Abstract
Differentiating between adenoid cystic carcinoma (AdCC) and polymorphous adenocarcinoma (PAC) can be difficult on small biopsies and cytologic specimens. As such, further characterization of their immunophenotype may aid in distinction. Previous studies have found AdCC to be SOX10+/GATA3 variable and PAC to be GATA3 negative. SOX10 expression in PAC has, as yet, not been established. We performed GATA3 and SOX10 immunohistochemistry on whole sections of 25 cases each of AdCC and PAC (including both classic PAC and the cribriform variant) to assess whether these markers are of diagnostic utility in distinguishing between these entities. SOX10 was found to be positive in 100% of PAC and AdCC whereas GATA 3 was immunoreactive in 45% of AdCCs and 20% of PAC. While this is the first series to compare SOX10 and GATA3 staining in these two tumor types, their frequent expression and similar staining patterns render them of limited value in discriminating between these neoplasms.
Keywords: Salivary gland, Adenoid cystic carcinoma, Polymorphous adenocarcinoma, GATA3, SOX10
Introduction
AdCC and PAC are two distinct salivary gland malignancies that can have overlapping clinical presentations and histopathology. Both tend to arise in the minor salivary glands, with AdCC being the most common tumor of the minor salivary glands and PAC occurring almost exclusively in the minor salivary glands of the oral cavity and oropharynx [1, 2]. Many microscopic characteristics are shared by these carcinomas, including prominent perineural invasion and architectural variability encompassing tubular, cribriform and solid growth patterns [3]. Although the “targetoid” arrangement of perineural invasion in PAC and the more regular and “tighter” cribriform pattern in AdCC can be helpful differentiating features, they may not be present in all specimens [4, 5]. Correct identification of these entities is crucial due to significant differences in prognosis and treatment. PAC is typically low grade with local recurrence, rare nodal metastases, and extremely rare distant metastases; whereas, AdCC is a slowly, but frequently, progressive cancer with high death rates due to disease.
SOX10, or SRY-related HMG-box 10 (SOX10), is a transcription factor associated with neural crest cells and their resultant mature cells such as melanocytes and Schwann cells [6, 7]. It has also been shown to be expressed in normal constituents of salivary glands such as myoepithelial cells, acinar cells, and intercalated ducts [6, 8]. Initial studies reported its utility in delineating two distinct subtypes of salivary gland tumors: acinic cell carcinoma, adenoid cystic carcinoma, epithelial myoepithelial carcinoma, myoepithelioma, and pleomorphic adenoma which are positive, versus salivary duct carcinoma (SDC), oncocytic carcinoma, oncocytoma, Warthin tumors and mucoepidermoid carcinoma which are negative [8]. Subsequently, Hsieh evaluated basal cell adenoma/adenocarcinoma, lymphoepithelial carcinoma, secretory carcinoma (SC), hyalinizing clear cell carcinoma, sialoblastoma and low grade SDC and determined that basal cell adenoma/adenocarcinoma, low grade SDC, sialoblastoma and SC were also immunoreactive [6]. To date, SOX10 has not been assessed in PAC. If negative, it could potentially serve as an adjunctive tool to discriminate between PAC and AdCC.
GATA3 is a zinc finger transcription factor of the GATA family which was named for the protein’s characteristic binding of A/TGATAA/G nucleotide sequences [9–11]. First characterized by its expression in T lymphocytes, in recent years it has been most usefully shown to be reactive in carcinomas of breast and urothelial origin [9, 12]. Schwartz et al. evaluated GATA3 expression in a variety of salivary neoplasms and demonstrated staining in SC, SDC, and Warthin’s tumor, as well as in 22% of AdCCs. None of the four PACs in their cohort, however, were positive [12].
As differentiating between these two entities is problematic and clinically important, and as few studies evaluating these stains specifically exist, we sought to examine GATA3 and SOX10 expression specifically in PACs and AdCC.
Materials and Methods
After institutional review board approval, the pathology information systems of Vanderbilt University Medical Center and Washington University School of Medicine were searched for cases of PAC and AdCC. Twenty-five cases of each were identified between 1994 and 2015. The hematoxylin and eosin-stained slides were reviewed from the corresponding resection specimens, and the interpretations were confirmed by at least one of three senior head and neck pathologists (KE, JL, or RC). AdCCs were diagnosed based on morphologic criteria outlined in the World Health Organization (WHO) classification of Head and Neck Tumors [13] and were graded by an experienced head and neck pathologist (KE) using the system described by Szanto et al. [14]. Briefly, Grade I tumors had predominantly tubular growth and no solid component; Grade II, had predominantly cribriform pattern and less than 30% solid growth; and Grade III contained > 30% solid areas. PAC cases were diagnosed by WHO criteria as well, and reflected the recent joining of classic PAC and cribriform adenocarcinoma under the single term PAC [15].
Immunohistochemistry for GATA3 and SOX10 was performed on whole tumor sections. Unstained slides from the formalin-fixed paraffin-embedded tissue blocks were used for staining on a BOND-MAX Automated IHC Stainer (Leica Biosystems, Buffalo Grove, Illinois). All steps besides dehydration, clearing and coverslipping were performed on the Bond Max. Slides were deparaffinized. For SOX10 staining, heat induced antigen retrieval was performed on the Bond Max using their Epitope Retrieval 2 solution for 10 min. The sections were incubated with anti-SOX-10 (Catalog #PA0813, Cell Marque, Rocklin, CA) for 1 h. For GATA-3 staining, heat induced antigen retrieval was performed on the Bond Max using their Epitope Retrieval 2 solution for 20 min. Slides were incubated with Ready-To-Use anti-GATA-3 (cat#PM405AA, BioCare Medical, Concord, CA) for 1 h. The Bond Refine Polymer detection system was used for visualization. Slides were then dehydrated, cleared, and coverslipped.
Nuclear staining was scored on a 0–16 scale (intensity of staining as 1–4 multiplied by extent of staining in quartiles as 0–4) by two study pathologists (AG and KE). A score of > 3 was considered positive. Scores were then classified as negative (score < 3), dim or focal (score 3–7), or strong and diffuse (score > 8). Cases with discrepant scores (a difference of > 2 points or over a cutoff) were re-reviewed until a consensus was reached.
De-identified clinical data regarding length of follow-up, recurrence and overall mortality were gathered from The Vanderbilt University Medical Center and Washington University School of Medicine electronic medical records (Epic Systems Corporation, Verona, WI). Recurrence was then compared with staining intensity. Calculations were tabulated using Excel (Microsoft Corporation, Redmond, WA).
Statistical analysis was performed using Prism 8 (GraphPad Software, San Diego, CA). Immunohistochemical staining scores were compared between AdCC and PAC and to evaluate outcomes data using the Mann–Whitney U test. SOX10 and GATA3 staining scores were compared between grades of AdCC using a one-way ANOVA test. For both tests a p value of < 0.05 was considered significant.
Results
SOX10 was present in 100% of PAC and AdCC. Reactivity was strong and diffuse with scores greater than 8 in 22 of 25 (88%) AdCC and in all (100%, n = 25) PACs. Three AdCC were focally positive (Table 1). GATA3 was expressed in 45% (10 of 25 cases) of AdCC, mostly in a weak and patchy distribution, with scores less than 8 in 80% of the cases. 20% (5 of 25) of PAC showed focal but strong GATA3 reactivity, specifically in areas with ductal differentiation (Fig. 1). The remaining PAC cases were negative.
Table 1.
Immunohistochemical staining results for SOX10 and GATA3 in adenoid cystic carcinoma (AdCC) and polymorphous adenocarcinoma (PAC)
| Staining pattern | SOX10 (p = 0.19) | GATA3 (p = 0.27) | ||
|---|---|---|---|---|
| Tumor type | AdCC n: 25 (%) |
PAC n: 25 (%) |
AdCC n: 25 (%) |
PAC n: 25 (%) |
| Strong and diffuse staining (score ≥ 8) | 22 (88) | 25 (100) | 2 (8) | 0 (0) |
| Dim or focal staining (score from 3 to 7) | 3 (12) | 0 (0) | 8 (32) | 5 (20) |
| Negative staining (score < 3) | 0 (0) | 0 (0) | 15 (60) | 20 (80) |
A p value of < 0.05 was considered significant
Fig. 1.
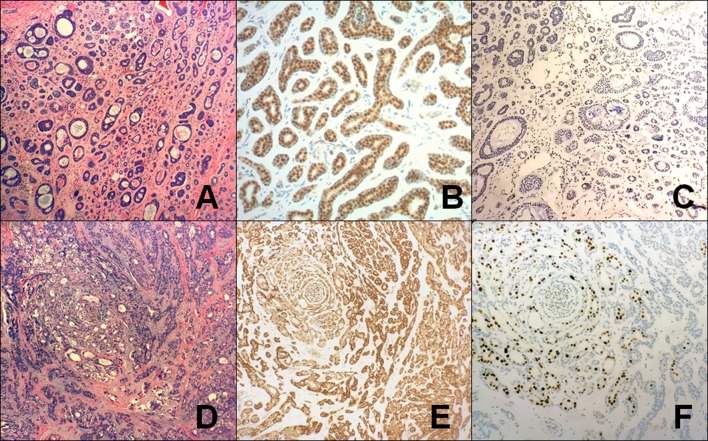
Characteristic findings in AdCC and PAC a H&E-stained AdCC excision specimen show a predominantly tubular (grade I) tumor. (40X); b SOX10 in AdCC showing strong, diffuse nuclear staining (40X); c GATA3 in AdCC showing no significant staining (40X); d H&E-stained PAC excision specimen showing a nested and tubular tumor with the characteristic “whorling” pattern on low power (40X); e SOX10 in PAC showing strong, diffuse nuclear staining (40X); f GATA3 in PAC showing focal, strong immunoreactivity in a subset of cells, specifically in areas with overt ductal differentiation (40X). (AdCC adenoid cystic carcinoma, PAC polymorphous adenocarcinoma)
The grade of AdCC and staining score was also investigated. Among the twenty-five AdCCs, 6 (24%) were grade I, 13 (52%) grade II, and 6 (24%) grade III. SOX10 expression ranged from dim or focal to strong and diffuse in all grades of AdCC. GATA 3 was negative to dim or focal in grades I and III and negative to strong and diffuse in grade II. The correlation of SOX10 and GATA 3 reactivity and grade is presented in Table 2. Staining intensity and distribution did not appear to correlate with grade.
Table 2.
Differential Sox10 and GATA 3 staining in adenoid cystic carcinomas based on grade
| Stain | SOX10 (p = 0.39) | GATA 3 (p = 0.23) | ||||
|---|---|---|---|---|---|---|
| Staining pattern | Negative (score < 3) | Dim or focal (score from 3 to 7) | Strong and diffuse (score > 8) | Negative (score < 3) | Dim or focal (score from 3-7) | Strong and diffuse (score > 8) |
|
Grade I n: 6 (%) |
0 (0) | 1 (17) | 5 (83) | 5 (83) | 1 (17) | 0 (0) |
|
Grade II n: 13 (%) |
0 (0) | 1 (8) | 12 (92) | 6 (46) | 5 (38) | 2 (15) |
|
Grade III n: 6 (%) |
0 (0) | 1 (17) | 5 (83) | 4 (67) | 2 (33) | 0 (0) |
A p value of < 0.05 was considered significant
Follow-up data was available for 84% (21 of 25) and 76% (19 of 25) of AdCC and PAC patients, respectively (average follow-up of 8.6 years). 52% (11 of 21) of AdCC and 37% (7 of 19 cases) of PAC experienced recurrence. Of PAC cases with follow-up, GATA 3 was negative in cases without recurrence (average score = 1.7) and weakly positive in cases with recurrence (average score 3.2), p = 0.14. GATA 3 expression was essentially negative in AdCCs with or without recurrence (average scores AdCC with recurrence 2.7, without 2.6, p = 0.71). SOX10 staining intensity was very strong in cases which recurred and which did not recur (average scores AdCC with recurrence 11.7, without 12.7, p = 0.66 and PAC with recurrence 12.7, without 13.9, p = 0.55). Overall, all-cause mortality for AdCC 5% (1 of 21) was while PAC was 16% (3 of 19 cases).
Discussion
There are several characteristic histologic features that can facilitate the proper recognition of PAC and AdCC. PAC is often arranged in concentrically streaming columns of cells which produce a whirling or “eye of the storm” appearance [16]. Its nuclei are monotonous and round to oval, with finely dispersed, pale to clear, somewhat vesicular chromatin. The cytoplasm, which may be of moderate amount, is amphophilic to eosinophilic [5]. This is in contradistinction to the angulated or peg-shaped, hyperchromatic nuclei and scant cytoplasm of AdCC tumor cells. Unlike the slate blue-gray background matrix of PAC, AdCC has a prominent acellular myxohyaline material that forms ‘cylinders’ within the tumor nests [17]. Finally, the tubules of AdCC have an outer myoepithelial layer (i.e. are biphasic) while those in PAC are usually lined by a monolayer of uniform ductal epithelial cells.
Differences notwithstanding, these tumors are often challenging to separate on small biopsies and their distinction has major prognostic implications. Patients with AdCC generally have poor long term survival marked by recurrence and distant metastatic rates of up to 67% and 46%, respectively [18]. The 5 year survival rate is ~ 55 to 89% while the 15- to 20 year survival is even poorer [18]. PAC behaves in a much more indolent fashion with death due to disease being extremely rare. A SEER database analysis showed a 5 year disease specific survival (DSS) for PAC of 98.6% [19]. While both PAC and AdCC are treated surgically by complete local excision, AdCC is also managed with post-operative radiation to improve local control [1, 20].
A number of studies have evaluated the ability of various immunohistochemical markers to distinguish between AdCC and PAC [3, 21, 22]. Most promising is the application of a combined p63/p40 panel wherein AdCC is p63+/p40+ and PAC is p63+/p40− [3, 22, 23]. p63 staining appears diffuse in PAC and peripheral (abluminal) in AdCC, highlighting myoepithelial but not ductal cells [3]. Less definitive results have been observed with c-kit. While some groups report strong and diffuse positivity in AdCC and rare to low level expression in PAC, others have found frequent reactivity [22, 24]. Likewise, a significantly higher Ki-67(MIB-1) proliferation index has been noted in AdCC compared to PAC, but exceptions to this observation have been made elsewhere [21, 22]. Saghravaman et al. reported S100 staining more frequently and intensely in PAC when compared to AdCC, but the average expression level between the two carcinomas was not significant [21]. Lastly, AdCC possesses a characteristic MYB-NFIB fusion which leads to high expression of MYB protein detectable by immunohistochemistry. Existing evidence, however, has shown that the sensitivity and specificity of the MYB immunostain is relatively low for AdCC and expression can also occur in PAC [25, 26].
Limited data is available on SOX10 and GATA3 immunoreactivity in PAC and AdCC. GATA3 has been largely recognized as a marker of carcinomas of the breast and urothelium [9, 12]. Due to similarities between breast and salivary gland, Schwartz et al. analyzed a broad spectrum of salivary gland neoplasms for GATA3 expression. Nine of 41 (22%) of AdCCs showed variable strength and intensity of staining, while all PACS (n = 4) were negative [12]. In Miettinen’s cohort of 17 AdCCs, 5 (29%) were positive, but details on the quality and extent of staining were not provided. In addition, no PACs were represented in their study [27]. Ohtomo was the first to examine SOX10 expression in both normal salivary gland and salivary gland tumors. Twenty-two of 23 (96%) AdCC were positive, labelling a high proportion of both luminal and abluminal cells [8]. Such expression was confirmed by Hsieh, who noted staining of nearly all cells in the 13 cases of AdCC tested [6].
On the basis of these observations, we sought to determine whether GATA3 and SOX10 could be of value in distinguishing AdCC and PAC. In our series, SOX10 stained all AdCCs and PACs in a strong and diffuse fashion. Such reactivity in AdCC is like that described by other authors [6, 8]. For the first time in the literature, PAC was tested for SOX10 and uniform expression was found. Similar to prior reports, we confirmed GATA3 positivity in AdCC, but at a higher proportion than previously reported. Additionally, staining for both markers did not vary significantly between grades of AdCC. Likewise, we demonstrated that intensity of staining did vary between tumors which recurred and those which did not.
In contrast to the findings of Schwartz of absent expression in PAC, we observed focal but strong GATA3 expression in 5 of 25 cases. These differences may be because many earlier investigations used tissue microarrays (TMA) [8, 12]. Though TMA has shown to be effective in evaluating salivary gland tumors, there are limitations due to the small sample sizes, tumor heterogeneity, and core loss [28–30]. It is reasonable to assume that the patchy GATA3 staining seen in both in AdCC and PAC could have been missed on these small tissue samples. Evaluating immunohistochemical stains on resection specimens, as performed here, ensures detection of staining as well as more thorough characterization of staining patterns.
In summary, these results confirm consistent SOX10 and variable GATA3 expression in AdCC. Moreover, they show that both markers are expressed by PAC and at a frequency equivalent to that seen in AdCC. Staining intensity did predict likelihood of recurrence in either carcinoma. As such, GATA3 and SOX10 do not appear to provide any consistent separation of AdCC and PAC, nor do they appear to provide any significant prognostic information. Further studies using immunohistochemistry and molecular tests are needed to help discriminate between these similar salivary gland carcinomas, especially when faced with limited tissue biopsies.
Acknowledgements
We acknowledge the Translational Pathology Shared Resource supported by NCI/NIH Cancer Center Support Grant 2P30 CA068485-14 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 5U24DK059637-1.
Compliance with Ethical Standards
Conflict of interest
The authors declare no competing financial interests. There is No conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51(7):652–661. doi: 10.1016/j.oraloncology.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Verma V, Mendenhall WM, Werning JW. Polymorphous low-grade adenocarcinoma of the head and neck. Am J Clin Oncol Cancer Clin Trials. 2014;37(6):624–626. doi: 10.1097/COC.0b013e31827e5537. [DOI] [PubMed] [Google Scholar]
- 3.Rooper L, Sharma R, Bishop JA. Polymorphous low grade adenocarcinoma has a consistent p63+/p40− immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015;9(1):79–84. doi: 10.1007/s12105-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman PD, Lumerman H. Lobular carcinoma of intraoral minor salivary gland origin. Report of twelve cases. Oral Surgery Oral Med Oral Pathol. 1983;56(2):157–165. doi: 10.1016/0030-4220(83)90282-7. [DOI] [PubMed] [Google Scholar]
- 5.McHugh JB, Visscher DW, Barnes EL. Update on selected salivary gland neoplasms. Arch Pathol Lab Med. 2009;133(11):1763–1774. doi: 10.5858/133.11.1763. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh MS, Lee YH, Chang YL. SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol. 2016;56:134–142. doi: 10.1016/j.humpath.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Castranova V, Asgharian B, Sayre P, Virginia W, Carolina N. Sox10—a marker for not only Schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue. A systematic analysis of 5134 tumors. Am J Surg Pathol. 2016;39(6):1922–2013. doi: 10.1097/PAS.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtomo R, Mori T, Shibata S, et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol. 2013;26(8):1041. doi: 10.1038/modpathol.2013.54. [DOI] [PubMed] [Google Scholar]
- 9.Labastie MC, Bories D, Chabret C, Gregoire JM, Chretien S, Romeo PH. Structure and expression of the human GATA3 gene. Genomics. 1994;21(1):1–6. doi: 10.1006/geno.1994.1217. [DOI] [PubMed] [Google Scholar]
- 10.Goyal A, Zhang G, Yang B. Differential expression patterns of GATA3 in usual and differentiated types of vulvar intraepithelial neoplasia: potential diagnostic implications. Mod Pathol. 2018. [DOI] [PubMed]
- 11.Ko LJ, Engel JD. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol. 1993;13(7):4011–4022. doi: 10.1128/mcb.13.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz LE, Begum S, Westra WH, Bishop JA. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013;7(4):311–315. doi: 10.1007/s12105-013-0442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenman G, Licitra L, Said-Al-Naief N, van Zante A, Yarbrough WG. Adenoid cystic carcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. Tumours of salivary glands. WHO classification of head and neck tumours. 4th edn. Lyon, France, International Agency for Research on Cancer, 2017.
- 14.Szanto PA, Luna MA, Tortoledo ME, et al. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54(6):1062–1069. doi: 10.1002/1097-0142(19840915)54:6<1062::aid-cncr2820540622>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Fonseca I, Assad A, Katabi N, Seethala R, Weinreb I, Wenig BM. Polymorphous adenocarcinoma. In: El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. Tumours of salivary glands. WHO classification of head and neck tumours. 4th ed. Lyon, France, International Agency for Research on Cancer, 2017.
- 16.Stenman G, Simpson RH, Hellquist H, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018;42(2):11–27. doi: 10.1097/PAS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 17.Seethala RR. Histologic grading and prognostic biomarkers in salivary gland carcinomas. Adv Anat Pathol. 2011;18(1):29–45. doi: 10.1097/PAP.0b013e318202645a. [DOI] [PubMed] [Google Scholar]
- 18.Xu B, Drill E, Ho A, et al. Predictors of outcome in adenoid cystic carcinoma of salivary glands: a clinicopathologic study with correlation between MYB fusion and protein expression. Am J Surg Pathol. 2017;41(10):1422–1432. doi: 10.1097/PAS.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu B, Drill E, Ho AA, et al. Polymorphous low-grade adenocarcinoma of the head and neck: a population-based study of 460 cases. Am J Surg Pathol. 2015;41(10):1644–1649. doi: 10.1002/lary.25266. [DOI] [PubMed] [Google Scholar]
- 20.Castle JT, Thompson LD, Frommelt RA, Wenig BM, Kessler HP. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86(2):207–219. [PubMed] [Google Scholar]
- 21.Saghravanian N, Mohtasham N, Jafarzadeh H. Comparison of immunohistochemical markers between adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma. J Oral Sci. 2009;51(4):509–514. doi: 10.2334/josnusd.51.509. [DOI] [PubMed] [Google Scholar]
- 22.Beltran D, Faquin WC, Gallagher G, August M. Selective immunohistochemical comparison of polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma. J Oral Maxillofac Surg. 2006;64(3):415–423. doi: 10.1016/j.joms.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Argyris PP, Wetzel SL, Greipp P, et al. Clinical utility of myb rearrangement detection and p63/p40 immunophenotyping in the diagnosis of adenoid cystic carcinoma of minor salivary glands: a pilot study This study was presented in the poster session of the 69th Annual Meeting of the AAOMP, Apri. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(3):282–289. doi: 10.1016/j.oooo.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Edwards PC, Bhuiya T, Kelsch RD. C-kit expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and monomorphic adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(5):586–593. doi: 10.1067/moe.2003.31. [DOI] [PubMed] [Google Scholar]
- 25.Brill LB, Kanner WA, Fehr A, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Mod Pathol. 2011;24(9):1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 26.Persson F, Fehr A, Sundelin K, Schulte B, Lon̈ing T, Stenman G. Studies of genomic imbalances and the MYB-NFIB gene fusion in polymorphous low-grade adenocarcinoma of the head and neck. Int J Oncol. 2012;40(1):80–84. doi: 10.3892/ijo.2011.1190. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen M, et al. GATA3 marker in surgical pathology—a systematic analysis of 2500 epithelial and non-epithelial tumors. Am J Surg Pathol. 2015;38(1):13–22. doi: 10.1097/PAS.0b013e3182a0218f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca FP, Benevenuto De Andrade BA, Carrinho Ayrosa Rangel AL, et al. Tissue microarray is a reliable method for immunohistochemical analysis of pleomorphic adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;17(1):81–88. doi: 10.1016/j.oooo.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Paiva-Fonseca F, de-Almeida OP, Ayroza-Rangel ALC, Agustin-Vargas P. Tissue microarray construction for salivary gland tumors study. Med Oral Patol Oral Cir Bucal. 2013;18(1):e1. doi: 10.4317/medoral.18204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwafuchi H, Mori N, Takahashi T, Yatabe Y. Phenotypic composition of salivary gland tumors: an application of principal [corrected] component analysis to tissue microarray data. Mod Pathol. 2004;17(7):803–810. doi: 10.1038/modpathol.3800122. [DOI] [PubMed] [Google Scholar]