010201)91:3<505::aid-cncr1029>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]