Abstract
A 19 year old otherwise healthy male presented with a history of acute onset left neck pain with subsequent swelling and development of a left neck mass that progressively enlarged over a two month period. Imaging studies revealed a solid heterogeneous mass with prominent calcifications displacing normal structures. The lesion was resected via transcervical approach and a diagnosis of calcifying fibrous tumor (CFT) was rendered. The clinical, radiographic, histologic and immunophenotypic features of CFT are discussed. CFT is a rare benign soft tissue tumor with distinctive histologic findings. They present as well-circumscribed but unencapsulated, paucicellular lesions consisting of hyalinized fibrous tissue with chronic lymphoplasmacytic inflammation and variable amounts of both psammomatous and dystrophic calcifications distributed throughout. They are found in numerous locations throughout the body, most often in the gastrointestinal tract or subcutaneous soft tissue, but are relatively uncommon in the neck. This article describes a case of CFT which presented as an enlarging neck mass in a young male.
Keywords: Calcifying fibrous tumor, Calcifying fibrous pseudotumor, Psammomatoid calcifications, Dystrophic calcifications, Neck mass
History
A 19 year old otherwise healthy male presented with a history of acute onset left neck pain with subsequent swelling and development of a left neck mass that progressively enlarged over a two month period. The patient also reported intermittent vocal fatigue, mild dysphagia, shortness of breath and a 20 lb weight loss in association with the onset of the neck mass. An initial series of fine needle aspiration and core needle biopsies were performed but were non-diagnostic.
Radiographic Features
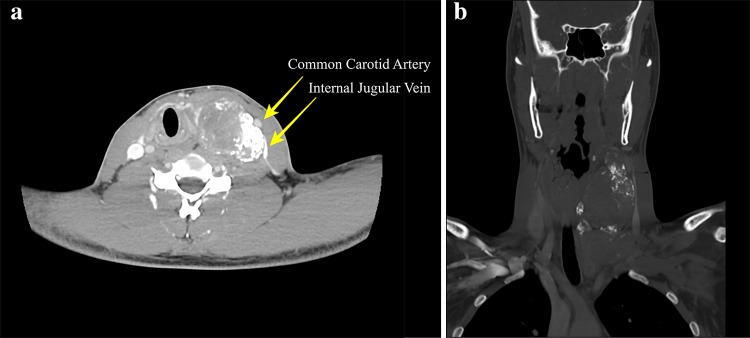
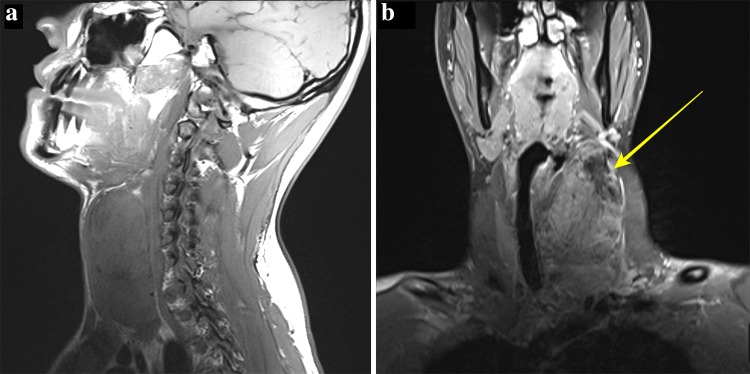
Imaging revealed a large, solid heterogeneous mass at the left neck measuring 4.5 × 5.7 × 9.8 cm. Contrast enhanced computerized tomography (CT) revealed a heterogeneously enhancing, circumscribed mass (Fig. 1a) with prominent eccentric calcifications visualized on bone window (Fig. 1b). The lesion displaced the left common carotid artery and internal jugular vein laterally but without splaying of the vasculature (Fig. 1a). Additionally, due to the mass effect, the laryngotracheal complex was shifted laterally. On Magnetic resonance imaging (MRI) the mass was also seen to be heterogeneous, with only mild enhancement (Fig. 2a). The superior portion was hypointense with a rim of enhancement, suggesting prior biopsy site or necrosis. Based on the CT and MRI findings a sympathetic trunk Schwannoma was favored with ganglioneuroma and malignant peripheral nerve sheath tumor considered less likely. Based on the absence of intense enhancement and lack of splaying of the carotid sheath vessels, a carotid body tumor was not favored.
Fig. 1.
Computer Tomography a Axial soft tissue window CT with contrast demonstrated a heterogeneous mass with numerous internal calcifications. The mass displaces the vasculature laterally indicating a carotid space occupying mass. b Coronal CT with bone windowing accentuates the diffuse calcifications present in this large carotid space tumor
Fig. 2.
Magnetic Resonance Imaging a Sagittal T1 sequence demonstrates a well-circumscribed heterogeneously isointense mass in the left neck with area of hypointensity superiorly. b Coronal T1 fat-saturated post-contrast sequence demonstrate mild enhancement with a hypointense area superiorly likely from necrosis or prior biopsy (arrow)
Diagnosis and Treatment
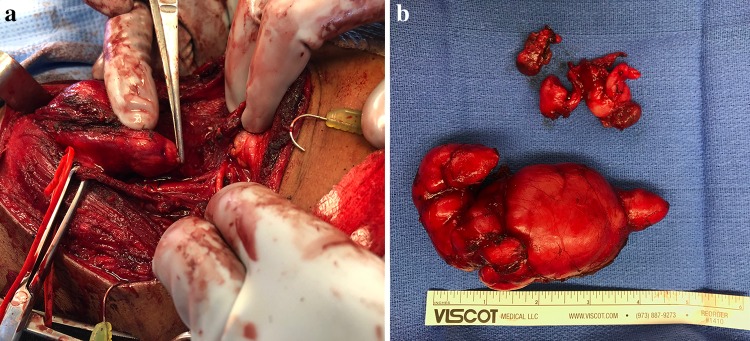
The initial FNA and core biopsies had provided non-diagnostic tissue samples and the patient then underwent transcervical resection of the mass. It was noted operatively to be within the carotid sheath and encompassed the sympathetic trunk with the common carotid artery overlying the mass. Grossly, the submitted tissue consisted of an 11.0 cm firm, multinodular mass (Fig. 3b). Microscopic examination revealed a sclerotic, hyalinized fibrous tumor with numerous psammomatoid and dystrophic calcifications. Distributed throughout the tissue was a lymphoplasmacytic chronic inflammatory infiltrate. There were occasional neurovascular bundles entrapped in the hyalinized fibrous tissue. Immunohistochemical staining was non-specific, with SMA and CD34 highlighting vascular elements, along with weakly CD34 positive lesional spindle cells. Immunohistochemical stains for desmin, ALK-1, EMA, AE1/AE3, S100, and EBER-ISH were all negative. The inflammatory infiltrate included a mixed population of CD3 and CD20 positive lymphocytes along with numerous CD138 positive plasma cells. Kappa and Lambda stains showed no light chain restriction. Based on the finding of a fibrosclerosing process with chronic inflammation and plasma cells, IgG and IgG4 immunostains were performed. An IgG:IgG4 ratio of 20–30% of the plasma cells was observed (Fig. 4). Based on the lesion’s presentation and initial consideration of a carotid body tumor, additional stains for CD56, chromogranin, neuron specific enolase and synaptophysin were performed and were negative. The microscopic and immunophenotypic features were characteristic of calcifying fibrous tumor (CFT).
Fig. 3.
a Intraoperative photograph showing the close proximity to adjacent structures. b Gross image of tumor following resection demonstrates a well-circumscribed, multinodular mass
Fig. 4.
a 100× magnification of CFT showing hyalinized fibrous tissue with variably sized calcifications and a background of chronic inflammatory cells. b 200× magnification of CFT with hyalinized fibrous tissue with calcifications, some with a laminated appearance, and scattered plasma cells
Discussion
CFT is a rare tumor with a distinctive histological presentation. Initially referred to as childhood fibrous tumor with psammoma bodies, the name calcifying fibrous pseudotumor was proposed in 1993 as additional cases revealed less of a predilection for children [1]. Current WHO classification uses the term “calcifying fibrous tumor” with “tumor” chosen to relay the rare but potential risk of recurrence [2].
The CFT was originally described as a subcutaneous soft tissue entity but has since been reported in a wide variety of locations including the gastrointestinal tract, pleura, abdominal cavity, and neck [2–4]. Some studies have shown that CFTs predominantly occur in the gastrointestinal tract, with the stomach being the primary location [4]. CFTs in the gastrointestinal tract differ from their soft tissue counterparts based on prominent lymphoid cuffs and increased perivascular lymphocytes relative to the soft tissue lesions [5]. CFTs are benign and appropriately treated with local resection. The risk of recurrence is small and there have been no reported deaths [2, 4]. Most lesions are found incidentally on physical exam but tumor growth may cause patients to present with mass effect or other site specific symptoms [2, 6]. Most CFTs are solitary lesions, however, there have been reports of multifocal tumors. In some studies, multifocality has been observed in up to 10% of cases and a case of two siblings with multiple CFTs has been described. This has led to speculation on a potential genetic susceptibility or environmental cause [3]. The median age at time of diagnosis was 33.58 years with CFTs being identified across all age ranges [4]. Additionally, depending on the study, CFTs either do not appear to show a gender predilection [7] or demonstrate a slight female predominance [4].
Imaging studies of CFTs reflect their histological composition well, characteristically revealing a well circumscribed mass containing scattered calcifications [4, 6]. Plain film radiography provides basic information on the anatomic location but offer little information on composition of the lesion [4]. A clearly circumscribed mass can be discerned on ultrasound [6], but ultrasound studies are limited due to acoustic shadowing from prominent calcifications and varying levels of fibrosis causing differences in echogenicity [8]. More advanced techniques such as CT and MRI provide much greater detail and are essential in surgical planning. Specifically, CT is better at detecting areas with early mineralization that may be missed on other imaging modalities [4] and can demonstrate the clear line of cleavage between the mass and the adjacent tissues characteristic of CFTs [6]. CFTs on MRI typically demonstrate a signal strength on T1 equal to that of surrounding muscle mixed with areas of lower T1 signal, while T2-weighted images show areas of low signal intensity due to extensive fibrosis [9]. CT can be an important modality to rule out other entities when a patient presents with an enlarging neck mass because of its ability to precisely locate the mass which is key to determining possible etiologies. Ultrasound may be indicated to determine a cystic versus solid process, as well as vascular flow characteristics, but CT or MRI are recommended prior to resection [6]. CT and MRI provide a three dimensional reconstruction of the lesion and better elucidate the relationship to surrounding structure which is important in surgical planning [6]. However, while imaging can play an important role in diagnosis of many lesions, the final diagnosis of CFT is dependent on microscopic examination of tissue.
Histologically, CFTs are usually well-circumscribed but unencapsulated, paucicellular lesions composed of hyalinized fibrous tissue and variable numbers of both psammomatous and dystrophic calcifications distributed throughout the lesion. Associated chronic lymphoplasmacytic inflammation is a consistent finding. Adjacent normal structures such as adipose tissue and neurovascular bundles can become trapped in the lesion [1, 2]. Lesional cells have been found to express Factor XIIIa and vimentin but lack staining for actin, desmin, S100, and cytokeratins [2]. There have been inconsistent results with regards to CD34 expression and the current understanding is that CD34 is variably immunoreactive in CFT [5, 6].
Some cases of CFT have shown an increased IgG:IgG4 ratio in the plasma cell population. One study found a ratio of 56% for IgG:IgG4 plasma cells [10]. This result is higher than the 40% required to consider immunoglobulin G4-related disease (IgG4-RD) and a potential association between IgG4-RD and CFT has been postulated [11]. However, not all cases of CFT have shown an increased IgG: IgG4 ratio in the plasma cells and serum IgG levels are not always available for comparison. Further studies are required to deduce the relationship between IgG4-RD and CFTs. It had been previously theorized that CFT represented a late stage of inflammatory myofibroblastic tumor (IMT) or occurred in association with IMTs [2–5]. While there is some histologic overlap, the WHO classifies CFT as a distinct entity and there are enough histologic and immunophenotypic differences to conclude that there is no evidence of an association between the two entities [2, 12].
In conclusion, CFT is an uncommon benign soft tissue lesion with distinctive histopathologic appearance. Characteristic findings include circumscription, paucicellular, hyalinized fibrous tissue, cytologically bland spindle cells, variable numbers of psammomatous and/or dystrophic calcifications and a chronic lymphoplasmacytic inflammatory infiltrate. Imaging will demonstrate a well circumscribed mass with scattered calcifications, however, while imaging is necessary in preparation for surgical removal, a definitive diagnosis depends on histologic tissue examination. CFT is managed by simple surgical removal and has an excellent survival rate. The risk of local recurrence is low and mostly likely due to incomplete surgical removal [7]. Long term survival is 100% with no reported deaths caused by CFT [4].
Compliance with Ethical Standards
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of retrospective case report, formal consent is not required. The tumor tissue included in the manuscript was obtained as part of the standard of care for the patient and retrospectively collected for the case report.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Army, Air Force, Department of Defense nor the U.S. Government.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kerry B. Baumann, Email: Kerry.b.baumann.mil@mail.mil
Michael I. Orestes, Email: michael.i.orestes.mil@mail.mil
Sarah M. Heaton, Email: sarah.m.heaton4.mil@mail.mil
Ryan E. Whiting, Email: ryan.e.whiting.mil@mail.mil
Nena C. Wendzel, Email: nena.c.wendzel.mil@mail.mil
Robert D. Foss, Email: robert.d.foss.ctr@mail.mil
References
- 1.Fetsch JF. Calcifying fibrous pseudotumor. Am J Surg Pathol. 1993;17(5):502–508. doi: 10.1097/00000478-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery E. Calcifying fibrous tumour. In: Fletcher CDM, Unni KK, Martens F, editors. Pathology & genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. pp. 77–78. [Google Scholar]
- 3.Chen KTK. Familial peritoneal multifocal calcifying fibrous tumor. Am J Clin Pathol. 2003;119:811–815. doi: 10.1309/MXC6TWELUUH420W0. [DOI] [PubMed] [Google Scholar]
- 4.Chorti A, Papavramidis TS, Michalopoulos A. Calcifying fibrous tumor, review of 157 patients reported in International Literature. Medicine. 2016;95(20):1–12. doi: 10.1097/MD.0000000000003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pezhouh MK, Rezaei MK, Shabihkhani M, Ghosh A, Belchis D, Montgomery EA, Voltaggio L. Clinicopathologic study of calcifying fibrous tumor of the gastrointestinal tract: a case series. Hum Pathol. 2017;62:199–205. doi: 10.1016/j.humpath.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Cao KX, Rosenberg AE, Hakim J, Masiakos PT. Axillary calcifying fibrous tumor (CFT) in an 8 year old girl. J Pediatr Surg. 2012;47:2341–2344. doi: 10.1016/j.jpedsurg.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Bell DM, Dekmezian RH, Husain SA, Luna MA. Oral calcifying fibrous pseudotumor: case analysis and review. Head Neck Pathol. 2008;2:343–347. doi: 10.1007/s12105-008-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jo BJ, Yoon SW, Ahn HJ, Kwon SW. Imaging findings of calcifying fibrous tumour of the liver. Br J Radiol. 2011;84:e31–e34. doi: 10.1259/bjr/30585776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinohara N, Nagano S, Yokouchi M, Arishama Y, Tabata K, Higashi M, Kitajima S, Yonezawa S, Komiya S. Bilobular calcifying fibrous pseudotumor in soleus muscle: a case report. J Med Case Rep. 2011;5:1–4. doi: 10.1186/1752-1947-5-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson BK, Balzer B, Goldwasser J, Dhall D. Calcifying fibrous tumor: an unrecognized IgG4-related. Acta Pathologica Microbiologica et Immunologica Scandinavica. 2014;123:72–76. doi: 10.1111/apm.12302. [DOI] [PubMed] [Google Scholar]
- 11.Kuo T, Chen T, Lee L. Sclerosing angiomatoid nodular transformation of the spleen (sant): clinicopathological study of 10 cases with or without abdominal disseminated calcifying fibrous tumors, and the presence of a significant number of IgG4+ plasma cells. Pathol Int. 2009;59:844–850. doi: 10.1111/j.1440-1827.2009.02456.x. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento A, Ruiz R, Hornick JL, Fletcher CD. Calcifying fibrous ‘Pseudotumor” clinicopathologic study of 15 cases and analysis of relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol. 2002;10(3):189–196. doi: 10.1177/106689690201000304. [DOI] [PubMed] [Google Scholar]