Abstract
Abstract
Mesenchymal stem cells (MSCs) based therapies are a major field of regenerative medicine. However, the success of MSC therapy relies on the efficiency of its delivery and retention, differentiation, and secreting paracrine factors at the target sites. Recent studies show that superparamagnetic iron oxide nanoparticles (SPIONs) modulate the regenerative effects of MSCs. After interacting with the cell membrane of MSCs, SPIONs can enter the cells via the endocytic pathway. The physicochemical properties of nanoparticles, including size, surface charge (zeta-potential), and surface ligand, influence their interactions with MSC, such as cellular uptake, cytotoxicity, homing factors, and regenerative related factors (VEGF, TGF-β1). Therefore, in-depth knowledge of the physicochemical properties of SPIONs might be a promising lead in regenerative and anti-inflammation research using SPIONs mediated MSCs. In this review, recent research on SPIONs with MSCs and the various designs of SPIONs are examined and summarized.
Graphic abstract
A graphical abstract describes important parameters in the design of superparamagnetic iron oxide nanoparticles, affecting mesenchymal stem cells. These physicochemical properties are closely related to the mesenchymal stem cells to achieve improved cellular responses such as homing factors and cell uptake.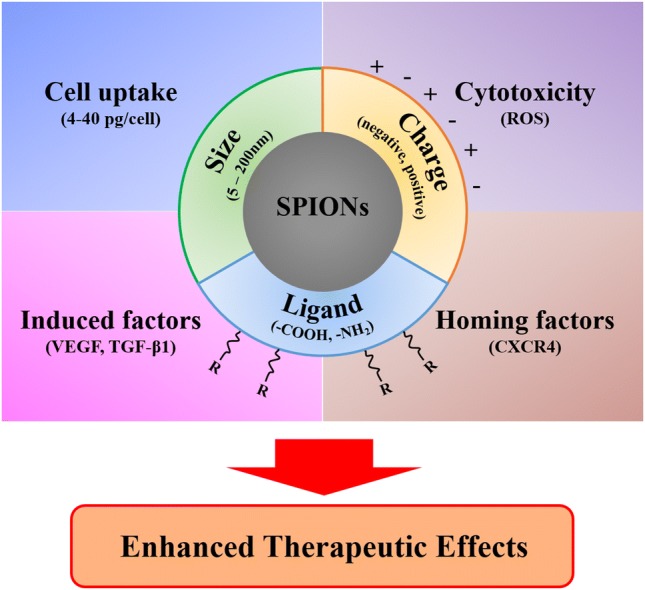
Keywords: Mesenchymal stem cells, Superparamagnetic iron oxide nanoparticles, Magnetic attraction, Physicochemical properties of nanoparticles, Inducing therapeutic factors
Introduction
Regenerative medicine is an expansive interdisciplinary science that aims to restore damaged or aging tissues by modeling their native morphology and functions [1–3]. During the last decades, regenerative medicine has developed tremendously and has led to a paradigm shift in stem cell biology. The discovery of mesenchymal stem cells (MSCs) from adult bone marrow, by Friedenstein [4] in 1974, had the appearance of fibroblasts, and has laid the new foundation of cell-based regenerative therapy.
MSCs are multipotent stromal cells that can differentiate into a variety of cell types, including osteoblasts, chondrocytes, myocytes, and adipocytes [5, 6]; and reside in bone marrow, cord cells, adipose tissue, molar cells, and amniotic fluid. MSCs can be readily expanded ex vivo for several passages without losing their self-renewal capacity [7, 8]. In addition to its multi-lineage differentiation capacity useful for regeneration, MSCs regulate immune and inflammatory responses by releasing paracrine factors and can alter their microenvironment. They stimulate tissue repair, communicate with other cells in the body, and migrate to the injury site [9–13]. For this reason, MSCs are one of the most used stem cells in regenerative cell-based therapies. However, the success of these regenerative therapies relies on several demanding factors, such as delivery and retention efficiency of MSCs and maintenance of therapeutic MSCs phenotype which have the ability to release anti-inflammation factors in the target organ [14].
The migration of MSCs to the injury sites, a process termed as “homing”, poses further difficulties to the MSC-based therapy [15]. For regenerative effects to be possible, successful homing of the MSCs to the diseased organ/tissue is required. Until now, even though many research groups have tried to unearth the mechanism of homing, it is not entirely understood. Various former studies have suggested chemokines and their receptors (e.g., SDF-1α and CXCR4) are essential factors that induce the homing of MSCs. The C-X-C chemokine receptor 4 (CXCR4) recognizes stromal-derived factor 1-alpha (SDF-1α) gradient, which occurs from injury sites and is expressed on the MSC membrane, and induce chemotaxis of MSCs [16]. Many factors can modulate the expression of CXCR4 receptors on MSCs. For example, cytokines such as interleukin (IL)-1 beta (IL-1β), and IL-6, can induce the expression of CXCR4 in cultured MSCs and improve the homing phenomenon in vitro and in vivo [17, 18]. Other strategies include hypoxia culture conditions. Hung et al. [19] demonstrated that short-term hypoxia conditions increased both RNA and protein expression of CX3CR1 and CXCR4 chemokine receptors; 24 h after which MSCs increased the homing phenomenon in response to the SDF-1α.
Superparamagnetic iron oxide nanoparticles (SPIONs) have been identified as a valuable tool to develop applications in medicine, including magnetic resonance imaging (MRI) [20], biosensor [21], cell separation [22], and drug delivery [23]. The SPIONs are biocompatible and stable and are degraded through the normal iron metabolism in the body [24, 25]. More recently, it was demonstrated that remote migration with external magnets (magnetic cell attraction) allow magnetically labeled cells to be retained at their site of implantation in vitro [26], in artificial tissues [27–29], in vivo [30] and meta-analysis [31]. Notably, these SPIONs conjugated to the surface of the stem cells and internalized into the cell. In summary, The SPIONs characterized by biocompatibility, and rapid response to an external magnetic field can be used as a key tool in the advanced biomedical applications including cell tracking in MRI, drug delivery, and magnetic cell attraction.
While the engineered magnetic nanoparticles allow specific cell targeting and internalization, the specific reaction could also induce cytotoxicity to the cell. Nano-cytotoxicity must, therefore, be carefully considered because certain features of nanoparticles might entail drawbacks or even dangers [32]. In previous studies, nanoparticle-induced toxicity was correlated with the level of cellular uptake, and the internalization process was deeply reliant on the physicochemical properties of the nanoparticles [33]. The size, stability, and coating of magnetic nanoparticles, as well as an excessive intracellular iron dose, can affect cell morphology, signaling process [34–36], and stem-cell differentiation [37, 38]. Interestingly, mild toxicity by SPIONs can increase the homing effect of MSCs. Huang et al. [39] reported that iron oxide nanoparticles induce overexpression of CXCR4 in MSCs, which affects the homing phenomenon of MSCs. In addition, other studies reported that the iron oxide nanoparticles that induce overexpression of CXCR4 can be used as drugs for enhanced regeneration [40, 41]. Therefore, as considering cellular physiology and iron oxide nanoparticle properties, the engineered iron oxide nanoparticles has become more important. This study aims to review the effects of SPIONs on MSCs for MRI tracking, magnetic attraction, enhanced chemotaxis, and releasing paracrine factors such as angiogenic, anti-apoptotic, and anti-inflammatory.
MSCs labeled with SPIONs for enhanced homing and regenerative effects
Tracking the path of stem cells via in vivo magnetic resonance imaging (MRI) is important to demonstrate the homing effect and to validate the regenerative effect noninvasively. In vivo tracking of the transplanted cells is necessary for better understanding their migration dynamics, differentiation processes, and regeneration potential, and to evaluate cell distribution and homing at target sites [42]. Numerous magnetic nanoparticles, such as iron oxide nanoparticles, have been considerably utilized as a tool for tracking stem cells. The iron oxide nanoparticles can provide high sensitivity and spatial 3D resolution in MRI. They can also highlight the labeled cells in their anatomical distribution, provide surrounding tissue information, and clinical applicability with nontoxicity and noninversion [43].
Superparamagnetic iron oxide nanoparticles (SPIONs) have demonstrated successful and efficient applications in medicine for biosensor, MRI, drug delivery for cancer, and cell separation due to low cytotoxicity, biocompatibility, and magnetic properties [20–22, 44]. SPIONs are, therefore, used to label stem cells to monitor cell fates in several disease models [45] (Table 1). Table 1 also summarizes the applicable dosages of magnetic nanoparticles for the magnetic functionalization of MSC without high cytotoxicity.
Table 1.
The experimental designs of mesenchymal stem cell-based therapy using SPIONs
| References | Purpose of study | Type of stem cells | Type of SPIONs | Animal | Target sites | Administration | Dose ([Fe] µg/mL) | Results |
|---|---|---|---|---|---|---|---|---|
| [46] | MRI tracking | hMSCs | Citrate-coated SPIONs, ferumoxide, and ferucarbotran | Rats | Muscle | Local injection | Citrate-coated SPIONs: 5 µg/mL, ferumoxide: 100 µg/mL, ferucarbotran: N/A | Greater MRI sensitivity of transplanted MSCs labelled with citrate SPIONs compared to Endorem (Feridex®) in low Fe concentration |
| [47] | MRI tracking | hUC-MSCs | SPIONs | Rats | Spinal cord | Local injection | 7 µg/mL | Noninvasive feasible MRI imaging of transplanted MSCs labelled with SPIONs in spinal cord until 3 weeks |
| [48] | MRI tracking | mMSCs | USPIONs with poly-l-lysine (PLL) | Mice | Brain | Intravenous injection | 50 µg/mL with PLL 1.5 µg/mL | Significant signal loss in T2-weighted images and effective decrease in transverse relaxation time at the injury site after intravenous injection because of transplanted MSCs labelled with USPIONs |
| [49] | MRI tracking | hMSCs | SPIONs loaded Poly(lactide-co-glycolide) MPs | Mice | Back | Local injection | 50 µg/mL | Enhanced MRI parameters with the relaxivity, residence time, and the R2 signal compared to free IO-NPs |
| [50] | Magnetic attraction | rMSCs | Poly-l-lysine (PLL) coated SPIONs | Rats | Spinal cord | Intrathecal injection | 154 µg/mL | Labelled transplanted MSCs were guided by a magnetic field near the lesion site in the rat spinal cord |
| [51] | Magnetic attraction | hNSCs | Ferumoxide with poly-l-lysine (PLL) | Rats | Brain | Intravenous injection | 25 µg/mL | Enhanced efficient delivery of transplanted NSCs to the target sites with magnetic guidance |
| [26] | Magnetic attraction, enhanced chemotaxis | mMSCs | SPIONs with rhodamine B | Mice | Olfactory bulb | Local injection | 15 µg/mL | Enhanced homing effects of MSCs in vivo with increased CXCR4 expression and permanent magnet guidance induced by iron oxide nanoparticles |
| [39] | Enhanced chemotaxis | mMSCs | Zinc-doped iron oxide nanoparticle with hyaluronic acid (HA) | Mice | Brain | Local injection | 6.03 µg/mL (108 µM) | Improved the homing effects to traumatic brain injury and glioblastoma models due to increased CXCR4 expression in MSCs by iron-based nanoparticles |
| [52] | Enhanced chemotaxis | rMSCs | Iron oxide nanoparticles with polydopamine (PDA) | Rats | Ear | Intravenous injection | 50 µg/mL | Increased expression of CXCR4, c-Met, and CCR1 membrane protein and cytokine (VEGF) by iron oxide nanoparticles with PDA |
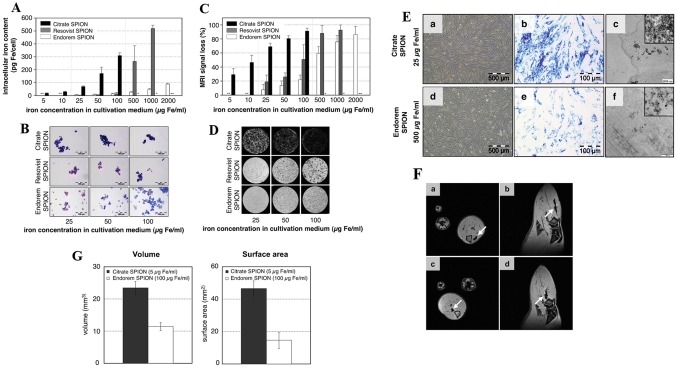
The distribution and migratory properties of transplanted MSCs are helpful in determining the metrics of homing efficiency. However, one of the major limitations is the presence of very low amounts (below 3%) of MSCs in the target organs after intravenous (IV) injection [53]. Developing engineered SPIONs are, therefore, requisite because unmodified bare SPIONs does not label stem cells efficiently. For example, Andreas et al. [47] made SPIONs with citrate for better labeling efficiency, and their in vivo MRI results suggested superior visualization comparing to commercial SPIONs (Feridex®, Resovist®) (Fig. 1).
Fig. 1.
Analysis of the intracellular iron content in cultivation medium using a ICP–OES, prussian blue staining b, and visualization with MRI in vitro c, d. e Intracellular Imaging of SPIONs after incubation with Citrate and Endoderm SPION using microscope, Prussian blue staining, and TEM. f In vivo MR imaging of Citrate SPION (a, b) and Endoderm SPION (c, d) labelled cells which showed hypointensity in rat muscle tissue in the axial and sagittal.
(Reprinted with from Andreas et al. [47]. Copyright (2012), with permission from Elsevier)
Some studies have suggested that MSCs were visualized by in vivo MRI about several weeks due to the SPIONs in the cells. According to Hu et al. [48] after SPIONs-labelled MSC transplantation into the rat spinal cord, they migrated into the host tissue, recovered function, and showed the hypointense signal on MRI until 3 weeks. These results suggest that long-term monitoring of in vivo human MSCs-labeled SPIONs for clinical implantation is possible.
Since MSCs lack phagocytosis, transfection agents [i.e., Poly-l-lysine, (PLL)] are required for the better uptake of SPIONs in MSCs. Mishra et al. [49] used Ultra-small SPIONs (USPIONs) with PLL for monitoring MSCs in Traumatic Brain Injury (TBI) mice model after IV injection. They used 12 nm USPIONs with PLL (50 µg:1.5 µg) to track stem cells by MR imaging. Furthermore, they reported that 100 MSCs labeled USPIONs are monitored in the MRI until 21 days. Similarly, Xu et al. [54] demonstrated SPIONs loaded poly (lactide-co-glycolide) (PLGA) microparticles to enhance several MR parameters; r2 relaxivity (fivefold), cellular internalization time (threefold) and R2 signal (twofold). However, despite the potential, MSC based therapy still has limitations owing to very low homing efficiency at the target sites.
Magnetic force can attract and keep the SPIONs at the target site. The magnetic attraction technique is suitable for stem cell-based therapy and has been used to increase the homing and retention of MSCs at the target sites. The magnetic attraction of MSCs can potentially circumvent some of its current limitations. As the static magnetic field is harmless, it can magnetically guide stem cells, minimizing interferences with biological barriers [55].
The MSCs internalize the nanoparticles (< 200 nm) by endocytosis or micropinocytosis [56]. Iron oxide nanoparticles smaller than 30 nm are superparamagnetic and have the magnetic properties only in the presence of an external magnet [50]. Song et al. confirmed that rats were exposed by a permanent magnet (0.32T) on their skull for a week followed by injecting the stem cells labeled with PLL coated SPIONs, which results in an almost fourfold increase in the magnet area as well as a noteworthy decrease at the infarct size. Similarly, Vanecek et al. [46] used a small permanent magnet (0.35T) to guide SPIONs with PLL by a magnetic field in the rat spinal cord. They demonstrated MSCs’ attraction to the magnet in vitro and in in vivo rat models. Magnetized MSCs gathered both into magnet area in vitro and at the spinal cord nearby the magnet in vivo were visualized by MRI, and also confirmed by Prussian blue staining and immunofluorescence imaging.
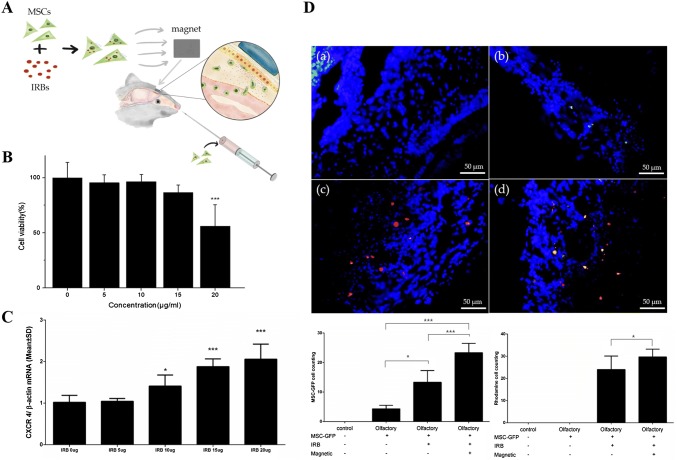
Interestingly, iron oxide nanoparticles are used not only for either MRI contrast agents or magnetic attraction techniques but also for enhancing the homing factors of stem cells such as CXCR4. Huang et al. [39] demonstrated that homing effects on traumatic brain injury (TBI) and glioblastoma model are improved by the stimulation of CXCR4-SDF-1α signaling pathway. Yun et al. [26] reported that the internalization of SPIONs into MSCs stimulate CXCR4 expression and CXCR4-SDF-1α signaling (Fig. 2).
Fig. 2.
a Schemes of mesenchymal stem cell homing with a permanent magnet in the olfactory mouse model. b In vitro cytotoxicity test of IRBs in MSCs. c Western blotting for expression of CXCR4, d immunofluorescence images of olfactory bulb for validating enhanced homing of magnetized MSCs in the olfactory injured mouse models. (a) Normal mice, (b) MSCs injection in olfactory injured mouse, (c) magnetized MSCs injection in olfactory injured mouse, (d) magnetized MSCs injection under magnetic field in olfactory injured mouse.
(Reprinted with from Yun et al. [26])
Moreover, their results suggested that MSCs with SPIONs migrated to injured olfactory tissue with the guidance of an external magnet, resulting in better homing effects of MSCs in vivo. Similarly, Li et al. [52] explained that MSCs internalized with Fe3O4@PDA show no adverse effect on MSC proliferation, biocompatibility, and viability. Further, they also proposed Fe3O4@PDA nanoparticles promoted not only the expression of CXCR4 chemokine receptor but also the migration-related proteins, c-Met, and C–C motif chemokine receptor 1 (CCR1). Remarkably, the iron oxide nanoparticles loaded MSCs expressed enhanced homing and anti-inflammatory abilities without the external magnetic attraction in vivo.
Kupffer cells show high phagocytic activities of commercialized SPIONs, including Feridex® and Resovist®. The SPIONs uptaken by immune cells were used to monitor the liver and spleen in clinical MR imaging. On the other hand, MSCs lack a phagocytic mechanism, and thus they do not have substantial cellular uptake [47]. Therefore, to increase the internalization of SPIONs into the MSCs, several strategies have been reported such as transfection agents [57], translocating peptides [58], and high-affinity ligands [59]. However, the strategies for the internalization efficiency of SPIONs can cause negative properties of SPIONs such as higher cytotoxicity, or lower stability. Therefore, the design strategies that allow efficient internalization of SPIONs by MSCs are also required to consider the potential side effects from the modified SPIONs.
Engineered magnetic nanoparticles for successful MSC labeling
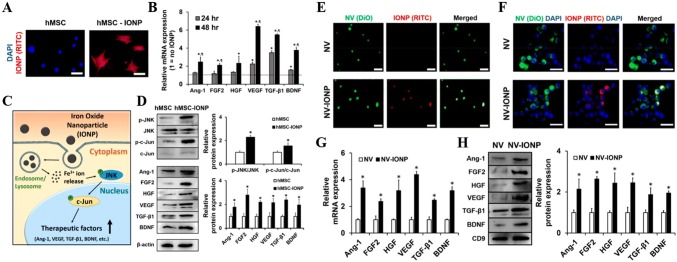
Several commercial products using SPIONs are approved by the FDA (i.e., ferumoxytol) owing to their biocompatibility and paramagnetic property (Feridex®, Resovist®) for MRI agents [60]. Even though other metals such as Gd, Cu, Co, Mn, and Ni also have paramagnetic properties, iron or iron oxide nanoparticles show much lower toxicity, so the iron-based nanoparticles have a potential for biomedical applications [61]. Recent studies reported that the internalized SPIONs in MSCs induced new biofunctional properties for MSCs’ homing by increasing CXCR4 [26, 39, 52, 62]. Some studies demonstrated that SPIONs induced the MSCs secreting the angiogenic, anti-apoptotic, and anti-inflammatory factors, such as angiopoietin-1 (Ang-1), transforming growth factor-beta 1 (TGF-β1), and vascular endothelial growth factor (VEGF) [40, 52, 62, 63] (Fig. 3). The internalization of SPIONs into MSCs is possible to change biofunctional properties, which can enhance chemotaxis and secretion of paracrine factors. Thus, understanding the correlation between the physicochemical properties of SPIONs in terms of size, zeta-potential, and surface material and the therapeutic capability of the MSCs induced by the SPIONs is very important [63]. Table 2 summarized the molecular factors induced by the physicochemical properties of SPIONs.
Fig. 3.
a RITC-IONP internalization in hMSCs with fluorescence miscopy images, b change in relative mRNA expression of anti-inflammatory, neurotrophic, angiogenic and antiapoptotic factors at 24 and 48 h in hMSC-IONP by qRT- PCR, c representative images of IONP internalization and signalling pathways for up-regulation of angiogenic (Ang-1, VEGF), anti-apoptotic (VEGF), neurotrophic (BNDF), and anti-inflammatory (TGF-β1) factors in hMSC. d Western blot conducted in hMSC and hMSC-IONP at 48 h to express intracellular signal and therapeutic factors, e NV (green) and NV-IONPs (red) were identified using confocal microscopy images. f Internalization images using fluorescent images of NV (green) and NV-IONP (red) in Raw264.7 macrophages. g, h qRT-PCR and western blot analysis IONP-induced factors (anti-inflammatory, neurotrophic, and antiapoptotic) mRNAs inside NV and NV-IONP respectively.
(Reprinted with permission from Kim et al. [64]. Copyright 2018 American Chemical Society)
Table 2.
The physicochemical properties of SPIONs in inducing cellular membrane protein and paracrine factors
| References | Size of SPIONs (diameter) | Zeta-potential | Surface material | Type of core | Exposure concentration | Reported uptake ([Fe]) | Viability | Induced factors |
|---|---|---|---|---|---|---|---|---|
| [62] | 48 nm (non-coating), 62 nm (coating) | N/A | Polydopamine (PDA) | Iron Oxide nanoparticle (Fe3O4) | 50 µg/mL | 40 pg/cell | 100% | CXCR4, CCR1, c-Met, TGF-β, IL-10 |
| [26] | 5.22 ± 0.9 nm | + 15.2 mV | Oleic acid | SPIONs (Fe3O4) | 15 µg[Fe]/mL | N/A | 95% | CXCR4 |
| [41] | 22 nm | N/A | Polyethylene glycol (PEG) | Magnetite (Fe3O4) | 40 µg/mL | 8.5 pg/cell | 100% | Cx43(H9C2), VEGF, bFGF, HGF, PAI-1, PEDF |
| [64] | 12 nm | N/A | Polyethylene glycol (PEG) | SPIONs | 40 µg/mL | N/A | 100% | Ang-1, FGF2, HGF, VEGF, TGF-β1, BNDF |
| [39] | 212.3 ± 5.7 nm | N/A | Hyaluronic acid (HA) | HA-Zn0.4Fe2.6O4 nanocluster | 6.03 µg/mL (108 µM) | N/A | 100% | CXCR4 |
| [40] | 15 nm | N/A | Poly-l-lysine (PLL) | Zn0.4Fe2.6O4 nanoparticle | 50 µg[metal]/mL | 4.6 pg/cell [metal] | N/A | Zinc-mediated Wnt signaling factors (WNT1, WNT3a), β-catenin, TuJ1, MAP2 A/B |
| [65] | 120–150 nm | − 5.5 mV | Dextran | Iron Oxide nanoparticle | 300 µg/mL | N/A | N/A | IL-15R, EGFR, CXCR4 |
Size effects of nanoparticles
SPIONs can be internalized by nonspecific or receptor-mediated (i.e., clathrin and caveolae) endocytosis [66]. Endocytosis depends on the size. Nanoparticles which have different diameters enter the MSCs via different pathways [67–69]. The exact size ranges of endocytic pathways are still controversial, but many studies have reported that nanoparticles below 200 nm diameter enter the cells via endocytic pathway [67, 70–74].
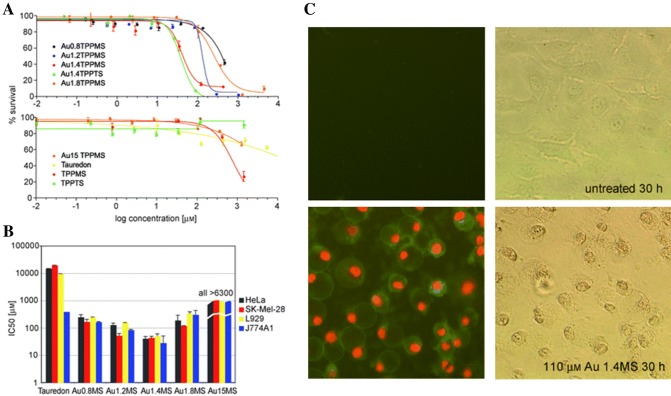
The mechanism of endocytosis depends on the size of the vesicles by which nanoparticles are wrapped and transported into the cells. Shang et al. demonstrated that nanoparticles with different sizes showed different particle dynamics on the MSCs membrane for being internalized [75]. Nanoparticles with size less than 10 nm accumulated on the membrane for being internalized, while nanoparticles with a size of 100 nm were directly internalized with the single-particle state, regardless of the surface charges. Particle size and surface area are important properties in the context of cytotoxicity. In general, the internalized nanoparticles form a new protein layer on the surface depending on the size [76]. Smaller nanoparticles have a larger surface area and a higher chance to interact with plasma protein or molecules, inducing higher cytotoxicity. Pan et al. [77] demonstrated that smaller gold nanoparticles (1.4 nm) are 60-fold more toxic than larger particles (15 nm) and caused rapid necrosis within 12 h (Fig. 4). Park et al. [78] also reported that 4 nm silver nanoparticles induced a higher level of reactive oxygen species (ROS) and IL-8 than 20 nm and 70 nm silver nanoparticles.
Fig. 4.
a MTT tests were performed adding different sizes of Au compounds after 48-hour in HeLa cells. b IC50 values of various Au compounds in HeLa, SK-Mel-28 melanoma cells, L929 fibroblasts, and J774A1 macrophage cells. Au1.4MS showed the lowest IC50 value across cell lines. c Fluorescence staining of HeLa cells with Annexin-V for apoptosis and PI for necrosis compared to control.
(Reprinted with permission from Pan et al. [77]. Copyright 2007 with permission from John Wiley and Sons)
Table 2 summarizes various SPIONs used for MSC based therapy to induce membrane protein (CXCR4, Cx43, c-Met, and CCR1) or paracrine factors (VEGF, TGF-β, and IL-10) in recent studies. The sizes of most SPIONs are between 5 nm and 200 nm to facilitate endocytosis and reduce cytotoxicity. Overall, small nanoparticles have substantial toxicity that may induce biochemical reactions such as ROS, and the endocytic pathway also depends on the nanoparticle size. Therefore, the size of nanoparticles can be an important factor in applying for MSCs.
Surface charge of nanoparticles
The surface charge of the nanoparticles can affect the endocytosis pathway and efficiency [79, 80]. As the cell membrane has a characteristic negative surface charge, the cationic nanoparticles favor an electrostatic interaction with the cells [81].
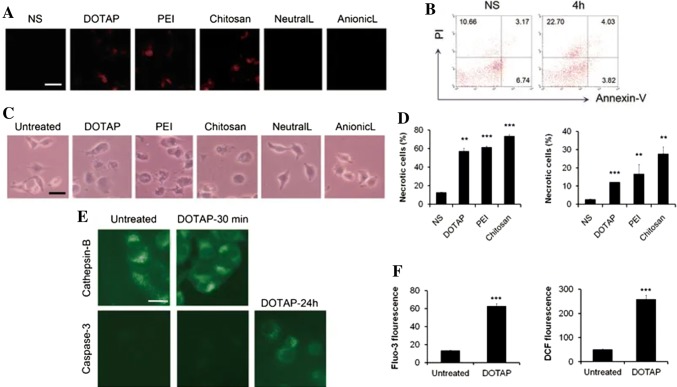
During the endocytosis pathway, positively charged nanoparticles have a chance to escape from endosome after internalization, and be localized at the cell cytosol and perinuclear space; and is known as the ‘proton-sponge’ effect. This effect indicates that sufficient positive charge of nanoparticles induce the chloride ion influx, leading to osmotic swelling to maintain a neutral charge in endosome and rupture of the endosome [82]. In spite of the advantage, several reports indicate that positively charged nanoparticles possess toxicity [83–86]. Moreover, Wei et al. [87] demonstrated that cationic nanoparticles including cationic liposomes, PEI, and chitosan interact with Na+/K+ -ATPase and cause inflammatory response leading to cell necrosis before the cell apoptosis (Fig. 5). Nevertheless, the positively charged nanoparticles have been commonly used in delivery carriers of negatively charged genes as non-viral vectors [88]. Therefore, the titration of cationic concentration is essential. On the other hand, some studies have reported that the anionic nanoparticles may be internalized through the interaction with the proteins in the membrane which possess the positive potential, and then they can be internalized into the cells via their repulsive interactions with negative cell membranes [89].
Fig. 5.
a Analysis of necrosis by propidium iodide in mouse lungs, b flow cytometry of Annexin-V and PI to detect necrotic cells induced by injecting cationic liposomes, c microscopic images of cell morphological changes induced various nanocarriers in vitro. d Quantification of flow cytometry to detect necrotic cells using PI and Annexin-V after nanocarriers treatment. e Immunofluorescence image of Cathepsin-B and Caspase-3 after treatment of DOTAP with time intervals. f ROS levels and Ca2+ concentration detected by H2DCF-DA and Fluo-3/AM with flow cytometry in A549 cells after DOTAP liposomes treatment.
(Reprinted with from Wei et al. [87]
Table 2 summarizes zeta-potential of the nanoparticles previously reported. Yun et al. [26] used commercial SPIONs for the enhanced homing phenomenon of MSCs with a magnet to olfactory bulb. The zeta-potential of the iron oxide nanoparticles with rhodamine B (IRB) was + 15.2 mV. Chung et al. reported that dextran-coated iron oxide nanoparticles as a tool to boost MSC migration, trans-differentiation into DA-like neurons, and protection effects for Parkinson’s disease [65]. The zeta-potential of the dextran-coated particle was − 5.5 mV. In summary, several studies emphasized the influence of zeta-potential on the nanoparticle for effective therapy using MSCs.
Surface ligand of nanoparticles
Chemical composition at the surface of nanoparticles changes the kinetics and the route of nanoparticle internalization [90]. The surface characteristics of nanoparticle are affected by the type and the thickness of the biomolecules which are adsorbed on the surface [91, 92]. Thus, the surface of nanoparticles can be modified to enhance interactions with the cell membrane. For improving the reaction, many studies used a transfection agent (TA) such as lipid and polycation [93–95]. Several lipid-based TAs are commercially available with SPION for MRI tracking. Küstermann et al. demonstrated that SPIONs and TAs are no significant toxicity proliferating murine embryonic stem cells (mESCs) [93]. However, the excess of SPIONs and TAs inhibited proliferation and increased cytotoxicity; therefore, a proper amount for using TAs and SPIONs are required. Poly-l-lysine (PLL) is the most common polycation TA used with commercial SPIONs, such as Resovist® and Feridex® [96–98]. The efficiency of PLL was affected by molecular weight, the concentration ratio of the Fe/PLL, and the reaction time [94, 98]. However, an excessive amount of polycations can lead to cell death with cell membrane pore and ionic dissymmetry [99, 100]. Therefore, the amount of PLL needs to be controlled for escaping unpredictable side effects.
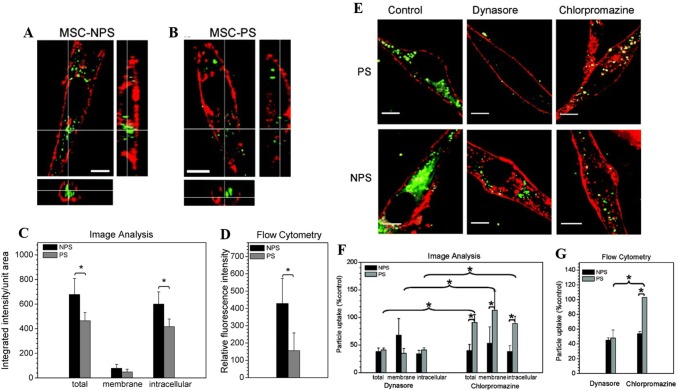
Interestingly, a few researchers have suggested that specific functional groups, such as carboxyl (–COOH) or amine (–NH2) play an essential role in internalization into MSCs [101, 102]. Even though cationic nanoparticle favors uptake compared to anionic nanoparticles, the functional groups select their internalization route (clathrin-mediated endocytosis) irrespective of their surface charge, aiding an efficient internalization into MSCs (Fig. 6).
Fig. 6.
Confocal image to show uptake of a amino-functionalized (NPS) and b non-functionalized (PS) cationic nanoparticles by MSCs. c Quantitative confocal imaging of membrane and intracellular fluorescence after NPS and PS nanoparticles treatment, and d fluorescence intensity after NPS and PS nanoparticles treatment by flow cytometry. e Confocal images to show uptake of NPS and PS nanoparticles in the presence of dynasore or chlorpromazine. f, g Quantitative image and flow cytometry analysis for particle uptake in presence of dynasore or chlorpromazine.
(Reprinted with permission from Jiang et al. [102]. Copyright (2010) American Chemical Society)
Besides, the specific coating materials for the SPIONs can be used (Table 2). Li et al. [62] modified the iron oxide nanoparticles (IONPs) with polydopamine (PDA). PDA is used for biomedical applications to coat nanoparticles due to biocompatibility and biodegradability [103, 104]. In this study, PDA-IONPs had the advantages of enhanced labeling efficiency and non-cytotoxicity with migration ability and secretion of vascular endothelial growth factor (VEGF). Han and Kim et al. used polyethylene glycol (PEG) coating agent to maintain the colloidal stability of nanoparticles in the field of an external magnet [41, 64]. PEG has the hydrophilic steric barrier, which is known for prolonging the half-life of nanoparticles in a biological fluid, avoiding aggregation [105]. Han et al. suggested that the internalization of aggregated IONPs to MSCs is lower than PEGlyation IONPs. Also, PLL [40] and dextran [65] were used for enhanced labeling efficiency without cytotoxicity. Hyaluronic acid (HA) [39] was used to improve the interaction with CD44 positive cells. In summary, the use of specific transfection agents and coating materials affect labeling efficiency. Further studies are required for the secretion migration and anti-inflammatory factors induced by the transfection agents and coating materials.
Conclusion
Here, we outlined recent studies on the physicochemical properties of SPIONs and the biological behaviors of MSCs. Previous studies reported that iron-based magnetic nanoparticles improve the expression of CXCR4 in MSCs. Follow-up research reported the nanoparticles to increase chemotaxis, angiogenesis, anti-apoptosis, anti-inflammation, and differentiation of MSCs. In general, as MSCs lack phagocytic activity, SPIONs should be designed to target the endocytic pathways, including micropinocytosis, clathrin-mediated endocytosis, and caveolae-mediated endocytosis. The physicochemical properties of SPIONs such as the size, zeta-potential, and surface ligand change the endocytic pathways, which affect additional regenerative factors including CXCR4, c-Met, Ang-1, VEGF and TGF-β.
Several studies have demonstrated that the nanoparticles smaller than 200 nm enter the cells via endocytosis, while those smaller than 5 nm have a larger surface area, and induce ROS, and cause considerably more toxicity. The cationic nanoparticles interact with cell membrane easily due to electrostatic interactions, but some studies have reported that positive nanoparticles are cytotoxic. The TAs such as lipid and polycation are commonly used to alter the surface of the nanoparticles for improved SPIONs internalization. However, the effects of TAs are different depending on the coating materials of SPIONs. The excessive polycation can induce cell membrane pores and ionic dissymmetry. Moreover, some studies have demonstrated that the specific surface functional groups of nanoparticles play a vital role in the interactions with the MSCs and the selection of the endocytic pathway. Therefore, the engineering physicochemical properties of magnetic nanoparticles are critical to the MSCs to enhance the regenerative effects and prevent undesirable cytotoxicity in the target site. In conclusion, precise validation of magnetic nanoparticles in terms of physicochemical properties is necessary before using them as therapeutic agents modulating the regenerative ability of MSCs.
Acknowledgements
This work was supported by Grants from the National Research Foundation of Korea (NRF), No. 2018R1D1A1B07042339 and 2019K2A9A2A08000123.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wan Su Yun, Email: ip9801@naver.com.
Susmita Aryal, Email: sabicaaryal@gmail.com.
Ye Ji Ahn, Email: ahnyeji@yonsei.ac.kr.
Young Joon Seo, Email: okas2000@yonsei.ac.kr.
Jaehong Key, Email: jkey@yonsei.ac.kr.
References
- 1.Peran M, Garcia MA, Lopez-Ruiz E, Bustamante M, Jimenez G, Madeddu R, Marchal JA. Functionalized nanostructures with application in regenerative medicine. Int J Mol Sci. 2012;13:3847–3886. doi: 10.3390/ijms13033847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe B, Nam SY. Synthesis and biocompatibility assessment of a cysteine-based nanocomposite for applications in bone tissue engineering. Biomed Eng Lett. 2016;6:271–275. doi: 10.1007/s13534-016-0239-x. [DOI] [Google Scholar]
- 3.Kim M, Gweon B, Koh U, Cho Y, Shin DW, Noh M, Shin JH. Matrix stiffness induces epithelial mesenchymal transition phenotypes of human epidermal keratinocytes on collagen coated two dimensional cell culture. Biomed Eng Lett. 2015;5:194–202. doi: 10.1007/s13534-015-0202-2. [DOI] [Google Scholar]
- 4.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678–683. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 7.Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;1068:352–366. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- 8.Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. Advancing cartilage tissue engineering: the application of stem cell technology. Curr Opin Biotechnol. 2005;16:503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz FF, Weiss DJ, Rocco PR. Prospects and progress in cell therapy for acute respiratory distress syndrome. Expert Opin Biol Ther. 2016;16:1353–1360. doi: 10.1080/14712598.2016.1218845. [DOI] [PubMed] [Google Scholar]
- 11.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 13.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 14.Heathman TR, Nienow AW, McCall MJ, Coopman K, Kara B, Hewitt CJ. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med. 2015;10:49–64. doi: 10.2217/rme.14.73. [DOI] [PubMed] [Google Scholar]
- 15.Kang SK, Shin IS, Ko MS, Jo JY, Ra JC. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968. doi: 10.1155/2012/342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 17.Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 19.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing XH, Yang L, Duan XJ, Xie B, Chen W, Li Z, Tan HB. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Joint Bone Spine. 2008;75:432–438. doi: 10.1016/j.jbspin.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Niemeyer M, Oostendorp RA, Kremer M, Hippauf S, Jacobs VR, Baurecht H, Ludwig G, Piontek G, Bekker-Ruz V, Timmer S, et al. Non-invasive tracking of human haemopoietic CD34(+) stem cells in vivo in immunodeficient mice by using magnetic resonance imaging. Eur Radiol. 2010;20:2184–2193. doi: 10.1007/s00330-010-1773-z. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm C, Bal L, Smirnov P, Galy-Fauroux I, Clement O, Gazeau F, Emmerich J. Magnetic control of vascular network formation with magnetically labeled endothelial progenitor cells. Biomaterials. 2007;28:3797–3806. doi: 10.1016/j.biomaterials.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Phanapavudhikul P, Shen S, Ng WK, Tan RB. Formulation of Fe3O4/acrylate co-polymer nanocomposites as potential drug carriers. Drug Deliv. 2008;15:177–183. doi: 10.1080/10717540801952597. [DOI] [PubMed] [Google Scholar]
- 24.Sakhtianchi R, Minchin RF, Lee KB, Alkilany AM, Serpooshan V, Mahmoudi M. Exocytosis of nanoparticles from cells: role in cellular retention and toxicity. Adv Colloid Interface Sci. 2013;201–202:18–29. doi: 10.1016/j.cis.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Anzai Y, Piccoli CW, Outwater EK, Stanford W, Bluemke DA, Nurenberg P, Saini S, Maravilla KR, Feldman DE, Schmiedl UP, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228:777–788. doi: 10.1148/radiol.2283020872. [DOI] [PubMed] [Google Scholar]
- 26.Yun WS, Choi JS, Ju HM, Kim MH, Choi SJ, Oh ES, Seo YJ, Key J. Enhanced homing technique of mesenchymal stem cells using iron oxide nanoparticles by magnetic attraction in olfactory-injured mouse models. Int J Mol Sci. 2018;19:1376. doi: 10.3390/ijms19051376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fayol D, Frasca G, Le Visage C, Gazeau F, Luciani N, Wilhelm C. Use of magnetic forces to promote stem cell aggregation during differentiation, and cartilage tissue modeling. Adv Mater. 2013;25:2611–2616. doi: 10.1002/adma.201300342. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto Y, Ito A, Fujita H, Nagamori E, Kawabe Y, Kamihira M. Functional evaluation of artificial skeletal muscle tissue constructs fabricated by a magnetic force-based tissue engineering technique. Tissue Eng Part A. 2011;17:107–114. doi: 10.1089/ten.TEA.2010.0312. [DOI] [PubMed] [Google Scholar]
- 29.Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, Bronk LF, Ananta JS, Mandelin J, Georgescu MM, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291–296. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudeurge A, Wilhelm C, Chen-Tournoux A, Farahmand P, Bellamy V, Autret G, Ménager C, Hagège A, Larghéro J, Gazeau F. Can magnetic targeting of magnetically labeled circulating cells optimize intramyocardial cell retention? Cell Transplant. 2012;21:679–691. doi: 10.3727/096368911X612440. [DOI] [PubMed] [Google Scholar]
- 31.Ahn YJ, Kong TH, Choi JS, Yun WS, Key J, Seo YJ. Strategies to enhance efficacy of SPION-labeled stem cell homing by magnetic attraction: a systemic review with meta-analysis. Int J Nanomed. 2019;14:4849–4866. doi: 10.2147/IJN.S204910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 33.Mojica Pisciotti ML, Lima E, Jr, Vasquez Mansilla M, Tognoli VE, Troiani HE, Pasa AA, Creczynski-Pasa TB, Silva AH, Gurman P, Colombo L, et al. In vitro and in vivo experiments with iron oxide nanoparticles functionalized with DEXTRAN or polyethylene glycol for medical applications: magnetic targeting. J Biomed Mater Res B Appl Biomater. 2014;102:860–868. doi: 10.1002/jbm.b.33068. [DOI] [PubMed] [Google Scholar]
- 34.Buyukhatipoglu K, Clyne AM. Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation. J Biomed Mater Res A. 2011;96:186–195. doi: 10.1002/jbm.a.32972. [DOI] [PubMed] [Google Scholar]
- 35.Soenen SJ, Nuytten N, De Meyer SF, De Smedt SC, De Cuyper M. High intracellular iron oxide nanoparticle concentrations affect cellular cytoskeleton and focal adhesion kinase-mediated signaling. Small. 2010;6:832–842. doi: 10.1002/smll.200902084. [DOI] [PubMed] [Google Scholar]
- 36.Soenen SJ, Himmelreich U, Nuytten N, Pisanic TR, 2nd, Ferrari A, De Cuyper M. Intracellular nanoparticle coating stability determines nanoparticle diagnostics efficacy and cell functionality. Small 2010; 6:2136–2145. 10.1002/smll.201000763. [DOI] [PubMed]
- 37.Fayol D, Luciani N, Lartigue L, Gazeau F, Wilhelm C. Managing magnetic nanoparticle aggregation and cellular uptake: a precondition for efficient stem-cell differentiation and MRI tracking. Adv Healthc Mater. 2013;2:313–325. doi: 10.1002/adhm.201200294. [DOI] [PubMed] [Google Scholar]
- 38.Chang YK, Liu YP, Ho JH, Hsu SC, Lee OK. Amine-surface-modified superparamagnetic iron oxide nanoparticles interfere with differentiation of human mesenchymal stem cells. J Orthop Res. 2012;30:1499–1506. doi: 10.1002/jor.22088. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, Zhang F, Wang Y, Sun X, Choi KY, Liu D, Choi JS, Shin TH, Cheon J, Niu G, et al. Design considerations of iron-based nanoclusters for noninvasive tracking of mesenchymal stem cell homing. ACS Nano. 2014;8:4403–4414. doi: 10.1021/nn4062726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yun S, Shin TH, Lee JH, Cho MH, Kim IS, Kim JW, Jung K, Lee IS, Cheon J, Park KI. Design of magnetically labeled cells (mag-cells) for in vivo control of stem cell migration and differentiation. Nano Lett. 2018;18:838–845. doi: 10.1021/acs.nanolett.7b04089. [DOI] [PubMed] [Google Scholar]
- 41.Han J, Kim B, Shin J-Y, Ryu S, Noh M, Woo J, Park J-S, Lee Y, Lee N, Hyeon T. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano. 2015;9:2805–2819. doi: 10.1021/nn506732n. [DOI] [PubMed] [Google Scholar]
- 42.Schafer R. Labeling and imaging of stem cells—promises and concerns. Transfus Med Hemother. 2010;37:85–89. doi: 10.1159/000287271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng Y, Huang J, Zhu T, Li R, Wang Z, Ma F, Zhu J. Stem cell tracking technologies for neurological regenerative medicine purposes. Stem Cells Int. 2017;2017:2934149. doi: 10.1155/2017/2934149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santra S, Kaittanis C, Grimm J, Perez JM. Drug/dye-loaded, multifunctional iron oxide nanoparticles for combined targeted cancer therapy and dual optical/magnetic resonance imaging. Small. 2009;5:1862–1868. doi: 10.1002/smll.200900389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jasmin, Torres AL, Jelicks L, de Carvalho AC, Spray DC, Mendez-Otero R. Labeling stem cells with superparamagnetic iron oxide nanoparticles: analysis of the labeling efficacy by microscopy and magnetic resonance imaging. Methods Mol Biol. 2012;906:239–252. doi: 10.1007/978-1-61779-953-2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanecek V, Zablotskii V, Forostyak S, Ruzicka J, Herynek V, Babic M, Jendelova P, Kubinova S, Dejneka A, Sykova E. Highly efficient magnetic targeting of mesenchymal stem cells in spinal cord injury. Int J Nanomed. 2012;7:3719–3730. doi: 10.2147/IJN.S32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andreas K, Georgieva R, Ladwig M, Mueller S, Notter M, Sittinger M, Ringe J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials. 2012;33:4515–4525. doi: 10.1016/j.biomaterials.2012.02.064. [DOI] [PubMed] [Google Scholar]
- 48.Hu SL, Lu PG, Zhang LJ, Li F, Chen Z, Wu N, Meng H, Lin JK, Feng H. In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J Cell Biochem. 2012;113:1005–1012. doi: 10.1002/jcb.23432. [DOI] [PubMed] [Google Scholar]
- 49.Mishra SK, Khushu S, Singh AK, Gangenahalli G. Homing and tracking of iron oxide labelled mesenchymal stem cells after infusion in traumatic brain injury mice: a longitudinal in vivo MRI study. Stem Cell Rev Rep. 2018;14:888–900. doi: 10.1007/s12015-018-9828-7. [DOI] [PubMed] [Google Scholar]
- 50.Taboada E, Rodriguez E, Roig A, Oro J, Roch A, Muller RN. Relaxometric and magnetic characterization of ultrasmall iron oxide nanoparticles with high magnetization. Evaluation as potential T1 magnetic resonance imaging contrast agents for molecular imaging. Langmuir. 2007;23:4583–4588. doi: 10.1021/la063415s. [DOI] [PubMed] [Google Scholar]
- 51.Song M, Kim YJ, Kim YH, Roh J, Kim SU, Yoon BW. Using a neodymium magnet to target delivery of ferumoxide-labeled human neural stem cells in a rat model of focal cerebral ischemia. Hum Gene Ther. 2010;21:603–610. doi: 10.1089/hum.2009.144. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Wei Z, Lv H, Wu L, Cui Y, Yao H, Li J, Zhang H, Yang B, Jiang J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int J Nanomed. 2019;14:573–589. doi: 10.2147/IJN.S184920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 54.Xu C, Miranda-Nieves D, Ankrum JA, Matthiesen ME, Phillips JA, Roes I, Wojtkiewicz GR, Juneja V, Kultima JR, Zhao W, et al. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly(lactide-co-glycolide) microparticles. Nano Lett. 2012;12:4131–4139. doi: 10.1021/nl301658q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin TH, Cheon J. Synergism of nanomaterials with physical stimuli for biology and medicine. Acc Chem Res. 2017;50:567–572. doi: 10.1021/acs.accounts.6b00559. [DOI] [PubMed] [Google Scholar]
- 56.Cores J, Caranasos TG, Cheng K. Magnetically targeted stem cell delivery for regenerative medicine. J Funct Biomater. 2015;6:526–546. doi: 10.3390/jfb6030526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH, Jr, Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 58.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 59.Gamarra LF, Pavon LF, Marti LC, Pontuschka WM, Mamani JB, Carneiro SM, Camargo-Mathias MI, Moreira-Filho CA, Amaro E., Jr In vitro study of CD133 human stem cells labeled with superparamagnetic iron oxide nanoparticles. Nanomedicine. 2008;4:330–339. doi: 10.1016/j.nano.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Li L, Jiang W, Luo K, Song H, Lan F, Wu Y, Gu Z. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595–615. doi: 10.7150/thno.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Wei Z, Li B, Li J, Lv H, Wu L, Zhang H, Yang B, Zhu M, Jiang J. In vivo migration of Fe3O4@ polydopamine nanoparticle-labeled mesenchymal stem cells to burn injury sites and their therapeutic effects in a rat model. Biomaterials science. 2019;7:2861–2872. doi: 10.1039/C9BM00242A. [DOI] [PubMed] [Google Scholar]
- 63.Duan X, Li Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small. 2013;9:1521–1532. doi: 10.1002/smll.201201390. [DOI] [PubMed] [Google Scholar]
- 64.Kim HY, Kumar H, Jo MJ, Kim J, Yoon JK, Lee JR, Kang M, Choo YW, Song SY, Kwon SP, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18:4965–4975. doi: 10.1021/acs.nanolett.8b01816. [DOI] [PubMed] [Google Scholar]
- 65.Chung TH, Hsu SC, Wu SH, Hsiao JK, Lin CP, Yao M, Huang DM. Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of Parkinson’s disease. Nanoscale. 2018;10:2998–3007. doi: 10.1039/c7nr06976f. [DOI] [PubMed] [Google Scholar]
- 66.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9:8655–8671. doi: 10.1021/acsnano.5b03184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shang L, Nienhaus K, Nienhaus GU. Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnology. 2014;12:5. doi: 10.1186/1477-3155-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 70.Wang T, Wang L, Li X, Hu X, Han Y, Luo Y, Wang Z, Li Q, Aldalbahi A, Wang L, et al. Size-dependent regulation of intracellular trafficking of polystyrene nanoparticle-based drug-delivery systems. ACS Appl Mater Interfaces. 2017;9:18619–18625. doi: 10.1021/acsami.7b05383. [DOI] [PubMed] [Google Scholar]
- 71.Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomed. 2014;9(Suppl 1):51–63. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Copolovici DM, Langel K, Eriste E, Langel U. Cell-penetrating peptides: design, synthesis, and applications. ACS Nano. 2014;8:1972–1994. doi: 10.1021/nn4057269. [DOI] [PubMed] [Google Scholar]
- 73.Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharm Sci. 2013;8:1–10. doi: 10.1016/j.ajps.2013.07.001. [DOI] [Google Scholar]
- 74.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/Bj20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shang L, Nienhaus K, Jiang X, Yang L, Landfester K, Mailander V, Simmet T, Nienhaus GU. Nanoparticle interactions with live cells: quantitative fluorescence microscopy of nanoparticle size effects. Beilstein J Nanotechnol. 2014;5:2388–2397. doi: 10.3762/bjnano.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliver Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 78.Park J, Lim DH, Lim HJ, Kwon T, Choi JS, Jeong S, Choi IH, Cheon J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun (Camb) 2011;47:4382–4384. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
- 79.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 80.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 81.Petri-Fink A, Chastellain M, Juillerat-Jeanneret L, Ferrari A, Hofmann H. Development of functionalized superparamagnetic iron oxide nanoparticles for interaction with human cancer cells. Biomaterials. 2005;26:2685–2694. doi: 10.1016/j.biomaterials.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 82.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Sharma A, Madhunapantula SV, Robertson GP. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin Drug Met. 2012;8:47–69. doi: 10.1517/17425255.2012.637916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lunov O, Syrovets T, Loos C, Nienhaus GU, Mailander V, Landfester K, Rouis M, Simmet T. Amino-functionalized polystyrene nanoparticles activate the NLRP3 inflammasome in human macrophages. ACS Nano. 2011;5:9648–9657. doi: 10.1021/nn203596e. [DOI] [PubMed] [Google Scholar]
- 85.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 86.Tousignant JD, Gates AL, Ingram LA, Johnson CL, Nietupski JB, Cheng SH, Eastman SJ, Scheule RK. Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid: plasmid DNA complexes in mice. Hum Gene Ther. 2000;11:2493–2513. doi: 10.1089/10430340050207984. [DOI] [PubMed] [Google Scholar]
- 87.Wei XW, Shao B, He ZY, Ye TH, Luo M, Sang YX, Liang X, Wang W, Luo ST, Yang SY, et al. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25:237–253. doi: 10.1038/cr.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 89.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 90.Musyanovych A, Dausend J, Dass M, Walther P, Mailander V, Landfester K. Criteria impacting the cellular uptake of nanoparticles: a study emphasizing polymer type and surfactant effects. Acta Biomater. 2011;7:4160–4168. doi: 10.1016/j.actbio.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 91.Ehrenberg MS, Friedman AE, Finkelstein JN, Oberdorster G, McGrath JL. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials. 2009;30:603–610. doi: 10.1016/j.biomaterials.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 92.Chen ZP, Xu RZ, Zhang Y, Gu N. Effects of proteins from culture medium on surface property of silanes-functionalized magnetic nanoparticles. Nanoscale Res Lett. 2009;4:204–209. doi: 10.1007/s11671-008-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kustermann E, Himmelreich U, Kandal K, Geelen T, Ketkar A, Wiedermann D, Strecker C, Esser J, Arnhold S, Hoehn M. Efficient stem cell labeling for MRI studies. Contrast Media Mol Imaging. 2008;3:27–37. doi: 10.1002/cmmi.229. [DOI] [PubMed] [Google Scholar]
- 94.Babic M, Horak D, Trchova M, Jendelova P, Glogarova K, Lesny P, Herynek V, Hajek M, Sykova E. Poly(l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 2008;19:740–750. doi: 10.1021/bc700410z. [DOI] [PubMed] [Google Scholar]
- 95.Arbab AS, Yocum GT, Rad AM, Khakoo AY, Fellowes V, Read EJ, Frank JA. Labeling of cells with ferumoxides–protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed Int J Devoted Dev Appl Magn Reson In vivo. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 96.Liu G, Gilad AA, Bulte JW, van Zijl PC, McMahon MT. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol Imaging. 2010;5:162–170. doi: 10.1002/cmmi.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 98.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JW, Frank JA. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation. 2003;76:1123–1130. doi: 10.1097/01.TP.0000089237.39220.83. [DOI] [PubMed] [Google Scholar]
- 99.Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, Frank JA. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004;3:24–32. doi: 10.1162/153535004773861697. [DOI] [PubMed] [Google Scholar]
- 100.Zauner W, Ogris M, Wagner E. Polylysine-based transfection systems utilizing receptor-mediated delivery. Adv Drug Deliver Rev. 1998;30:97–113. doi: 10.1016/S0169-409x(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 101.Jiang XE, Musyanovych A, Rocker C, Landfester K, Mailander V, Nienhaus GU. Specific effects of surface carboxyl groups on anionic polystyrene particles in their interactions with mesenchymal stem cells. Nanoscale. 2011;3:2028–2035. doi: 10.1039/c0nr00944j. [DOI] [PubMed] [Google Scholar]
- 102.Jiang XE, Dausend J, Hafner M, Musyanovych A, Rocker C, Landfester K, Mailander V, Nienhaus GU. Specific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cells. Biomacromol. 2010;11:748–753. doi: 10.1021/bm901348z. [DOI] [PubMed] [Google Scholar]
- 103.Zhang R, Wang S, Yang Y, Deng Y, Li D, Su P, Yang Y. Modification of polydopamine-coated Fe3O4 nanoparticles with multi-walled carbon nanotubes for magnetic-μ-dispersive solid-phase extraction of antiepileptic drugs in biological matrices. Anal Bioanal Chem. 2018;410:3779–3788. doi: 10.1007/s00216-018-1047-1. [DOI] [PubMed] [Google Scholar]
- 104.Chen Y, Zhang F, Wang Q, Lin H, Tong R, An N, Qu F. The synthesis of LA-Fe3O4@ PDA-PEG-DOX for photothermal therapy–chemotherapy. Dalton Trans. 2018;47:2435–2443. doi: 10.1039/C7DT04080F. [DOI] [PubMed] [Google Scholar]
- 105.Jones M, Leroux J. Polymeric micelles—a new generation of colloidal drug carriers. Eur J Pharm Biopharm. 1999;48:101–111. doi: 10.1016/s0939-6411(99)00039-9. [DOI] [PubMed] [Google Scholar]