Abstract
Cancer progression can be understood as the result of deregulation of tumors’ immune microenvironments. Recent studies of the alterations of microenvironments highlight their significant influence on the prognosis of patients with head and neck squamous cell carcinoma (HNSCC). It is necessary to better characterize tumor-infiltrating lymphocytes by focusing, in particular, on the tumor escape mechanisms from immune surveillance. One of the best described tumor immune system evasion mechanisms is the expression of co-stimulation molecules that constitute so-called “immune checkpoints”. These molecules regulate the immune response by either activating or inhibiting its effects. The programmed cell death 1 (PD-1) surface protein is an inhibitory co-stimulation molecule that induces exhaustion of activated T-lymphocytes (TLs, T cells) through binding with its ligands, PD-L1 and PD-L2. Half of HNSCCs exhibit PD-L1 expression with higher expression identified in human papillomavirus (HPV) positive tumors. Numerous studies have shown differences between the microenvironments of HPV+ and HPV− cancers. Notably, infiltrations of exhausted CD4+ PD1+ and CD8+ PD1+ T cells are far higher in the microenvironment of HPV+ tumors. The FDA has approved the use of molecules that target PD-1 for the treatment of HNSCC. The first results of clinical trials with anti-PD-1 blockers in HNSCC show improved patient survival, particularly long-term survival without recurrence. However, discordant results were sometimes observed, and improvements in defining cellular predictive markers are necessary. With the development of immunotherapies, pathologists play a role in the selection of patients who are eligible for specific treatments and assessment of their prognosis in greater detail. An automated, quantitative in situ imaging system that integrates both multispectral imaging and automated slide scanning could be developed in pathology laboratories. The evaluation of PD-L1 expression has only been used to stratify the administration of first-line immunotherapy. The validation of these tests and their routine interpretation is essential. No specific recommendation is adopted for HPV+ HNSCC.
Keywords: Head and neck cancer, Immunotherapy, PD-L1, Immune checkpoint, Papillomavirus, HPV, PD-1
Why is Microenvironment Important?
It is now acknowledged that the immune system plays a major role in a tumor’s microenvironment. The theory of immunosurveillance was first stated by Paul Ehrlich at the beginning of the twentieth century and later clarified by Robert Schreiber in his theory of the three Es [1]. This theory refers to three successive phases of tumor development: (i) elimination: there is a strong and effective immune response leading to the destruction of tumor cells; (ii) equilibrium: the immune reaction allows control of tumor proliferation which, however, persists; (iii) escape: the immune system can no longer fight against tumor proliferation making tumor growth possible. Occurrence, growth, persistence, and progression of a tumor may be considered as a successful escape from the anti-tumoral immune system. Immune evasion is regarded as a key hallmark of cancer, generating an environment permissive for survival and progression. In the past few years, modulation of this system has been regarded as a promising approach among treatment options offered to cancer patients. Currently, one of the best described mechanisms of immune system escape is the expression of inhibition co-stimulatory molecules. These co-stimulation molecules are called “immune checkpoints”. They can activate or inhibit molecules and make it possible to regulate the immune response. An adequate immune response results from a balance between activating and inhibiting signals.
Why Could Co-inhibitory Molecules be Selected as Immunotherapy Targets?
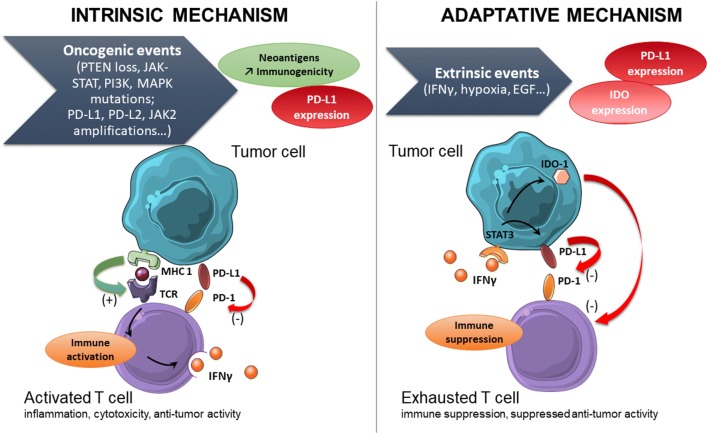
The surface protein programmed cell death 1 (PD-1) is known to be an inhibitory co-stimulation molecule that acts after the activation of T-lymphocytes and persists in the presence of stimuli. CD4 and CD8 T-lymphocytes (TLs, T cells), B-lymphocytes (BLs), macrophages, or natural killer (NK) cells express this protein. PD-1 is often viewed as an “exhaustion marker” since CD8+ PD-1+ T cells are no longer able to proliferate and release anti-tumor cytokines such as interferon γ (IFNγ) [2, 3]. However, PD-1 may also be interpreted as an activation marker since it is induced after T cell activation [4]. The PD-1 ligands are PD-L1 (programmed death-ligand 1) and PD-L2 (programmed death-ligand 2). PD-L1 is expressed by activated lymphocytes, macrophages, dendritic cells, and mast cells, but also by non-immune cells such as epithelial cells (Fig. 1). It is important to note that some tumor cells can also express PD-L1 in the presence of IFNγ. IFNγ, secreted by active T cells and NK cells, stimulates an adaptive reaction of the tumor cell to express PD-L1 (Fig. 2) [5]. In this context, PD-L1 expression may be interpreted as an immune system activation marker as well as a resistance marker to anti-tumor immunity. Alternatively, following intrinsic oncogenic events, PD-L1 self-expression may result from various pathways of tumor cell activation including chromosomal alterations, gene amplifications, or mutations (Fig. 2). Such events contribute to a strategy of resistance to immune surveillance and are associated with a poor prognosis [6].
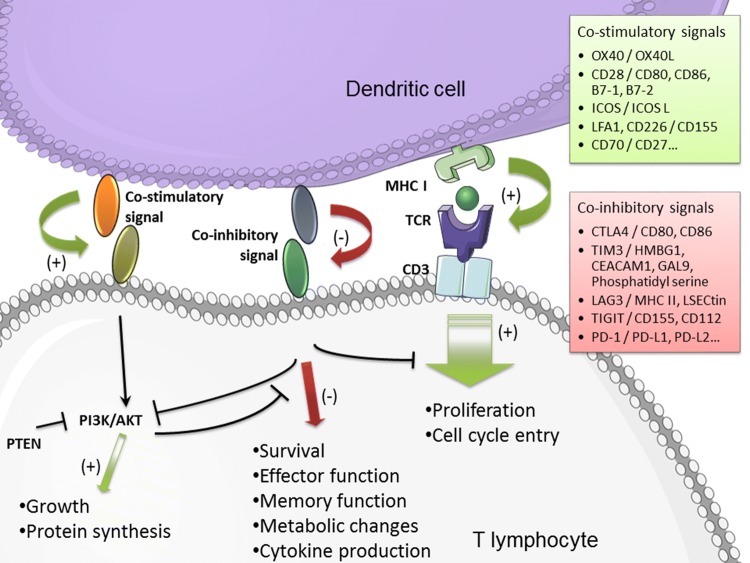
Fig. 1.
Immune checkpoint activities. T cell activation, via the TCR signaling, is balanced by numerous co-stimulatory and co-inhibitory signals. From Outh-Gauer et al. 2018 [7] (modified)
Fig. 2.
Interaction between tumor cells and immune cells concerning PD-L1/PD1 synapse. PD-L1 expression on cancer cells can be induced by the presence of interferon γ or due to intrinsic oncogenic events. Therefore, PD-L1 self-expression may result from activation of various tumor cell pathways, chromosomal alterations, gene amplifications, or mutations. From Outh-Gauer et al. 2018 [7] (modified)
The other PD-1 ligand, PD-L2, is less well-known. Its constitutive expression is weak, and it remains expressed almost exclusively by antigen-presenting cells such as dendritic cells and macrophages. Stimulation of inhibitory co-stimulation molecules such as PD-1 cause induction of non-functional TLs called anergic T cells. These lymphocytes cannot express their auxiliary (CD4+) or cytotoxic (CD8+) activities against tumor cells [7]. Although every kind of immune cell may have anti-tumoral activity, main findings in the recent years were directed towards the adaptive immune system, focusing on TL- and BL-derived antibodies. Immunosuppressive cells that express these inhibitory co-stimulating molecules have additional immunosuppressive functions. The regulatory TLs (CD4+ , FoxP3+), which secrete immunosuppressive cytokines, deplete the extra-cellular medium into interleukin-2 (it stimulates the activation of CD4+ T lymphocytes) and sequester the CD80/CD86 molecules present on the surface of antigen-presenting cells (CD80/CD86 normally stimulates T lymphocytes) [8]. Despite these immunosuppressive effects, intratumoral infiltration by regulatory TLs is correlated with a good prognosis in HNSCC [9].
The M2-type macrophages stimulate angiogenesis, immunosuppression (via the secretion of IL-10, TGF-β, and the depletion of metabolites necessary for T lymphocytes), proliferation, and tumor invasion (via the induction of an epithelio-mesenchymal transition as well as the maintenance of tumor stem cells) [10, 11]. These macrophages are theoretically the opposite of type M1 macrophages, which are pro-inflammatory (production of IL6, 12, 23, and TNFα as well as overexpression of MHC class I and II) and therefore allow stimulation of the anti-tumor immune response [10].
What does HNSCC Microenvironment Look Like?
According to 2012 data, the incidence and mortality of cancers of the upper aerodigestive tract are seventh globally. The main risk factors are tobacco, alcohol (as a co-factor in tobacco use) and human papillomavirus (HPV) infection. Treatments are dependent on the TNM classification and location of the cancer. Surgery, chemotherapy, and/or radiotherapy are most commonly used. The prognosis is guarded with a 5-year survival of less than 50%. Many head and neck cancer genes are involved in cell proliferation and cell cycle control, WNT-catenin signaling, cell survival, and epigenetic regulation [12]. Genetic changes in head and neck cancers present an opportunity for immunotherapy with immune checkpoint inhibitors. In situ study of the co-inhibitory molecule PD-1 and its ligand, PDL-1, has received particular attention over the past few years. Zandberg and Strome [13] reviewed results obtained from PD-L1 staining in HNSCC, mostly of the oropharynx, and showed that despite high technical heterogeneity, due to different staining antibodies and interpretation protocols, PD-L1 is expressed in approximately half of oropharyngeal squamous cell carcinomas (OSCCs) (Table 1). PD-L2 was detected in 64% of cases in a cohort of 146 HNSCC patients [14]. Furthermore, Ahmadi et al. demonstrated a link between PD-L1 expression in oral SCC and smoking, gender, and p53 expression. PD-L1 expression was observed in 70 cases (27.5%) and more commonly in females (odds ratio [OR] 2.19; P = 0.005) [15]. PD-L1 expression did not seem to correlate with lymph node invasion, TNM staging, or survival among a cohort of surgically and adjuvant radiotherapy and/or chemotherapy treated OSCC patients [16]. Finally, PD-L1 expression seems to be significantly higher in metastases compared to primary OSCC (P < 0.05) [17].
Table 1.
PD-L1 expression among HPV- or non-HPV-induced HNSCC patients (2019)
| Localization | Number of patients | % of tumors expressing PD-L1 | % of HPV+ tumors expressing PD-L1 | % of HPV− tumors expressing PD-L1 | References |
|---|---|---|---|---|---|
| OC, HP, L, PNS | 24 | 66 | Strome et al. (2002) [51] | ||
| OC, HP, OP | 64 | 51 | 62 | 41 | Badoual et al. (2013) [4] |
| OP | 181 | 46.4 | 49.2 | 34.1 | Kim et al. (2016) [52] |
| OP | 27 | 59 | 70 | 29 | Chen et al. (2012) [53] |
| OP | 45 | 87 | Festino et al. (2016) [54] | ||
| OC, OP, HP | 64 | 51.5 | 62.5 | 40 | Gooden et al. (2011) [55] |
| OC, HP, L, PNS | 50 | 64 | Partlová et al. (2015) [29] | ||
| OC | 21 | 71 | Hsu et al. (2015) [41] | ||
| OP | 133 | 68 | 71 | 61 | Li et al. (2016) [56] |
| OC, OP, HP, L | 38 | 58 | Clinical trial NCT03463161 [57] | ||
| OC, OP, HP, L | 361 | 57.3 | 52 | 56 | Ferris et al. (2016) [58] |
| OC, OP, HP, L | 882 | 85 | Burtness et al. (2019) [59] | ||
| OP, HP, L | 10 | 80 | 75 | 100 | Elbers et al. (2019) [60] |
From Outh-Gauer et al. (2018) [7] (modified). From Zandberg DP and Strome SE (modified) [60]
HNSCC head and neck squamous cell carcinoma, HP hypopharynx, HPV human papillomavirus, L larynx, OC oral cavity, OP oropharynx, PNS paranasal sinus
Intra-tumor regulatory T cells may exert stronger immunosuppressive properties than circulating regulatory T cells in the peripheral blood, as suggested by higher expression levels of immune-checkpoint receptors PD-1, CTLA-4, and TIM-3 and immunosuppressive molecules including LAP and CD39 ectonucleotidase [18]. Taken altogether, these results illustrate that a single molecule, such as PD-1 or PD-L1, can confer different pathophysiologic meanings and prognostic values depending on the tumor context and involved regulation pathways.
What is the Role of HPV Infection in HNSCC Microenvironment?
In 2017, the WHO classification [19] recognized human papillomavirus positive (HPV+) OSCCs as an increasingly distinct clinical subgroup of patients [20]. Genetic, epigenetic, and molecular features differ between HPV+ and HPV− OSCC tumors. A high somatic mutation rate is frequently observed among all HNSCCs (mean value approximately 180 somatic mutations per mega base) [21] with a comparable mutational burden between HPV+ and HPV− tumors [22, 23]. PIK3CA mutations predominate in HPV-related tumors while TP53 is the most often mutated in non-HPV-induced SCC (41%) [21, 24]. Such differences can be explained by varying oncogenic pathways. HPV-induced cancers involve E6 and E7 viral oncogenes which impair the cellular cycle via p53 inactivation [25]. PTEN (phosphatase and tensin homolog) loss is frequent, independent of HPV status [24].
Therefore, immunogenicity of tumors can result from expression of tumor self-antigens, mutated protein-derived neoantigens, or virus-induced antigens. Researchers and pathologists have also described differences in tumor microenvironments that are associated with variance in outcomes [4, 26, 27]. In particular, infiltrations of exhausted CD4+ PD1+ and CD8+ PD1+ T cells are far higher in the microenvironment of HPV+ tumors. Similar observations have been made with FoxP3+ T cell infiltration. These differences could be driven by the presence of interferon (IFN), known to be produced after HPV replication in cancer cells stroma [28]. PD-1 is expressed by CD4+ and CD8+ lymphocytes. It is a marker for activation or exhaustion, and the expression of other inactive immune checkpoints markers (Tim-3, CTLA) in a same T cell is associated with deep anergy. Macrophages (CD68+), tumor cells (cytokeratin+). BLs, and TLs express PDL-1 and PD-L2. PD-L1 staining was reported in more than 50% of tumor cells with a close co-localization of PD-1 T cells and PD-L1+ tumor cells, suggesting a strong interaction between these cells. However, no correlation was reported in terms of PD-L1 and PD-L2 expression between HPV+ and HPV- cancer populations [14]. In another study, PD-L1-labeled cells, either tumor cells or CD68+ tumor-associated macrophages, were predominantly located at the tumor margin whereas anti-PD-1 antibody mostly labeled CD8+ T cells within the tumor among HPV+ oropharyngeal carcinomas [27].
The differences between HPV+ and HPV− can be also demonstrated in other fields. For example, HPV-induced peritumoral immune cells exhibit more CD8+ IFNγ TL, CD8+ IL-17+ TL, and myeloid dendritic cells, as well as higher pro-inflammatory cytokines levels. Additionally, Cox-2 mRNA levels decrease whereas PD-1 mRNA levels increase [29]. The immune landscape of HNSCC has been a rationale for the evaluation of immunotherapies that target immune checkpoints like PD-1/PD-L1 and CTLA4 [30]. Moreover, several clinical trials have evaluated the efficacy of alternative, de-escalated therapeutic protocols for the management of patients with HPV+ OSCC in order to spare them high-grade toxicities induced by aggressive radiation and chemoradiation therapies [31]. For example, the De-ESCALaTE HPV and RTOG 1016 clinical trials have compared a conventional platinum-based chemoradiotherapy arm to an experimental arm where cisplatin was replaced by the cetuximab anti-EGFR antibody [32, 33]. Unfortunately, the cisplatin arm performed poorly compared to the conventional arm. The latter remains the recommended therapeutic option for management of locally advanced HPV+ HNSCC. Even within the HPV+ OSCC population, however, there are known differences in survival and prognosis with two distinct groups of patients that display different rates of distant metastatic spread [34, 35].
More recently, it has been reported that quantification of CD103, a marker of resident memory T cells, represents an independent prognostic marker in HPV+ HNSCC. Thus, in the light of recent clinical reports and our own observations, we believe additional biomarkers are required to further stratify HPV+ patients into prognostic subgroups to allow for tailored therapy. Stratification strategies based on markers that predict response to treatment are crucial and urgently needed in order to discriminate between the two groups of HPV+ OSCC. HPV-positive tumors are enriched in markers of T-regulatory cells (Tregs) and HPV-negative tumors in protumorigenic M2 macrophages [36].
How can the Pathologist Help Understand the Microenvironment?
The first data evaluating the expression of PD-L1 by immunohistochemistry in a predictive framework comes from the analysis of pre-treatment biopsies testing an anti-PD-1 in various solid tumors. Tumor heterogeneity, reproducibility, and the reliability of staining were the first limitations to the interpretation of these results. Many studies have shown that PD-L1 status can vary between different lesions in the same patient and also be modified after anti-cancer treatments. These findings are reported in many cancers and are not specific to HNSSC. However, although immunohistochemical staining with anti-PD-L1 has limitations, many clinical trials have shown correlation between the expression of PD-L1 and efficacy of anti-PD-1/PD-L1 treatments. It is important to note, however, that a number of patients with a negative PD-L1 tumor also showed treatment response, while some patients with a positive PD-L1 tumor expression had no immune response. Additionally, the relationship between the positivity of PD-L1 and long-term benefit of anti-PD-1/PD-L1 treatment, in terms of overall survival and progression-free survival, is less established than between expression and response. The precise measurement of PD-L1 expression remains a challenge. Beyond the pre-analytical contingencies related to the development of immunohistochemical staining, the uncoordinated development of multiple antibodies, kits, and PD-L1 immunohistochemistry platforms was significantly involved in complicating its assessment as a biomarker (Table 2). Several standardized FDA-approved assays are commercially available to date: the Ventana PD-L1 SP263 assay (Ventana Medical Systems Inc.), 28-8 PharmDx (Agilent Technologies/Dako) [31], 22C3 PharmDx (Agilent Technologies/Dako), and Ventana PD-L1 SP142 Assay (Ventana Medical Systems Inc.). The 22C3 PharmDx and 28-8 PharmDx are performed on the Autostainer Link 48 platform (Agilent Technologies, Santa Clara, CA, USA) and the Ventana PD-L1 SP263 and SP142 Assays on the Ventana ULTRA platform (Ventana Medical Systems, Tucson, AZ, USA). Nevertheless, many other antibodies are available and can be used in other platforms, so scoring methods and positivity thresholds can radically vary from one kit or antibody to another. These different techniques are therefore not always comparable or transferable routinely without harmonization and validation. The Blueprint Working Group and other working groups have compared different antibodies, autostainers, and staining scores for lung cancer [37]. Further complicated interpretations, morphologic identification of the different cell types expressing PD-L1 (macrophages, tumor cells, etc.) can be difficult. The distinction between macrophages and tumor cells is sometimes delicate, especially in solid tumor nests.
Table 2.
Anti PD-L1 antibodies scoring and platforms
| Primary antibody clone | 28-8 (Dako) | 22C3 (Dako) | SP142 (Ventana) | SP263 (Ventana) | 73-10 (Dako) | E1L3N (cell signaling) |
|---|---|---|---|---|---|---|
| PD-L1 testing methods | ||||||
| Platform |
Dako Autostainer Link 48 |
Dako Autostainer Link 48 |
Ventana BenchMark Ultra |
Ventana BenchMark Ultra |
Dako Autostainer Link 48 |
Leica bond-III Autostainer |
| Instrument and detection systems | EnVision FLEX visualization system | EnVision FLEX visualization system | OptiView DAB IHC detection kit and OptiView amplification kit | OptiView DAB IHC detection kit | EnVision FLEX visualization system |
Refine detection kit Mixed DAB |
| Interpretative scoring | > 1%, > 5% | TC or IC: > 1%, > 50% | TC: ≥ 5%, IC: ≥ 5% | TC: ≥ 25% | TC: ≥ 1% | TC or IC: ≥ 1, ≥ 5, ≥ 10 or ≥ 50% |
| Agent | Nivolumab | Pembrolizumab | Atezolizumab | Durvalumab | Avelumab | |
In HNSCC, is There Enough Information to Claim Equivalence of Different Clones for PD-L1 Immunohistochemistry?
For now, few studies have been performed to compare the different anti PD-L1 clones available. Similar to lung cancers, the preliminary results of Ratcliffe et al. showed good agreement between the 22C3, 28-8, and SP263 assays, [38]. In their study, lower agreement for SP142 was found. The same data are described in a study comparing 22C3 and SP142 performances in oropharyngeal cancer where moderate agreement was observed between the two antibodies [39]. Conversely, a moderate to high correlation was described in an assay comparing SP263 and SP142 staining for hypopharyngeal squamous cell carcinomas [40]. These results emphasize the need for new “real life data” comparing clones and platforms, like for lung cancers or melanoma.
What do Pathologists have to Know About Checkpoint Inhibitors in HNSCC?
The US Food and Drug Administration (FDA) has approved Nivolumab (Opdivo © Bristol-Myers-Squibb) and Pembrolizumab (Keytruda © Merck Sharp & Dohme) which target PD-1 for the treatment of patients with recurrent and/or metastatic HNSCC with platinum-refractory cancer. The FDA first approved Pembrolizumab in 2016 following the results of the KEYNOTE 012 cohort expansion phase I clinical trial with no comparative group [41]. Nivolumab was subsequently approved based on the phase III CHECKMATE 141 Pivotal trial. Nivolumab was delivered with no regards to the PD-L1 status. Most of the major trials on immunotherapy have demonstrated a positive correlation between PD-L1 positivity and clinical outcome, especially using Combine Positive Score (CPS). In 2019, the FDA approved Pembrolizumab for first-line treatment of patients with metastatic or unresectable, recurrent HNSCC [42]. Approval was based on the KEYNOTE-048 (NCT02358031) assay. The indications for HNSCC are: (i) in combination with platinum and FU for the first-line treatment of patients with metastatic or with unresectable, recurrent HNSCC; (ii) as a single agent for the first-line treatment of patients with metastatic or with unresectable, recurrent HNSCC with tumors expressing PD-L1 with a CPS ≥ 1 as determined by an FDA-approved test; (iii) as a single agent for the treatment of patients with recurrent or metastatic HNSCC with disease progression or after platinum-containing chemotherapy. Durvalumab (IMFINZI © Medimmune/AstraZeneca), a fully human monoclonal antibody that blocks PD-L1 binding to its receptors PD-1 and CD80, was evaluated in the HAWK study [43]. Median progression-free survival (PFS) and median overall survival (OS) were 2.1 months and 7.1 months, respectively. Overall response rate was higher in HPV-positive cancer patients than in HPV-negative cancer patients (30% versus 10%). In other studies, no difference in patient’s survival between HPV positive and HPV negative tumors was observed.
Numerous trials are ongoing, particularly for HNSCC. The first aim is to determine the optimal use of these therapies by evaluating optimal doses, selecting responder patients, and avoiding side effects such as possible hyperprogression. Hyperprogression phenomenon is characterized by an acceleration of tumor growth kinetics and has recently been reported in 29% of 34 recurrent and/or metastatic HNSCC patients undergoing anti-PD-1 immunotherapy [44]. Further assessment of the mechanisms and causality of this phenomenon is necessary. The second aim is to optimize the treatment by combining immunotherapy with chemotherapy or radiotherapy. Some trials mix immunotherapy and therapeutic vaccination for HPV+ HNSCC. These approaches are promising and could result in new, dedicated therapeutic approaches, especially when combined with blockade of PD-(L)1 [4, 45, 46].
How are PD-L1 Evaluation and Scoring Performed?
How can Anti PDL-1 Immunochemistry be Evaluated? Why Use Combine Positive Score instead of Tumor Proportion Score?
As discussed previously, PD-L1 immunochemistry is still the best marker to select patients who are eligible for immunotherapy. Several cell types, both immune and tumor cells, express PD-L1. It is necessary for pathologists to be aware of the different scoring systems available. A minimum of 100 viable tumor cells must be present on the PD-L1 stained slide for the specimen to be considered adequate for evaluation. Depending on the trials, different scoring systems with varying cut-offs are used to evaluate PD-L1 staining. PD-L1 protein expression can be determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity. Another scoring system is the Combined Positive Score (CPS) which divides the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) by the total number of viable tumor cells, multiplied by 100. Although the result of the calculation can exceed 100, the maximum score is defined as CPS 100. Any perceptible and convincing partial or complete linear membrane staining (≥ 1+) of viable tumor cells that is perceived as distinct from cytoplasmic staining is considered PD-L1-positive and included in the scoring. Any membrane and/or cytoplasmic staining (≥ 1+) of lymphocytes and macrophages within tumor nests and/or adjacent supporting stroma is considered PD-L1 staining and should be included in the CPS numerator. The evaluation is performed at 20 × magnification. To be considered positive, the staining of the tumor cells has to be convincing partial or complete linear membrane (at any intensity) located on viable invasive tumor cells (Fig. 3). The same criteria are used for the evaluation of the immune cells staining. A screening of the whole slide is required [47]. In some publications, the percentage of positive immune cells (IC) is provided; further research is needed to evaluate the role of the PD-L1+ immune infiltration as a routine marker. Other immune checkpoint inhibitors such as CTLA4 are used in trials. These molecules could become other useful immune markers to stratify tumors and predict the therapeutic response.
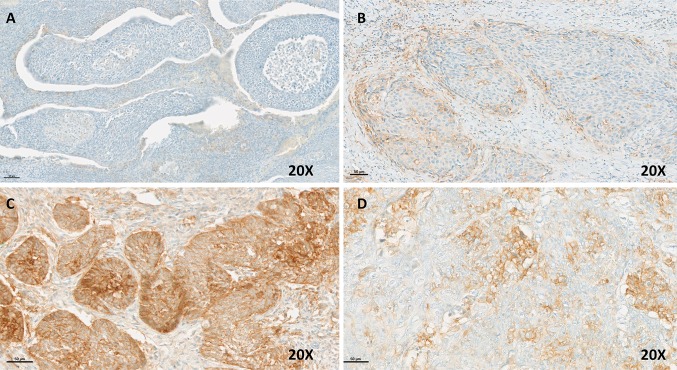
Fig. 3.
OPSCC immunochemistry with PD-L1 antibody (22C3; Dako, Glostrup, Denmark). a no staining, TPS, IC, and CPS = 0. b TPS = 3%, IC = 10%, CPS = 3. c TPS = 100%, IC = 20%, CPS = 110. d TPS = 20%, IC = 2%, CPS = 20
Nowadays, CPS scoring is the gold standard for PD-L1 in situ evaluation, as this score has been validated in different trials and will be required for specific treatments (first line for patients with metastatic or unresectable recurrent HNSCC with a CPS ≥ 1). No other recommendation or information about PD-L1 staining description is required; however, our team has added the TPS score to pathology reports to allow for a better description of PD-L1 staining.
Does In Situ RNA Hybridization Play a Role in Routine PD-L1 Detection?
Tumor cell mRNA staining using RNAScope® demonstrated statistical significance (at α = 0.05) in the PD-L1 high (TCIHC ≥ 25%) vs the PD-L1 low (TCIHC < 25%) groups for HNSCC. The number of punctate dots/tumor cells was significantly higher in the PD-L1 high vs the PD-L1 low groups for HNSCC [48]. In this study, a positive relationship was observed between PD-L1 mRNA and PD-L1 protein expression where 67.3% of PD-L1 IHC positive patient samples were PD-L1 RNAScope positive and 88.2% of PD-L1 IHC negative patient samples were also PD-L1 RNAScope negative. To date, this technique is dedicated to research and not used routinely.
Is there Sufficient Evidence to Justify Clinical Utility in Quantitating Subpopulations of T Cell Infiltrates in a Multiplex Fashion in Conjunction with PD-L1 Assessment, or is it Still Too Early?
A fluorescent multiparametric platform allows multiparametric in situ analysis of immune cell infiltration. A fluorescent spectral scanner coupled to a software can automatically identify various cellular profiles by their size, shape, and morphology. Several companies developing analysis software programs across the world may be cited, such as Definiens®, Tribvn®, Owkins®, Visiopharm® in Europe, and Indica Labs® (with Halo), Inform® in the United States. These programs allow for analyses of several parameters including cell shape, cell quantification, and vascular networks and add efficiency and reproducibility. The digital analysis of images will eventually make it possible to homogenize results and improve patient care, but a pathologist must validate the results. In fact, even though the interpretation is automatized, the diversity of tissues, cells and staining require a pathologist to confirm the final diagnosis.
These results, combined with the capacity to integrate and process multiple fluorescent signals, will help to better understand the immune cell role within the tumor microenvironment network. This technique allows the observation of biomarkers expressed by different cells or co-expressed by the same cells. The use of immunofluorescence is particularly adapted for the multiple co-localization studies to understand the role of immune cells in HNSCC and explore the interaction between the different immune cells (Fig. 4). The combination of several stains on a single slide allows the acquisition of spatial and functional data in order to achieve the identification of “signatures” in the microenvironment, and improve the development of new patient management strategies.
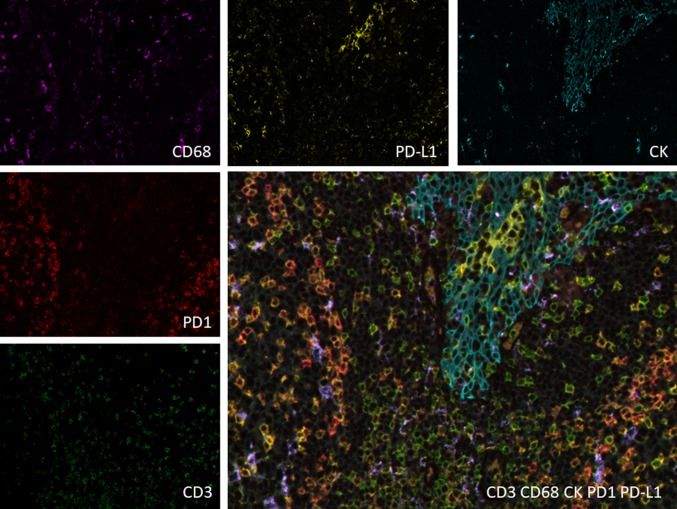
Fig. 4.
Example of Opal™ multiplex immunofluorescent staining to highlight the microenvironment. Anti-cytokeratin (CK) antibody stains epithelial cells, anti CD3 antibody is present at the surface of T cells (some are CD4+), and the anti CD68 antibody stains macrophages. PD-L1 is mostly expressed by epithelial cells but can be expressed by other cells
Currently, there are no recommendations for associating the subpopulations of CD8+ T cells with PD-L1 before immunotherapy. However, during pilot clinical studies, tumor infiltration with CD8+ T cells or resident memory CD8+ T cells before or after treatment were correlated with response to immunotherapy [49, 50].
Conclusion
We have demonstrated the important role that immunotherapy plays in the management of cancers currently and predict that these therapies will be increasingly pertinent to the treatment of HNSCC. While immunotherapy has long been considered a marginal therapeutic option for cancer treatment, there is now evidence substantiating the major importance of the immune system in tumor control. The various molecules targeted by these therapies modulate the microenvironment and enhance its anti-tumor activity. The most commonly used molecules are anti-checkpoint antibodies. The blockade of the PD-1 and PD-L1 co-inhibitory molecules restores an anti-tumor immune system by the impairment of tumor immunosuppression strategies. Encouraging clinical results have been achieved in HNSCC treatments, however, many remaining challenging must be overcome in order to optimize efficacy.
The pathologist’s role will be in diagnostics, patient selection, and performance of predictive markers, and close interaction with clinicians will be necessary to guide the decision-making processes. Anti PD-L1 immunostaining is now (or will be) required by physicians for specific treatment indications only. Even if most laboratories are trained to perform and interpret PD-L1 immunostaining, there is no current indication to perform it routinely in patients with HNSCC. Pathologist awareness of the quantification, stratification, and optimization of the staining is required for appropriate interpretation of the study. Furthermore, pathologists will have opportunities to develop new strategies examining the microenvironment, for example using multiplex staining or other tests such as in situ RNAseq, in situ nanostring, or immunoligation.
Acknowledgements
We thank Manon Labrande for language editing and reviewing, and Servier Medical Art for providing the art material used for Figs. 1 and 2.
Compliance with Ethical Standards
Conflict of interest
Cécile Badoual has received research grants from Servier, has been member of advisory board BMS, Astrazeneca, MSD and has speaker honorarium from Merck, BMS, Roche, Astrazeneca. Eric Tartour has received research grants from GSK, Vaxeal, Servier, has been member of advisory board BMS, Astra-Zeneca and has speaker honorarium from Merck, BMS, Roche. Sophie Outh-Gauer, Aurélien Morini, Charles Lépine, Alain Jung declares that he has no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389–395. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 5.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol (Baltimore, Md 1950) 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 6.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7–H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C, et al. Immunotherapy in head and neck cancers: a new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev. 2018;65:54–64. doi: 10.1016/j.ctrv.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badoual C. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 10.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.She L, Qin Y, Wang J, Liu C, Zhu G, Li G, et al. Tumor-associated macrophages derived CCL18 promotes metastasis in squamous cell carcinoma of the head and neck. Cancer Cell Int. 2018;18:120. doi: 10.1186/s12935-018-0620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18:269–282. doi: 10.1038/nrc.2018.11. [DOI] [PubMed] [Google Scholar]
- 13.Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014;50:627–632. doi: 10.1016/j.oraloncology.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Yearley J, Gibson C, Yu N. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res An Off J Am Assoc Cancer Res. 2017;23:3158–3167. doi: 10.1158/1078-0432.CCR-16-1761. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadi N, Gao K, Chia N, Kwon MS, Palme CE, Gupta R, et al. Association of PD-L1 expression in oral squamous cell carcinoma with smoking, sex, and p53 expression. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:631–638. doi: 10.1016/j.oooo.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Ukpo OC, Thorstad WL, Lewis JS. B7–H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2013;7:113–121. doi: 10.1007/s12105-012-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moratin J, Metzger K, Safaltin A, Herpel E, Hoffmann J, Freier K, et al. Upregulation of PD-L1 and PD-L2 in neck node metastases of head and neck squamous cell carcinoma. Head Neck. 2019;41:2484–2491. doi: 10.1002/hed.25713. [DOI] [PubMed] [Google Scholar]
- 18.Jie H-B, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer. 2013;109:2629–2635. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. In: World Health Organization classification of tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
- 20.Gillison ML, Broutian T, Pickard RKL, Tong Z, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes DN, Grandis JR, El-Naggar AK. The Cancer Genome Atlas: integrated analysis of genome alterations in squamous cell carcinoma of the head and neck. J Clin Oncol. 2013;31:6009. [Google Scholar]
- 23.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman R, Gatalica Z, Knezetic J, Reddy S, Nathan C-A, Javadi N, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1625–1638. doi: 10.1002/hed.24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 26.Jung AC, Briolat J, Millon R, de Reyniès A, Rickman D, Thomas E, et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
- 27.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Partlová S, Bouček J, Kloudová K, Lukešová E, Zábrodský M, Grega M, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology. 2015;4:e965570. doi: 10.4161/21624011.2014.965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalieri S, Rivoltini L, Bergamini C, Locati LD, Licitra L, Bossi P. Immuno-oncology in head and neck squamous cell cancers: news from clinical trials, emerging predictive factors and unmet needs. Cancer Treat Rev. 2018;65:78–86. doi: 10.1016/j.ctrv.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Mirghani H, Casiraghi O, Amen F, He M, Ma X-J, Saulnier P, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol Springer Nature. 2015;28:1518–1527. doi: 10.1038/modpathol.2015.113. [DOI] [PubMed] [Google Scholar]
- 32.Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393:51–60. doi: 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. doi: 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with HPV-related head and neck cancer. J Neurooncol. 2013;112:449–454. doi: 10.1007/s11060-013-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saloura V, Izumchenko E, Zuo Z, Bao R, Korzinkin M, Ozerov I, et al. Immune profiles in primary squamous cell carcinoma of the head and neck. Oral Oncol. 2019;96:77–88. doi: 10.1016/j.oraloncology.2019.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsao MS, Kerr KM, Kockx M, Beasley M-B, Borczuk AC, Botling J, et al. PD-L1 Immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scott M, Scorer P, et al. Agreement between programmed cell death ligand-1 diagnostic assays across multiple protein expression cutoffs in non–small cell lung cancer. Clin Cancer Res. 2017;23:3585–3591. doi: 10.1158/1078-0432.CCR-16-2375. [DOI] [PubMed] [Google Scholar]
- 39.De Meulenaere A, Vermassen T, Creytens D, Aspeslagh S, Deron P, Duprez F, et al. Importance of choice of materials and methods in PD-L1 and TIL assessment in oropharyngeal squamous cell carcinoma. Histopathology. 2018;73:500–509. doi: 10.1111/his.13650. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Hahn E, Slodkowska E, Eskander A, Enepekides D, Higgins K, et al. Reproducibility of PD-L1 immunohistochemistry interpretation across various types of genitourinary and head/neck carcinomas, antibody clones, and tissue types. Hum Pathol. 2018;82:131–139. doi: 10.1016/j.humpath.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Hsu C, Lee S-H, Ejadi S, Even C, Cohen R, Le Tourneau C, et al. Antitumor activity and safety of pembrolizumab in patients with PD-L1-positive nasopharyngeal carcinoma: interim results from a phase 1b study. Ann Oncol. 2015;26:ix94.1–ix94. [Google Scholar]
- 42.FDA. FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma [Internet]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma. Accessed 20 Feb 2020.
- 43.Zandberg DP, Jarkowski A, Emeribe UA, Goswami T, Melillo G. A Phase 2, multicenter, single-arm, global study of MEDI4736 monotherapy in patients with recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): HAWK ( NCT02207530) J Clin Oncol. 2015;33:TPS6086. [Google Scholar]
- 44.Saâda-Bouzid E, Defaucheux C, Karabajakian A, Palomar Coloma V, Servois V, Paoletti X, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2017;28:1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 45.Nizard M, Roussel H, Diniz MO, Karaki S, Tran T, Voron T, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Commun. 2017;8:15221. doi: 10.1038/ncomms15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16–related cancer. JAMA Oncol. 2019;5:67. doi: 10.1001/jamaoncol.2018.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dako. PD-L1 IHC 22C3 pharmDx interpretation manual—head and neck squamous cell carcinoma (HNSCC) [Internet]. https://www.agilent.com/cs/library/usermanuals/public/29314_22c3_pharmDx_hnscc_interpretation_manual_us.pdf. Accessed 20 Feb 2020.
- 48.Duncan DJ, Scott M, Scorer P, Barker C. Assessment of PD-L1 mRNA and protein expression in non-small cell lung cancer, head and neck squamous cell carcinoma and urothelial carcinoma tissue specimens using RNAScope and immunohistochemistry. Ahmad A, editor. PLoS ONE. 2019;14:e0215393. doi: 10.1371/journal.pone.0215393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen P-L, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. CD103+ tumor-resident CD8+ T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-PD-1 treatment. Clin Cancer Res. 2018;24:3036–3045. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 51.Strome SE, Savva A, Brissett AE, Gostout BS, Lewis J, Clayton AC, et al. Squamous cell carcinoma of the tonsils: a molecular analysis of HPV associations. Clin Cancer Res An Off J Am Assoc Cancer Res. 2002;8:1093–1100. [PubMed] [Google Scholar]
- 52.Kim HS, Lee JY, Lim SH, Park K, Sun J-M, Ko YH, et al. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat Off J Korean Cancer Assoc. 2016;48:527–536. doi: 10.4143/crt.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, et al. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217:385–393. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, et al. Cancer treatment with anti-PD-1/PD-L1 agents: is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76:925–945. doi: 10.1007/s40265-016-0588-x. [DOI] [PubMed] [Google Scholar]
- 55.Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Li M, Lian Z, Zhu H, Kong L, Wang P, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Target Oncol. 2016;11:753–761. doi: 10.1007/s11523-016-0451-8. [DOI] [PubMed] [Google Scholar]
- 57.Epacadostat shows value in two SCCHN trials. Cancer Discov. 2017;7:OF2. 10.1158/2159-8290.CD-NB2017-100. [DOI] [PubMed]
- 58.Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 60.Elbers JBW, Al-Mamgani A, Tesseslaar MET, van den Brekel MWM, Lange CAH, van der Wal JE, et al. Immuno-radiotherapy with cetuximab and avelumab for advanced stage head and neck squamous cell carcinoma: Results from a phase-I trial. Radiother Oncol. 2019;142:79–84. doi: 10.1016/j.radonc.2019.08.007. [DOI] [PubMed] [Google Scholar]