Abstract
Acute pancreatitis (AP) is a common gastrointestinal disorder. Approximately 15%-20% of patients develop severe AP. Systemic inflammatory response syndrome and multiple organ dysfunction syndrome may be caused by the massive release of inflammatory cytokines in the early stage of severe AP, followed by intestinal dysfunction and pancreatic necrosis in the later stage. A study showed that 59% of AP patients had associated intestinal barrier injury, with increased intestinal mucosal permeability, leading to intestinal bacterial translocation, pancreatic tissue necrosis and infection, and the occurrence of multiple organ dysfunction syndrome. However, the real effect of the gut microbiota and its metabolites on intestinal barrier function in AP remains unclear. This review summarizes the alterations in the intestinal flora and its metabolites during AP development and progression to unveil the mechanism of gut failure in AP.
Keywords: Acute pancreatitis, Gut microbiota, Short-chain fatty acids, Intestinal barrier
Core tip: Acute pancreatitis (AP) is a common clinical acute abdomen disease, and its incidence is increasing year by year. There are several reviews on the pathophysiology, therapeutic options and clinical trials of AP. However, the real effect of the gut microbiota and its metabolites on intestinal barrier function in AP remains unclear. This review summarizes the alterations in the intestinal flora and its metabolites during AP development and progression to unveil the mechanism of gut failure in AP.
INTRODUCTION
Acute pancreatitis (AP) is a common gastrointestinal disorder. It is a local inflammatory response of the pancreas caused by abnormal activation of pancreatic enzymes by a variety of causes. AP is classified into mild AP (MAP), moderately severe AP (MSAP), and severe AP (SAP) based on the Atlanta Classification of 2012 revision[1]. Approximately 15%-20% of patients develop SAP[2,3], and both systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) may be caused by the massive release of inflammatory cytokines in the early stage of SAP, followed by intestinal dysfunction and pancreatic necrosis in the later stage[4,5]. Most bacteria causing necrotic infection of pancreatic tissue are from the intestinal flora, such as Escherichia coli and Enterococci[6]. Therefore, the intestinal flora may play an important role in the development of SAP.
The gastrointestinal tract, the largest organ in the human body, provides a broad colonization surface for the flora. It contains 150 times the total number of human genes[7]. The human intestinal flora has more than 1500 species and more than 50 phyla, with the largest number of Firmicutes, followed by Bacteroidetes, and other common phyla are Proteobacteria, Actinomyces, Actinobacteria, Fusobacteria and Verrucomicrobia[7]. In recent years, with the development of metagenomic research, people have become increasingly aware that the intestinal flora plays an important role in human health and diseases, including gastrointestinal diseases, such as inflammatory bowel disease[8], irritable bowel symptoms[9], colon cancer[10], and extragastrointestinal diseases, such as Alzheimer’s disease[11], coronary heart disease[12], obesity[13], and diabetes[14]. Some studies have found early dysbiosis of the intestinal flora during the occurrence and development of SAP. In addition to intestinal bacteria, their metabolites, such as short-chain fatty acids (SCFAs), also affect the progression of AP.
This review summarizes the alterations in intestinal flora and its metabolites during the development and progression of AP to unveil the mechanism of gut failure in AP and finally provide a potential therapeutic target for AP.
CHANGES IN THE INTESTINAL FLORA DURING AP
In recent years, an increasing number of studies have found that the intestinal flora changes during the development of AP, which may be related to the severity of the disease. During the AP process, abnormal secretion of trypsin and destruction of pancreatic structure lead to abnormal pancreas secretion, which can cause changes in intestinal homeostasis and the intestinal flora[15,16]. Patients with AP had a greater abundance of the phyla Bacteroidetes and Proteobacteria with lower abundance of Firmicutes and Actinobacteria than healthy controls[17]. Tan et al[18] found that the microbial composition shifted significantly between patients with AP and healthy controls (HCs). The abundance of potentially pathogenic bacteria such as Enterobacteriaceae and Enterococcus was significantly increased, and that of beneficial bacteria such as Bifidobacterium was significantly decreased in both the MAP and SAP groups. The abundance of Enterobacteriaceae and Enterococcus increased by 3.2% and 9.3%, respectively, whereas Bifidobacterium abundance decreased by 9.2% in the SAP group compared to that in the MAP group. Our results also showed differences between the AP and HC groups; furthermore, the microbial composition changed further with the worsening of AP, and the abundance of beneficial bacteria such as Blautia was decreased in SAP compared with that in MAP and MSAP. It was suggested that the gut microbiota is an important mediator during AP and that its dysbiosis is associated with AP severity[19].
As there were significant changes in the abundance and structure of the intestinal flora in AP patients, researchers continued to study the changes in intestinal flora during AP using animal models. Animal experimental evidence also demonstrated similar intestinal microbiota changes in AP. Chen et al[20] applied 16S rRNA high-throughput sequencing analysis to study intestinal microbiota changes in rats in a sham-operated group (SO group) and an acute necrotizing pancreatitis (ANP) group. The SO and ANP groups showed structural segregation, and the microbiota diversity of the ANP group significantly decreased. At the phylum level, the abundance of Saccharibacteria and Tenericutes decreased significantly. At the genus level, the abundance of Escherichia-Shigella and Phascolarctobacterium increased significantly, while the abundance of Candidatus_Saccharimonas, Prevotellaceae_UCG-001, Lachnospiraceae_UCG-001, Ruminiclostridium_5 and Ruminococcaceae_UCG-008 decreased significantly. At the same time, the amount of antimicrobial peptides (AMPs) secreted by panspermia cells decreased significantly and was negatively correlated with the abundance of Escherichia coli and Shigella. Deficiencies in Paneth cell AMPs were reported to be associated with intestinal barrier failure, leading to bacterial translocation[21]. Ye et al[22] found that obesity could aggravate AP, deteriorate intestinal permeability and aggravate intestinal inflammation. They analysed the faecal microbiota composition and found that obese rats with AP had lower bacterial richness than rats with normal weight. Studies have suggested that faecal bacterial richness is a major marker of gut health[23,24]. Our animal research revealed that antibiotic-treated mice and germ-free mice exhibited alleviated pancreatic injury after AP induction and that subsequent faecal microbiota transplantation in turn exacerbated disease. Moreover, our previous results were supported by animal research, which also found that gut microbiota-depleted AP rats displayed less pancreatic injury and lower levels of interleukin (IL)-17A, tumour necrosis factor-α and IL-1beta in the plasma than AP rats with an intact microbiota[25]. Many recent studies have shown that this may be related to IgA, a key immune protein that is mainly located in the small intestine and protects the intestinal barrier from pathogenic bacteria. The diversity of bacteria can stimulate the body to produce different IgA and combine with the bacteria[26]. Through the combination with bacteria, it can modify the metabolism of bacteria and eliminate the mucosal inflammation response[27], which maintain immune homeostasis. The production of IgA depends on bacterial diversity. Deficiency of IgA in the gut lumen was associated with altered microbiota composition in the small intestine[28], increased susceptibility to induced colitis, and higher bacterial translocation to mesenteric lymph nodes after Salmonella typhimurium challenge, which suggested that IgA played a crucial role in the immune regulation between the intestinal flora and the host. Taken together, these studies reveal that the intestinal flora changes during AP and that these changes may be related to the severity of disease.
GUT MICROBIOTA MAY PROMOTE AP PROGRESSION BY AFFECTING INTESTINAL MUCOSAL BARRIER FUNCTION
Normal gut bacteria play a crucial role in maintaining gut mucosal integrity. However, gut mucosal ischaemia and reperfusion during AP can damage gut barrier integrity and lead to gut bacterial translocation to other locations, causing local and systemic infections[29,30]. Studies have revealed that intestinal mucosal barrier injury is one of the major complications of AP. A meta-analysis showed that 59% of AP patients had associated intestinal barrier injury[31], with increased intestinal mucosal permeability, leading to intestinal bacterial translocation, pancreatic tissue necrosis and infection, and the occurrence of MODS. It has been shown that the initial onset of caerulein-driven AP is dependent on the activation of NOD1 in acinar cells by commensal bacteria translocated from the gut, which further induces the expression of inflammatory mediators[32]. The intestinal flora can affect intestinal mucosal barrier function in various ways. First, the biological barrier is composed mainly of the normal intestinal flora and can regulate the intestinal microecological balance. In general, the intestinal flora coexists harmoniously with the human body and does not cause intestinal inflammatory reactions. However, when the intestinal flora is out of balance, the intestinal mucosal barrier can be destroyed by affecting intestinal inflammation and the immune response. Tan et al[18] found that serum IL-6 content was positively correlated with the abundance of Enterobacteriaceae and Enterococcus and negatively correlated with Bifidobacterium abundance, whereas plasma endotoxin content was positively correlated with Enterococcus abundance. This finding suggests that the inflammatory response may be related to intestinal flora imbalance. Second, the intestinal flora can also influence the mechanical barrier of the intestinal mucosa. Zhu et al[33] reported that mice receiving berberine promoted the expression of ZO-1 and Occludin in the intestinal mucosa by increasing the abundance of the beneficial bacteria Akkermansia in the intestinal tract, thus thickening the mucous layer of the intestinal mucosa and maintaining the function of the intestinal barrier. Third, Akkermansia muciniphila highly produces the pilus-like protein Amuc_1100, which is involved in host immune homeostasis of the intestinal mucosa and improves intestinal barrier function. In summary, the intestinal flora can affect AP progression by influencing the biological, mechanical and immune barriers of the intestinal mucosa[34].
POSSIBLE MECHANISM BY WHICH THE INTESTINAL FLORA AFFECTS THE INTESTINAL MUCOSAL BARRIER
In recent years, with a better understanding of intestinal microecology, studies have shown that not only the intestinal flora itself but also the metabolites of the intestinal flora participate in the regulation of body activities and metabolism. The metabolites of the intestinal flora consist mainly of SCFAs, indole derivatives, polyamines, organic acids, and vitamins. SCFAs are the most common metabolites of the gut microbiota. They include mainly acetate, propionate and butyrate, while formate, valerate, caproate, etc., are in the minority[35]. Acetate and propionate are produced mainly by Firmicutes and Bacteroidetes, which are the most prevalent bacteria, constituting 80% to 90% of the gut microbiota[24]. Acetate and propionate are produced mainly by Bacteroidetes, and Firmicutes are the primary contributors of butyrate[36]. Our previous study results showed that AP patients had intestinal flora imbalance and decreased SCFA content in the early stage of the disease, and the bacteria producing SCFAs and the SCFA contents in SAP patients were significantly reduced compared to those in MAP patients. With an understanding of SCFAs, it has been found that they can maintain intestinal mucosal barrier function.
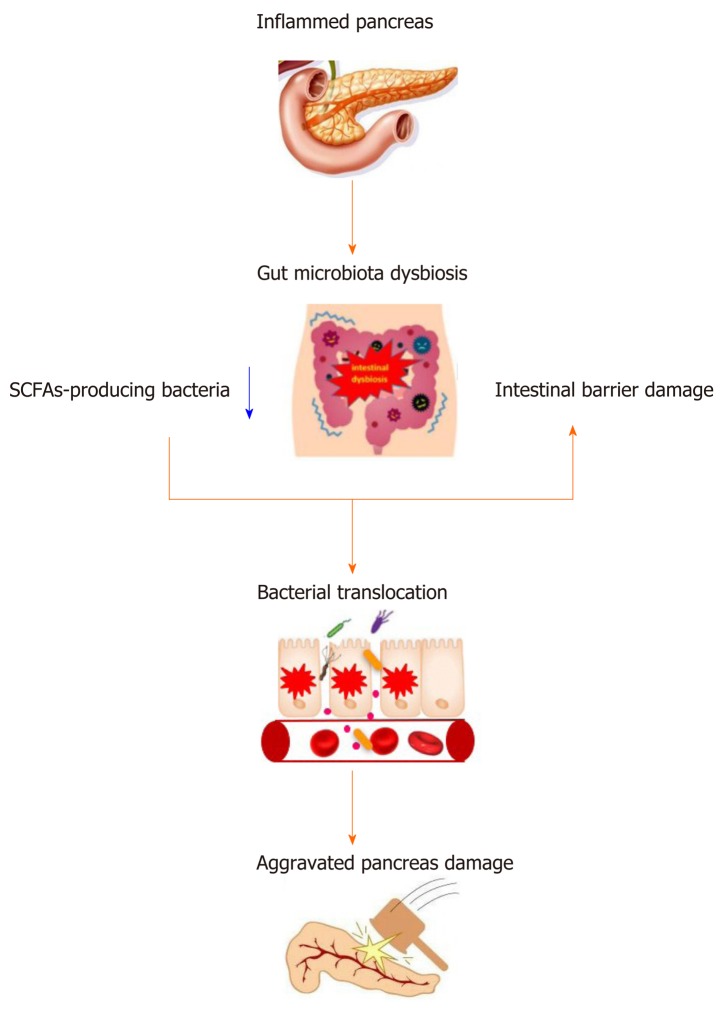
SCFAs are the main energy source of intestinal epithelial cells (IECs), and SCFAs can promote the proliferation and differentiation of IECs, reduce cell apoptosis, and play an important role in maintaining the mechanical barrier of the intestinal mucosa[37]. Studies have also shown that SCFAs can promote intestinal epithelial tight junction protein synthesis, increase the protein expression of Zo-1 and Occludin, inhibit intestinal permeability, and enhance the intestinal mucosa mechanical barrier function[38]. Moreover, SCFAs can enhance the intestinal mucosal immune barrier. Antibacterial peptides are small molecular peptides with broad-spectrum antimicrobial activities that are produced by IECs. SCFAs can promote antibacterial peptide production, including lysozyme, defensin and mucin gene expression, and increase the secretion of AMPs to enhance the immunity of the intestinal mucosa[39]. In addition, studies have found that supplementing SCFAs can increase intestinal cross-epithelial resistance, reduce intestinal mucosal permeability, and strengthen the function of the intestinal chemical barrier[40]. SCFAs can also regulate the intestinal biological barrier. SCFAs can reduce the pH of the intestinal tract, which is conducive to the growth of probiotics, while inhibiting the growth and colonization of pathogenic bacteria, such as Escherichia coli and Shigella[41]. A study revealed that butyrate could ameliorate caerulein-induced AP and intestinal injury[42]. Therefore, SCFAs play an important role in the maintenance of intestinal mucosal barrier function. During AP, gut microbiota dysbiosis with the reduction of SCFAs and intestinal barrier damage further aggravates pancreas damage and promotes the progression of AP (Figure 1).
Figure 1.
The relationship between acute pancreatitis and gut microbiota. SCFAs: Short-chain fatty acids.
REGULATION OF THE INTESTINAL FLORA MAY ALLEVIATE DAMAGE TO THE INTESTINAL MUCOSAL BARRIER DURING AP
Changes in the intestinal microbial community lead to alterations of intestinal barrier function, resulting in bacterial overgrowth and impaired immunity[43]. In 2002, a randomized double-blind controlled trial studied the efficacy of probiotic lactobacilli in the treatment of AP. The results showed that the incidence of infectious complications, such as infectious pancreatic necrosis and pancreatic abscess, was significantly lower in the probiotic treatment group than in the control group, suggesting that probiotics can improve the prognosis of AP to some extent[44]. Probiotics can enhance epithelial barrier function by dampening the proinflammatory cytokine and chemokine response, accelerating reconstitution, and altering commensal microbiota in the absence of a functional mucus barrier. However, a few years later, a study obtained the opposite result[45]. Patients who received probiotics had an increased risk of death[46]. Therefore, we need to assess the general situation of patients and then provide appropriate treatment. Lutgendorff et al[47] reported that probiotic pre-treatment beginning five days prior to the induction of AP diminished AP-induced intestinal barrier dysfunction and prevented oxidative stress via mechanisms involving mainly mucosal glutathione biosynthesis in rats. Faecal microbiota transplantation (FMT) is a method of reconstructing the normal intestinal flora and an important means of treating various diseases caused by intestinal flora disorders. During treatment, the functional flora from a faecal sample from a healthy donor is transplanted into the intestinal tract of patients, and the intestinal flora with normal functions is reconstructed to treat intestinal and extraintestinal diseases. Li et al[48] used ceftriaxone sodium to alleviate intestinal mucosal barrier injury in mice and found that after FMT treatment, intestinal mucosal injury in mice was effectively alleviated, inflammatory cell infiltration was reduced, and the secretory IgA (SIgA, an important component of the intestinal immune barrier) concentration was increased, suggesting that FMT played a certain role in the treatment of intestinal mucosal barrier injury. Our results showed that in gut microbiota-depleted mice treated with normal mouse faeces, AP induction can further damage the intestinal mucosal barrier compared to that in untreated AP mice. In summary, regulation of the intestinal flora may alleviate damage to the intestinal mucosal barrier during AP.
CONCLUSION
In summary, damage to the intestinal mucosal barrier can cause intestinal bacteria to migrate to the blood or other tissues and organs to accelerate the progression of and aggravate AP. Changes in the structure and quantity of the intestinal flora during AP are closely related to damage to the intestinal mucosal barrier, and regulating the intestinal flora to improve intestinal mucosal barrier injury may be an effective method for AP treatment. Although FMT has certain therapeutic effects on some intestinal diseases and parenteral diseases related to intestinal flora imbalance, there is a lack of basic research and clinical trials on AP, and its efficacy and safety need to be identified and confirmed to find an effective way to treat injury to the intestinal mucosal barrier during AP.
Footnotes
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Peer-review started: January 12, 2020
First decision: January 19, 2020
Article in press: April 15, 2020
P-Reviewer: Bramhall SR, Kochhar R, Manenti A S-Editor: Wang YQ L-Editor: Webster JR E-Editor: Zhang YL
Contributor Information
Xue-Yang Li, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Cong He, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
Yin Zhu, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China. zhuyin27@sina.com.
Nong-Hua Lu, Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang 330006, Jiangxi Province, China.
References
- 1.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Portelli M, Jones CD. Severe acute pancreatitis: pathogenesis, diagnosis and surgical management. Hepatobiliary Pancreat Dis Int. 2017;16:155–159. doi: 10.1016/s1499-3872(16)60163-7. [DOI] [PubMed] [Google Scholar]
- 3.Tenner S, Baillie J, DeWitt J, Vege SS American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1415; 1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 4.Maheshwari R, Subramanian RM. Severe Acute Pancreatitis and Necrotizing Pancreatitis. Crit Care Clin. 2016;32:279–290. doi: 10.1016/j.ccc.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Schietroma M, Pessia B, Carlei F, Mariani P, Sista F, Amicucci G. Intestinal permeability and systemic endotoxemia in patients with acute pancreatitis. Ann Ital Chir. 2016;87:138–144. [PubMed] [Google Scholar]
- 6.Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas. 2003;26:122–129. doi: 10.1097/00006676-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer M. Recent Research on Fecal Microbiota Transplantation in Inflammatory Bowel Disease Patients. Gastroenterol Hepatol (NY) 2019;15:44–47. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Xu CM, Liu YX, Wang XQ, Zhang L, Li M, Zhu SW, Xie ZJ, Wang PH, Duan LP, Zhu HQ. Characteristic dysbiosis of gut microbiota of Chinese patients with diarrhea-predominant irritable bowel syndrome by an insight into the pan-microbiome. Chin Med J (Engl) 2019;132:889–904. doi: 10.1097/CM9.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong TNY, Wang X, Nakatsu G, Chow TC, Tipoe T, Dai RZW, Tsoi KKK, Wong MCS, Tse G, Chan MTV, Chan FKL, Ng SC, Wu JCY, Wu WKK, Yu J, Sung JJY, Wong SH. Association Between Bacteremia From Specific Microbes and Subsequent Diagnosis of Colorectal Cancer. Gastroenterology. 2018;155:383–390.e8. doi: 10.1053/j.gastro.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, Esmaeili Taba SM, Salami M. Does Severity of Alzheimer's Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double Blind Clinical Trial. Front Neurol. 2018;9:662. doi: 10.3389/fneur.2018.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Wang N, Ma Y, Wen D. Hydroxytyrosol Improves Obesity and Insulin Resistance by Modulating Gut Microbiota in High-Fat Diet-Induced Obese Mice. Front Microbiol. 2019;10:390. doi: 10.3389/fmicb.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Yue R, Chen Y, Yang M, Huang X, Shui J, Peng Y, Chin J. Gut Microbiota, a Potential New Target for Chinese Herbal Medicines in Treating Diabetes Mellitus. Evid Based Complement Alternat Med. 2019;2019:2634898. doi: 10.1155/2019/2634898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahuja M, Schwartz DM, Tandon M, Son A, Zeng M, Swaim W, Eckhaus M, Hoffman V, Cui Y, Xiao B, Worley PF, Muallem S. Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity. Cell Metab. 2017;25:635–646. doi: 10.1016/j.cmet.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tilg H, Adolph TE. Beyond Digestion: The Pancreas Shapes Intestinal Microbiota and Immunity. Cell Metab. 2017;25:495–496. doi: 10.1016/j.cmet.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XM, Zhang ZY, Zhang CH, Wu J, Wang YX, Zhang GX. Intestinal Microbial Community Differs between Acute Pancreatitis Patients and Healthy Volunteers. Biomed Environ Sci. 2018;31:81–86. doi: 10.3967/bes2018.010. [DOI] [PubMed] [Google Scholar]
- 18.Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, Yuan H, Liu C, Li Y, Xu K. Dysbiosis of Intestinal Microbiota Associated With Inflammation Involved in the Progression of Acute Pancreatitis. Pancreas. 2015;44:868–875. doi: 10.1097/MPA.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y, Zhao J, Xia L, He W, Liu L, Luo C, Shu X, Cai Q, Chen Y, Lu N. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol. 2019;54:347–358. doi: 10.1007/s00535-018-1529-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Huang C, Wang J, Zhou H, Lu Y, Lou L, Zheng J, Tian L, Wang X, Cao Z, Zeng Y. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats. PLoS One. 2017;12:e0176583. doi: 10.1371/journal.pone.0176583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. 2012;55:1154–1163. doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 22.Ye C, Liu L, Ma X, Tong H, Gao J, Tai Y, Huang L, Tang C, Wang R. Obesity Aggravates Acute Pancreatitis via Damaging Intestinal Mucosal Barrier and Changing Microbiota Composition in Rats. Sci Rep. 2019;9:69. doi: 10.1038/s41598-018-36266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng J, Lou L, Fan J, Huang C, Mei Q, Wu J, Guo Y, Lu Y, Wang X, Zeng Y. Commensal Escherichia coli Aggravates Acute Necrotizing Pancreatitis through Targeting of Intestinal Epithelial Cells. Appl Environ Microbiol. 2019:85. doi: 10.1128/AEM.00059-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunker JJ, Erickson SA, Flynn TM, Henry C, Koval JC, Meisel M, Jabri B, Antonopoulos DA, Wilson PC, Bendelac A. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017:358. doi: 10.1126/science.aan6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC. IgA Function in Relation to the Intestinal Microbiota. Annu Rev Immunol. 2018;36:359–381. doi: 10.1146/annurev-immunol-042617-053238. [DOI] [PubMed] [Google Scholar]
- 28.Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, Antonopoulos DA, Bendelac A. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity. 2015;43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 30.Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, Gooszen HG, Akkermans LM, Kroese AB. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery. 2009;145:157–167. doi: 10.1016/j.surg.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46 Suppl:S46–S51. doi: 10.1097/MCG.0b013e3182652096. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji Y, Watanabe T, Kudo M, Arai H, Strober W, Chiba T. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity. 2012;37:326–338. doi: 10.1016/j.immuni.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Zhang D, Zhu H, Zhu J, Weng S, Dong L, Liu T, Hu Y, Shen X. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe-/- mice. Atherosclerosis. 2018;268:117–126. doi: 10.1016/j.atherosclerosis.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M, Boeren S, Satokari R, Mercenier A, Palva A, Smidt H, de Vos WM, Belzer C. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12:e0173004. doi: 10.1371/journal.pone.0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, Theil PK, Purup S, Hald S, Schioldan AG, Marco ML, Gregersen S, Hermansen K. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients. 2018:10. doi: 10.3390/nu10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23:60–75. doi: 10.3748/wjg.v23.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian L, Zhou XQ, Jiang WD, Liu Y, Wu P, Jiang J, Kuang SY, Tang L, Tang WN, Zhang YA, Xie F, Feng L. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2017;66:548–563. doi: 10.1016/j.fsi.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 40.Wang CC, Wu H, Lin FH, Gong R, Xie F, Peng Y, Feng J, Hu CH. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 2018;24:40–46. doi: 10.1177/1753425917741970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 42.Pan X, Fang X, Wang F, Li H, Niu W, Liang W, Wu C, Li J, Tu X, Pan LL, Sun J. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharmacol. 2019;176:4446–4461. doi: 10.1111/bph.14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lumsden A, Bradley EL., 3rd Secondary pancreatic infections. Surg Gynecol Obstet. 1990;170:459–467. [PubMed] [Google Scholar]
- 44.Oláh A, Belágyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–1107. doi: 10.1046/j.1365-2168.2002.02189.x. [DOI] [PubMed] [Google Scholar]
- 45.Kumar M, Kissoon-Singh V, Coria AL, Moreau F, Chadee K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2017;312:G34–G45. doi: 10.1152/ajpgi.00298.2016. [DOI] [PubMed] [Google Scholar]
- 46.Venkatesan T. Probiotic Prophylaxis in Predicted Severe Acute Pancreatitis: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutr Clin Pract. 2008;23:662–663. doi: 10.1177/0884533608326323. [DOI] [PubMed] [Google Scholar]
- 47.Lutgendorff F, Nijmeijer RM, Sandström PA, Trulsson LM, Magnusson KE, Timmerman HM, van Minnen LP, Rijkers GT, Gooszen HG, Akkermans LM, Söderholm JD. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS One. 2009;4:e4512. doi: 10.1371/journal.pone.0004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Liang P, Li Z, Wang Y, Zhang G, Gao H, Wen S, Tang L. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front Microbiol. 2015;6:692. doi: 10.3389/fmicb.2015.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]