Abstract
BACKGROUND
Hepatic encephalopathy (HE) is a reversible neuropsychiatric complication of liver cirrhosis and occurs in up to 50% of cirrhotic patients. Studies examining the prognostic significance of HE are limited despite the high prevalence in cirrhosis.
AIM
To define the clinical outcomes of patients after an episode of HE treated with current standards-of-care.
METHODS
All patients hospitalised with HE requiring Rifaximin to 3 tertiary centres over 46-mo (2012–2016) were identified via pharmacy dispensing records. Patients with hepatocellular carcinoma and those prescribed Rifaximin prior to admission were excluded. Medical records were reviewed to determine baseline characteristics and survival. The Kaplan-Meier method was used to calculate survival probability. Univariate survival analysis was performed with variables reaching statistical significance included in a multivariate analysis. The primary outcome was 12-mo mortality following commencement of Rifaximin.
RESULTS
188 patients were included. Median age was 57 years (IQR 50-65), 71% were male and median model for end stage liver disease and Child Pugh scores were 25 (IQR 18-31) and 11 (IQR 9-12) respectively. The most common causes of cirrhosis were alcohol (62%), hepatitis C (31%) and non-alcoholic fatty liver disease (20%). A precipitating cause for HE was found in 92% patients with infection (43%), GI bleeding (16%), medication non-compliance (15%) and electrolyte imbalance (14%) the most common. During a mean follow up period of 12 ± 13 mo 107 (57%) patients died and 32 (17%) received orthotopic liver transplantation. The most common causes of death were decompensated chronic liver disease (57%) and sepsis (19%). The probability of survival was 44% and 35% at 12- and 24-mo respectively. At multivariate analysis a model for end stage liver disease > 15 and international normalised ratio reached statistical significance in predicting mortality.
CONCLUSION
Despite advances made in the management of HE patients continue to have poor survival. Thus, in all patients presenting with HE the appropriateness of orthotopic liver transplantation should be considered.
Keywords: Hepatic encephalopathy, Cirrhosis, Portal hypertension, Prognosis, Rifaximin, Lactulose
Core tip: The development of hepatic encephalopathy in cirrhotic patients continues to be associated with an extremely poor prognosis despite current standards-of-care and newer therapeutic options such as Rifaximin. In this study, the probability of survival at 12-mo was 44% after an episode of acute hepatic encephalopathy requiring hospital admission. Thus, in all patients with hepatic encephalopathy the appropriateness of urgent liver transplantation assessment should be considered.
INTRODUCTION
Prognosis in patients with liver cirrhosis varies significantly depending on whether a patient has compensated or decompensated cirrhosis[1,2]. In patients with compensated cirrhosis, median survival is greater than 12 years. By contrast, in patients experiencing a decompensation, commonly defined by ascites, hepatic encephalopathy (HE), variceal haemorrhage and jaundice, survival is far shorter at two years or less[3-5].
HE is defined as a reversible neuropsychiatric complication of liver cirrhosis. It represents the second most common decompensating event after ascites and will occur in 30%-45% of cirrhotic patients during their lifetime[6,7]. HE manifests as a wide spectrum of neuropsychiatric abnormalities and motor disturbance, ranging from mild alterations in cognitive function to coma and death[8,9]. The clinical features of HE define the grade of encephalopathy, with the West Haven criteria most commonly employed to stage the severity of disease[10]. Plasma ammonia levels are typically elevated in patients with HE, however this is not a reliable sign and is not used in the diagnosis of HE[11]. The current treatment priorities in HE are to identify and reverse the underlying precipitants, which include sepsis, gastrointestinal bleeding, medications such as benzodiazepines, opiates and anti-histamines, acid-base disturbances, renal impairment and constipation[2]. Traditionally, pharmacological therapies have aimed to decrease plasma ammonia levels. Lactulose, which decreases colonic pH and plasma ammonia levels has been the mainstay of treatment for many years. More recently, Rifaximin, a broad-spectrum semisynthetic derivative of rifamycin with minimal systemic absorption, has been added to the therapeutic armamentarium in addition to the use of lactulose[12-14]. Multiple other therapeutic options require further trials to clearly define their role in the management of HE[15-18].
The natural history and prognosis of patients with ascites and variceal bleeding has been extensively studied, however, despite its prevalence there is a paucity of literature related to the prognostic significance of HE. Two sentinel studies published prior to the development of rifaximin demonstrated that development of HE was associated with an extremely poor survival in patients with cirrhosis who did not receive liver transplantation[3,19]; Bustamante et al[19] demonstrated a survival probability of 42% and 23% at one and three years respectively in cirrhotic patients after development of a first episode of acute HE. In the post-Rifaximin era, there is a paucity of literature investigating the prognosis of cirrhotic patients following an episode of HE[20]. To our knowledge, survival of patients with a presentation of HE in the era of rifaximin has yet to be studied in an Australian real-world cohort.
In this study, we evaluated the clinical outcomes and probability of survival amongst cirrhotic patients who presented with acute HE requiring hospital admission and were commenced on rifaximin. In addition, we aimed to identify factors associated with mortality.
MATERIALS AND METHODS
Patient selection and data collection
All patients admitted with HE to three Australian tertiary centres, including one transplant centre, over a 42-mo period from May 2012 to March 2016 who were prescribed rifaximin were identified retrospectively via pharmacy dispensing records. Inclusion criteria were that rifaximin must have been commenced during an inpatient admission for HE and continued upon discharge from hospital. Patients with hepatocellular carcinoma (HCC) diagnosed prior to or during the index admission with HE were excluded from the study. Diagnosis of HCC was established by standard imaging techniques (CT Quad Phase Liver or MRI Liver) and/or serum alpha foetoprotein and/or histological examination. The Human Research Ethics Committee at Monash Health approved the study as audit activity and the committee provided a waiver for informed consent.
For each patient, medical records were manually reviewed to collect baseline demographic data, medical comorbidities, aetiology of liver disease, medication use, laboratory results, evidence of decompensated liver disease, precipitating causes of HE and previous and current treatments of HE. Patient outcome data up to 48-mo following the index admission was collected. Death was determined through hospital medical records and confirmed with a patient’s Local Medical Officer if required. Each medical record was independently reviewed by two reviewers and any discrepancies in data were referred to a third reviewer. All patients were risk stratified using the model for end stage liver disease (MELD) and Child-Pugh (CP) scores; when calculating the CP score, the serum albumin level prior to intravenous albumin administration was used. The diagnosis and grade of HE was determined using established West Haven criteria[9].
All patients were followed-up from the date of rifaximin commencement until the date of death, liver transplantation or last known survival up to 48-mo following index admission. The primary outcome was 12-mo survival following the commencement of rifaximin. The secondary outcome was to identify patient-specific prognostic factors at the time of commencement of rifaximin that would be useful in determining suitability for a liver transplant.
Treatment protocols for hepatic encephalopathy
Patients with cirrhosis and HE are admitted under a specialist Gastroenterology or Liver Transplant Unit. In all patients treatment of HE consists of identification and correction of possible precipitating factors. Intravenous albumin (1.5 g/kg per day) is typically administered consistent with evidence that it shortens the duration of acute HE[21]. In our centres, regular Lactulose (administered orally or rectally in the setting of reduced mental state) is given as first-line therapy and rifaximin is typically reserved for patients with recurrent or refractory HE.
Statistical analysis
Survival probability curves were calculated using the Kaplan-Meier method. Univariate survival analysis was performed using the Cox proportional hazards model to analyse each considered variable, which included demographic data, maximal grade of HE, precipitating factors of HE, MELD and CP scores and clinical and laboratory data at the time of HE. Variables which reached statistical significance (P ≤ 0.05) in the univariate analysis were subsequently included in a multivariate analysis to identify variables independently associated with survival. We used the stepwise Cox regression procedure (variables entered if P ≤ 0.10, variables removed if P ≥ 0.15). Statistical analysis was carried out using R for windows (version 1.1.419) through the survival package as well as through MedCalc (version 19.0.7).
RESULTS
Total 365 patients with acute HE necessitating hospital admission were prescribed rifaximin during the study period. Total 177 (48%) patients were excluded from the study, leaving a total of 188 patients for analysis (Figure 1). Reasons for exclusion included: pre-existing use of rifaximin prior to admission in 134 (37%) patients, the presence of HCC in 41 (11%) patients and no identifiable start date for rifaximin in 2 (0.5%) patients.
Figure 1.
Recruitment flowchart. HE: Hepatic encephalopathy; HCC: Hepatocellular carcinoma.
Characteristics of patients
There were 133 males and 55 females with a median age of 57 years (IQR 50–65). All patients had established cirrhosis based on hospital records compiled from previous histological and radiology data. The most common aetiologies of cirrhosis were: Alcohol (70 patients), non-alcoholic fatty liver disease (24 patients) and hepatitis C (20 patients) (Table 1). Four patients had previously received a transjugular intrahepatic portosystemic shunt procedure. The median CP score was 11 (IQR 9-12) and 3, 39 and 120 patients had Child A, B and C cirrhosis respectively on admission; 26 patients had insufficient documentation to accurately calculate a CP score. The median MELD score was 25 (IQR 18-31) with 77% patients having a MELD score ≥ 15. Baseline patient characteristics and laboratory data are shown in Tables 1 and 2.
Table 1.
Baseline characteristics of study patients
| Parameter | n (%) |
| Age (yr) | 57 (IQR 50–65) |
| Male | 133 (71) |
| Current smoker | 70 (37) |
| Co morbidities | |
| IHD | 16 (9) |
| DM | 64 (34) |
| CCF | 16 (9) |
| Previous CVA | 15 (8) |
| COPD | 14 (7) |
| Non-HCC malignancy | 20 (11) |
| CKD | 39 (21) |
| Ascites | 138 (73) |
| Aetiology of cirrhosis | |
| Alcohol | 117 (62) |
| HBV | 6 (3) |
| HCV | 59 (31) |
| NASH | 37 (20) |
| PSC | 6 (3) |
| AIH | 4 (2) |
| PBC | 1 (1) |
| Other | 11 (6) |
| Child Pugh score | 11 (IQR 9-12) |
| CPA | 3 (2) |
| CPB | 39 (21) |
| CPC | 120 (64) |
| Unknown | 26 (14) |
| MELD | 25 (IQR 18-31) |
| Hepatic encephalopathy | |
| Grade 1 | 22 (5) |
| Grade 2 | 93 (23) |
| Grade 3 | 38 (57) |
| Grade 4 | 9 (14) |
| Unknown grade | 26 |
| Previous episode of encephalopathy | 75 |
| Median duration of encephalopathy (d) | 7 (IQR 2-9) |
Continuous variables are presented as median (interquartile range). IHD: Ischemic heart disease; DM: Diabetes mellitus; CCF: Congestive cardiac failure; CVA: Cerebrovascular event; COPD: Chronic obstructive pulmonary disease; HCC: Hepatocellular cancer; CKD: Chronic kidney disease; HCV: Hepatitis C; HBV: Hepatitis B; NASH: Non-alcoholic steatohepatitis; PSC: Primary sclerosing cholangitis; AIH: Autoimmune hepatitis; PBC: Primary biliary cirrhosis; CPA: Child's Pugh A; CPB: Childs Pugh B; CPC: Childs Pugh C; MELD: Model for end-stage liver disease.
Table 2.
Baseline laboratory parameters
| Parameters | mean ± SD |
| Haemoglobin (g/L) | 90 ± 20 |
| Platelet (× 109/L) | 86.1 ± 64 |
| White cell count (× 109/L ) | 8 ± 6 |
| Bilirubin (µmol/L) | 151 ± 168 |
| Albumin (g/L) | 25 ± 7 |
| ALT (U/L) | 135 ± 468 |
| ALP (U/L) | 166 ± 106 |
| GGT (U/L) | 154 ± 262 |
| INR | 2.1 ± 1.0 |
| Urea (mmol/L) | 11 ± 7 |
| Creatinine (micromol/L) | 161 ± 138 |
| Sodium (mmol/L) | 133 ± 7 |
| Potassium (mmol/L) | 4.5 ± 1.0 |
ALT: Alanine transaminase; ALP: Alkaline Phosphatase; GGT: Gamma glutamyl transferase; INR: international normalised ratio.
A likely precipitant of decompensated cirrhosis with acute HE was identified in 173 (92%) patients (Table 3); in many patients this was felt to be multi-factorial with more than one precipitant identified. Alone or in combination, the most commonly identified causes for HE were: Infection (including spontaneous bacterial peritonitis) in 81 (43%) patients, gastrointestinal bleeding in 31 (16%), constipation in 35 (19%) and non-compliance with prescribed medications in 29 (15%). In relation to the severity of HE, the West Haven HE grades were available in 162 (86%) patients (Table 1), with 22 (14%), 93 (57%), 38 (23%) and 9 (5%) patients recording a maximal HE grade of 1, 2, 3 and 4 respectively. Thirty-three (18%) patients required admission to an intensive care ward. In addition to rifaximin, 166 (88%) patients received either oral or rectal lactulose with a mean dose of 177 mL, 13 (7%) patients received macrogol (polyethylene glycol “3350”) and 19 patients received other forms of aperients.
Table 3.
Precipitating factors for hepatic encephalopathy (alone or in combination with other factors)
| Precipitating factors | n (%) |
| Infection including SBP | 81 (43) |
| Gastrointestinal bleeding | 31 (16) |
| Constipation (opiate-induced) | 10 (5) |
| Constipation (not opiate induced) | 25 (13) |
| Benzodiazepine use | 10 (5) |
| Noncompliance to regular medications | 29 (15) |
| Electrolyte imbalance | 27 (14) |
| Other | 24 (13) |
| Unknown | 15 (8) |
SBP: Spontaneous bacterial peritonitis.
Documentation of resolution of encephalopathy was identified in 133 patients prior to discharge with a median duration to resolution of symptoms of 7 d (IQR 2–9 d). Of the remaining 55 patients, 20 died prior to resolution of HE and in the other 35 documentation was insufficient to determine whether HE has resolved at the time of discharge.
Mortality and prognostic factors
Within a mean follow-up period of 12 ± 13 mo, 107 (57%) patients died and 32 (17%) received liver transplantation. 42 patients died during or within 30-d of the index admission in which rifaximin was commenced. Causes of death included liver failure in 61 (57%) patients, sepsis in 19 (18%), unknown cause in 12 (11%), non-HCC malignancy in 4 (4%), cerebrovascular accidents in 4 (4%), gastrointestinal bleeding in 4 (4%), ischemic gut in 1 (1%) and cardiopulmonary arrest in 2 (1%) patients. The probably of survival in the entire cohort was 44% at 12-mo, 35% at 24-mo and 29% at 36-mo (Figure 2).
Figure 2.
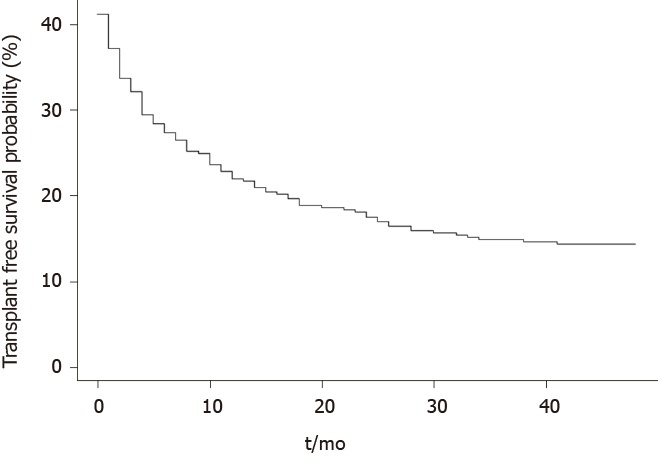
Transplant-free survival probability following commencement of rifaximin.
Twenty-seven variables were included in the univariate analysis, of which 10 were significantly associated with a poor prognosis: Hepatitis C infection, infection as the precipitant for HE, serum bilirubin, urea, creatinine, international normalised ratio (INR), white cell count, CP score, MELD and a MELD score ≥ 15 (vs ≤ 15). These variables were subsequently introduced into the multivariate analysis. The multivariate analysis (performed in the 159 patients in whom all variables were available) identified two variables as statistically significant, independent prognostic factors: A MELD score ≥ 15 and INR (Table 4).
Table 4.
Hazard ratio for the different variables investigated by univariate analysis and multivariate analysis as possible prognostic factors in 188 cirrhotic patients presenting with hepatic encephalopathy and commenced on rifaximin
| Variable | Univariate hazard ratio using cox regression (95%CI)1 | Multivariate hazard ratio (95%CI)1 |
| Age | 1.014 (0.99, 1.03) | |
| Sex (male vs female) | 1.087 (0.75, 1.58) | |
| Aetiology of cirrhosis2 | ||
| HBV infection | 0.76 (0.28, 2.05) | |
| HCV infection | 0.62 (0.42, 0.91)a | |
| Alcohol | 0.92 (0.65, 1.30) | |
| Precipitating factors2 | ||
| Gastrointestinal bleed | 0.75 (0.46, 1.22) | |
| Infection | 1.49 (1.03, 2.15)a | |
| Diuretic therapy | 1.47 (0.94, 2.32) | |
| Constipation | 1.19 (0.73, 1.95) | |
| Ascites at the time of HE2 | 1.11 (0.77, 1.59) | |
| Maximal grade of HE (grade 3 and 4 vs grade 1 and 2) | 0.80 (0.53, 1.21) | |
| Serum values3 | ||
| Bilirubin | 1.001 (1, 1.002)a | |
| ALT | 1 (0.99, 1.003) | |
| GGT | 1 (1, 1) | |
| Albumin | 0.97 (0.95, 1.002) | |
| Urea | 1.05 (1.02, 1.09)a | |
| Creatinine | 1.001 (1, 1.002)a | |
| Sodium | 0.99 (0 .97, 1.03) | |
| Potassium | 1.16 (0.96, 1.40) | |
| INR | 1.5 (1.21, 1.85)a | 1.27 (1.04, 1.54)a |
| Hb | 0.98 (0.97, 1.01) | |
| WCC | 1.06 (1.02, 1.10)a | |
| Plt | 1.00 (0.99, 1.01) | |
| Child Pugh Score (C vs A/B) | 1.57 ( 1.02, 2.41)a | |
| MELD | 1.03 (1.01, 1.06)a | |
| MELD (≥ 15 vs ≤ 15) | 2.41 ( 1.20. 4.85)a | 2.17 (1.07, 4.43)a |
In brackets: 95% confidence interval.
Presence vs absence.
Hazard ratio per unit increase.
P ≤ 0.05. HE: Hepatic encephalopathy; HCV: Hepatitis C; HBV: Hepatitis B; ALT: Alanine transaminase; GGT: Gamma glutamyl transferase; INR: international normalised ratio; Hb: Haemoglobin; WCC: White cell count; Plt: Platelet count; MELD: Model for end-stage liver disease.
DISCUSSION
Hepatic encephalopathy remains a common complication in patients with liver cirrhosis. Our study demonstrates that development of HE necessitating hospital admission in cirrhotic patients is associated with a short life expectancy in the absence of liver transplantation despite current standards-of-care. The cumulative survival at 12-, 24- and 36-mo were 44%, 35% and 29% respectively with the majority of patients dying from complications of decompensated liver disease or liver failure. At multi-variate analysis the variables significantly associated with mortality were a MELD score ≥ 15 and INR.
Our study represents one of the largest real-world studies to investigate the prognostic significance of HE in the era of rifaximin. Study inclusion criteria were broad and simple, including all cirrhotic patients admitted with acute HE and commenced on rifaximin to three metropolitan tertiary centres in Australia, including one transplant centre, with a total catchment area of approximately two million people. The study population consisted of patients with decompensated cirrhosis managed on a specialist Gastroenterology ward who received treatment consistent with recent practice guidelines. Study results thus represent real-world data and should be widely applicable to other treating centres.
The results of this study correlate with sentinel studies from the pre-rifaximin era. Bustamante et al[19] demonstrated a similar 12-mo survival probability of 42% amongst patients experiencing their first presentation with HE where lactulose was the primary pharmacological management option. In addition, Stewart et al[3] demonstrated that higher grades of HE corresponded to increased mortality rates with an overall survival of less than 20% at 36-mo in patients presenting with Grade 3 HE[3,19].
Following the introduction of rifaximin various studies have sought to assess whether the survival probability has improved in cirrhotic patients following an episode of HE. Sharma et al[8] demonstrated in a randomised control trial that the 10-d survival following the commencement of rifaximin plus lactulose for the management of HE was superior to patients receiving lactulose alone. A larger retrospective cohort study by Kang et al[22], of 421 patients with HE of whom 145 received rifaximin found rifaximin use to be independently associated with a decreased risk of death. Despite a similar median CP score (10 vs 11), this study demonstrated a survival probability at 12-, 24-, 36- and 48-mo of 70%, 68%, 64% and 63% respectively[22], significantly higher than the cumulative survival found in our cohort. The likely reason for this discrepancy in survival is that in the Kang et al[22] study, patients were enrolled after discharge from the index HE admission and patients who died within 2-d of recovery were excluded. Consistent with the study by Bustamante et al[19], we elected to enrol patients during the index admission in which rifaximin was commenced and patients in our cohort had a 22% 30-d mortality. Furthermore, in Australia, prescribing guidelines necessitate that rifaximin be used only in recurrent or refractory episodes of HE and thus it is typically employed as a second-line agent after Lactulose. Consistent with this, 40% of our patient cohort had experienced an episode of HE prior to the index admission.
Within our cohort, multiple clinical and standard laboratory variables were significantly associated with a poor prognosis at univariate analysis. Five laboratory variables were independently associated with a poor prognosis: Increased serum bilirubin, urea, creatinine, INR and decreased white cell count. Of these variables, bilirubin, renal function and INR are commonly utilised in prognostic risk stratification algorithms and have clear relationships with poor prognosis in patients with liver cirrhosis[23,24]. In addition, Childs Pugh C class cirrhosis and a MELD score ≥ 15 were both associated with a poor prognosis which is in keeping with their known value in prognosticating survival in advanced liver disease[23,24]. The prognostic significance of leukopaenia in HE requires further investigation. Other studies have not found a low white cell count to be a significant prognostic factor[19], however Qamar et al[25] demonstrated that leukopenia in patients with compensated cirrhosis predicted and increased mortality. Following multivariate analysis, a MELD score ≥ 15 and INR were found to be independently associated with a poor prognosis. A MELD score ≥ 15 was selected as the cut-off given data that patients with a MELD > 15 have higher mortality and shortened survival compared to those who proceed to liver transplantation assessment[26]. All measured components of the MELD were found to be prognostically significant within the univariate analysis but only an elevated INR was independently significant at multivariate analysis.
Interestingly, in our study the grade of HE did not reach statistical significance in predicting mortality in either univariate of multivariate analysis. This finding is discordant with some previous studies including the paper by Bustamante et al[19], in which higher grades of HE were found to be significant at univariate analysis but not on multivariate analysis. In addition, Bajaj et al[13] performed a large retrospective analysis of patients with alcohol-related cirrhosis and HE, finding that higher grades of HE were associated with a higher 30-d mortality. By comparison, Stewart et al[3] found on multivariate analysis that in hospitalised patients with HE, the presence of HE was a strong predictor of mortality however there was no difference detected between Grades 2 and 3 HE. One possible reason for our findings may be a type-2 error with insufficient patient numbers to demonstrate a statistically significant difference between Grades of encephalopathy. In our cohort, 80% patients had a maximum encephalopathy grade of 2 or 3 with few patients diagnosed with Grades 1 or 4.
In our cohort, the vast majority of patients had an identifiable precipitant for the development of HE. Overwhelmingly, HE occurred in patients with advanced, decompensated cirrhosis and portal hypertension and was most commonly associated with other co-existing complications of decompensated cirrhosis such as ascites with spontaneous bacterial peritonitis and gastrointestinal bleeding. This is consistent with previous observations that HE occurs relatively late in the natural history of cirrhosis and previous studies have also demonstrated an association between MELD score and the development of HE[14]. Indeed, it has been postulated that for HE to occur, decreased hepatic function and portosystemic shunting are necessary to allow putative toxic molecules to reach the cerebral circulation[3].
Our study has certain limitations including its retrospective design, meaning all data collection was ascertained through existing clinical records which were generated by multiple health practitioners in a non-standardised fashion. Inherent with retrospective studies, not all data points were available in all patients which potentially affects the statistical analysis. Errors were minimised by using a small number of data collectors who entered information into a standardised database and each medical record was independently reviewed by two researchers. Secondly, our study population was recruited from tertiary centres and consisted entirely of patients with decompensated liver cirrhosis with portal hypertension. All patients required hospital admission for acute HE and 73% had concurrent ascites. The median Child Pugh score of 11 and MELD score of 25 reflects that our population had advanced liver disease and were unwell at the time of hospital admission. Patients with advanced liver disease have a poor prognosis irrespective of the development of encephalopathy. The 30-d mortality in this study was 22% which is higher than that recorded by patients with acute variceal bleeding in recent studies[27,28] and again reflects that acute HE is associated with a very guarded prognosis.
Finally, due to the retrospective nature of the study it was not possible to accurately assess nutritional therapy during the acute course of encephalopathy and this is obviously an important factor in any patient with decompensated cirrhosis.
In conclusion, the development of HE in patients with cirrhosis still confers an extremely poor prognosis with low probability of transplant-free survival despite current standards-of-care. In all cirrhotic patients, development of HE should prompt consideration of the appropriateness of urgent liver transplantation assessment. Further prospective studies are required to investigate whether there is a survival benefit of rifaximin in patients with advanced cirrhosis and encephalopathy.
ARTICLE HIGHLIGHTS
Research background
Hepatic encephalopathy (HE) is a common neuropsychiatric complication in patients with liver cirrhosis and represents the second most common decompensating event after ascites. The current treatment approach for HE includes the reversal of identifiable underlying precipitants and the use of ammonia-lowering agents such as lactulose and rifaximin.
Research motivation
Previous sentinel studies have demonstrated that development of HE is associated with extremely poor transplantation-free survival. There remains a paucity of literature examining the natural history and prognosis of HE in the post-rifaxamin era.
Research objectives
We aimed to evaluate the clinical outcomes and survival probability of cirrhotic patients who developed acute HE requiring admission to hospital and were treated with rifaxamin in addition to current standards-of-care. In addition, we aimed to identify factors at the time of HE that could predict mortality and highlight the need to consider liver transplantation.
Research methods
We performed a retrospective, multi-centre analysis of 188 patients admitted with HE and commenced on rifaxmin with a mean follow-up period of 12 ± 13 mo. Survival probability curves were calculated using the Kaplan-Meier method. Univariate survival analysis was performed using the Cox proportional hazards model. Variables which reached statistical significance (P ≤ 0.05) were subsequently included in a multivariate analysis to identify factors independently associated with survival using the stepwise Cox regression procedure.
Research results
In patients with acute HE requiring hospital admission and treated with current standards-of-care, the probability of survival remains poor with a 1- and 3-year survival probability of 44% and 29% respectively. The majority of patients have an identifiable precipitant for HE and the most common cause of death was liver failure or complications of decompensated cirrhosis. Baseline international normalised ratio and a model for end stage liver disease score ≥ 15 reached statistical significance on multivariate analysis to predict mortality.
Research conclusions
Despite advances in treatment, the development of acute HE in cirrhotic patients continues to confer an extremely poor prognosis and a low probability of survival in the absence of liver transplantation. Both international normalised ratio, a marker of synthetic liver dysfunction, and model for end stage liver disease score, which is well-validated to prognosticate survival in advanced liver disease, were able to independently predict survival probability at the time of admission.
Research perspectives
The development of HE in a cirrhotic patient is an extremely serious complication that typically occurs late in the disease process and confers an extremely poor prognosis. Inpatient management of HE with current standards-of-care can successfully resolve the episode of HE in the majority of cases but has limited ability to affect the natural sequalae of the advanced disease state. In all cirrhotic patients, the development of HE should prompt consideration of the appropriateness of liver transplantation. Further prospective studies would be useful to investigate the survival benefits of rifaxamin in patients with advanced cirrhosis and HE.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Human Research Ethics Committee at Monash Health as audit activity.
Informed consent statement: The Human Research Ethics Committee at Monash Health provided a waiver for informed consent. Reference number: RES-19-0000-296Q.
Conflict-of-interest statement: We have no financial relationships to disclose.
Manuscript source: Invited Manuscript
Peer-review started: December 31, 2019
First decision: February 19, 2020
Article in press: May 1, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manenti A, Silva GF S-Editor: Zhang H L-Editor: A E-Editor: Li X
Contributor Information
Anuj Bohra, Department of Gastroenterology, Monash Health, Clayton 3168, Victoria, Australia. anujbohra@hotmail.com.
Thomas Worland, Department of Gastroenterology, Monash Health, Clayton 3168, Victoria, Australia; Department of Gastroenterology, Austin Health, Heidelberg 3084, Victoria, Australia.
Samuel Hui, Department of Gastroenterology, Monash Health, Clayton 3168, Victoria, Australia.
Ryma Terbah, Department of Gastroenterology, Austin Health, Heidelberg 3084, Victoria, Australia.
Ann Farrell, Department of Gastroenterology, Monash Health, Clayton 3168, Victoria, Australia.
Marcus Robertson, Department of Gastroenterology, Monash Health, Clayton 3168, Victoria, Australia; Department of Gastroenterology, Austin Health, Heidelberg 3084, Victoria, Australia; Department of Medicine, School of Clinical Sciences, Monash University, Clayton 3168, Victoria, Australia.
References
- 1.Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407–1414. doi: 10.1111/j.1478-3231.2012.02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 3.Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl. 2007;13:1366–1371. doi: 10.1002/lt.21129. [DOI] [PubMed] [Google Scholar]
- 4.Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 5.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Romero-Gómez M, Boza F, García-Valdecasas MS, García E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96:2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 7.Dharel N, Bajaj JS. Definition and nomenclature of hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S37–S41. doi: 10.1016/j.jceh.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 9.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 10.Ferenci P. Hepatic encephalopathy. Gastroenterol Rep (Oxf) 2017;5:138–147. doi: 10.1093/gastro/gox013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003;38:441–446. doi: 10.1016/s0168-8278(02)00436-1. [DOI] [PubMed] [Google Scholar]
- 12.American Association for the Study of Liver Diseases, European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 14.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, Teperman L, Hillebrand D, Huang S, Merchant K, Shaw A, Bortey E, Forbes WP. Rifaximin Treatment in Hepatic Encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 15.Sushma S, Dasarathy S, Tandon RK, Jain S, Gupta S, Bhist MS. Sodium benzoate in the treatment of acute hepatic encephalopathy: a double-blind randomized trial. Hepatology. 1992;16:138–144. doi: 10.1002/hep.1840160123. [DOI] [PubMed] [Google Scholar]
- 16.Rahimi RS, Singal AG, Cuthbert JA, Rockey DC. Lactulose vs polyethylene glycol 3350--electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174:1727–1733. doi: 10.1001/jamainternmed.2014.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martí-Carvajal AJ, Gluud C, Arevalo-Rodriguez I, Martí-Amarista CE. Acetyl-L-carnitine for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2019;1:CD011451. doi: 10.1002/14651858.CD011451.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentile S, Guarino G, Romano M, Alagia IA, Fierro M, Annunziata S, Magliano PL, Gravina AG, Torella R. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3:184–191. doi: 10.1016/s1542-3565(04)00667-6. [DOI] [PubMed] [Google Scholar]
- 19.Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890–895. doi: 10.1016/s0168-8278(99)80144-5. [DOI] [PubMed] [Google Scholar]
- 20.Bannister CA, Orr JG, Reynolds AV, Hudson M, Conway P, Radwan A, Morgan CL, Currie CJ. Natural History of Patients Taking Rifaximin-α for Recurrent Hepatic Encephalopathy and Risk of Future Overt Episodes and Mortality: A Post-hoc Analysis of Clinical Trials Data. Clin Ther. 2016;38:1081–1089.e4. doi: 10.1016/j.clinthera.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Sharma BC, Singh J, Srivastava S, Sangam A, Mantri AK, Trehanpati N, Sarin SK. Randomized controlled trial comparing lactulose plus albumin versus lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol. 2017;32:1234–1239. doi: 10.1111/jgh.13666. [DOI] [PubMed] [Google Scholar]
- 22.Kang SH, Lee YB, Lee JH, Nam JY, Chang Y, Cho H, Yoo JJ, Cho YY, Cho EJ, Yu SJ, Kim MY, Kim YJ, Baik SK, Yoon JH. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther. 2017;46:845–855. doi: 10.1111/apt.14275. [DOI] [PubMed] [Google Scholar]
- 23.Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, Testa E, Risso D, Colla G, Testa R. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. 2003;52:134–139. doi: 10.1136/gut.52.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albers I, Hartmann H, Bircher J, Creutzfeldt W. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269–276. doi: 10.3109/00365528909093045. [DOI] [PubMed] [Google Scholar]
- 25.Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Makuch R, Rendon G Portal Hypertension Collaborative Group. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689–695. doi: 10.1016/j.cgh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The Survival Benefit of Liver Transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 27.Robertson M, Ng J, Abu Shawish W, Swaine A, Skardoon G, Huynh A, Deshpande S, Low ZY, Sievert W, Angus P. Risk stratification in acute variceal bleeding: Comparison of the AIMS65 score to established upper gastrointestinal bleeding and liver disease severity risk stratification scoring systems in predicting mortality and rebleeding. Dig Endosc. 2019 doi: 10.1111/den.13577. [DOI] [PubMed] [Google Scholar]
- 28.Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, Keough A, Llop E, González A, Seijo S, Berzigotti A, Ma M, Genescà J, Bosch J, García-Pagán JC, Abraldes JG. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412–419.e3. doi: 10.1053/j.gastro.2013.10.018. [DOI] [PubMed] [Google Scholar]


