Abstract
The classification of sinonasal adenocarcinoma (SNAC) is complex. The high-grade, non-intestinal SNAC group is particularly heterogeneous, with tumors showing widely variable morphology. SMARCB1 (INI-1)-deficient sinonasal carcinoma is a newly described, aggressive tumor that usually resembles sinonasal undifferentiated carcinoma (SNUC) or non-keratinizing squamous cell carcinoma; however, glandular differentiation has been rarely reported and this feature may be under-recognized. We present a dedicated series of 12 SMARCB1-deficient SNACs. All tumors had an oncocytoid/plasmacytoid cytomorphology with variable degrees of glandular differentiation consisting of tubules and cribriform structures with foci of intracellular or intraluminal mucin. Three of 12 tumors exhibited foci of yolk sac tumor-like histologic features. The tumors were uniformly high-grade, with nuclear pleomorphism, elevated mitotic rates and frequent necrosis. By immunohistochemistry, all tumors were entirely SMARCB1-deficient, and 10 of 12 were CK7-positive. Occasional expression of CDX2 (4 of 12), CK20 (3 of 12), and p40 (3 of 10) was seen. Expression of yolk sac markers was variably present in tumors that harbored yolk sac-like areas but also tumors that did not: glypican-3 (10 of 11), SALL4 (6 of 11), HepPar-1 (4 of 11), PLAP (1 of 10), and AFP (1 of 11). SMARCB1-deficient sinonasal carcinoma, particularly the oncocytoid/plasmacytoid form, can demonstrate variable degrees of glandular differentiation. This unexpected morphology combined with variable immunohistochemical results may lead to misdiagnoses of high-grade intestinal or non-intestinal SNAC, myoepithelial carcinoma, or even yolk sac tumor or metastatic hepatocellular carcinoma.
Keywords: INI-1, SMARCB1, Adenocarcinoma, Sinonasal, SMARCB1-deficient sinonasal carcinoma, Yolk sac tumor
Introduction
The classification of sinonasal adenocarcinoma (SNAC) is complex. In addition to salivary-type SNACs which are analogous to their major salivary gland counterparts, there are also so-called surface-type adenocarcinomas which are further classified by grade and the presence or absence of intestinal differentiation [1.] The high-grade non-intestinal SNAC group is particularly heterogeneous, with tumors showing tremendously variable morphology [2, 3.] The classification of this group, similar to sinonasal undifferentiated carcinoma, is undergoing refinement as tumors with distinct immunohistochemical and molecular signatures are gradually being recognized. For example, it has been demonstrated that a subset of sinonasal adenocarcinomas are not truly surface derived but rather have a seromucinous gland phenotype [4] and there are rare case reports of SNAC that are human papillomavirus (HPV)-related [3.]
SMARCB1 (INI-1)-deficient sinonasal carcinoma is a newly described, aggressive tumor that was first reported in 2014 independently by two groups [5, 6.] Since then, additional case reports and case series have been published which confirms this as distinct tumor entity [7–11.] Most tumors demonstrate either a basaloid “blue cell” tumor morphology reminiscent of sinonasal undifferentiated carcinoma or non-keratinizing squamous cell carcinoma, but others have a more oncocytoid/plasmacytoid or “pink cell” tumor morphology [5, 6, 8, 10.] There are also rare reported cases of SMARCB1-deficient carcinomas with glandular differentiation, sarcomatoid change and even yolk sac differentiation [8, 11.] Herein we present a dedicated series of SMARCB1-deficient SNACs.
Methods
All cases of SMARCB1-deficient SNACs were retrieved and reviewed from the authors’ files. A total of 12 cases demonstrated clear-cut morphologic evidence of glandular differentiation and were included in this study. Five of these cases have been previously reported [5, 8, 10.] Immunohistochemistry for SMARCB1, CK7, CK20, S100, p40, CDX2, glypican-3, SALL4, HepPar-1, PLAP, and AFP was performed. As these cases were sourced from multiple institutions, immunohistochemical protocols were not standardized for all cases. Patient demographic data as well as histologic and immunohistochemical features were recorded.
Results
Twelve cases of SMARCB1-deficient SNAC were found, which are summarized in Table 1. They occurred in 10 men and 2 women ranging from 21 to 82 years (mean, 57 years) and arose in the nasal cavity (n = 6), maxillary sinus (n = 1), ethmoid sinus [1] or multiple sinonasal tract subsites (n = 4). Five cases were prospectively identified as SMARCB1-deficient carcinoma, while 5 were originally diagnosed as high-grade non-intestinal adenocarcinoma, 1 as clear cell carcinoma and 1 as sinonasal undifferentiated carcinoma.
Table 1.
Clinical and pathologic characteristics of SMARCB1-deficient adenocarcinomas
| Case | Age | Sex | Location | Glandular morphology (% glandular component) |
Non-glandular morphology | Original diagnosis | Reference if previously published |
|---|---|---|---|---|---|---|---|
| 1 | 71 | F | Maxillary sinus | Oncocytoid, myxoid, yolk sac-like (30%) | Oncocytoid, basaloid, spindle cell | High-grade non-intestinal adenocarcinoma | [6, 10] |
| 2 | 51 | M | Nasal cavity | Oncocytoid, myxoid (5%) | Oncocytoid | SMARCB1-deficient carcinoma | [10] |
| 3 | 79 | F | Nasal cavity | Oncocytoid, cribriform (100%) | N/A | SMARCB1-deficient carcinoma | [10] |
| 4 | 58 | M | Frontal sinus | Oncocytoid, myxoid, yolk sac-like (30%) | Oncocytoid | SMARCB1-deficient carcinoma | N/A |
| 5 | 82 | M | Maxillary and ethmoid sinuses | Oncocytoid, myxoid (50) | Oncocytoid | SMARCB1-deficient carcinoma | N/A |
| 6 | 40 | M | Nasal cavity | Oncocytoid, cribriform, tubuloglandular (75%) | Oncocytoid | High-grade non-intestinal adenocarcinoma | [14] |
| 7 | 50 | M | Nasal cavity | Oncocytoid, cribriform, tubular (50%) | Oncocytoid | High-grade non-intestinal adenocarcinoma | [14] |
| 8 | 39 | M | Nasal cavity, maxillary and frontal sinuses | Oncocytoid, cribriform (50) | Oncocytoid | High-grade non-intestinal adenocarcinoma | N/A |
| 9 | 64 | M | Nasal cavity | Oncocytoid, myxoid (100%) | N/A | Sinonasal undifferentiated carcinoma | N/A |
| 10 | 58 | M | Ethmoid sinus | Clear cell, yolk sac-like, focal oncocytoid (90%) | Oncocytoid, clear cell | Clear cell carcinoma | N/A |
| 11 | 71 | M | Nasal cavity, ethmoid sinus | Oncocytoid, tubular, myxoid, focal signet ring cells (70%) | Oncocytoid | High-grade non-intestinal adenocarcinoma | N/A |
| 12 | 21 | M | Nasal cavity | Oncocytoid, cribriform (70%) | Oncocytoid, squamoid | SMARCB1-deficient carcinoma | N/A |
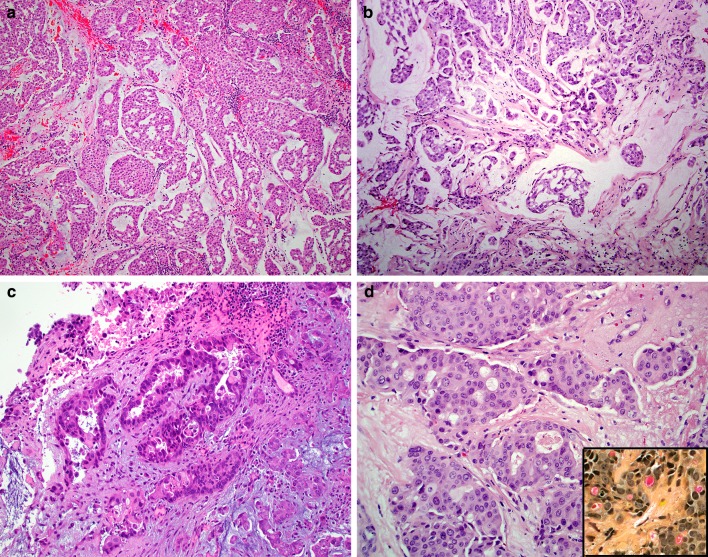
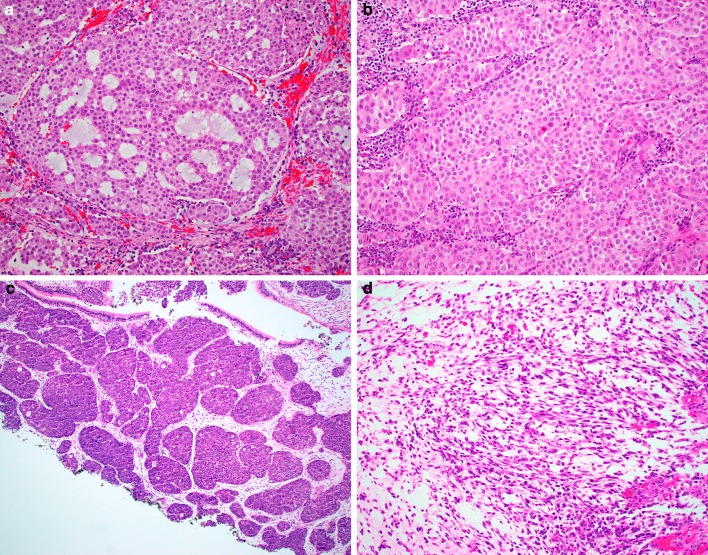
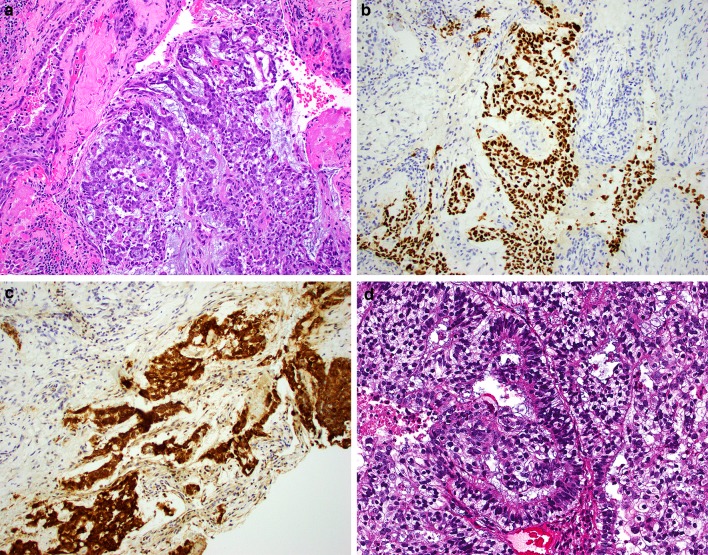
Histologically, the extent of glandular differentiation in the SMARCB1-deficient adenocarcinomas ranged from 5 to 100% (mean 58%) of tumor volume, consisting of cribriform structures and tubules with foci of intracellular and/or intraluminal mucin (Fig. 1a–d). Myxoid stromal changes were common, seen in 7 of 12 cases. All tumors demonstrated prominent oncocytoid/plasmacytoid cytomorphology in both the glandular and non-glandular areas (Fig. 2a–b). Uncommon features included foci of basaloid (n = 1), spindled (n = 1), squamoid (n = 1) or clear cell (n = 1) morphology (Fig. 2c–d). Three cases demonstrated focal but overt yolk sac tumor-like histologic features with a microcystic/reticular pattern in a highly myxoid stromal background (Fig. 3). No cases had a component of a better-differentiated carcinoma, and no other germ cell tumor-like patterns were identified. The tumors were uniformly high-grade, with all cases exhibiting nuclear pleomorphism, elevated mitotic rates, and tumor necrosis.
Fig. 1.
The SMARCB1-deficient sinonasal adenocarcinomas were made up of oncocytoid/plasmacytoid tumor cells in rounded nests with cribriform architecture and myxoid stromal changes (a–b). Overt glandular differentiation was seen in the form of punched-out luminal spaces (c–d). A mucicarmine stains confirms the presence of intracytoplasmic mucin (inset)
Fig. 2.
Oncocytoid/plasmacytoid cytomorphology was seen in all cases not only in the glandular tumor component (a), but also the non-glandular areas (b) of the SMARCB1- deficient sinonasal adenocarcinomas. Uncommon features included basaloid (c) and spindle cell (d) patterns, each seen in one case
Fig. 3.
Three of the SMARCB1-deficient sinonasal adenocarcinomas exhibited yolk sac tumor-like morphology, here in the form of microcystic and reticular growth patterns in a myxoid stroma (a). These tumors were strongly positive for yolk sac markers like SALL4 (b) and glypican-3 (c). One tumor was reminiscent of the secretory endometrium-like pattern of yolk sac tumor, and a structure resembling a Schiller-Duvall body was identified (d)
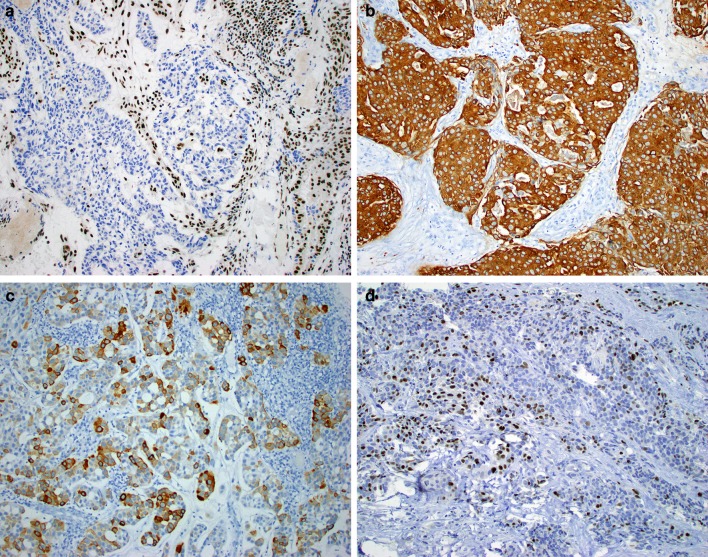
The immunohistochemical staining patterns are summarized in Table 2. By immunohistochemistry, all tumors were entirely SMARCB1-deficient, by definition (Fig. 4a). Ten of 12 were CK7-positive (Fig. 4b). Occasional expression of CK20 (3 of 12) (Fig. 4c), CDX2 (4 of 12), and p40 (3 of 10) (Fig. 4d) was seen. All 11 cases tested were negative for S100. Variable expression of yolk sac immunohistochemical markers was seen, including glypican-3 (10 of 11), SALL4 (6 of 11), HepPar1 (4 of 11), PLAP (1 of 10), and AFP (1 of 11). Interestingly, while the expression of these markers was strongly present in the three cases exhibiting yolk sac-like morphology (Fig. 2b–c), it was also seen in some cases that lacked clear yolk sac-like histologic features.
Table 2.
Immunohistochemical profile of the glandular component of SMARCB1-deficient sinonasal adenocarcinomas
| Case | SMARCB1 | CK7 | CK20 | CDX2 | p40 | S100 | AFP | Glypican-3 | PLAP | SALL4 | HepPar-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lost | F+ | – | – | – | – | – | + | F+ | + | – |
| 2 | Lost | F+ | – | – | – | – | – | – | – | – | – |
| 3 | Lost | + | – | – | – | – | ND | ND | ND | ND | ND |
| 4 | Lost | – | – | – | – | – | F+ | + | ND | + | – |
| 5 | Lost | + | + | – | ND | – | – | F+ | – | + | – |
| 6 | Lost | + | – | – | – | – | – | F+ | – | – | – |
| 7 | Lost | + | – | – | ND | ND | – | F+ | – | – | – |
| 8 | Lost | + | F+ | – | – | – | – | F+ | – | – | + |
| 9 | Lost | F+ | – | + | F+ | – | – | F+ | – | F+ | – |
| 10 | Lost | – | – | F+ | – | – | – | + | – | + | F+ |
| 11 | Lost | + | – | F+ | F+ | – | – | + | – | – | + |
| 12 | Lost | + | F+ | ND | F+ | – | ND | ND | ND | ND | ND |
ND not done, F+ focally positive
Fig. 4.
By immunohistochemistry, the SMARCB1-deficient sinonasal adenocarcinomas were consistently negative for SMARCB1 (12 of 12 cases) with retained expression in benign lymphocytes and stromal cells (a), and positive for CK7 (11 of 12 cases) (b), but were otherwise highly variable, with occasional expression of CK20 (c), p40 (d), and other markers
Discussion
SMARCB1-deficient sinonasal carcinoma is a distinct tumor entity that has characteristic histologic appearance albeit with some variability. The predominant morphology is that of a basaloid “blue cell” tumor; as such the majority of cases reported in the literature were previously diagnosed as sinonasal undifferentiated carcinoma [7, 8, 10.] This basaloid morphology can also have an exophytic surface component with downward growth into the mucosal glands mimicking a non-keratinizing squamous cell carcinoma, basaloid squamous cell carcinoma and even carcinoma-ex-Schneiderian papilloma [8.] The second most common morphologic pattern, however, is the oncocytic/plasmacytoid (rhabdoid) or “pink cell” tumor, in which the tumor cells have abundant eosinophilic cytoplasm and eccentrically placed nuclei [8.] This “pink cell” pattern is more reminiscent of other tumors in the family of SMARCB1-deficient malignancies such as rhabdoid tumor of kidney/soft tissue or epithelioid sarcoma.
As demonstrated in this study, SMARCB1-deficient carcinomas with this oncocytic/plasmacytoid pattern can have foci of glandular differentiation with overt tubule formation, cribriform areas as well as intraluminal and intracellular mucin. The extent of the glandular differentiation is highly variable, with some cases showing very focal areas whereas in other cases it is the predominating pattern. Myxoid stromal alterations were also a common finding in this group. Five of 12 of our cases were prospectively diagnosed, whereas the other seven were found through retrospective review; these cases were previously classified as a high-grade non-intestinal adenocarcinoma, clear cell carcinoma, or sinonasal undifferentiated carcinoma. Similar to sinonasal undifferentiated carcinoma which is considered a heterogeneous group of tumors and a diagnosis of exclusion, the high-grade non-intestinal adenocarcinoma category is also composed of tumors with a variety of morphologies, and both categories are undergoing refinement with separation of distinct diagnostic entities that are defined by more specific morphologic and immunohistochemical profile and reproducible molecular alterations [3, 7, 12.]
In their large series of sinonasal high-grade non-intestinal adenocarcinomas, Stelow et al. reported a variety of morphologic patterns including blastomatous, apocrine, oncocytic/mucinous and poorly differentiated/undifferentiated [3.] Their histologic description of the oncocytic/mucinous pattern is reminiscent of the SMARCB1-deficient adenocarcinomas in our series, though these reported cases reportedly arose in association with oncocytic Schneiderian papillomas, an association we did not find [3.] The importance of separation of these new tumor types such as the SMARCB1-deficient SNAC from the high-grade non-intestinal adenocarcinoma group is important for further characterizing the morphologic spectrum, better predicting biologic behavior, and most importantly offering the opportunity for use of targeted therapies as they are developed [12.] SMARCB1-deficient sinonasal carcinomas in general appear to be aggressive neoplasms, with frequent local invasion into the brain and/or skull base. The majority of patients have died of disease (mean, 15 months) [5–7.] On the other hand, there are currently phase II clinical trials underway for both adults and pediatric patients with SMARCB1-negative tumors using an oral EZH2 inhibitor (https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/tazemetostat). Correctly identifying all forms of SMARCB1-deficient sinonasal carcinoma will potentially allow patients to benefit from such targeted therapies.
While the oncocytoid/plasmacytoid morphology and glandular differentiation seen in SMARCB1-deficient SNAC is distinct, there can be focal expression of both CK20 and CDX2 in these tumors, creating a potential diagnostic pitfall with sinonasal intestinal-type adenocarcinomas. However, in general, sinonasal intestinal-type adenocarcinomas will have more diffuse expression of CDX-2 and CK20 and morphology that tends to parallel both normal and neoplastic intestine [1.] Nonetheless, when considering an adenocarcinoma in the sinonasal tract it may be helpful to employ SMARCB1 immunohistochemistry in order to identify SMARCB1-deficient adenocarcinomas, especially as the full spectrum of morphology continues to be unraveled.
Recently, Zamecnik et al. reported a single case of SMARCB1-deficient sinonasal carcinoma with yolk sac differentiation [11.] This case had both basaloid and oncocytic/plasmacytoid patterns with transitions to areas that were described to resemble the microcystic/reticular type of yolk sac tumor [11.] There was also immunohistochemical expression of AFP, glypican-3 and CDX-2, limited to these areas of yolk sac-like morphology [11.] Our series further explores the yolk sac-like morphology and immunohistochemical phenotype. Three of our cases had focal areas that resemble the reticular/microcystic pattern of a yolk sac tumor, and arguably the oncocytoid pattern seen in all our cases is highly reminiscent of the hepatoid pattern that has been described in yolk sac tumors [13.] Indeed, the expression of glypican-3, a marker of both yolk sac and hepatic phenotype, was present in almost all of our cases. Some of our cases also demonstrated expression of SALL4, whereas PLAP and AFP were rare, occurring in only one case each. The rhabdoid cells seen in SMARCB1-deficient carcinomas which are characterized by eccentric nuclei and pale-eosinophilic perinuclear inclusions are somewhat reminiscent of the PAS-positive hyaline globules that are seen with yolk sac tumors. Despite these immunohistochemical and morphologic similarities, gonadal yolk sac tumors have retained expression of SMARCB1 [13] and extragonadal yolk sac tumors are exceptionally rare in the sinonasal tract [14–16.] Since the rare case reports of sinonasal yolk sac tumors were all reported prior to the recognition of SMARCB1-deficient carcinomas, it is actually quite plausible that some or all of these cases may represent SMARCB1-deficient SNACs. If the diagnosis of an extragonadal yolk sac tumor is being considered in the sinonasal tract, it would be prudent to perform immunohistochemistry for SMARCB1. Metastatic hepatocellular carcinoma also enters the differential for SMARCB1-deficient SNAC, but the patient’s history combined with the status of SMARCB1 will easily resolve this differential diagnosis.
The myxoid stromal changes of SMARCB1-deficient sinonasal adenocarcinomas together with cribriform/epithelial cell morphology also may raise consideration for a myoepithelial carcinoma. SMARCB1 loss has been documented in subset of soft tissue myoepithelial carcinomas but has not been fully studied in myoepithelial carcinomas of the salivary gland. S100 may be helpful in resolving this differential diagnosis as all eleven of our tested cases were negative for S100.
In summary, SMARCB1-deficient sinonasal carcinoma are histologically defined by either a basaloid or oncocytoid/plasmacytoid pattern, but the morphologic spectrum continues to expand as additional cases are identified. SMARCB1-deficient sinonasal carcinoma, particularly the oncocytoid/plasmacytoid form, can demonstrate varying degrees of glandular differentiation. This unexpected morphology combined with variable immunohistochemical results may lead to misdiagnoses of high-grade intestinal or non-intestinal SNAC, myoepithelial carcinoma, or even yolk sac tumor or metastatic hepatocellular carcinoma. In this limited series, it appears that SMARCB1-deficient carcinomas with glandular differentiation show a predilection for male patients, and in contrast to non-glandular tumors, may occur with greater frequency in the nasal cavity.
Funding
This study was partially funded by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center.
Compliance with Ethical Standards
Conflict of interest
Authors have no conflicts of interest or disclosures as it pertains to this manuscript.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standard.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leivo I. Sinonasal adenocarcinoma: update on classification, immunophenotype and molecular features. Head Neck Pathol. 2016;10:68–74. doi: 10.1007/s12105-016-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stelow EB, Mills SE, Jo VY, et al. Adenocarcinoma of the upper aerodigestive tract. Adv Anat Pathol. 2010;17:262–269. doi: 10.1097/PAP.0b013e3181e3bf80. [DOI] [PubMed] [Google Scholar]
- 3.Stelow EB, Jo VY, Mills SE, et al. A histologic and immunohistochemical study describing the diversity of tumors classified as sinonasal high-grade nonintestinal adenocarcinomas. Am J Surg Pathol. 2011;35:971–980. doi: 10.1097/PAS.0b013e31821cbd72. [DOI] [PubMed] [Google Scholar]
- 4.Purgina B, Bastaki JM, Duvvuri U, et al. a subset of sinonasal non-intestinal type adenocarcinomas are truly seromucinous adenocarcinomas: a morphologic and immunophenotypic assessment and description of a novel pitfall. Head Neck Pathol. 2015;9:436–446. doi: 10.1007/s12105-015-0615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agaimy A, Koch M, Lell M, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014;38:1274–1281. doi: 10.1097/PAS.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell D, Hanna EY, Agaimy A, et al. Reappraisal of sinonasal undifferentiated carcinoma: SMARCB1 (INI1)-deficient sinonasal carcinoma: a single-institution experience. Virchows Arch. 2015;467:649–656. doi: 10.1007/s00428-015-1853-1. [DOI] [PubMed] [Google Scholar]
- 8.Agaimy A, Hartmann A, Antonescu CR, et al. SMARCB1 (INI-1)-deficient sinonasal carcinoma: a series of 39 cases expanding the morphologic and clinicopathologic spectrum of a recently described entity. Am J Surg Pathol. 2017;41:458–471. doi: 10.1097/PAS.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasserman JK, Dickson BC, Perez-Ordonez B, et al. INI1 (SMARCB1)-deficient sinonasal carcinoma: a clinicopathologic report of 2 cases. Head Neck Pathol. 2017;11:256–261. doi: 10.1007/s12105-016-0752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakkar A, Antony VM, Pramanik R, et al. SMARCB1 (INI1)-deficient sinonasal carcinoma: a series of 13 cases with assessment of histologic patterns. Hum Pathol. 2019;83:59–67. doi: 10.1016/j.humpath.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Zamecnik M, Rychnovsky J, Syrovatka J. Sinonasal SMARCB1 (INI1) deficient carcinoma with yolk sac tumor differentiation: report of a case and comparison with INI1 expression in gonadal germ cell tumors. Int J Surg Pathol. 2018;26:245–249. doi: 10.1177/1066896917741549. [DOI] [PubMed] [Google Scholar]
- 12.Guilmette J, Sadow PM. High-grade sinonasal carcinoma: classification through molecular profiling. Arch Pathol Lab Med. 2019 doi: 10.5858/arpa.2018-0224-RS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prat J, Bhan AK, Dickersin GR, et al. Hepatoid yolk sac tumor of the ovary (endodermal sinus tumor with hepatoid differentiation): a light microscopic, ultrastructural and immunohistochemical study of seven cases. Cancer. 1982;50:2355–2368. doi: 10.1002/1097-0142(19821201)50:11<2355::AID-CNCR2820501122>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Mei X, Xia Y, Sasano H, et al. Sinonasal yolk sac (Endodermal sinus) tumor in an adult female—a case report and review of the literature. APMIS. 2015;123:810–814. doi: 10.1111/apm.12409. [DOI] [PubMed] [Google Scholar]
- 15.Filho BC, McHugh JB, Carrau RL, et al. Yolk sac tumor in the nasal cavity. Am J Otolaryngol. 2008;29:250–254. doi: 10.1016/j.amjoto.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Mishra A, El-Naggar AK, DeMonte F, et al. Endodermal sinus tumor of the paranasal sinuses. Head Neck. 2008;30:539–543. doi: 10.1002/hed.20711. [DOI] [PubMed] [Google Scholar]