Abstract
Claudins are integral to the structure and function of tight junctions. Altered claudin expression has been shown to affect disease behavior and patient prognosis in various neoplasms. The objectives of this study were to analyze the claudin-1, -4 and -7 expression in odontogenic tumors and characterize their expression pattern in distinct tumor cell types in relation to the recurrence potential. Sixty-nine cases of odontogenic tumors, including 43 ameloblastomas (AM), 17 adenomatoid odontogenic tumors (AOT), 6 ameloblastic fibromas (AF) and 3 ameloblastic carcinomas (AC) were investigated for claudin-1, -4 and -7 expression immunohistochemically. The staining was analyzed semi-quantitatively and categorized into 4 levels, based on the percentage of positively stained neoplastic epithelial cells. Claudin-1 was expressed in all AOT and AF cases, whereas most AC (66.7%) showed no expression. The claudin-1 staining was moderate-to-intense in the odontogenic epithelium of AF. In contrast, its staining of ameloblast-like cells and stellate reticulum-like cells in AM was weak. Claudin-7 expression was noted in all tumor types studied, while the expression of claudin-4 was limited and mainly localized in the squamous differentiated cells of AM and AC. AM showed significantly higher claudin-4, but lower claudin-7 expression than AOT. In addition, AC showed diminished claudin-1 immunoreactivity, compared to AOT. Low claudin-1 expression in AM was significantly associated with the increased clinical recurrence. The loss of claudin-1 may underlie the locally invasive nature of AM.
Keywords: Claudin, Ameloblastoma, Adenomatoid odontogenic tumor, Ameloblastic fibroma, Ameloblastic carcinoma
Introduction
Differentiated epithelial cells are characterized by their ability to form polarity architecture and cell–cell adhesion. These features help create a protective barrier between internal human tissues and surrounding environment. At the most apical portion, tight junctions (zonula occludens) serve this property by regulating the diffusion of ions and molecules along paracellular channels [1]. Emerging studies have shown that tight junction is also involved in the intracellular signaling and regulation of the epithelial cell proliferation, polarity and differentiation [2].
Tight junctions are composed of 3 transmembrane components, i.e., claudins, occludins and junctional adhesion molecules. Claudins are the major transmembrane proteins, inherent to the structure and function of tight junctions. They localize exclusively on tight junctions and form the indispensable backbone of tight junctions by creating intercellular sealing strands. In addition, zona occludens combine with other intracellular membrane proteins to help construct frameworks connecting these transmembrane proteins with the actin cytoskeleton. To date, 27 claudin members are identified in mammals [3]. They show diverse expression pattern among different cell types and tissues. Some claudin members are expressed solely in specific cell or tissue types. Epithelial cells typically express multiple claudins and different claudin combinations influence the tight junction formation. In addition, claudins may interact with other proteins and participate in the cell–cell and cell-extracellular matrix communication to control cellular proliferation and migration [4].
Dysregulation of claudin expression has been documented in various epithelial neoplasms and associated with the altered tumor behavior and patient prognosis [5, 6]. Recent data substantiate the functionally related, albeit diverse roles of differential claudin expression in tumorigenesis. In breast carcinomas [7, 8] and esophageal squamous cell carcinoma [9, 10], the diminished expression of claudin-1 was associated with the increased tumor recurrence, and the claudin-7 loss was correlated with their enhanced metastasis potential. In addition, the decreased claudin-1 and claudin-7 expression was noted in the higher grade of prostatic adenocarcinoma [11]. In other cancers, including colon adenocarcinoma, cervical and oral squamous cell carcinoma, claudin-1 was shown to overexpress and associate with the aggressive cancer phenotypes [12–14]. In addition, studies showed that the upregulation of claudin-4 was correlated with poor clinical outcomes in endometrial and gastric adenocarcinoma [15, 16]. The expression of these proteins also showed differential expression pattern in distinct neoplastic subtypes of the same tumor [5].
Odontogenic tumors constitute a unique group of benign and malignant neoplasms arising from the epithelium and/or ectomesenchyme of developing dental apparatus. They often show varying histopathologic subtypes and diverse clinical behavior. Ameloblastoma (AM), the most common clinically significant odontogenic tumor, is composed of neoplastic ameloblast-like cells and stellate reticulum-like cells reminiscent to those of enamel organ. It demonstrates various histopathologic patterns and is known to behave in a locally aggressive manner with a relatively high recurrence rate [17]. Its malignant counterpart, the ameloblastic carcinoma (AC), is a rare entity with frequent metastasis [18]. In contrast, adenomatoid odontogenic tumor (AOT) poses excellent prognosis without recurrence. Neoplastic epithelial cells in (AOT), are entirely encapsulated and arrange in distinct duct-like structures, whorled mass and spindle epithelial strands [19]. Ameloblastic fibroma (AF) represents a true mixed odontogenic epithelial and ectomesenchymal neoplasm with varying clinical behavior [20].
A previously study reported the strong claudin-1 and claudin-7 expression in enamel organs and ameloblasts of developing human tooth, while claudin-4 demonstrated weak expression, mainly limited to the outer enamel epithelium. In contrast, their expression in ameloblastoma was noted primarily in the stellate reticulum-like cells and squamous differentiation area [21]. In addition, claudins was differentially expressed in the lining of odontogenic keratocyst, dentigerous cyst and radicular cyst [22]. These data suggested that claudins may play a role during the normal odontogenesis as well as the development of odontogenic tumors. To the best of our knowledge, the expression of claudins has not been studied in other types of odontogenic tumors. Therefore, the objectives of this study were to comparatively analyze the claudin-1, -4 and -7 expression in different types of odontogenic tumors and characterize their expression pattern in distinct tumor cell types together with the association with tumor recurrence.
Materials and Methods
Tissue Samples
Sixty-nine cases of odontogenic tumors, including 43 ameloblastomas (AM), 17 adenomatoid odontogenic tumors (AOT), 6 ameloblastic fibromas (AF) and 3 ameloblastic carcinomas (AC) were included. All microscopic sections were re-examined to confirm the diagnosis, based on the World Health Organization criteria. Patient information, including age, sex and anatomical site, was recorded. The study was approved by the local Human Research Ethics Committee.
Immunohistochemical Methods
The immunohistochemical staining was performed with Leica Microsystems Bond-Max Autostainer. The 5-µm thick sections were deparaffinized with Bond Dewax Solution. The antigen retrieval was performed for claudin-1 and -7 staining by incubating slides with the Bond Epitope Retrieval Solution 2 for 30 min at 95 °C. For claudin-4 staining, slides were incubated with the Bond Epitope Retrieval Solution 1 for 20 min at 95 °C.
The primary antibodies used were the polyclonal anti-claudin-1 (1:200 dilution), monoclonal anti-claudin-4 (1:500 dilution) and monoclonal anti-claudin-7 (1:500 dilution) antibodies (Invitrogen, Camarillo, CA). The immunohistochemical procedure was performed using the Bond Polymer Refine Detection kit (Leica Microsystems). A 3% hydrogen peroxide was then applied for 5 min. The primary antibodies were applied at room temperature for 50 min, followed by 12-min incubations with the Post Primary Polymer and the Polymer Poly-HRP IgG, respectively. The sections were reacted with diaminobenzidine for 3 min and counterstained with hematoxylin. The Bond Wash Solution was used to rinse between each step. As positive controls, colonic mucosa samples were used. Negative controls were prepared using isotype-matched antibodies.
Immunostaining Assessment and Statistical Analysis
The immunohistochemical assessment was performed and agreed upon by two pathologists who were blinded to all patient clinical data. The positive immunoreactivity localized at the plasma membrane of neoplastic cells was evaluated. Overall, the percentage of positive neoplastic cells was semi-quantitatively assessed and categorized into one of the following groups: 0 = no positive cells; 1 + = positive cells detected ≤ 25% of tumor; 2 + = positive cells detected between 26 and 50% of tumor; 3 + = positive cells detected between 51 and 75% of tumor and 4 + = positive cells detected more than 75%. For distinct comparison, the expression levels were further grouped into low expression (levels 0 and 1 +) and high expression (levels 2 + , 3 + and 4 +). The staining intensity was examined in each tumor cell type and classified into four levels: level 0 = no staining; level + = mild staining; level ++ = moderate staining and level +++ = intense staining.
The results were statistically analyzed using the IBM SPSS Statistics version 22 (IBM Corporation, NY) for Windows. The continuous variables were expressed as means + standard deviation (SD). Comparative analyses of different claudin expression among groups were performed using Kruskal–Wallis test, followed by post hoc pairwise comparison using the Bonferroni method. The Mann–Whitney U test was used to analyze the association between claudin expression and recurrence status of ameloblastoma patients. Correlations among claudin expression levels were examined with Spearman’s correlation coefficient. A P value less than 0.05 was considered statistically significant.
Results
Characteristics of 69 patients enrolled were presented in Table 1. The average age of AM, AOT, AF and AC patients were 36.9 ± 17.8, 21.4 ± 12.9, 14.8 ± 6.5 and 28.7 ± 5.5 years, respectively. The male-to-female ratios of respective lesions were 1:1.4, 1:7.5, 0:6 and 2:1. The majority of AM, AF and AC affected the posterior mandible, whereas most AOT presented on the anterior maxilla. Table 2 showed the levels of immunohistochemical staining of claudins in 4 types of odontogenic tumors. The staining intensity of distinct cell types in each tumor was detailed in Table 3.
Table 1.
Patient characteristics
| Tumors | Sex | Age | Location | |||||
|---|---|---|---|---|---|---|---|---|
| Maxilla | Mandible | |||||||
| Male | Female | Mean ± SD | Range | Ant | Post | Ant | Post | |
| AM (43) | 18 | 25 | 36.9 ± 17.8 | 8–83 | 2 | 3 | 7 | 31 |
| AOT (17) | 2 | 15 | 21.4 ± 12.9 | 6–59 | 9 | 2 | 4 | 2 |
| AF (6) | 0 | 6 | 14.8 ± 6.5 | 8–25 | 0 | 1 | 1 | 4 |
| AC (3) | 2 | 1 | 28.7 ± 5.5 | 25–35 | 0 | 0 | 1 | 2 |
Table 2.
Levels of claudin-1, -4 and -7 expression in odontogenic tumors
| Claudins | Odontogenic tumors | Immunohistochemical staining, n (%) | Expression levels, n (%) | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Level 0 | Level 1+ | Level 2+ | Level 3+ | Level 4+ | Low | High | |||
| Claudin-1 |
AM AOTa AF ACa |
1 (2.3) 0 (0) 0 (0) 2 (66.7) |
10 (23.3) 0 (0) 3 (50.0) 1 (33.3) |
11 (25.6) 2 (11.8) 0 (0) 0 (0) |
13 (30.2) 10 (58.8) 0 (0) 0 (0) |
8 (18.6) 5 (29.4) 3 (50.0) 0 (0) |
11 (25.6) 0 (0) 3 (50.0) 3 (100) |
32 (74.4) 17 (100) 3 (50.0) 0 (0) |
0.005 |
| Claudin-4 |
AMb,c AOTb AFc AC |
17 (39.5) 14 (82.4) 6 (0) 2 (66.7) |
20 (46.5) 3 (17.6) 0 (0) 1 (33.3) |
4 (9.3) 0 (0) 0 (0) 0 (0) |
2 (4.7) 0 (0) 0 (0) 0 (0) |
0 (0) 0 (0) 0 (0) 0 (0) |
37 (86.0) 17 (100) 6 (100) 3 (100) |
6 (14.0) 0 (0) 0 (0) 0 (0) |
0.002 |
| Claudin-7 |
AMd AOTd AF AC |
2 (4.7) 1 (5.9) 1 (16.7) 0 (0) |
6 (14.0) 1 (5.9) 1 (16.7) 0 (0) |
8 (18.6) 1 (5.9) 1 (16.7) 1 (33.3) |
18 (41.7) 2 (11.8) 0 (0) 2 (66.7) |
9 (20.9) 12 (70.6) 3 (50.0) 0 (0) |
8 (18.6) 2 (11.8) 2 (33.3) 0 (0) |
35 (81.4) 15 (88.2) 4 (66.7) 3 (100) |
0.046 |
Analyses of comparison were performed using Kruskal–Wallis test
Tumor pairs labelled by letters a, b, c and d showed statistically significant differences in the expression of the designated claudins using post hoc pairwise comparison tests
Table 3.
Immunohistochemical staining intensity of claudins in different tumor cell types
| Tumors | Type of tumor cells | Claudin-1 | Claudin-4 | Claudin-7 |
|---|---|---|---|---|
| AM (43) | Ameloblast-like cells | 0/+ | 0 | 0/+ |
| Stellate reticulum-like cells | + | 0 | 0/+ | |
| Squamous cells | +++ | +++ | ++ | |
| Granular cells | ++ | ++ | +++ | |
| AOT (17) | Spindle epithelial cells | +++ | 0/+ | ++ |
| Ductal-like structures | 0 | 0 | ++ | |
| Whorled masses | 0 | 0 | 0/+ | |
| AF (6) | Columnar epithelium | ++ | 0 | ++ |
| Stellate reticulum-like cells | +++ | 0 | ++ | |
| Dental papilla-like stroma | 0 | 0 | 0 | |
| AC (3) | Ameloblast-like cells | 0 | 0 | 0/+ |
| Stellate reticulum-like cells | 0 | 0 | + | |
| Squamous cells | + | + | + |
Claudin-1
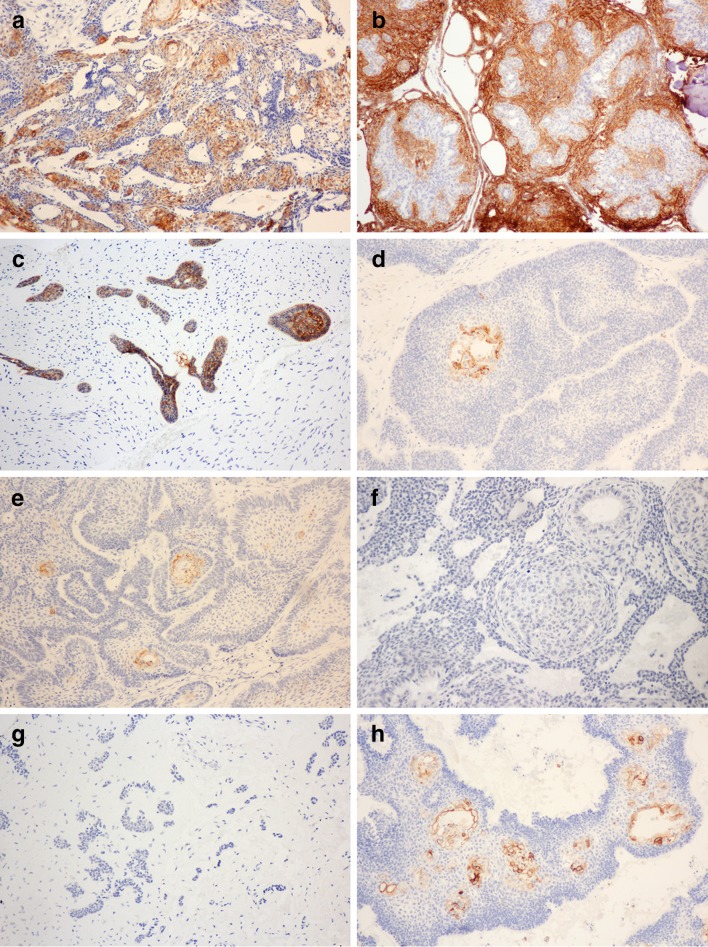
AM demonstrated a wide range of claudin-1 expression levels, varied from no staining (level 0; 2.3%) to positive immunoreactivity in more than 75% of neoplastic cells (level 4 + ; 18.6%). The majority of AM expressed claudin-1 between 51 and 75% of neoplastic cells (level 3 + ; 30.2%), followed by between 26 and 50% (level 2 + , 25.6%). Squamous epithelial cells within the ameloblastic units corresponding to the acanthomatous histopathologic pattern showed the most intense staining, followed by granular cells in the granular cell pattern. The immunoreactivity in the peripheral ameloblast-like cells and central stellate reticulum-like cells are generally weak (Fig. 1a). Low expression of claudin 1 was significantly associated with the increased recurrence of AM (P = 0.024) (Table 4).
Fig. 1.
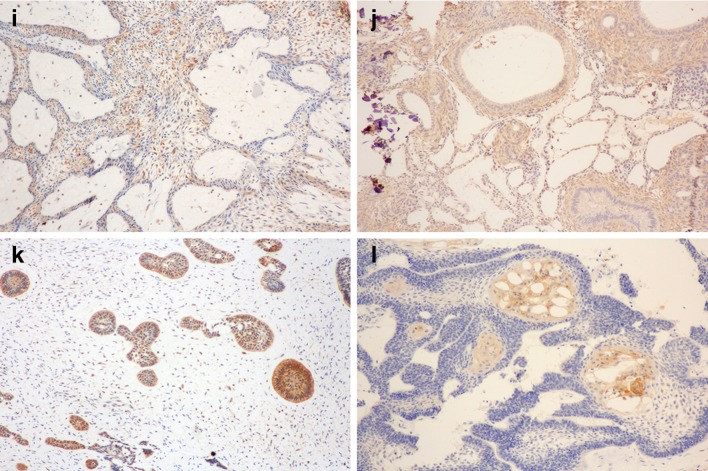
Expression of claudins in odontogenic tumors. Claudin-1 expression in a AM, b AOT, c AF, d AC; Claudin-4 expression in e AM, f AOT, g AF, h AC; Claudin-7 expression in i AM, j AOT, k AF, l AC
Table 4.
Relationship between the claudin expression and the recurrence status of ameloblastoma patients
| Ameloblastoma | Immunohistochemical staining, n (%) | P-value | |||||
|---|---|---|---|---|---|---|---|
| Level 0 | Level 1+ | Level 2+ | Level 3+ | Level 4+ | |||
| Claudin-1 | Recurrence (12) | 1 (8.3) | 5 (41.7) | 2 (16.7) | 4 (33.3) | 0 (0) | 0.024 |
| No recurrence (31) | 0 (0) | 5 (16.1) | 9 (29.0) | 9 (29.0) | 8 (25.8) | ||
| Claudin-4 | Recurrence (12) | 4 (33.3) | 6 (50.0) | 1 (8.3) | 1 (8.3) | 0 (0) | 0.574 |
| No recurrence (31) | 13 (41.9) | 14 (45.2) | 3 (9.7) | 1 (3.2) | 0 (0) | ||
| Claudin-7 | Recurrence (12) | 0 (0) | 3 (25.0) | 2 (16.7) | 5 (41.7) | 2 (16.7) | 0.629 |
| No recurrence (31) | 2 (6.5) | 3 (9.7) | 6 (19.4) | 13 (41.9) | 7 (22.6) | ||
Claudin-1 was expressed in all AOT and AF cases. Most AOTs (58.8%) showed positive staining between 51 and 75% of neoplastic cells (level 3 +), followed by staining more than 75% (level 4 +) in 29.4% of cases. Strong claudin-1 immunoreactivity was noted on the cell membrane of spindle-shaped epithelial cells arranging in anastomosing strands in AOT, whereas tumor cells forming duct-like structures or whorled masses did not express this protein (Fig. 1b). AF showed claudin-1 expression at two different levels. Half of the cases expressed claudin-1 more than 75% of epithelial tumor cells (level 4 +), and another half showed positive staining between 26 and 50% (level 2 +). The immunoreactivity was moderate-to-intense and localized in both the peripheral columnar epithelium and central stellate reticulum-like cells of the odontogenic epithelium (Fig. 1c).
In contrast to the staining pattern of benign odontogenic tumors, the majority of AC (66.7%) did not expression claudin-1. The positive staining was weak and only noted in scattered squamous cells within the ameloblastic units (Fig. 1d). Kruskal–Wallis test demonstrated statistically significant difference in claudin-1 expression among lesions (P = 0.005) and the pairwise comparison indicated that the claudin-1 expression level was significantly different between AOT and AC (P = 0.005).
Claudin-4
Claudin-4 showed limited expression in the odontogenic tumors studied. All AF cases and the majority of AOT (82.4%) and AC (66.7%) did not express this protein (Fig. 1g, f). AM showed the expression of claudin-4 in 60.5% of cases, however, the majority of which (46.5%) stained less than 25% of tumor cells (level 1 +).
In AM and AC, claudin-4 expression was noted in the squamous epithelial cells within the ameloblastic units. Granular cells in AM were also immunoreactive. No staining was noted in the ameloblast-like cells or reticulum-like cells (Fig. 1e, h). In claudin-4-positive AOTs, the staining was weak and present only in the spindle epithelial cells. The significant difference in claudin-4 expression was noted among lesions (P = 0.002) and the adjusted pairwise comparison reported the statistically significant difference between AM and AOT (P = 0.013) and between AM and AF (P = 0.037). No associated between the claudin-4 expression and the recurrence status of ameloblastoma was observed.
Claudin-7
In contrast to claudin-4, claudin-7 was frequently expressed in all tumors studied (Fig. 1i–l). The majority of AM (62.6%) and AC (66.7%) expressed claudin-7 in more than 50% of tumor cells. In addition, 70.6% of AOT and 50% of AF showed positive immunoreactivity in more than 75% of tumor cells (level 4 +). The expression of claudin-7 could be found with varying intensity across all neoplastic epithelial-typed cells. In general, the epithelial component of AF, and the epithelial strands as well as duct-like structures in AOT moderately immunoreacted to claudin-7. The ameloblast-like cells, stellate reticulum-like cells in AM and AC, as well as the whorled epithelial cells in AOT showed no or weak staining. The levels of claudin-7 expression were significantly different among lesions (P = 0.046) and the pairwise comparison indicated with statistically significant difference between AM and AOT (P = 0.034). The level of claudin-7 expression was not associated with the recurrence status of ameloblastoma patients.
Correlation among claudin-1, -4 and -7 expression
Spearman correlation analyses were used to evaluate the relationships among different claudins in odontogenic tumors (Table 5). Due to the limited number of AC cases and the lack of claudin-4 immunoreactivity in AF, their expression levels were not included in the correlation analyses. Results indicated that the expression levels of claudin-1 and claudin-7 were significantly correlated in AM (P = 0.001) and AF (P = 0.007). In contrast, AOT, demonstrated diverse expression pattern of claudins.
Table 5.
Correlation among claudin-1, -4 and -7 expression in odontogenic tumors
| Tumors | Claudin expression | Spearman’s rho | P-value |
|---|---|---|---|
| AM (43) | Claudin-1–Claudin-4 | − 0.031 | 0.843 |
| Claudin-1–Claudin-7 | 0.481 | 0.001 | |
| Claudin-4–Claudin-7 | 0.186 | 0.232 | |
| AOT (17) | Claudin-1–Claudin-4 | 0.376 | 0.136 |
| Claudin-1–Claudin-7 | 0.039 | 0.882 | |
| Claudin-4–Claudin-7 | 0.020 | 0.941 | |
| AF (6) | Claudin-1–Claudin-7 | 0.933 | 0.007 |
Discussion
In this study, we first report the differential expression of claudins in odontogenic tumors. Claudin-7 is widely expressed in the majority of odontogenic tumor types studied, whereas claudin-4 demonstrates restricted expression pattern. AM shows significantly greater claudin-4 but lower claudin-7 expression than AOT. In addition, significantly higher claudin-4 is also noted in AM than AF. These differences in claudin-4 could be owing to that its expression is solely restricted to the squamous and granular cells in the acanthomatous and granular cell ameloblastomas, respectively. All other tumor cell types demonstrate no-to-minimal expression of this protein. Our data suggests that claudin-4 may play a limited role in the overall development of odontogenic tumors.
Claudin-7 expression is observed in varying intensity in all odontogenic epithelial cell types. More than half of AOT and AF cases express claudin-7 in more than 75% of neoplastic epithelial cells. In AM, the intense staining was noted in the squamous and granular cells. Interestingly, we noted the differences between the claudin-7 staining intensity within the odontogenic epithelial components of AF/AOT and AM/AC. In AF, the peripheral columnar cells and central stellate-reticulum-like cells shows moderate claudin-7 staining, whereas the ameloblastic units of AM and AC show no-to-weak expression. In addition, AOT also demonstrates moderate claudin-7 intensity within the spindle epithelial strands and duct-like structures. The odontogenic epithelium in AF is considered primitive in origin, and in some cases, particularly in children with small asymptomatic lesion, may represent the early stages of developing odontoma [23]. Furthermore, regarding AOT, a recent immunoprofiling study suggested that it may represent a hamartoma rather than true neoplasm [24]. From these data, it is tempted to speculate that the diminished claudin-7 expression may underlie the neoplastic potential in AM and AC. Future studies focusing on the functionally-related aspect of claudin-7 should help clarify its potential role in the odontogenic tumorigenesis.
A previous study reported the distinct immunoprofiles of different epithelial cell types in AOT. It has been noted that the spindle-shaped epithelial cells often co-express vimentin and cytokeratins (CK) 5, 14, whereas CK19 is negative, suggesting that these cells are more primitive than those cuboidal or columnar cell type within the rosette/whorl-like areas [25]. Notably, we observe the intense claudin-1 staining within the cell membrane of these spindle-shaped epithelial cells forming sheets or interlacing strands in AOT, whereas the duct-like, whorled or rosette structures show no immunoreactivity. Previous ultrastructural studies reported the presence of gap junctions and desmosomes in the epithelial cells of AOT. Our finding indicates that tight junctions are also intact in these spindle epithelial strands.
The defect in structures and functions of tight junctions is believed to be one of the major mechanisms in the advanced progression of epithelial neoplasms. This process could lead to the disruption of cellular cohesion, allowing the diffusion of nutrient and growth factors which sequentially induces tumor cell proliferation, differentiation and invasiveness [26]. Claudin-1 demonstrates a noteworthy pattern of expression in odontogenic tumors with regards to their recurrence potential. All AOT cases express this protein and 88.2% of which stain claudin-1 in more than 50% of neoplastic cells. Half of AF cases express claudin-1 in more than 75% of neoplastic cells and another half show less than 25% of positive staining. The recurrence of AOT is exceedingly rare, while AF generally exhibits varying clinical behavior with overall recurrence rate of approximately 16% following conservative excision. In contrast, most AC cases do not express this protein. Interestingly, in AM the expression level of claudin-1 is diverse and the low claudin-1 expression is significantly associated with the higher recurrence rate.
The loss of claudin-1 has been reported in several cancers, such as those of breast, lung and liver, and is associated with the aggressive clinical and/or pathologic characteristics [7, 27, 28]. A recent meta-analysis showed that low claudin-1 expression in breast, colorectal, esophageal and lung cancers was significantly associated with worse patient survival [29]. The loss of claudin-1 in colorectal cancer was shown to be correlated with the increased tumor size, and vascular invasion [30]. Overexpressing claudin-1 in a lung adenocarcinoma cell line resulted in the inhibition of cell migration, invasion and metastasis. Conversely, knockdown of claudin-1 enhanced cancer cell invasive and metastatic potential [27]. Our data implicate that the loss of claudin-1 negatively impact the clinical behavior of odontogenic tumors and claudin-1 may act to suppress the invasive potential of AM.
Several studies attempted to decipher the mechanisms underlying the loss of claudin-1 in tumors. It was shown that the Snail superfamily of zinc-finger transcription factor may be involved for this phenomenon. Slug and Snail, the members of Snail superfamily, are established E-cadherin repressors and believed to be responsible for the epithelial-mesenchymal transition of several human cancers. In epithelial cells, the overexpression of both transcription factors was shown to directly downregulate claudin-1 [31]. In estrogen receptor (ER)-positive breast cancer, the methylation of claudin-1 CpG promotor island is associated with claudin-1 loss [32]. In addition, gene expression can be influenced by the presence of microRNAs, which can exert their activity by abrogating mRNA translation or utilizing RNA interference. Recent studies showed that microRNA may play a role in regulating claudin-1 expression in a tumor type-specific manner. In ovarian cancer, miR-155 was shown to target and attenuate claudin-1 mRNA and protein expression [33], whereas miR-155 overexpression in colorectal cancers alternatively upregulated this protein [34]. In non-small-cell lung carcinoma cell line, the miR-375 overexpression was associated with the decreased CLDN1 mRNA as well as protein expression and worse patient survival [35]. It could be of interest for future studies to investigate whether these factors may affect in the differential expression of claudin-1 in AMs with distinct clinical behavior.
In summary, we report the frequent expression of claudin-7 in neoplastic epithelial cells of odontogenic tumors studied, whereas the expression of claudin-4 is relatively limited. Our results show the trend toward the decreasing claudin-1 expression from AOT, AF, AM to AC with the statistically significant difference observed between AOT and AC. Notably, recurrent AMs exhibit diminished claudin-1 expression, compared to the non-recurrent cases. These data suggest that claudin-1 may play a role in the locally aggressive potential of AM.
Funding
This study was funded by a Research Fund, Faculty of Dental Medicine, Rangsit University.
Compliance with Ethical Standards
Conflict of interest
All authors disclose no commercial associations or financial interest that might pose a conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ekarat Phattarataratip, Email: Ekarat.P@chula.ac.th.
Kraisorn Sappayatosok, Email: kraisorn.s@rsu.ac.th.
References
- 1.Singh AB, Uppada SB, Dhawan P. Claudin proteins, outside-in signaling, and carcinogenesis. Pflug Arch. 2017;469(1):69–75. doi: 10.1007/s00424-016-1919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Severson EA, Parkos CA. Mechanisms of outside-in signaling at the tight junction by junctional adhesion molecule A. Ann N Y Acad Sci. 2009;1165:10–18. doi: 10.1111/j.1749-6632.2009.04034.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsukita S, Tanaka H, Tamura A. The Claudins: from tight junctions to biological systems. Trends Biochem Sci. 2019;44(2):141–152. doi: 10.1016/j.tibs.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, et al. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63(4):622–634. doi: 10.1136/gutjnl-2012-304241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon MJ. Emerging roles of claudins in human cancer. Int J Mol Sci. 2013;14(9):18148–18180. doi: 10.3390/ijms140918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh AB, Dhawan P. Claudins and cancer: fall of the soldiers entrusted to protect the gate and keep the barrier intact. Semin Cell Dev Biol. 2015;42:58–65. doi: 10.1016/j.semcdb.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Morohashi S, Kusumi T, Sato F, Odagiri H, Chiba H, Yoshihara S, et al. Decreased expression of claudin-1 correlates with recurrence status in breast cancer. Int J Mol Med. 2007;20(2):139–143. [PubMed] [Google Scholar]
- 8.Sauer T, Pedersen MK, Ebeltoft K, Naess O. Reduced expression of Claudin-7 in fine needle aspirates from breast carcinomas correlate with grading and metastatic disease. Cytopathology. 2005;16(4):193–198. doi: 10.1111/j.1365-2303.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto K, Kusumi T, Sato F, Kawasaki H, Shibata S, Ohashi M, et al. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed Res. 2008;29(2):71–76. doi: 10.2220/biomedres.29.71. [DOI] [PubMed] [Google Scholar]
- 10.Usami Y, Chiba H, Nakayama F, Ueda J, Matsuda Y, Sawada N, et al. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol. 2006;37(5):569–577. doi: 10.1016/j.humpath.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan GM, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Jr, Ross JS. Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Hum Pathol. 2007;38(4):564–569. doi: 10.1016/j.humpath.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115(7):1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sappayatosok K, Phattarataratip E. Overexpression of claudin-1 is associated with advanced clinical stage and invasive pathologic characteristics of oral squamous cell carcinoma. Head Neck Pathol. 2015;9(2):173–180. doi: 10.1007/s12105-014-0559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang WN, Li W, Wang XL, Hu Z, Zhu D, Ding WC, et al. CLDN1 expression in cervical cancer cells is related to tumor invasion and metastasis. Oncotarget. 2016;7(52):87449–87461. doi: 10.18632/oncotarget.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konecny GE, Agarwal R, Keeney GA, Winterhoff B, Jones MB, Mariani A, et al. Claudin-3 and claudin-4 expression in serous papillary, clear-cell, and endometrioid endometrial cancer. Gynecol Oncol. 2008;109(2):263–269. doi: 10.1016/j.ygyno.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resnick MB, Gavilanez M, Newton E, Konkin T, Bhattacharya B, Britt DE, et al. Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol. 2005;36(8):886–892. doi: 10.1016/j.humpath.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Bilodeau EA, Prasad JL, Alawi F, Seethala RR. Molecular and genetic aspects of odontogenic lesions. Head Neck Pathol. 2014;8(4):400–410. doi: 10.1007/s12105-014-0588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzitelli A, Smoll NR, Chae MP, Rozen WM, Hunter-Smith DJ. Incidence and overall survival of malignant ameloblastoma. PLoS ONE. 2015;10(2):e0117789. doi: 10.1371/journal.pone.0117789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chrcanovic BR, Gomez RS. Adenomatoid odontogenic tumor: an updated analysis of the cases reported in the literature. J Oral Pathol Med. 2019;48(1):10–16. doi: 10.1111/jop.12783. [DOI] [PubMed] [Google Scholar]
- 20.Chrcanovic BR, Brennan PA, Rahimi S, Gomez RS. Ameloblastic fibroma and ameloblastic fibrosarcoma: a systematic review. J Oral Pathol Med. 2018;47(4):315–325. doi: 10.1111/jop.12622. [DOI] [PubMed] [Google Scholar]
- 21.Bello IO, Soini Y, Slootweg PJ, Salo T. Claudins 1, 4, 5, 7 and occludin in ameloblastomas and developing human teeth. J Oral Pathol Med. 2007;36(1):48–54. doi: 10.1111/j.1600-0714.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 22.Siar CH, Abbas SA. Claudin expression and tight junction protein localization in the lining epithelium of the keratocystic odontogenic tumors, dentigerous cysts, and radicular cysts. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(5):652–659. doi: 10.1016/j.oooo.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Buchner A, Vered M. Ameloblastic fibroma: a stage in the development of a hamartomatous odontoma or a true neoplasm? Critical analysis of 162 previously reported cases plus 10 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(5):598–606. doi: 10.1016/j.oooo.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Reichart PA, Philipsen HP, Khongkhunthian P, Sciubba JJ. Immunoprofile of the adenomatoid odontogenic tumor. Oral Dis. 2017;23(6):731–736. doi: 10.1111/odi.12572. [DOI] [PubMed] [Google Scholar]
- 25.Leon JE, Mata GM, Fregnani ER, Carlos-Bregni R, de Almeida OP, Mosqueda-Taylor A, et al. Clinicopathological and immunohistochemical study of 39 cases of adenomatoid odontogenic tumour: a multicentric study. Oral Oncol. 2005;41(8):835–842. doi: 10.1016/j.oraloncology.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Escudero-Esparza A, Jiang WG, Martin TA. The Claudin family and its role in cancer and metastasis. Front Biosci (Landmark Ed). 2011;16:1069–1083. doi: 10.2741/3736. [DOI] [PubMed] [Google Scholar]
- 27.Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, et al. Claudin-1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 2009;179(2):123–133. doi: 10.1164/rccm.200803-456OC. [DOI] [PubMed] [Google Scholar]
- 28.Higashi Y, Suzuki S, Sakaguchi T, Nakamura T, Baba S, Reinecker HC, et al. Loss of claudin-1 expression correlates with malignancy of hepatocellular carcinoma. J Surg Res. 2007;139(1):68–76. doi: 10.1016/j.jss.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 29.Pyo JS, Kim NY, Cho WJ. Prognostic role of Claudin-1 immunohistochemistry in malignant solid tumors: a meta-analysis. J Pathol Transl Med. 2019 doi: 10.4132/jptm.2019.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim NY, Pyo JS, Kang DW, Yoo SM. Loss of claudin-1 expression induces epithelial-mesenchymal transition through nuclear factor-kappaB activation in colorectal cancer. Pathol Res Pract. 2019;215(3):580–585. doi: 10.1016/j.prp.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, et al. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J. 2006;394(Pt 2):449–457. doi: 10.1042/BJ20050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Cello F, Cope L, Li H, Jeschke J, Wang W, Baylin SB, et al. Methylation of the claudin 1 promoter is associated with loss of expression in estrogen receptor positive breast cancer. PLoS ONE. 2013;8(7):e68630. doi: 10.1371/journal.pone.0068630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin W, Ren Q, Liu T, Huang Y, Wang J. MicroRNA-155 is a novel suppressor of ovarian cancer-initiating cells that targets CLDN1. FEBS Lett. 2013;587(9):1434–1439. doi: 10.1016/j.febslet.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Zhang GJ, Xiao HX, Tian HP, Liu ZL, Xia SS, Zhou T. Upregulation of microRNA-155 promotes the migration and invasion of colorectal cancer cells through the regulation of claudin-1 expression. Int J Mol Med. 2013;31(6):1375–1380. doi: 10.3892/ijmm.2013.1348. [DOI] [PubMed] [Google Scholar]
- 35.Yoda S, Soejima K, Hamamoto J, Yasuda H, Nakayama S, Satomi R, et al. Claudin-1 is a novel target of miR-375 in non-small-cell lung cancer. Lung Cancer. 2014;85(3):366–372. doi: 10.1016/j.lungcan.2014.06.009. [DOI] [PubMed] [Google Scholar]