Abstract
The multi-disciplinary field of microfluidics has the potential to provide solutions to a diverse set of problems. It offers the advantages of high-throughput, continuous, rapid and expeditious analysis requiring minute quantities of sample. However, even as this field has yielded many mass-manufacturable and cost-efficient point-of-care devices, its direct and practical applications into the field of disease diagnostics still remain limited and largely overlooked by the industry. This review focuses on the phenomenon of hydrodynamic focusing and its potential to materialize solutions for appropriate diagnosis and prognosis. The study aims to look beyond its intended cytometric applications and focus on unambiguous disease detection, monitoring, drug delivery, studies conducted on DNA and highlight the instances in the scientific literature that have proposed such approach.
Keywords: Drug delivery, DNA, Blood, Point-of-care, Flow cytometer
Introduction
Microfluidics is a relatively new, interdisciplinary field that has the potential to solve many diverse problems that are proving to be a challenge when attempted to be being solved using existing conventional technologies [1]. It is essentially the manipulation of fluid using sub-millimeter sized microscale devices [2]. This emerging field has been marked by many profound applications and has served as a viable alternative to many macro-scale devices that are labor-intensive and tedious, involving complex process flows [3]. It is characterized by the usage of minute quantities of samples and reagents with the subsequent reduction in costs and analysis time. The field of microfluidics is an interdisciplinary one and hence from this inherent nature, it has the potential to solve a broad spectrum of problems [4]. It is imminent that microfluidics is poised to predominate many existing and conventional technologies in the near future.
However, another fact remains imminent, which is, that even after focusing highly on family planning policies by developing nations [5], the population staggeringly and disproportionately continues to rise. This is a typical case for many of the developing nations, which are also resource deficient [6]. These two factors work in tandem to claim more lives through diseases alone. In the last century alone, smallpox was responsible for 300–500 million deaths [7, 8], the Spanish flu affected 500 million people worldwide, with 20–50 million fatalities [9]. Until the last century, such diseases proved to be fatal until their cure was found, and the technique of vaccination was mastered. However, even with the tremendous growth of science and technology and with an increased understanding of human anatomy, we still find ourselves to be surrounded by countless other ailments. Early detection of widespread ailments such as cancer [10], malaria [11] or HIV [12] can play a pivotal role in ensuring minimal mortality rate. Many of these diseases affect at the cellular level, and the symptoms, with their associated ambiguity, provide only a rough initial guess to the identification. For instance, both pale or yellowish skin, as well as fatigue, can indicate anemia or jaundice; however, proper identification of the disease can only be ascertained after conducting further tests, often requiring a blood sample.
Accurate diagnosis of a patient can be performed only after knowing the ailment. Earlier, medical care had to be provided to the patient in the absence of information about the disease. This often proved to be unnecessary and at times, counterproductive. Testing was not carried out in the vicinity of the patient and hence used up time in logistics which adversely affected the mortality rates. Technologies that employed decentralized, simple and automated methods of testing could make a massive difference to this scenario. It is here that the inception of point of care technology to the clinical and biomedical field offered profound advantages and the potential to solve many problems simultaneously [13]. Point of care technology or POCT is simply the investigation or testing of specimens near the patient and making results available at the time of consultation. Devices based on POCT do not require expansive laboratories, skilled personnel and are not labor-intensive. Reminiscent of the advantages offered by the field of microfluidics, it in addition to them provides practical devices that are self-sufficient and expeditious. They have the potential of yielding results with accuracy comparable to those of labs and these applications have appropriately earned them the title of lab on a chip. It has been reported that many of these applications are superior to many of the commercial immuno sensing systems. These can render accurate prognosis and reduce the cases of overtreatment [14]. These devices are gaining relevance in developed countries for primary care and outpatient clinics. These devices can also help to save lives on a large scale by preventing epidemics during natural disasters, on-going battle-fields as well as developing countries [15].
Some POCT devices have successfully proliferated the markets, such as the blood glucose test. POCT devices are highly customizable to suit the needs of the developing as well as the developed nations of the world. Due to their versatility, they can offer solutions to a wide variety of diseases. This is particularly beneficial since the developing regions continue to battle highly contagious diseases and the developed regions try to contain non-communicable diseases. This versatility acts as a two-pronged attack against the outbreak of many deadly diseases in the world [16]. POCT devices can analyze various biomarkers such as proteins, nucleic acids, cells and metabolites. Proteins when analyzed using immunoassay based devices can yield information regarding various cancers, and also aid in chalking out a personalized treatment plan for the patient [17]. Analysis of nucleic acids can signal towards the presence of sexually transmitted infectious vectors. Metabolites such as glucose, creatinine and urea nitrogen can indicate ailments such as diabetes and liver disease [18]. In a resource-deficient setting, paper microfluidics method promises a respite from all the challenges and problems surrounding disease diagnostics [19]. Lateral flow immunoassay technology is another approach which has a strong presence in the disease diagnostics landscape in the developing countries. Tests utilizing this method are able to detect communicable diseases such as HIV, Strep A/B, malaria and meningitis [20].
Numerous Point-of-care disease diagnostic techniques are based on studying properties of cells. Conventionally, cells analysis is carried out using a flow cytometer. Even after miniaturization of this method to suit POCT applications, hydrodynamic focusing continues to govern the fluid flow at the fundamental level. However, to appreciate the importance of hydrodynamic focusing, which forms the core of flow cytometers, an overview of cell cytometry becomes important. Cell cytometry pertains directly to the studies conducted on cells to quantify properties related to cells. These properties can be as simple as cell count measurement or as indiscernible as cell granularity, rigidity and morphology. The techniques dedicated to cell cytometry have been used by biologists to gauge the excess or lack thereof of the requisite number of various cell sub-populations. This was earlier done using hemocytometer, [21] which was completely dependent on the dexterity of the investigator. There are mainly three widely used techniques which are Coulter counters, Optical flow cytometers and digital image analysis. Based on the electrical and impedance properties of the cells, Coulter counters came into existence having the capability to provide resolution even smaller than 1 µm [22]. These electronic cell counters were the first to replace the hemocytometer successfully. Furthermore, due to the difference in the behavior of cells to optical stimuli such as laser, meaningful deductions can be conducted based on the refraction spectrum or fluorescence when utilizing highly specific dyes for distinction. Optical flow cytometers work on this principle to give reliable cell counts as well as other parameters that give further insight into the ailment affecting the individual. This technology has the ability to distinguish cells based on 14 different properties [23]. Many parallel innovations are constantly improving flow cytometry, and its dominance in the field of biomedical research is certain [24]. It offers a window to analyze cells based on their morphology as well as cellular content. The microfluidic flow cytometers can also detect circulating tumor cells and simultaneously offer high-throughput screening. The accuracy and reliability of these flow cytometers have further been vindicated by their acceptance from the Food and Drug Administration. However, both of the above devices suffer from disadvantages owing to their large size hindering portability as well as costs that reduce their accessibility to the general public. Another alternative involves digital image analysis, which with the help of photo-microscope and accompanying software detects cell size as well as fluorescence [25]. With the rapid growth in micro-fabrication techniques, microfluidic analogues are beginning to provide advantages that were otherwise unachievable by the other well established commercial alternatives and hence are paving the way for point-of-care diagnosis [21].
As propounded earlier that disease diagnostics relies heavily on cell properties [26–31] and micro fluidics deals with manipulation of fluids at the microscale [32], characteristic of typical cellular diameters, the phenomenon of hydrodynamic focusing serves as this link between these two fields. Persistent and spasmodic outbreaks still pose a disproportionate impact on economically deficient as well as developing nations [33–36]. Tuberculosis and malaria claim 2 million [37] and 1 million lives annually [38]. Microfluidic hydrodynamic focusing comes under the umbrella of novel diagnostic methods that have an immense potential of changing the mortality rate statistics for the better [39]. It is estimated that a rapid, user-friendly test for TB can save 400,000 lives and a test devoid of laboratory infrastructure for malaria can save 300,000 lives annually [40]. A review of the scientific literature becomes imminent because of the exponentially growing human population and with the simultaneous deficiency of resources and time; prompt diagnosis is proving to be arduous [41–43]. There exist plethora of other versatile alternatives for cell sorting, encompassing intricate methods based upon optical and magnetic properties [44–48], dielectrophoresis [49–53], standing surface acoustic waves [54–58] to as simple as size-based filtration [59–63]. However, hydrodynamic focusing does not require additional assembly for generating external forces and exploits channel geometry to affect and ultimately manipulate the fluid flow to achieve the measurement of the desired properties such as the frequency, size and shape of the target particles [64–66]. The vast and ever-expanding applications of this phenomenon will also be disseminated to understand how one basic method can manifest itself to give rise to profound ways of solving real-life practical problems pertaining primarily to the field of disease diagnostics. It then becomes imperative to understand it to pave the way for possible future innovations that may have far-reaching repercussions.
This review will commence with the presentation of the theoretical formulation of two-dimensional hydrodynamic focusing, in brief, followed by an introduction of the three-dimensional variant. The importance of the controlling parameters will be focused upon to give an idea as to how the variation in them laid the foundation for the diverse applications covered here. Most of the prominent applications will be focused upon, which have presented a novel microfluidic setup that breaks the barrier of the already established cytometric applications. These include studies that have attempted to detect or indicate the onset of many diseases, followed by drug delivery, which is an equally important aspect of disease diagnostics. Further, the studies conducted on DNA are covered, as they have the potential to provide a profound outlook towards the union of Bio-medical and microfluidics fields. The study will culminate with the impact of such studies as well as their possible repercussions towards revolutionizing the conventional outlook towards these fields.
Hydrodynamic flow focusing
Hydrodynamic focusing is a useful technique for sample focusing and control [67–69]. It may be characterized as the squeezing of the sample fluid of interest utilizing another fluid, which is also known as the sheath fluid. This phenomenon has been studied in great detail and has yielded a myriad of applications [68–70]. However, most of the applications have centered around the investigation of cell properties using flow cytometry [71]. Flow cytometric studies require the cells to line-up in a single-file line; this is possible by achieving the sample fluid cross-sectional diameter as close as possible to the cell diameter. There exist mainly two basic approaches for cell focusing, namely, sheath flow focusing and sheathless flow focusing [71, 72]. Sheathless flow focusing makes use of external forces such as dielectrophoresis, acoustic, magnetic and inertial. On the other hand, Sheath flow focusing utilizes one or more sheath fluid and is also known as hydrodynamic focusing. The desired output, which is the final focused width of the sample fluid, mainly depends on the fluid properties and is a function of flow conditions and fluid properties. Hydrodynamic focusing has been successfully replicated in microfluidic devices and has proven to interrogate cell properties in an efficient manner.
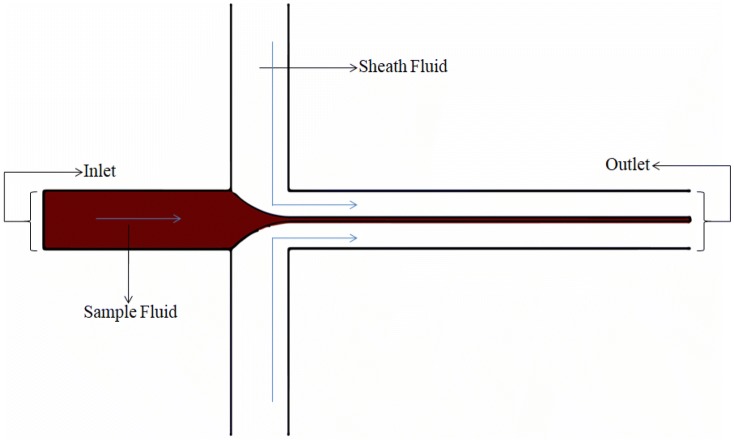
Depending on the number of dimensions in which the sample fluid is being squeezed, hydrodynamic focusing can further be subdivided into either two-dimensional (2D) or three-dimensional (3D) flow focusing. The simplest and primitive type is the 2D flow focusing. In this type of fluid focusing the sample fluid retains one of its original dimensions while its other dimension is being subjected to reduction due to the compressive action achieved using a sheath fluid. However, due to its associated simple device architecture, it has the disadvantage of collocating particles in a singular plane of width close to the cellular diameter and hence makes it cytometric applications limited due to its restricted depth of field. Even with its limitations, it has been studied extensively by many research groups [65, 73–76] and has served to give an empirical insight into the flow physics of focusing obtained using hydrodynamic forces. Since the actual model does not differ much from the theoretical model with its underlying assumptions, a reliable quantification in the form of expression of the focused sample width is obtained. It has also served as a basis to study and understand the three-dimensional focusing phenomenon. It is found that the focused width depends only on the flow rate ratio, viscosity ratio and the aspect ratio for which the dependencies are explicitly demonstrated in our work, Tripathi et al. [67] Other no table research groups have also attempted to delineate this principle such as the works of Lee et al. [77], Knight et al. [78], Stiles et al. [79], based on the same parameters, to varying extents of inquest. The two-dimensional hydrodynamic focusing is illustrated in Fig. 1; the fluid to be subjected to focusing is made to flow in a single channel and is then suddenly exposed to the sheath fluids. For highlighting the approximate profile of the fluid undergoing focusing, the sheath fluid has been rendered colorless.
Fig. 1.
A simplified schematic view of the two-dimensional hydrodynamic focusing phenomenon
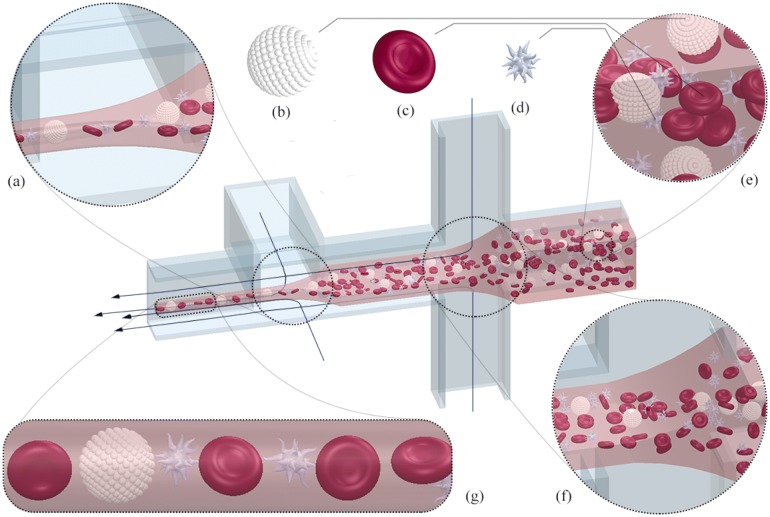
In the 3D focusing method, the sample fluid is surrounded and acted upon by the sheath fluid from all sides, with both its dimensions decrementing simultaneously or successively to better capture and manipulate cells in a single-file line. This gives exceptionally improved estimates of cell populations and avoids adhesion with channel walls. The empirical model, which involves more complex flow physics than the 2D counterpart, has been formulated [80, 81]. The 3D flow focusing is an area of active research and has been successfully realized by many research groups [82–88]. The integrated microfluidic flow cytometer proposed by Mao et al. [89] exploits hydrodynamic focusing, and the ingenious device architecture forces the secondary Dean vortices to work in tandem to align cells in a single line for their subsequent observation. In one such study conducted by Tripathi et al. [70], 3D focusing has been achieved using only one sheath inlet in conjunction with ingenious channel geometry utilizing two bends of opposite curvature. The theoretical complexity and manufacturability hurdles have not impeded the advancement of three-dimensional hydrodynamic focusing devices, and this, in turn, has opened up many avenues of achieving the desired output in equally numerous and versatile ways as was in the two-dimensional case. It was concluded from the work of Chung et al. [90] that upon the correlation of the results between Poiseuille flow model and CFD simulations, and subsequent visualization using laser scanning microscope that the focused sample stream obtained from three dimensional focusing had more stability and showed a count versus intensity trend more akin to normal distribution in contrast to the two dimensional focusing scenario. Figure 2 depicts the phenomenon of 3D flow focusing phenomenon in its entirety. It also gives an idea of how the fluid is manipulated on being subjected to successive hydrodynamic focusing.
Fig. 2.
The phenomenon of hydrodynamic focusing, manipulating the blood sample and its cellular constituents such as leukocytes (b), erythrocytes (c), and thrombocytes (d) by successive application of hydrodynamic focusing as shown in (a, f). The resultant single file line (g) obtained after focusing. e Blood matrix before focusing
Applications of hydrodynamic focusing
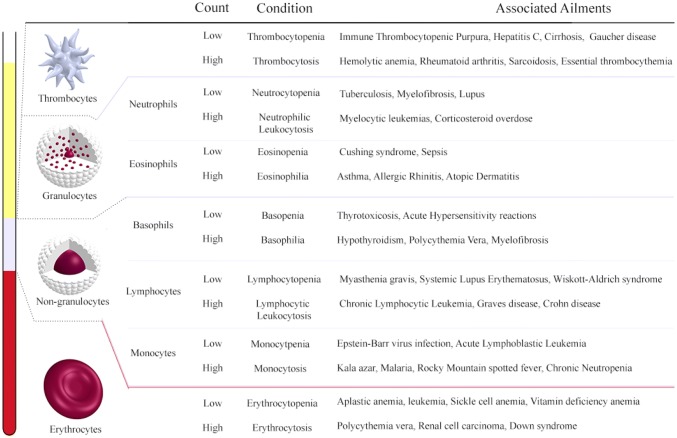
One of the first applications of two-dimensional hydrodynamic focusing by Knight et al. [91] (Fig. 3) aimed neither at the manipulation of particles for flow cytometry nor cell sorting but instead to reduce the mixing times at nanoscale which otherwise relied heavily on the diffusion phenomenon yielding impractical mixing times. Due to the small focusing width obtained, the diffusion time required by the particles was reduced significantly; this was because of the small length scales involved. The various facets of hydrodynamic focusing were deduced numerically using the circuit model that exploits the analogy between the proportionality of volumetric flow rate and the applied pressure in a microchannel with the Ohm’s law. Since the method can gauge and analyze the physical properties of the cell, such as the frequency, shape and other biomechanical properties such as deformability and fragility, it is possible to narrow down and aid in the accurate identification of the ailment and hence offer appropriate treatment in a faster and efficient manner. One of the parameters measured by the optical setup in the hydrodynamic focusing microfluidic device is the cell count. Figure 4 depicts the associated diseases with the corresponding cell count for each of the principal constituents of blood. Further subsections will elaborate on those applications that have impacted the regimes of disease diagnostics, drug delivery, studies conducted on DNA as well as other notable instances. These studies have left an indelible mark on the way the particular fields in question are perceived and have the potential to pave the way for viable alternatives to the conventional methodologies.
Fig. 3.
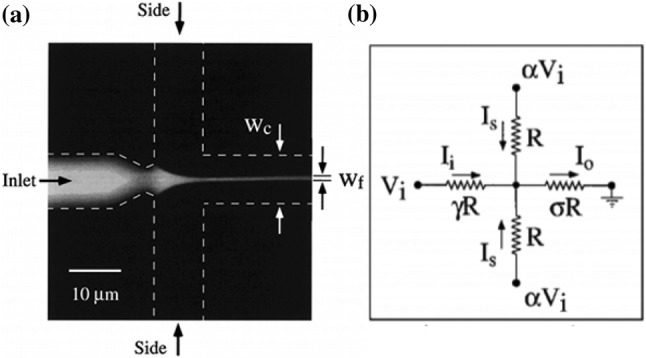
a Hydrodynamic focusing in a cross-flow microchannel showing channel dimension and focused width. b The corresponding resistive circuit analogy. Adapted from [78] by permission from the American Physical Society
Fig. 4.
The major constituents of blood and the inference of the probable ailments inferred from their respective cell count
Disease diagnostic applications
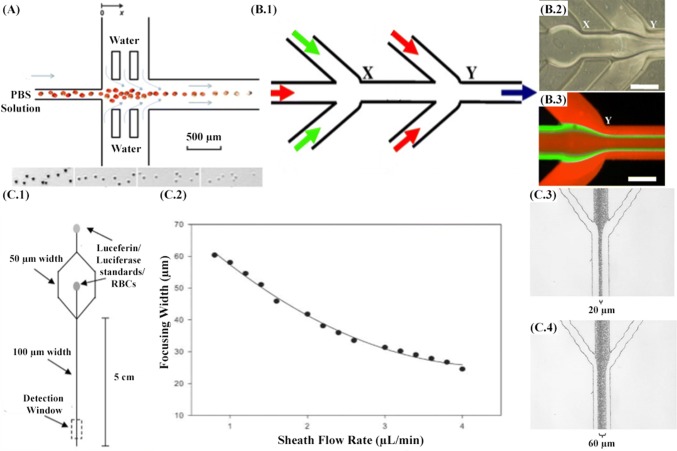
This section focuses explicitly on disease diagnostic applications, which have been realized using hydrodynamic flow focusing. Non-communicable diseases such as diabetes mellitus, myocardial infarction, hypertension and sickle cell anemia have wreaked havoc on the health of developing as well as developed nations. Zhan et al. (Fig. 5a) [92] have proposed a microfluidic device with hydrodynamic focusing as its central pillar to investigate an underlying erythrocyte property that is common to all the diseases mentioned above. An understanding of the biomechanics of erythrocytes, determined by the membrane integrity and cytoskeletal structure, is required to better recognize the onset and subsequent progression of such diseases. The importance of hydrodynamic focusing is two-fold in this study as it used for quick dilution of the buffer and affecting lysis of erythrocytes during the flow. Even the slightest variations in the deformability as well as surface area to volume ratio can signal succumbing to diabetes mellitus as the change in the ratio between phospholipids and cholesterol causes a decrease in erythrocyte deformability, or increased cell rigidity due to decrease of phospholipids and phosphatidylethanolamine and increase of sphingomyelinin hypertension patients or polymerization of hemoglobin in sickle cell anemia due incorrect replacement of valine with glutamate. This study aims to establish a link between osmotic lysis kinetics and cell biomechanics.
Fig. 5.
A Microfluidic device design showing the osmotic lysis of erythrocytes. The inset images from left to the right show the cell images at various locations (x = 1.5 mm, x = 13.5 mm, x = 22.5 mm, and x = 37.5 mm). Adapted from [92] by permission from the Royal Society of Chemistry. B.1 Schematic of the 5-inlet MF system. B.2 Optical micrograph of the flow pattern at the two junctions (X and Y) of the MF system. B.3 Fluorescence micrograph of flow pattern at junction Y. The volumetric flow rates used for rhodamine, fluorescein, and rhodamine were 200, 20, and 200 µL/min, respectively. The red and green color is rhodamine and fluorescein, respectively. Scale bar = 250 µm. Adapted from [94] by permission from Elsevier. C.1 Microchip design used to deform erythrocytes and detect ATP via chemiluminescent reaction with luciferin/luciferase. C.2 Width of the focused stream as a function of the sheath flow rate. C.3 Micrograph demonstrating a focusing width of approximately 60 mm (cross-sectional area = 3480 sq. mm). C.4 Micrograph demonstrating a focusing width of approximately 20 mm (cross-sectional area = 1160 sq. mm). Adapted from [93] by permission from the Royal Society of Chemistry
The lysis kinetics data captured by this study vindicates the variation in the cell fragility in response to chemical, heating and glucose treatment. Smart channel geometry can meticulously lead to expeditious focusing of microparticles including cells. Moehlenbrock et al. [93] (Fig. 5c) have surpassed the typical applications limited to cell counts. Their methods aim to capture the released ATPs due to deformation and lysis of erythrocytes. These results can be compared and contrasted with individuals suffering from pulmonary hypertension. This condition can be present due to diseases like scleroderma, chronic obstructive lung disease, etc. The microfluidic device through hydrodynamic focusing is able to induce deformation, thereby quantifying the ATP release. This mechanism can offer a deeper understanding of the stimuli that trigger this onset and has the capability to detect and treat the associated ailments. It also becomes important to measure the release data as the ATP can initiate the synthesis of nitric oxide, which is a potent vasodilator. The flow rates of the accompanying sheaths of luciferin/luciferase mixture can be varied to control the deformation and, subsequently, the amount of the ATP released. It was found out from the study that increasing the mechanical deformation results in an increase in the amount of ATP released. The effectiveness of hydrodynamic focusing as an approach for cancer treatment has been suggested by Koh et al. [94] (Fig. 5b). The biocompatibility, effective drug encapsulation, permeability and retention, thereby ensuring long circulation times have been utilized to ensure targeted delivery of oligodeoxyribonucleotides, which discombobulate the target cancer cells by Watson–Crick base pairing. They are used in conjunction with liposomes due to their low permeability, selectivity and degradation. Through this study, it is aimed to demonstrate how hydrodynamic focusing can be used for the generation of uniformly distributed monodispersed lipopolyplex nanoparticles. The illustration of the 5 inlet microfluidic device shows the inlets of protamine and lipid streams into the oligodeoxyribonucleotide stream.
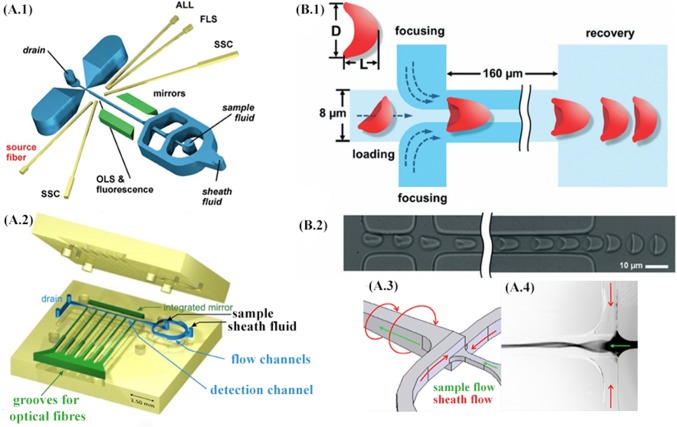
The possibility of leukocyte differentiation to narrow down and identify ailments related to these subsets of cells has been realized in practice and its performance furthermore compared with conventional flow cytometers as predicated in the study by Frankowski et al. [95] refer Fig. 6A.1, A.2. The method uses fluorescence-based segregation of fresh venous blood samples using solutions of antibodies to accurately detect and quantify various lymphocyte populations, including CD4/CD8 concentration ratios, one of the primary indicators for HIV disease. Cascaded hydrodynamic focusing is employed, and the coefficient of variation as a function of relative sample flow rate and fluorescence intensity was chosen to contrast the performance empirically. Hydrodynamic focusing on both the dimensions was achieved using a single inlet for sheath flow. The signal-to-noise ratio was smaller for the microfluidic alternative; however, it was found that performance was comparable. In yet another study by Frankowski et al. [96] as illustrated in Fig. 6A.3, A.4, the earlier device geometry was modified to introduce spin focusing in conjunction with hydrodynamic focusing channel architecture. The new device has the capability of detecting CD3+ and CD4+T lymphocytes. Both the cascaded hydrodynamic focusing and spin focusing method in conjunction with hydrodynamic focusing were compared, and it was observed that resolving dim fluorescent particles and differentiation, the former resolved better than the latter. Platelets are another critical constituent of blood. They are smaller discoid cell fragments, circulating in the blood, often in a quiescent or activated state. An activated state can be triggered in response to diseases ranging from cardiovascular disease to metastatic cancer, as well as diabetes. The activated state cannot be directly discerned from the cells themselves but can be recognized by the von Willebrand factor, a platelet-activating protein. The macro-scale alternative of the parallel-plate flow chamber, with its low aspect ratio for protein disposition, inadvertently wastes a lot of sample volume. Here, in the work of Kent et al. [97], hydrodynamic focusing is used to manipulate the sample fluid in a thin layer, which is then made to interact with a protein functionalized surface. The platelet surface adhesion parameters can be interpreted to offer a clearer view of the onset of many diseases. The device architecture is simple and can offer the possibility of mass-production and hence, a point-of-care device. Many of the applications have directly analyzed the blood sample of the patient; however, treatment can also inadvertently potentiate new ailments or increase the severity of the existing one. Blood transfusion, one of the integral instruments of a vast majority of diagnoses, has the potential to deteriorate the health of the subject due to the decrement in stored red blood cell quantity. Red blood cells due to their waste products and enzymatic reactions are damaged and can lead to haemolysis, reduced in vivo recovery, energy and membrane loss, altered oxygen release, reduced adenosine tri-phosphate and nitric oxide secretion and introduction of toxic products in the fluid via shedding. Toxic products such as lysophopholipids can cause acute lung injury; free iron can lead to infections, and subsequent inflammation and shed micro-vesicles can use nitric oxide and as a consequence, initiate thrombosis. Some measures of red blood cell storage quality such as hemoglobin concentration and its effects can be analyzed and deduced easily; however, cell rigidity measurements are not as simple, and their affects on the anatomy still largely ambiguous [98]. Zheng et al. [99] have demonstrated the application of hydrodynamic focusing to measure stored erythrocyte deformability. Since measurements on mechanical properties can differ significantly under different deformation modes, folding was chosen, and quantified using deformation index as defined in the Fig. 6b for healthy RBCs. In addition to this time, constant or the recovery rate obtained by plotting deformation index as a function of time and circularity, measured optically, were also characterized. The inlet channel had RBCs near the vicinity of the channel wall, and the focusing channels were required in order to orient the cells in a standing position. It was found from the scatter plots that time constant decreases for blood samples stored for a longer time and associated changes in standard deviation for circularity. Due to the folding mode of deformation, no significant difference in the deformation index was found for samples as a function of storage time, thereby making time constant and circularity measurements viable parameters for the quantification of stored blood quality. Table 1 summarizes the various important aspects of each of these studies objectively and attempts to establish a common feature against which appropriate comparisons can be made.
Fig. 6.
A.1 The layout of the microfluidic prototype chip with cascaded hydrodynamic focusing. Grooves to insert optical fibers are indicated in yellow and integrated mirrors in green color (ALL: axial light loss, FLS: forward light scatter; SSC: side scatter). Fluorescence is measured in parallel to orthogonal light scatter (OLS). Adapted from [95] with permission from Wiley Online Library. A.2 The layout of the microfluidic structure featuring spin hydrodynamic focusing (the top and bottom parts before assembly). The hollow cavities of the microfluidic chip, including flow channels (blue color), integrated mirror, and six grooves to insert optical fibers (green), are “detached” from the body (yellow) to facilitate the overview on the layout. A.3 Operation principle with sheath and sample flow directions. A.4 Fluorescence image (inverted greyscale) of rhodamine dye 6G used as a sample fluid and excited with a mercury arc lamp. Adapted from [96] with permission from the Multidisciplinary Digital Publishing Institute. B.1 Schematic of the microfluidic device for studying deformability changes of stored RBCs. Hydrodynamic focusing centers the cell and adjusts it to the ‘standing’ orientation. The RBC is ‘folded’ into a parachute-like shape when pressure-driven through the 8 μm × 8 μm central channel. Deformation index (DI = L/D) is defined to measure RBC deformation (top-left). The time constant (tc) of each cell is determined by fitting DI value changes during the cell shape recovery process to an exponential function after the cell exits the microchannel. B.2 Experimental images are showing the centering, orienting, folding, and shape recovering of an RBC. Adapted from [99] with permission of the Royal Society of Chemistry. (Color figure online)
Table 1.
Summarization of the prominent applications of the phenomenon of hydrodynamic focusing for disease diagnostic applications
| Research groups | Cell/macromolecule targeted | Parameters analyzed | Diagnostic applications |
|---|---|---|---|
| Zhan et al. [92] | Erythrocytes | Release profiles as a function of time and distance traveled. | Diabetes mellitus, myocardial infarction, hypertension, sickle cell anemia |
| Moehlenbrock et al. [93] | Erythrocytes, ATP | ATP release data as a function of RBC deformability | Pulmonary hypertension scleroderma, chronic obstructive lung disease |
| Koh et al. [94] | Liposomes | Average particle size, zeta potential, and morphology of the lipopolyx particles. | Cancer treatment |
| Frankowski et al. [95] [96] | Leukocyte subsets: CD3+, CD4+, CD8+, CD19+, CD3− | Fluorescent intensity detection, scatter plot and pulse height distribution | Malaria, Kala Azar, Hypothyroidism, Tuberculosis, HIV |
| Kent et al. [97] | Thrombocytes | Adhesion results from surface coverage of VWF coating | Cardiovascular diseases, cancer metastasis, diabetes |
| Zheng et al. [102] | Erythrocytes | Deformation index via the folding mode, time constant or recovery rate | Reliable quantification of stored blood quality for effective treatment |
Drug delivery applications
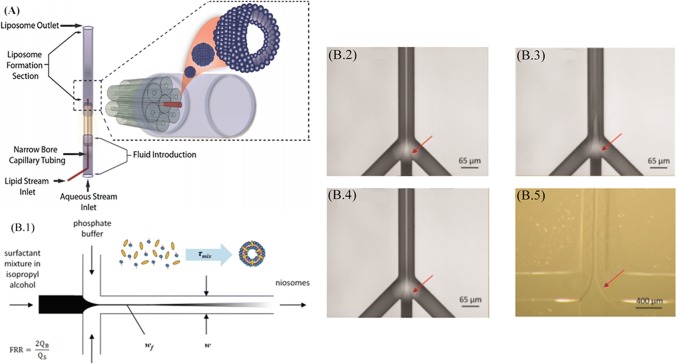
This section will highlight the drug delivery applications that have been implemented using the hydrodynamic flow focusing phenomenon. It has been used to generate drug carriers for targeted drug delivery by the synthesis of bio-macromolecules. Hood et al. [100] have shown via a method to produce nanoscale liposomes using three-dimensional hydrodynamic focusing, exploiting the radial geometry of the concentric capillary array as depicted in Fig. 7a. With the inherent advantage of high throughput as well as the control over the size and frequency of the process over the conventional liposome preparation techniques such as bulk scale alcohol injection and sonication. Here, by the use of a circular array of tubes and precise control over the flow rate of the buffer, the required concentrations for the sustenance of the lipids are achieved, which then coalesce into liposomes. The size of the liposomes and their polydispersity are a function of the invariable design parameters constituting the device architecture as well as variable operational parameters such as the flow rates of the sample and sheath buffers. This is an economical and expeditious approach to promote the conventionalization of microfluidic devices in the industry with the principle of hydrodynamic focusing at its helm. The study of Lo et al. [101], shown in Fig. 7b focuses on the synthesis of niosomes as a carrier of treatment agents for pharmaceutical and cosmetic applications or contrast agents for clinical imaging applications instead of their biological analogue liposomes. Niosomes are synthetic membrane vesicles formed by the self assemblage of a non-ionic surfactant, preferably in a mixture of cholesterol and diacetyl phosphate. They can be produced by the conventional macroscale bulk method of mixing of two liquid, which is, however, time-exhaustive and also offers poor control over polydispersity in size with a detrimental effect on the consistency of niosome dosage or image quality. However, with the application of hydrodynamic focusing, controlled mixing was achieved in just seconds offering very low variance in the niosome size distribution. The size of a niosome can influence the circulation time in the body or the image quality. This study has a direct bearing on the utilization of niosomes to promote the development and utilization of biomimetic colloidal systems for nanomedicine applications.
Fig. 7.
A 3D HF to synthesize liposomes. Adapted from [100] with permission of the Royal Society of Chemistry. B.1 Niosome self-assembly. A central stream (sorbitan ester, cholesterol, and dicetyl phosphate) in isopropyl alcohol focused by adjacent streams (phosphate buffer). B.2–5 The central stream contains ether. B.2 Span 20/IPA, B.3 Span 60/IPA, B.4 Span 80/IPA mixture by two adjacent PBS streams (65 μm × 120 μm). B.5 PDMS microchannel (400 μm × 56 μm). Adapted from [101] with the permission of the American Chemical Society
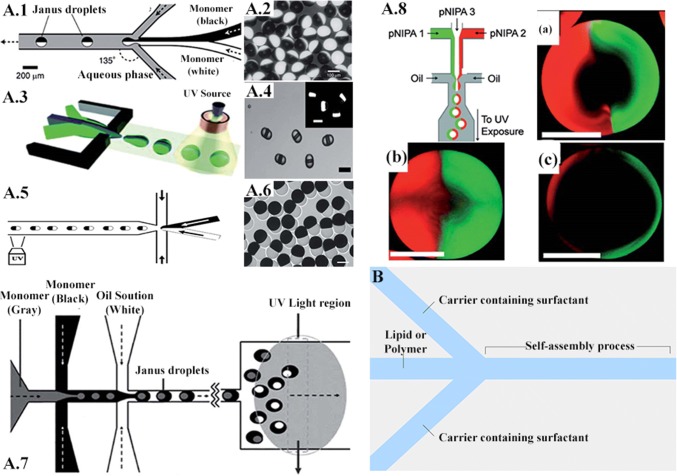
The study conducted by Damiati et al. [102] tries to establish a relation between hydrodynamic focusing and the applications centered on drug delivery. This is evidenced by the potential to create many unique and customizable carriers. In contrast to the conventional methods which offer little control over the drug carrier topography, these microfluidic applications can yield desirable properties in the carrier. This can be achieved the strategic placement of channel confluences. However, even the variation of flow rates for the same geometry can result in entirely different carriers in relation to their structural and chemical properties. It is also capable of ensuring stability and uniformity of the carriers. Compared to other alternatives, this is a straight-forward and inexpensive technique. Some platforms suitable for preparing self-assembled particulate drug delivery systems include hydrodynamic flow focusing (HFF). Self-assembled carriers are commonly generated through HFF by controlling the mixing rates between the fluid streams based on the microchannel shapes, flow rates, and diffusion coefficients of different miscible streams. Increasing the ratio of the flow rate of solvent to that of water induces the slow mixing and generation of large nanoparticles. The HFF method usually produces self-assembled drug delivery systems smaller than 1 μm, which might allow better delivery across the physiological barriers. Moreover, in the microfluidic HFF system, the narrow width of the core stream offers fast diffusion due to the small length scale. One example of exploiting microfluidic platforms to produce a smart drug delivery system is the generation of Janus particles. Janus particles are being increasingly studied because of the integrity of their incompatible constitution comprising of both hydrophilic and hydrophobic nature. The particles are synthesized using droplet microfluidics, which is a direct application of hydrodynamic focusing. The constitution can be binary as well as ternary, with the latter being achieved using successive stages of hydrodynamic focusing. These particles offer diverse applications because of their structure–property relationship, specificity towards biomolecules and non-specific absorption [103]. This unique feature allows two different reactions to occur on the same particle. The technique of hydrodynamic focusing is preferred as it offers unparallel control over the monodispersity, morphology and multi-functionality of the particles. Janus particles can potentially be used to deliver multiple agents with different solubility. Xie et al. [104] fabricated a nano-precipitation system that enables the one-step generation of Janus polymeric nanoparticles composed of poly(lactic-co-glycolic acid) (PLGA) to encapsulate paclitaxel and doxorubicin hydrochloride (hydrophobic and hydrophilic drugs, respectively) on different sides of the particle (Fig. 8).
Fig. 8.
A.1 Schematic of the channel and flow configuration. A.2 Optical micrograph of monodisperse bicolor polymeric particles. A.3 Schematic illustration of the generation ternary droplets. A.4 Optical micrograph of the resulting droplets. A.5 Schematic diagram of the microfluidic device used for the synthesis of hybrid Janus microspheres. A.6 Optical image of synthesized hybrid Janus microspheres with dumbbell shapes. A.7 Schematic representation of PDMS double emulsion device. A.8 Schematic of a microfluidic device forming aqueous droplets from three independent semidilute PNIPAAm solutions. Right after droplet break up, the center phase (colorless PNIPAAm) is assembled in the core of the droplets, whereas the left- and right-flowing phases (green- and red-tagged PNIPAAm) form a Janus-shaped shell. Resulting droplets (a–c) on varying the flow ratios of the inlet channels. Adapted from [105] with permission of the Royal Society of Chemistry. B Microfluidic platform for the generation of self‐assembled drug and gene carriers: schematic of a simple hydrodynamic device with hydrodynamic flow focusing
The study conducted by Wang et al. [106] on the quantitative and expeditious delivery of exogenous molecules into cells is an indication where the applications of hydrodynamic focusing can prove to satisfactory drug delivery mechanisms. Calcein AM was used which on reaction generated fluorescence, possible only after its penetration into the interior of the cell due to hydrodynamic focusing. The relation between parameters such as flow rate ratio of the sample fluid to the sheath fluid and the corresponding enzymatic activity were studied to investigate and hence quantify any correlation between the two. The target cells were Chinese hamster ovary cells; upon their preparation, the final concentration was 106 cells/ml in the culture medium. In addition to this, the walls of the microchannel were coated with a glycoprotein called fibronectin to favor cell adhesion. The cell-permeant dye, calcein AM was diluted to 20 μg/ml. All the channels are of uniform dimensions, with the cross-sectional area being 100 μm wide and 33 μm deep. To ensure dynamic control over the flow ratios, syringe pumps were utilized. The results were also simulated on the commercial software, FLUENT. It was observed that on increasing the sheath flow rate in comparison to the sample flow rate, the width showed a decreasing trend. The microfluidic technique called PARTCELL was implemented to analyze the effect of the dimensions of the carrier stream on the amount of assimilation of molecules into the cell. By selective labeling of different regions of the cell, the intracellular mixing phenomenon can be observed. Upon performing the numerical simulation and their subsequent experimental verification, it was observed that the higher the flow rate ratio, the lesser is the concentration. The peak concentration was proposed to be the resultant of two factors, as the flow rate is increased, focusing width decreases, thereby reducing the diffusion length involved, as well as shortening the diffusion time.
Applications for studies on DNA
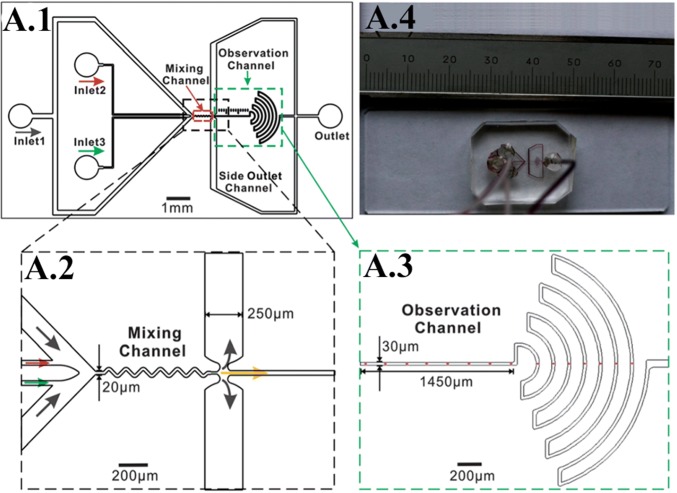
One of the most basic entities on which the whole of clinical research and medicine rest upon are the elusive and perplexing properties of the DNA molecule. A basic understanding of its reaction to stimuli can unearth a lot of about how diseases diagnostics should be approached and about our outlook of the various diseases that continue to cause epidemics as well as newly emerging risks that still have no cure available. Li et al. [107], depicted in Fig. 9a, provide a profound example of how an established phenomenon such as hydrodynamic focusing can further refine and aid in the development of another equally important field, such as determining the kinetics of DNA–protein interaction. A greater understanding of the reaction kinetics of processes such as DNA hybridization, DNA–protein binding, and protein-protein interaction lie at the very basis of the plethoric cellular activities that are responsible for the regulation of gene expression, repair, and replication of DNA damage. In contrast to turbulent mixers that require higher reagent consumption, the laminar micromixer can achieve complete mixing using hydrodynamic focusing. In addition to this, the wide range of observation time from sub-milliseconds to seconds could render accurate grasping of the folding process. The channel architecture was such that it employed dual hydrodynamic focusing to overcome the hurdle of the inability of hydrodynamic focusing to capture the interaction kinetics of bio-macromolecules. There is a three-fold order of magnitude reduction in the consumption of the samples and four-fold orders of magnitude increment in the observation time window. Hence, this application can serve as a much-needed glance at accurately determining the kinetics of macromolecules including immune-recognition. One such study was done by Wong et al. [108] on the deformation of DNA molecules in response to hydrodynamic focusing. The study brings forth a method to characterize the rheological properties of DNA molecules. According to the classical theory, long-chain polymers can be thought of as beads connected in series via springs. Viscous drag also acts on the beads in conjunction with the springs. The above-mentioned theoretical model yields a set of relaxation times, which are indicative of the time required for the polymer to assume its equilibrium state. Other methods, such as the utilization of magnetic tweezers to elongate the polymer, prove to be very laborious. Hydrodynamic focusing, on the other hand, provides a platform to analyze the motion of the molecule. In addition to this, the stretching of the DNA molecules leads to an increase in the contact area for their association with oligo probes. The study also provides a mechanical point of view towards various biochemical reactions.
Fig. 9.
A.1 Schematic of the micromixer. The device aids in conducting studies on DNA kinetics using hydrodynamic flow focusing. The images A.2, A.3 are the enlarged views of the region of interest, A.4 is the actual fabricated device. Adapted from [107] with permission of the Royal Society of Chemistry
Conclusion
The field of microfluidics is an interdisciplinary one. Forces that are otherwise almost immeasurable at the macroscale govern the fluid flow at the microscale due to scaling effects. There is no doubt that this field is at a nascent stage and is poised to change the landscape of many present technologies. It has the potential to solve many problems that continue to exist even after continued efforts towards their eradication. It is in this context that diseases, a clear and present danger, having the potential to mutate into different forms and affect millions of lives should be solved from the microfluidics point of view.
Initial investigation into the state of the human anatomy was paved by hemocytometer, which was laborious and highly time-consuming and had a significant dependence on the skill of the investigator. With the advent of many innovations and technological advancements, Coulter counter and optical flow cytometers gained prominence and have entirely changed the outlook of the medical industry. Digital image analysis is also another alternative that utilizes the same principle as the hemocytometer, but the role of the investigator is assigned to a software. However, these bench-top instruments are quite costly, require large spaces, skilled medical personnel, and often limited to only cell cytometric studies. These disadvantages, in turn, hinder their portability, increase analysis times, infrastructure costs as well as access to the general public primarily in resource deficient areas. Point-of-care technology aims to remove these impediments from the path of rapid and accurate diagnosis. Its focus is to deliver accurate results at the site of the patient. Early detection and constant monitoring of various biomarkers can significantly make a huge difference between life and death in cases of cancer and HIV. Even though the advantages offered by the current commercial alternatives have not been dwarfed by a microfluidic device, but from the overview of many of the studies conducted in this area, it is clear that a viable alternative is taking form. It is also observed that challenges such as incompatibility between different components performing activities such as sample collection, sample preparation, reactions related to various biomarkers, signal transformation and finally result display. Only very few permutations from numerous possible alternatives offers a meaningful and feasible device architecture. Ambiguous and highly subjective objectives fail to attract funding. Lack of confidence in investors is also an issue that has impeded these devices from coming into the market [109].
An appropriate analogy can be derived from easy to use and widely available, economic glucometer that can easily display the blood sugar level as and when required by the diabetes patients. This has made a massive change in the treatment of such illnesses as the patient can easily monitor and take corrective steps, if required, such as administering insulin injections to prevent hormonal misbalance and safe-guard other organs from the ill-effects of the disease. There are only limited applications of this phenomenon, specifically towards the identification of a particular disease. Most of the applications and the associated microfluidic devices revolve around cytometry. The results obtained from such techniques are only able to portray a generic immunological profile and lack the specificity of acting as an independent point-of-care device to identify an ailment. The applications are limited as most of them focus on the frequency of the target cell, and other parameters, such as color, deformability, fragility, intracellular constituents, and morphology, are not analyzed. These often-overlooked parameters contain more vital and unambiguous indications of a disease. It is required that a more efficient transfer of knowledge occurs between the bio-medical and microfluidic fields. The bio-medical field has the potential to find profound ways of identifying new and more reliable biomarkers for suggesting new approaches to disease diagnosis. On the other hand, the field of microfluidics has the immense potential to devise mechanisms to capture the requisite data to give meaningful results in the form of practical devices. For instance, the device proposed by Moehlenbrock et al. [93] was able to find a novel application by assessing ATP release data brought about by cell lysis due to hydrodynamic focusing. Even the applications focusing on deformability of RBCs can give indications of diseases such as malaria, instead of being limited to finding the quality of the stored RBC sample. Isolation of biomarkers such as circulating tumor cells can also pave the way for early identification and monitoring of various cancers, instead of going for invasive procedures such as a biopsy. However, applications are limited by benign tumors that require localized sample collection.
Applications are not limited only to disease identification but also to the synthesis of specialized macromolecules for drug delivery. Liposomes and niosomes can be easily synthesized using hydrodynamic focusing, and their morphology and structure can be easily controlled using flow rates. The application suggested by Koh et al. [94] caters to this need as the synthesized lipopolyx has the capability to reduce target cell expression, in this case, cancer. With the introduction of Janus particles, it is now possible to realize specific biochemical reactions that offer an unprecedented leap from the traditional drug administering methods. Binary, ternary, or phase within a phase droplet can be prepared by simple manipulation of hydrodynamic focusing methods as well as controlling the flow rates. These synthesized macromolecules can act as vessels for carrying specific enzymes, which may have different phases and deliver them directly to the target cells without hindering other bodily functions. It becomes imperative to find an appropriate assemblage of drugs to be able to derive a method for packing them in a macromolecule using hydrodynamic focusing and come up with practical solutions to targeted drug delivery.
Hydrodynamic focusing is a simple phenomenon requiring uncomplicated device architecture, which can be manufactured expeditiously using conventional manufacturing technologies. As it is devoid of external forces and only utilizes hydrodynamic manipulation, the overall cost and complexity of the point-of-care device are reduced substantially. These offer the benefits of mass manufacturable and economical designs. The phenomenon by itself has been studied rigorously in both the two dimensional and three-dimensional domains; however, there remains a lot to be achieved precisely in the disease diagnostic applications. This limitation is mainly posed by the absence of a complete understanding of the disease and, subsequently, improper implementation in the form of a microfluidic device. Cytometric applications cannot fill this need by themselves, and there is an immediate need for exploiting hydrodynamic, focusing on actual manipulation of the sample. Cell count data can only offer a limited view into the immunological profile of the subject; other parameters need to be explored by devising newer device architectures. Most of the applications covered here indicate an impending change in the outlook and methodology towards diagnosis. From the literature survey, as well as the various applications studied in this review, it can be inferred that this simple phenomenon has the potential to revolutionize the field of disease diagnostics.
Acknowledgements
The authors would like to acknowledge the aid provided by the Birla Institute of Technology and Science, Pilani, KK Birla Goa Campus via ‘Research Initiation Grant’.
Author contribution
Manuscript: AR, Writing: AR, Reviewing and editing the final manuscript: ST, Writing the original draft: AR, Figure preparation: AR, Resources: ST.
Compliance with ethical standard
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nguyen NT. Fundamentals and applications of microfluidics. 2. Norwood: Artech House; 2006. [Google Scholar]
- 2.Bruus H. Theoretical microfluidics. Oxford: Oxford University Press; 2008. [Google Scholar]
- 3.Stone HA, Kim S. Microfluidics: basic issues, applications, and challenges. AIChE J. 2001;47(6):1250–1254. [Google Scholar]
- 4.Tabeling P. Introduction to microfluidics. Oxford: OUP Oxford University Press Inc.; 2005. [Google Scholar]
- 5.Mauldin WP, Ross JA. Family planning programs: efforts and results, 1982–89. Stud Fam Plan 1991. 1991;22(6):350–367. [PubMed] [Google Scholar]
- 6.Bangdiwala SI, Fonn S, Okoye O, Tollman S. Workforce resources for health in developing countries. Public Health Rev. 2010;32(1):296. [Google Scholar]
- 7.Koplow DA. Smallpox: the fight to eradicate a global scourge. J Clin Investig. 2003;112(12):1775. [Google Scholar]
- 8.Bailey P. The top 10: epidemic hall of infamy. In: Summer 2006: epidemics on the horizon. U C Davis magazine. 2008. http://magazinearchive.ucdavis.edu/issues/su06/feature_1b.html. Accessed 24 Aug 2019.
- 9.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93(14):1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 11.Landier J, Parker DM, Thu AM, Carrara VI, Lwin KM, Bonnington CA, Pukrittayakamee S, Delmas G, Nosten FH. The role of early detection and treatment in malaria elimination. Malar J. 2006;15(1):363. doi: 10.1186/s12936-016-1399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaaijer HL, Exel-Oehlers PV, Kraaijeveld T, Altena E, Lelie PN. Early detection of antibodies to HIV-1 by third-generation assays. Lancet. 1992;340(8822):770–772. doi: 10.1016/0140-6736(92)92303-w. [DOI] [PubMed] [Google Scholar]
- 13.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–144. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 14.Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE. Point of care diagnostics: status and future. Anal Chem. 2011;84(2):487–515. doi: 10.1021/ac2030199. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Lee SH. Lab on a chip for in situ diagnosis: from blood to point of care. Biomed Eng Lett. 2013;3(2):59–66. doi: 10.1007/s13534-013-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin CD, Chin SY, Laksanasopin T, Sia SK. Low-cost microdevices for point-of-care testing. In: Issadore D, Westervelt RM, editors. Point-of-care diagnostics on a chip. Heidelberg: Springer; 2013. pp. 3–21. [Google Scholar]
- 17.Rusling JF, Kumar CV, Gutkind JS, Patel V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst. 2010;135(10):2496–2511. doi: 10.1039/c0an00204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung W, Han J, Choi JW, Ahn CH. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron Eng. 2015;132:46–57. [Google Scholar]
- 19.Yetisen AK, Akram MS, Lowe CR. Paper based microfluidic point-of-care diagnostic devices. Lab Chip. 2013;13(12):2210–2251. doi: 10.1039/c3lc50169h. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Zapatero-Rodríguez J, Estrela P, O’Kennedy R. Point-of-care diagnostics in low resource settings: present status and future role of microfluidics. Biosens J. 2015;5(3):577–601. doi: 10.3390/bios5030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vembadi A, Menachery A, Qasaimeh MA. Cell cytometry: review and perspective on biotechnological advances. Front Bioeng Biotechnol. 2019;7:147. doi: 10.3389/fbioe.2019.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Yamamoto T. Quantification of virus particles using nanopore-based resistive-pulse sensing techniques. Front Microbiol. 2016;7:1500. doi: 10.3389/fmicb.2016.01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkerson MJ. Principles and applications of flow cytometry and cell sorting in companion animal medicine. Vet Clin North Am Small Anim Pract. 2012;42(1):53–71. doi: 10.1016/j.cvsm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Adan A, Alizada G, Kiraz Y, Baran Y, Nalbant A. Flow cytometry: basic principles and applications. Crit Rev Biotechnol. 2017;37(2):163–176. doi: 10.3109/07388551.2015.1128876. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Harrison PJ, Wieslander H, Pielawski N, Kartasalo K, Partel G, Solorzano L, Suveer A, Klemm AH, Spjuth O, Sintorn IM. Deep learning in image cytometry: a review. Cytom A. 2019;95(4):366–380. doi: 10.1002/cyto.a.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbluth MJ, Lam WA, Fletcher DA. Analyzing cell mechanics in hematologic diseases with microfluidic biophysical flow cytometry. Lab Chip. 2008;8(7):1062–1070. doi: 10.1039/b802931h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nash GB, Johnson CS, Meiselman HJ. Mechanical properties of oxygenated red blood cells in sickle cell (HbSS) disease. Blood. 1984;63(1):73–82. [PubMed] [Google Scholar]
- 28.Iwashita T, Kruger GM, Pardal R, Kiel MJ, Morrison SJ. Hirschsprung disease is linked to defects in neural crest stem cell function. Science. 2003;301(5635):972–976. doi: 10.1126/science.1085649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50(3):481–492. [PubMed] [Google Scholar]
- 30.Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater. 2005;1(1):15–30. doi: 10.1016/j.actbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 2011;124(1):9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442(7101):368. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 33.Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71((2_suppl)):147–155. [PubMed] [Google Scholar]
- 34.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutayeb A, Boutayeb S. The burden of non-communicable diseases in developing countries. Int J Equity Health. 2005;4(1):2. doi: 10.1186/1475-9276-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006;100(3):191–199. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, Lalvani A. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11(3):1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 38.Guinovart C, Navia MM, Tanner M, Alonso PL. Malaria: burden of disease. Curr Mol Med. 2006;6(2):137–140. doi: 10.2174/156652406776055131. [DOI] [PubMed] [Google Scholar]
- 39.Lee WG, Kim YG, Chung BG, Demirci U, Khademhosseini A. Nano/Microfluidics for diagnosis of infectious diseases in developing countries. Adv Drug Deliv Rev. 2010;62(4–5):449–457. doi: 10.1016/j.addr.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKeon J. Estimating the global health impact of improved diagnostic tools for the developing world. In: RAND health. 2007. https://www.rand.org/pubs/research_briefs/RB9293/index1.html. Accessed 24 Aug 2019.
- 41.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(3):293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 42.Hartley D. Rural health disparities, population health, and rural culture. Am J Public Health. 2004;94(10):1675–1678. doi: 10.2105/ajph.94.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrott GSJ, Holland DF. Population trends and problems of public health. Milbank Q. 1940;83(4):569–608. doi: 10.1111/j.1468-0009.2005.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams JD, Kim U, Soh HT. Multitarget magnetic activated cell sorter. Proc Natl Acad of Sci USA. 2008;105(47):18165–18170. doi: 10.1073/pnas.0809795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saliba AE, Saias L, Psychari E, Minc N, Simon D, Bidard FC, Mathiot C, Pierga JY, Fraisier V, Salamero J, Saada V. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. Proc Natl Acad of Sci USA. 2010;107(33):14524–14529. doi: 10.1073/pnas.1001515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pamme N, Wilhelm C. Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip. 2006;6(8):974–980. doi: 10.1039/b604542a. [DOI] [PubMed] [Google Scholar]
- 47.Xia N, Hunt TP, Mayers BT, Alsberg E, Whitesides GM, Westervelt RM, Ingber DE. Combined microfluidic-micromagnetic separation of living cells in continuous flow. Biomed Microdevices. 2006;8(4):299. doi: 10.1007/s10544-006-0033-0. [DOI] [PubMed] [Google Scholar]
- 48.Inglis DW, Riehn R, Austin RH, Sturm JC. Continuous microfluidic immunomagnetic cell separation. Appl Phys Lett. 2004;85(21):5093–5095. [Google Scholar]
- 49.Alshareef M, Metrakos N, Juarez Perez E, Azer F, Yang F, Yang X, Wang G. Separation of tumor cells with dielectrophoresis-based microfluidic chip. Biomicrofluidics. 2013;7(1):11803. doi: 10.1063/1.4774312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du E, Dao M, Suresh S. Quantitative biomechanics of healthy and diseased human red blood cells using dielectrophoresis in a microfluidic system. Extreme Mech Lett. 2014;1:35–41. doi: 10.1016/j.eml.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyun KA, Jung HI. Microfluidic devices for the isolation of circulating rare cells: a focus on affinity-based, dielectrophoresis, and hydrophoresis. Electrophoresis. 2013;34(7):1028–1041. doi: 10.1002/elps.201200417. [DOI] [PubMed] [Google Scholar]
- 52.de la Rosa C, Tilley PA, Fox JD, Kaler KV. Microfluidic device for dielectrophoresis manipulation and electrodisruption of respiratory pathogen Bordetella pertussis. IEEE Trans Biomed Eng. 2008;55(10):2426–2432. doi: 10.1109/TBME.2008.923148. [DOI] [PubMed] [Google Scholar]
- 53.Adekanmbi EO, Srivastava SK. Dielectrophoretic applications for disease diagnostics using lab-on-a-chip platforms. Lab Chip. 2016;16(12):2148–2167. doi: 10.1039/c6lc00355a. [DOI] [PubMed] [Google Scholar]
- 54.Qi A, Friend JR, Yeo LY, Morton DA, McIntosh MP, Spiccia L. Miniature inhalation therapy platform using surface acoustic wave microfluidic atomization. Lab Chip. 2009;9(15):2184–2193. doi: 10.1039/b903575c. [DOI] [PubMed] [Google Scholar]
- 55.Yeo LY, Friend JR. Surface acoustic wave microfluidics. Ann Rev Fluid Mech. 2014;46:379–406. [Google Scholar]
- 56.Ding X, Peng Z, Lin SCS, Geri M, Li S, Li P, Chen Y, Dao M, Suresh S, Huang TJ. Cell separation using tilted-angle standing surface acoustic waves. Proc Natl Acad Sci USA. 2014;111(36):12992–12997. doi: 10.1073/pnas.1413325111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sivanantha N, Ma C, Collins DJ, Sesen M, Brenker J, Coppel RL, Neild A, Alan T. Characterization of adhesive properties of red blood cells using surface acoustic wave induced flows for rapid diagnostics. Appl Phys Lett. 2014;105(10):103704. [Google Scholar]
- 58.Destgeer G, Sung HJ. Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves. Lab Chip. 2015;15(13):2722–2738. doi: 10.1039/c5lc00265f. [DOI] [PubMed] [Google Scholar]
- 59.Bhagat AAS, Bow H, Hou HW, Tan SJ, Han J, Lim CT. Microfluidics for cell separation. Med Biol Eng Comput. 2010;48(10):999–1014. doi: 10.1007/s11517-010-0611-4. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Liu CC, Li H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens Actuators B Chem. 2008;130(1):216–221. [Google Scholar]
- 61.Li X, Chen W, Liu G, Lu W, Fu J. Continuous-flow microfluidic blood cell sorting for unprocessed whole blood using surface-micromachined microfiltration membranes. Lab Chip. 2014;14(14):2565–2575. doi: 10.1039/c4lc00350k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mach AJ, Di Carlo D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol Bioeng. 2010;107(2):302–311. doi: 10.1002/bit.22833. [DOI] [PubMed] [Google Scholar]
- 63.Choi S, Song S, Choi C, Park JK. Continuous blood cell separation by hydrophoretic filtration. Lab Chip. 2007;7(11):1532–1538. doi: 10.1039/b705203k. [DOI] [PubMed] [Google Scholar]
- 64.Sundararajan N, Pio MS, Lee LP, Berlin AA. Three-dimensional hydrodynamic focusing in polydimethylsiloxane (PDMS) microchannels. J Microelectromech S. 2004;13(4):559–567. [Google Scholar]
- 65.Wu Z, Nguyen NT. Hydrodynamic focusing in microchannels under consideration of diffusive dispersion: theories and experiments. Sens Actuators B Chem. 2005;107(2):965–974. [Google Scholar]
- 66.Daniele MA, Boyd DA, Mott DR, Ligler FS. 3D hydrodynamic focusing microfluidics for emerging sensing technologies. Biosens Bioelectron. 2015;67:25–34. doi: 10.1016/j.bios.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 67.Tripathi S, Chakravarty P, Agrawal A. On non-monotonic variation of hydrodynamically focused width in a rectangular microchannel. Curr Sci. 2014;107(8):1260–1274. [Google Scholar]
- 68.Dziubinski M. Hydrodynamic focusing in microfluidic devices. In: Kelly R, editor. Advances in microfluidics. London: IntechOpen; 2012. pp. 29–54. [Google Scholar]
- 69.Golden JP, Justin GA, Nasir M, Ligler FS. Hydrodynamic focusing—a versatile tool. Anal Bioanal Chem. 2013;402(1):325–335. doi: 10.1007/s00216-011-5415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tripathi S, Kumar A, Kumar YBV, Agrawal A. Three-dimensional hydrodynamic flow focusing of dye, particles and cells in a microfluidic device by employing two bends of opposite curvature. Microfluid Nanofluidics. 2016;20(2):34. [Google Scholar]
- 71.Ligler FS, Kim JS. The microflow cytometer. New York: Jenny Stanford Publishing; 2010. [Google Scholar]
- 72.Tripathi S, Kumar YBV, Prabhakar A, Joshi SS, Agrawal A. Passive blood plasma separation at the microscale: a review of design principles and microdevices. J Micromech Microeng. 2015;25(8):083001. [Google Scholar]
- 73.Yang AS, Hsieh WH. Hydrodynamic focusing investigation in a micro-flow cytometer. Biomed Microdevices. 2007;9(2):113–122. doi: 10.1007/s10544-006-9003-9. [DOI] [PubMed] [Google Scholar]
- 74.Kunstmann-Olsen C, Hoyland JD, Rubahn HG. Influence of geometry on hydrodynamic focusing and long-range fluid behavior in PDMS microfluidic chips. Microfluid Nanofluidics. 2012;12(5):795–803. [Google Scholar]
- 75.Rodriguez-Trujillo R, Mills CA, Samitier J, Gomila G. Low cost micro-Coulter counter with hydrodynamic focusing. Microfluid Nanofluidics. 2007;3(2):171–176. [Google Scholar]
- 76.Simonnet C, Groisman A. Two-dimensional hydrodynamic focusing in a simple microfluidic device. Appl Phys Lett. 2005;87(11):114104. [Google Scholar]
- 77.Lee GB, Chang CC, Huang SB, Yang RJ. The hydrodynamic focusing effect inside rectangular microchannels. J Micromech Microeng. 2006;16(5):1024. [Google Scholar]
- 78.Knight JB, Vishwanath A, Brody JP, Austin RH. Hydrodynamic focusing on a silicon chip: mixing nanoliters in microseconds. Phys Rev Lett. 1998;80(17):3863. [Google Scholar]
- 79.Stiles PJ, Fletcher DF. Hydrodynamic control of the interface between two liquids flowing through a horizontal or vertical microchannel. Lab Chip. 2004;4(2):121–124. doi: 10.1039/b315524b. [DOI] [PubMed] [Google Scholar]
- 80.Shivhare PK, Bhadra A, Sajeesh P, Prabhakar A, Sen AK. Hydrodynamic focusing and interdistance control of particle-laden flow for microflow cytometry. Microfluid Nanofluidics. 2016;20(6):86. [Google Scholar]
- 81.Sadeghi A. Micromixing by two-phase hydrodynamic focusing: a 3d analytical modeling. Chem Eng Sci. 2018;176:180–191. [Google Scholar]
- 82.Amini H, Sollier E, Masaeli M, Xie Y, Ganapathysubramanian B, Stone HA, Di Carlo D. Engineering fluid flow using sequenced microstructures. Nat Commun. 2013;4:1826. doi: 10.1038/ncomms2841. [DOI] [PubMed] [Google Scholar]
- 83.Sundararajan N, Pio MS, Lee LP, Berlin AA. Three-dimensional hydrodynamic focusing in polydimethylsiloxane (PDMS) microchannels. J Microelectromech Syst. 2004;13(4):559–567. [Google Scholar]
- 84.Simonnet C, Groisman A. High-throughput and high-resolution flow cytometry in molded microfluidic devices. Anal Chem. 2006;78(16):5653–5663. doi: 10.1021/ac060340o. [DOI] [PubMed] [Google Scholar]
- 85.Chang CC, Huang ZX, Yang RJ. Three-dimensional hydrodynamic focusing in two-layer polydimethylsiloxane (PDMS) microchannels. J Micromech Microeng. 2007;17(8):1479. [Google Scholar]
- 86.Kennedy MJ, Stelick SJ, Perkins SL, Cao L, Batt CA. Hydrodynamic focusing with a microlithographic manifold: controlling the vertical position of a focused sample. Microfluid Nanofluidics. 2009;7(4):569. [Google Scholar]
- 87.Lin SC, Yen PW, Peng CC, Tung YC. Single channel layer, single sheath-flow inlet microfluidic flow cytometer with three-dimensional hydrodynamic focusing. Lab Chip. 2012;12(17):3135–3141. doi: 10.1039/c2lc40246g. [DOI] [PubMed] [Google Scholar]
- 88.Ha BH, Lee KS, Jung JH, Sung HJ. Three-dimensional hydrodynamic flow and particle focusing using four vortices Dean flow. Microfluid Nanofluidics. 2014;17(4):647–655. [Google Scholar]
- 89.Mao X, Nawaz AA, Lin SCS, Lapsley MI, Zhao Y, McCoy JP, El-Deiry WS, Huang TJ. An integrated, multiparametric flow cytometry chip using “microfluidic drifting” based three-dimensional hydrodynamic focusing. Biomicrofluidics. 2012;6(2):024113. doi: 10.1063/1.3701566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chung S, Park SJ, Kim JK, Chung C, Han DC, Chang JK. Plastic microchip flow cytometer based on 2- and 3-dimensional hydrodynamic flow focusing. Microsyst Technol. 2003;9:525–533. [Google Scholar]
- 91.Knight JB, Vishwanath A, Brody JP, Austin RH. Hydrodynamic focusing on a silicon chip: mixing nanoliters in microseconds. Phys Rev Lett. 1998;80:3863. [Google Scholar]
- 92.Zhan Y, Loufakis DN, Bao N, Lu C. Characterizing osmotic lysis kinetics under microfluidic hydrodynamic focusing for erythrocyte fragility studies. Lab Chip. 2012;12(23):5063–5068. doi: 10.1039/c2lc40522a. [DOI] [PubMed] [Google Scholar]
- 93.Moehlenbrock MJ, Price AK, Martin RS. Use of microchip-based hydrodynamic focusing to measure the deformation-induced release of ATP from erythrocytes. Analyst. 2006;131(8):930–937. doi: 10.1039/b605136g. [DOI] [PubMed] [Google Scholar]
- 94.Koh CG, Zhang X, Liu S, Golan S, Yu B, Yang X, Guan J, Jin Y, Talmon Y, Muthusamy N, Chan KK. Delivery of antisense oligodeoxyribonucleotidelipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J Control Release. 2010;141(1):62–69. doi: 10.1016/j.jconrel.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frankowski M, Bock N, Kummrow A, Schädel-Ebner S, Schmidt M, Tuchscheerer A, Neukammer J. A microflow cytometer exploited for the immunological differentiation of leukocytes. Cytom A. 2011;79(8):613–624. doi: 10.1002/cyto.a.21083. [DOI] [PubMed] [Google Scholar]
- 96.Frankowski M, Theisen J, Kummrow A, Simon P, Ragusch H, Bock N, Schmidt M, Neukammer J. Microflow cytometers with integrated hydrodynamic focusing. Sensors. 2013;13(4):4674–4693. doi: 10.3390/s130404674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kent NJ, O’Brien S, Basabe-Desmonts L, Meade GR, MacCraith BD, Corcoran BG, Kenny D, Ricco AJ. Shear-mediated platelet adhesion analysis in less than 100 μl of blood: toward a POC platelet diagnostic. IEEE Trans Biomed Eng. 2010;58(3):826–830. doi: 10.1109/TBME.2010.2090659. [DOI] [PubMed] [Google Scholar]
- 98.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107(1):1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 99.Zheng Y, Chen J, Cui T, Shehata N, Wang C, Sun Y. Characterization of red blood cell deformability change during blood storage. Lab Chip. 2013;14(3):577–583. doi: 10.1039/c3lc51151k. [DOI] [PubMed] [Google Scholar]
- 100.Hood RR, DeVoe DL, Atencia J, Vreeland WN, Omiatek DM. A facile route to the synthesis of monodisperse nanoscale liposomes using 3D microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip. 2014;14(14):2403–2409. doi: 10.1039/c4lc00334a. [DOI] [PubMed] [Google Scholar]
- 101.Lo CT, Jahn A, Locascio LE, Vreeland WN. Controlled self-assembly of monodisperse niosomes by microfluidic hydrodynamic focusing. Langmuir. 2010;26(11):8559–8566. doi: 10.1021/la904616s. [DOI] [PubMed] [Google Scholar]
- 102.Damiati S, Kompella U, Damiati S, Kodzius R. Microfluidic devices for drug delivery systems and drug screening. Genes (Basel) 2018;9(2):103. doi: 10.3390/genes9020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schick I, Lorenz S, Gehrig D, Tenzer S, Storck W, Fischer K, Strand D, Laquai F, Tremel W. Inorganic Janus particles for biomedical applications. Beilstein J Nanotechnol. 2014;5(1):2346–2362. doi: 10.3762/bjnano.5.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie H, She ZG, Wang S, Sharma G, Smith JW. One-step fabrication of polymeric Janus nanoparticles for drug delivery. Langmuir. 2012;28(9):4459–4463. doi: 10.1021/la2042185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lone S, Cheong IW. Fabrication of polymeric Janus particles by droplet microfluidics. RSC Adv. 2014;4(26):13322–13333. [Google Scholar]
- 106.Wang F, Wang H, Wang J, Wang HY, Rummel PL, Garimella SV, Lu C. Microfluidic delivery of small molecules into mammalian cells based on hydrodynamic focusing. Biotechnol Bioeng. 2008;100(1):150–158. doi: 10.1002/bit.21737. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Xu F, Liu C, Xu Y, Feng X, Liu BF. A novel microfluidic mixer based on dual-hydrodynamic focusing for interrogating the kinetics of DNA–protein interaction. Analyst. 2013;138(16):4475–4482. doi: 10.1039/c3an00521f. [DOI] [PubMed] [Google Scholar]
- 108.Wong PK, Lee YK, Ho CM. Deformation of DNA molecules by hydrodynamic focusing. J Fluid Mech. 2003;497:55–65. [Google Scholar]
- 109.Chin CD, Linder V, Sia SK. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip. 2012;12(12):2118–2134. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]