Abstract
Ceramides are significant metabolic products of sphingolipids in lipid metabolism and are associated with insulin resistance and hepatic steatosis. In chronic inflammatory pathological conditions, hypoxia occurs, the metabolism of ceramide changes, and insulin resistance arises. Hypoxia-inducible factors (HIFs) are a family of transcription factors activated by hypoxia. In hypoxic adipocytes, HIF-1α upregulates pla2g16 (a novel HIF-1α target gene) gene expression to activate the NLRP3 inflammasome pathway and stimulate insulin resistance, and adipocyte-specific Hif1a knockout can ameliorate homocysteine-induced insulin resistance in mice. The study on the HIF-2α—NEU3—ceramide pathway also reveals the role of ceramide in hypoxia and insulin resistance in obese mice. Under obesity-induced intestinal hypoxia, HIF-2α increases the production of ceramide by promoting the expression of the gene Neu3 encoding sialidase 3, which is a key enzyme in ceramide synthesis, resulting in insulin resistance in high-fat diet-induced obese mice. Moreover, genetic and pathophysiologic inhibition of the HIF-2α—NEU3—ceramide pathway can alleviate insulin resistance, suggesting that these could be potential drug targets for the treatment of metabolic diseases. Herein, the effects of hypoxia and ceramide, especially in the intestine, on metabolic diseases are summarized.
Keywords: Ceramide, Intestinal hypoxia, Insulin resistance, Diabetes mellitus, Hypoxia-inducible factors, Obesity
Core tip: Hypoxia is an essential risk factor that promotes insulin resistance in a variety of tissues, such as adipocytes, intestines, and the liver. In hypoxic adipocytes, hypoxia-inducible factor-1α upregulates pla2g16 gene expression to activate the NLRP3 inflammasome pathway, leading to insulin resistance. In obese animals or people, increased ceramide further results in insulin resistance under hypoxia. In intestinal epithelial cells, hypoxia-inducible factor-2α is activated and accumulates under hypoxia in high-fat diet-fed mice, which upregulates the target gene Neu3, accelerating the process of insulin resistance.
INTRODUCTION
Diabetes mellitus is caused by abnormal secretion or utilization of insulin, resulting in disorders of carbohydrate, protein, and fat metabolism. Hyperglycemia is the primary symptom that can induce visual lesions and impair the kidney, heart, brain, and other organs. Diabetes is characterized by high morbidity and mortality, which brings serious economic and medical burdens to modern society. According to the latest report of the International Diabetes Federation, there were 463 million patients with diabetes in the world in 2019, which is expected to reach 700 million in 2045 at a growth rate of 51%. According to the Global Burden of Disease Study 2013, in 2013, globally, 1.47 million people died because of diabetes and its complications[1]. In 2019, the International Diabetes Federation estimates that 4.2 million people worldwide died from diabetes every year, which was one of the three major causes of noncommunicable diseases worldwide[2].
Insulin resistance is another essential clinical feature of diabetes mellitus. Both weight gain and obesity are important risk factors for metabolic diseases such as type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD)[3]. It is universally known that low-grade inflammation, abnormal glucose and lipid metabolism, endoplasmic reticulum stress, and oxidative stress are involved in insulin resistance[4]. Recently, it was reported that the regulator of hypoxia [hypoxia-inducible factor (HIF)] and the corresponding changes in lipid metabolism, especially ceramide, promote the progression of insulin resistance and NAFLD[5].
HYPOXIA-INDUCIBLE FACTOR IN HYPOXIA
Normoxia refers to physiological oxygen levels (PO2) in normal tissue in a healthy state, but the oxygen content of different tissues varies in the physiological state, creating a wider range of oxygen levels (range from 13 kPa in the pulmonary vein to 2.7 kPa in the interstitial spaces), such as the intestinal mucosa PO2 being significantly lower than that of the lung mucosa[6]. Hypoxia refers to the phenomenon of insufficient oxygen in tissues or blood relative to physiological conditions.
HIF is a pivotal intracellular transcriptional regulator in response to hypoxia in metazoan development, physiology, and disease pathogenesis[7]. Most species that breathe oxygen express the highly conserved transcription complex HIF-1[8]. HIF-1, a heterodimer composed of an alpha and a beta subunit, belongs to the Per-Arnt-Sim (PAS) subfamily of the basic helix-loop-helix (bHLH) family of transcription factors. The structure of HIF consists of the following three parts: An N-terminal basic helix-loop-helix domain for deoxyribonucleic acid binding, a central region PAS domain that facilitates heterodimerization, and a C-terminus for recruitment of transcriptional coregulatory proteins[9]. There are six members of the human HIF family: HIF-1α, HIF-1β, HIF-2α, HIF-2β, HIF-3α, and HIF-3β. Many cells express HIF-1α and HIF-2α, especially intestinal epithelial cells[10].
There are two major regulatory mechanisms under normoxia. One way is the degradation of HIFα protein. Hydroxylated by the prolyl hydroxylase domain, HIFα binds to the E3 ubiquitin ligase complex containing the von Hippel-Lindau disease tumor suppressor protein, resulting in expeditious degradation of HIFα. The other way is suppression of transcriptional activity. After hydroxylation by HIFα asparaginyl residue with factor inhibiting HIF1 enzyme, the interaction of HIFα with the transcriptional coactivator cAMP-response element binding protein-binding protein and histone acetyltransferase p300 is incapacitated, thus impeding transcription. However, in hypoxia, HIFα subunits remain stabilized and are not hydroxylated by prolyl hydroxylase domain and factor inhibiting HIF1, which are O2-dependent oxygenases, resulting in the accumulation of HIFα and the upregulation of target gene expression[5,11].
HYPOXIA AND INSULIN RESISTANCE
Metabolic syndrome is a clustering of central obesity, insulin resistance, dysglycemia, and a proatherogenic plasma lipid profile, which are associated with the risk of developing cardiovascular disease and T2DM and are presumably caused by chronic inflammation[12,13]. Low-grade inflammation was due to hypoxia, lipids and metabolites, reactive oxygen species, and endoplasmic reticulum stress[4]. At the onset of obesity, resident M2 macrophages contribute to tissue and vascular remodeling to help adipocytes accommodate the new environment of overnutrition to protect adipose tissue from hypoxia and ischemia. Owing to the imbalance between M1 macrophages and M2 macrophages (decrease in protective M2 macrophages and increase in deleterious M1 macrophages), obesity promotes the development of hypoxia in adipose tissue. Moreover, M1 macrophages generate reactive oxygen species and nitrogen monoxide (NO), which influence endothelial cells, com-promising the angiogenesis needed to confront hypoxia[14]. In hypoxic tissues, for example, adipose tissue, chronic low-grade inflammation enhances the expression of HIF-1α, which stimulates inflammatory genes to amplify the “meta-inflammatory” reaction, leading to insulin resistance[15,16].
Increased uncoupled respiration by saturated fatty acids binding to adenosine diphosphate/adenosine-triphosphate translocase 2 (ANT2) is the original event in hypoxic adipocytes. A mass of free fatty acids provokes an ANT2-dependent increase in uncoupled mitochondrial respiration and oxygen consumption in obese/high-fat diet (HFD) mice, which stimulates the production of HIF-1α and relative hypoxia in adipocytes. Increased HIF-1α stimulates NO production by inducing iNOS expression. Then, insulin resistance could emerge by NO nitrosylation of the insulin-signaling molecule protein kinase B (Akt/PKB), which suppresses Akt-phosphorylated activation[17]. In addition, abundant HIF-1α increases lactate production in hypoxic adipocytes, giving rise to higher fasting blood glucose levels and accumulation of basal hepatic glucose[18]. Simultaneously, the activation of ANT2 plays a crucial role in furthering adipose inflammation and fibrosis and metabolic dysfunction. Nevertheless, adipocyte-specific ANT2 knockout seems to be effective in preventing inflammation and fibrosis in adipose tissue and improving glucose tolerance and insulin sensitivity in mice[19,20].
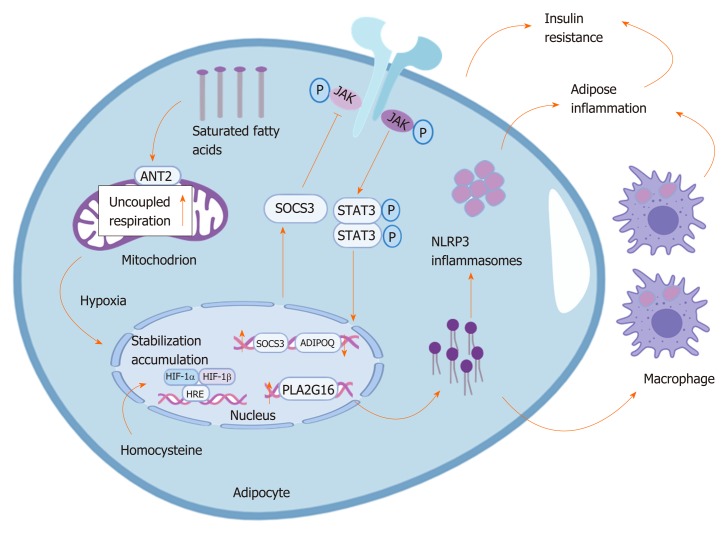
At length, two pathways dominate the accumulation of HIF-1α to generate insulin resistance in hypoxic adipocytes: The JAK-signal transducer and activator of transcription 3 (STAT3) signaling pathway and the phospholipase A2 group 16-lysophosphatidylcholine pathway. In the JAK-STAT3 signaling pathway, stabilization and accumulation of HIF-1α enhance the expression of suppressor of cytokine signaling 3 in the nucleus. Suppressor of cytokine signaling 3 protein phosphorylates STAT3, which downregulates the expression of adiponectin (encoded by ADIPOQ)[21,22]. In the phospholipase A2 group 16-lysophosphatidylcholine pathway, HIF-1α mediates homocysteine-induced adipose pla2g16 (a novel HIF-1α target gene) gene expression to elevate lysophosphatidylcholine (lyso-PC), which acts as a second signal activator in homocysteine-induced activation of the NLRP3 inflammasome pathway. Lysophosphatidylcholine (lyso-PC) not only further activates NLRP3 inflammasomes in adipocytes but also stimulates adipose tissue macrophage NLRP3 inflammasomes in a paracrine manner to induce insulin resistance[23] (Figure 1).
Figure 1.
Accumulation of hypoxia-inducible factor 1α induces insulin resistance in hypoxic adipocytes. Owing to excess saturated fatty acids binding to adenosine diphosphate/adenosine-triphosphate translocase 2 in mitochondria, which increases uncoupled respiration leading to hypoxia in adipose tissue, the stabilized and accumulated hypoxia-inducible factor 1α (HIF-1α) further regulates relative target genes. On the one hand, HIF-1α induces expression of suppressor of cytokine signalling 3, which in turn activates signal transducer and activator of transcription 3, and dimerized signal transducer and activator of transcription 3 enters the cell nucleus and inhibits the transcription of ADIPOQ, resulting in insulin resistance. On the other hand, HIF-1α up-regulates the expression of pla2g16 to increase the level of lyso-PC, which in turn activates NLRP3 inflammasomes and stimulates NLRP3 inflammasomes in macrophages of adipose tissue, promoting insulin resistance. ANT2: Adenosine diphosphate/adenosine-triphosphate translocase 2; HIF-1α: Hypoxia-inducible factor 1α; SOCS3: Suppressor of cytokine signalling 3; JAK: Janus kinase; STAT3: Signal transducer and activator of transcription 3; HRE: Hypoxia-inducible factor regulating element; P: Phosphate; lyso-PC: Lyso-phosphatidylcholine.
CERAMIDE AND INSULIN RESISTANCE
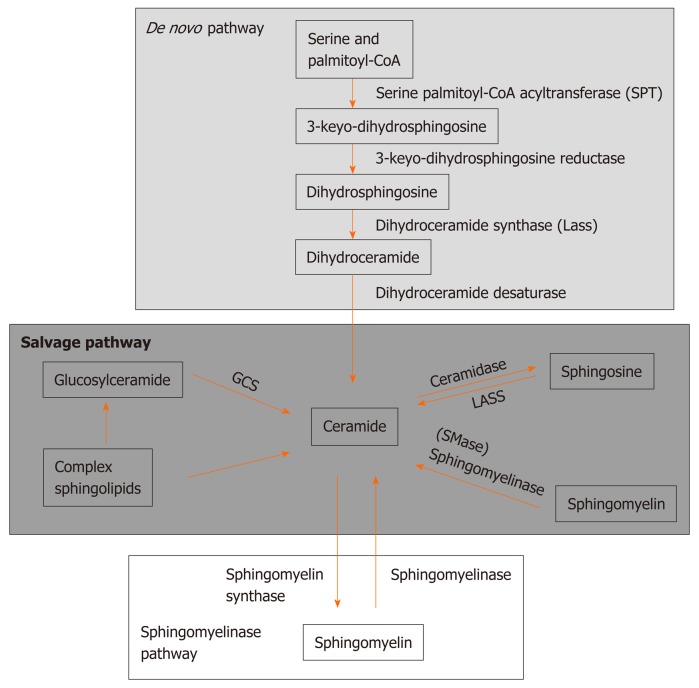
Ceramides, a family of waxy lipid molecules that are composed of sphingosine and fatty acid, are important pathogenic lipids in obesity-related disorders. Starting with saturated fatty acids and palmitate intake, de novo synthesis of ceramide undergoes four major steps. This begins with the condensation of palmitate and serine to form 3-keto-dihydrosphingosine. This reaction is catalyzed by the enzyme serine palmitoyl transferase and is the rate-limiting step of the pathway. In turn, 3-keto-dihydrosphingosine is reduced to dihydrosphingosine, followed by acylation through (dihydro) ceramide synthase to produce dihydroceramide. Then, ceramide synthesis is catalyzed by dihydroceramide desaturase[24]. Ceramide is also produced through the sphingomyelinase and salvage pathways. Via hydrolysis of sphingomyelin, which is catalyzed by the enzyme sphingomyelinase, ceramide can be generated. In addition, the salvage pathway reutilizes long-chain sphingoid bases to form ceramide through the action of ceramide synthase[25] (Figure 2).
Figure 2.
Ceramide synthesis pathways. Ceramides are synthesize through three ways, namely, de novo pathway, salvage pathway, and sphingomyelinase pathway. The de novo synthesis of ceramide commences with the condensation of serine and palmitate via action of serine palmitoyl-coenzyme A acyltransferase, followed by the continuous action of 3-keto-dihydrosphingosine reductase, dihydroceramide synthases, and dihydroceramide desaturase. In the sphingomyelinase pathway, ceramide can be produced from hydrolysis of sphingomyelin through the action of either acid or neutral sphingomyelinase, and ceramide also can synthesize sphingomyelin through the action of sphingomyelin synthase. The salvage pathway is more complex than the other two pathways. Glucosylceramide, complex sphingolipids, sphingosine, and sphingomyelin can generate ceramide from the action of diverse enzymes such as glucosylceramide synthase, LASS, and sphingomyelinase. SPT: Serine palmitoyl-coenzyme A acyltransferase; SMase: Sphingomyelinase; GCS: Glucosylceramide synthase.
In obese rodents, the production of ceramide increased compared with that in lean controls[26], especially glucosylceramide[27]. Similarly, studies performed in insulin-resistant human subjects demonstrate aberrant ceramide accumulation[28]. In subjects with T2DM, investigators observed elevations in serum ceramide compared with healthy control subjects[29]. It was reported that exercising training improved insulin sensitivity in obese and T2DM patients, and decreased the level of plasma ceramide especially C16:0 and C14:0. C16:0 was reduced from 2.5 nmol/mL to 1.75 nmol/mL and C14:0 reduced from 0.213 nmol/m to 0.185 nmol/mL[30]. In another clinical trial, it was found that in the group treated with berberine, the weight, body mass index, and ceramide of patients with T2DM significantly decreased compared with the lifestyle intervention group[31]. However, due to the small sample size and limitations of ceramide detection methods, there is no consistent clinical data on the specific ceramide concentration in obese or diabetic patients.
Risk factors that associate with obesity, such as saturated fatty acids and inflammatory cytokines, selectively promote sphingolipid synthesis enzymes. Moreover, lipidomic profiling reveals the relationship between sphingolipid levels and metabolic diseases, and sphingolipid is shown to be involved in insulin resistance, pancreatic beta cell failure, cardiomyopathy, and vascular dysfunction in in vivo and in vitro studies[32,33]. Adiponectin modulates ceramide by controlling its rate of degradation[34].
Mechanism of ceramide synthesis affecting insulin resistance
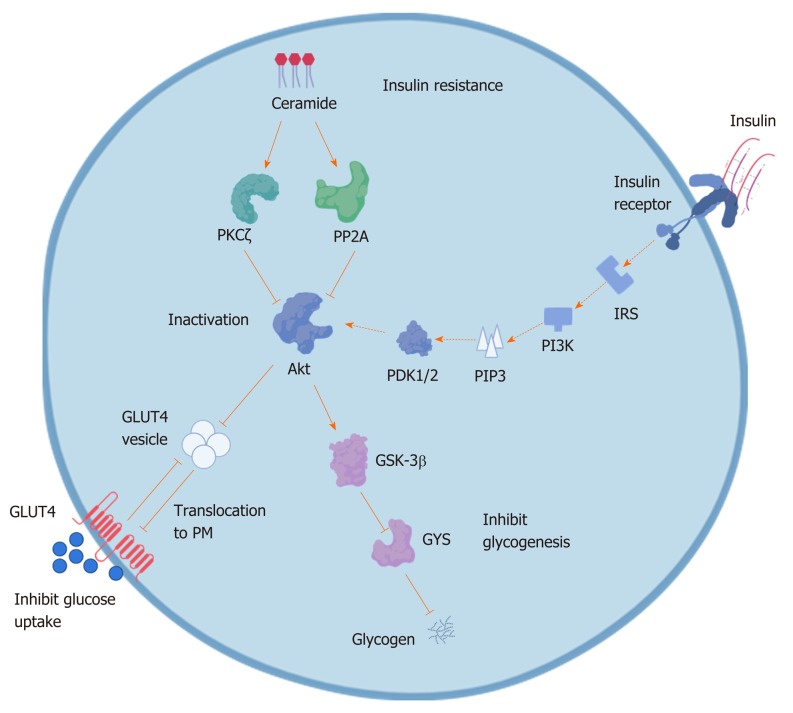
Ceramide is produced in response to almost all stress stimuli, including those associated with obesity (e.g., chemotherapy, inflammatory agonists, and saturated fatty acids). Aberrant accumulation of ceramide may lead to the activation of several signals, which may impair normal cellular function, especially insulin[34,35]. How does ceramide synthesis affect insulin resistance in metabolic disease? By blocking translocation of the glucose transporter 4 through the inhibition of Akt/PKB activation, ceramides inhibit insulin-stimulated glucose uptake and glycogen synthesis in adipocytes and isolated skeletal muscle[36,37]. Ceramides block the activation of Akt/PKB through two key regulatory mechanisms. First, ceramide activates the atypical protein kinase C isoform protein kinase Cç and stabilizes interactions between Akt/PKB and protein kinase Cç by recruiting the enzymes to detergent-resistant membrane fractions[38]. The enzyme’s PH domain of Akt/PKB reduces its affinity for phosphoinositides, resulting in inactivation of Akt/PKB and preventing the translocation of Akt/PKB to the plasma membrane[39,40]. The second mechanism is that activation of protein phosphatase 2A (PPA2, the primary phosphatase responsible for dephosphorylating Akt/PKB) dephosphorylates Akt/PKB. The effects of ceramide on Akt/PKB can be prevented by adding okadaic acid or overexpressing the SV40 small T antigen to inhibit PPA2[41] (Figure 3).
Figure 3.
The mechanism of ceramide inducing insulin resistance. Ceramide inactivates protein kinase B (Akt) through stimulating the activity of protein kinase Cç isoform and protein phosphatase 2A which phosphorylates and inhibits the translocation of Akt. The inactivation of Akt prevents from translocation of glucosetransporter4 vesicle to plasma membrane, resulting in inhibiting glucose uptake. Simultaneously, inactivated Akt in turn activates glycogen synthase 3, leading to inactivation of glycogen synthase and thus inhibition of glycogen synthesis and resulting in insulin resistance. PKB: Protein kinase B; PKCç: Protein kinase Cç; PP2A: Protein phosphatase 2A; GLUT4: Glucosetransporter4; PM: Plasma membrane; GSK-3: Glycogen synthase 3; GYS: Glycogen synthase; IRS: Insulin receptor substrate; PI-3K: Phosphoinositide 3-kinase; PIP3: Phosphatidylinositol-3,4,5-trisphosphate; PDK1/2: 3-phosphoinositide-dependent protein kinase 1/2.
HYPOXIA AND CERAMIDE
HIFα is stabilized and activated under hypoxic conditions[42]. It seems that hypoxia may enhance the level of ceramide in the majority of tissues. Hypoxia leads to ceramide upregulation in NT-2 neuronal precursor cells due to the actions of acid sphingomyelinase and ceramide synthase (LASS-5) to a large extent[43]. In addition, in resistant pulmonary arteries, hypoxia induces increased ceramide and reactive oxygen species[44]. Hypoxia activates neutral sphingomyelinases (nSMases), which are key enzymes in ceramide synthesis, enhancing the production of ceramide and the subsequent ceramide-triggered activation of protein kinase C ζ, which is an early and essential event in the signaling cascade of acute hypoxic pulmonary arteries. Inhibition of nSMase (GW4869) can prevent p47phox phosphorylation induced by hypoxia[45]. Likewise, palmitoyltransferase (SPT) and glucosylceramide synthase (GCS) are the pivotal enzymes of ceramide synthesis, which may regulate the cellular level of ceramide, deciding the fate of the cell exposed to hypoxia. The hypoxia-induced increase in ceramide is partially attributed to the transcriptional upregulation of SPT2. Specific siRNA of SPT2 or GCS can reduce ceramide[46]. Therefore, ceramide synthase inhibitors may be an efficient way to restrain ceramide synthase against hypoxic injury[47].
RELATIONSHIP BETWEEN INTESTINAL HYPOXIA AND CERAMIDE INCREASES WITH INSULUN RESISTANCE
Intestinal mucosal barrier and hypoxia
The intestine is one passageway that communicates between the environment and the external environment of the human body and plays an essential role in the absorption of nutrients and protection from chemical and physical injury. The function of absorption and protection is benefited by the intestinal mucosal barrier, which involves the external physical barrier and internal functional immune barrier[48]. The physical barrier mainly consists of cells and extracellular components, and the cellular components compromise intestinal epithelial cells and the inherent layer. Intestinal epithelial cells consist of absorption (absorbent intestinal cells and M cells) and secretion lines (Pan cells, cup cells, tuft cells, and intestinal endocrine cells)[49], and the inherent layer includes dendritic cells, macrophages, epithelial lymphocytes, regulatory T cells, and B lymphocytes[50]. The functional immune barrier consists of the chemical barrier (antimicrobial peptides, digestive secretions, cytokines, inflammatory mediators, etc.), intestinal microbiota barrier, and immune function barrier[51]. The barrier functions of the intestinal mucous membrane are regulated by the availability of oxygen[52].
Intestinal tissue oxygen has several characteristics. First, the intestinal epithelium is located between the underlying mucosa with high oxygen content and the anaerobic lumen of the intestine, forming a cliffy oxygen gradient under physiological conditions[53]. Second, slight changes in blood flow can cause a significant variation in intestinal oxygen, such as an increase in blood flow volume after feeding (5% of total blood flow increased to 30%), which leads to a change in blood flow of the intestinal mucosa being the reason for the distinct change in local oxygen levels[54]. Intestinal epithelial cells have better adaptability and regulation of hypoxia than other tissues, and physiologic hypoxia might be an adaptive regulation mechanism for the steep oxygen gradient[55].
Intestinal hypoxia is divided into physiological hypoxia and pathological hypoxia. Physiological hypoxia refers to a relatively low PO2 state present in mucosal epithelial cells even at baseline levels because the intestinal mucosa has a wealth of blood vessels, even if a slight reduction in blood flow can lead to a greater reduction in oxygen transport to the intestinal epithelial cells[52,56]. Pathological intestinal hypoxia widely exists in cancer, acute lung injury, inflammatory bowel disease and metabolic diseases[5,54,57]. Intestinal hypoxia is usually associated with the destruction of the intestinal mucosal barrier, such as that occurs in inflammatory intestinal diseases from the reduction in blood supply due to inflammatory immersion, edema, and vasoconstriction, leading to limited oxygen transport of the intestinal epithelium and aggregation of polymorphonuclear cells. At the same time, a large number of neutrophils rapidly deplete local oxygen through respiratory action, leading to hypoxia in the intestines[54]. The HIF-2α—NEU3—ceramide pathway may explain the relationship between intestinal hypoxia and insulin resistance.
The HIF-2α—NEU3—ceramide pathway
In intestinal epithelial cells, HIF-2α is activated and accumulates by hypoxia in HFD mice, which upregulates the target gene Neu3 encoding sialidase 3. Sialidase 3 hydrolyses gangliosides to form ceramides in the salvage pathway[25]. The HIF-2α—NEU3—ceramide pathway can promote the development of metabolic diseases, such as NAFLD, obesity, and insulin resistance. Ceramides are synthesized through three different pathways: De novo pathway, sphingomyelinase (SMase) pathway, and salvage pathway[58]. Increased levels of ceramide cause obesity, insulin resistance, and hepatic steatosis owing to upregulation of fatty acid synthesis. Nevertheless, the target genes of the three ceramide synthesis pathways, including Degs2, Smpd1, Smpd3, Smpd4, Enpp7, Neu3, Glb1, and Gba2, were substantially downregulated, further resulting in the reduction in ceramide in intestine-specific HIF-2α ablation mice, which significantly ameliorates HFD-induced obesity and hepatic steatosis and improves insulin sensitivity in mice. In addition, treatment with a pharmacological specific inhibitor of HIF-2α (PT2385) or inhibitor of NEU3 (N-acetyl-2,3-didehydro-N-acetyl-neuraminic acid, DANA, or naringin) lessens serum levels of ceramides, reduces obesity and fatty liver, and enhances insulin sensitivity[33,59].
The components of the intestinal barrier are abundant. HIF-1α derived from intestinal epithelial cells is important for intestinal intraepithelial lymphocytes and intestinal flora homeostasis. Whether other mechanisms are involved in insulin resistance under hypoxia requires more research to confirm.
CONCLUSION
The global incidence of T2DM has obviously increased in recent decades with economic development and lifestyle changes, especially in developed countries. Chronic inflammation, hypoxia, and the metabolism of ceramide are closely related to insulin resistance. Many studies have shown that HIFα regulates insulin resistance, for example, in adipocyte-specific Hif1a knockout mice, homocysteine-induced insulin resistance is ameliorated, the NLRP3 inflammasome is inhibited, and the production of ceramide is decreased[23]. Meanwhile, intestine-specific Hif2a ablation mice show improved HFD-induced insulin resistance[33].
Ceramide is a significant metabolic product of sphingolipids and contributes to insulin resistance and hepatic steatosis[60]. Under hypoxia, HIF-2α can induce ceramide in adipocytes and intestines, resulting in insulin resistance in HFD-induced obesity mice. As a result of a cliffy oxygen gradient in intestinal tissue and inflammatory changes in the intestinal mucosal barrier, hypoxia occurs in the intestine. Intestinal hypoxia may lead to HFD-induced insulin resistance. A study on the HIF-2α—NEU3—ceramide pathway revealed the role of ceramide in hypoxia and insulin resistance in obese mice.
In summary, hypoxia is a key feature of the progression of metabolic disease and HIF signaling, which can strongly influence metabolic disease by both genetic and pathophysiologic inhibition. Recent discoveries have identified exciting effects of pharmacologic inhibitors of HIF-2α or inhibitors of key enzymes (sialidase 3, NEU3) in ceramide synthesis. This may become a promising approach to the treatment of metabolic diseases, including insulin resistance and NAFLD.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors who contributed their efforts in this manuscript.
Peer-review started: December 30, 2019
First decision: March 27, 2020
Article in press: April 24, 2020
P-Reviewer: Balaban YH, Tomizawa M S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Zhang YL
Contributor Information
Qing-Song Xia, Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Fu-Er Lu, Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Fan Wu, Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Zhao-Yi Huang, Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Hui Dong, Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Li-Jun Xu, Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Jing Gong, Department of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China. jgongtcm@126.com.
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federation ID. IDF Diabetes Atlas, 9th edition 2019. Brussels, Belgium. Available from: https://www.diabetesatlas.org/en/sections/proven-and-effective-actions.html. [Google Scholar]
- 3.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 4.Dali-Youcef N, Mecili M, Ricci R, Andrès E. Metabolic inflammation: connecting obesity and insulin resistance. Ann Med. 2013;45:242–253. doi: 10.3109/07853890.2012.705015. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15:21–32. doi: 10.1038/s41574-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagundes RR, Taylor CT. Determinants of hypoxia-inducible factor activity in the intestinal mucosa. J Appl Physiol (1985) 2017;123:1328–1334. doi: 10.1152/japplphysiol.00203.2017. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 8.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Zhang L, Erbel PJ, Gardner KH, Ding K, Garcia JA, Bruick RK. Functions of the Per/ARNT/Sim domains of the hypoxia-inducible factor. J Biol Chem. 2005;280:36047–36054. doi: 10.1074/jbc.M501755200. [DOI] [PubMed] [Google Scholar]
- 10.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 12.Ye J, McGuinness OP. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304:E466–E477. doi: 10.1152/ajpendo.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Pouw Kraan TC, Chen WJ, Bunck MC, van Raalte DH, van der Zijl NJ, van Genugten RE, van Bloemendaal L, Baggen JM, Serné EH, Diamant M, Horrevoets AJ. Metabolic changes in type 2 diabetes are reflected in peripheral blood cells, revealing aberrant cytotoxicity, a viral signature, and hypoxia inducible factor activity. BMC Med Genomics. 2015;8:20. doi: 10.1186/s12920-015-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JA, Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, Quehenberger O, Johnson RS, Olefsky JM. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157:1339–1352. doi: 10.1016/j.cell.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leake I. ANT2 mediates hypoxia and inflammation in obesity. Nat Rev Endocrinol. 2019;15:64. doi: 10.1038/s41574-018-0140-z. [DOI] [PubMed] [Google Scholar]
- 20.Seo JB, Riopel M, Cabrales P, Huh JY, Bandyopadhyay GK, Andreyev AY, Murphy AN, Beeman SC, Smith GI, Klein S, Lee YS, Olefsky JM. Knockdown of Ant2 Reduces Adipocyte Hypoxia and Improves Insulin Resistance in Obesity. Nat Metab. 2019;1:86–97. doi: 10.1038/s42255-018-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, Kim JH, Li F, Qu A, Gavrilova O, Shah YM, Gonzalez FJ. Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J Biol Chem. 2013;288:3844–3857. doi: 10.1074/jbc.M112.426338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanatani Y, Usui I, Ishizuka K, Bukhari A, Fujisaka S, Urakaze M, Haruta T, Kishimoto T, Naka T, Kobayashi M. Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes. 2007;56:795–803. doi: 10.2337/db06-1039. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SY, Dong YQ, Wang P, Zhang X, Yan Y, Sun L, Liu B, Zhang D, Zhang H, Liu H, Kong W, Hu G, Shah YM, Gonzalez FJ, Wang X, Jiang C. Adipocyte-derived Lysophosphatidylcholine Activates Adipocyte and Adipose Tissue Macrophage Nod-Like Receptor Protein 3 Inflammasomes Mediating Homocysteine-Induced Insulin Resistance. EBioMedicine. 2018;31:202–216. doi: 10.1016/j.ebiom.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 25.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yetukuri L, Katajamaa M, Medina-Gomez G, Seppänen-Laakso T, Vidal-Puig A, Oresic M. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bijl N, Scheij S, Houten S, Boot RG, Groen AK, Aerts JM. The glucosylceramide synthase inhibitor N-(5-adamantane-1-yl-methoxy-pentyl)-deoxynojirimycin induces sterol regulatory element-binding protein-regulated gene expression and cholesterol synthesis in HepG2 cells. J Pharmacol Exp Ther. 2008;326:849–855. doi: 10.1124/jpet.108.139394. [DOI] [PubMed] [Google Scholar]
- 28.Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Górska M, Dobrzyń A, Baranowski M. Concentrations of sphingosine and sphinganine in plasma of patients with type 2 diabetes. Med Sci Monit. 2005;11:CR35–CR38. [PubMed] [Google Scholar]
- 30.Kasumov T, Solomon TP, Hwang C, Huang H, Haus JM, Zhang R, Kirwan JP. Improved insulin sensitivity after exercise training is linked to reduced plasma C14:0 ceramide in obesity and type 2 diabetes. Obesity (Silver Spring) 2015;23:1414–1421. doi: 10.1002/oby.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang X, Wang Z, Zhang J, Yan H, Bian H, Xia M, Lin H, Jiang J, Gao X. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease. J Transl Med. 2016;14:266. doi: 10.1186/s12967-016-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie C, Yagai T, Luo Y, Liang X, Chen T, Wang Q, Sun D, Zhao J, Ramakrishnan SK, Sun L, Jiang C, Xue X, Tian Y, Krausz KW, Patterson AD, Shah YM, Wu Y, Jiang C, Gonzalez FJ. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat Med. 2017;23:1298–1308. doi: 10.1038/nm.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summers SA, Goodpaster BH. CrossTalk proposal: Intramyocellular ceramide accumulation does modulate insulin resistance. J Physiol. 2016;594:3167–3170. doi: 10.1113/JP271676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann K, Dixit VM. Ceramide in apoptosis--does it really matter? Trends Biochem Sci. 1998;23:374–377. doi: 10.1016/s0968-0004(98)01289-4. [DOI] [PubMed] [Google Scholar]
- 37.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282:12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 39.Stratford S, DeWald DB, Summers SA. Ceramide dissociates 3'-phosphoinositide production from pleckstrin homology domain translocation. Biochem J. 2001;354:359–368. doi: 10.1042/0264-6021:3540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 42.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 43.Jin J, Hou Q, Mullen TD, Zeidan YH, Bielawski J, Kraveka JM, Bielawska A, Obeid LM, Hannun YA, Hsu YT. Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation-induced Bax redistribution to mitochondria in NT-2 cells. J Biol Chem. 2008;283:26509–26517. doi: 10.1074/jbc.M801597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno L, Moral-Sanz J, Morales-Cano D, Barreira B, Moreno E, Ferrarini A, Pandolfi R, Ruperez FJ, Cortijo J, Sanchez-Luna M, Villamor E, Perez-Vizcaino F, Cogolludo A. Ceramide mediates acute oxygen sensing in vascular tissues. Antioxid Redox Signal. 2014;20:1–14. doi: 10.1089/ars.2012.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frazziano G, Moreno L, Moral-Sanz J, Menendez C, Escolano L, Gonzalez C, Villamor E, Alvarez-Sala JL, Cogolludo AL, Perez-Vizcaino F. Neutral sphingomyelinase, NADPH oxidase and reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. J Cell Physiol. 2011;226:2633–2640. doi: 10.1002/jcp.22611. [DOI] [PubMed] [Google Scholar]
- 46.Kang MS, Ahn KH, Kim SK, Jeon HJ, Ji JE, Choi JM, Jung KM, Jung SY, Kim DK. Hypoxia-induced neuronal apoptosis is mediated by de novo synthesis of ceramide through activation of serine palmitoyltransferase. Cell Signal. 2010;22:610–618. doi: 10.1016/j.cellsig.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Crowder CM. Cell biology. Ceramides--friend or foe in hypoxia? Science. 2009;324:343–344. doi: 10.1126/science.1173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gehart H, Clevers H. Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol. 2019;16:19–34. doi: 10.1038/s41575-018-0081-y. [DOI] [PubMed] [Google Scholar]
- 50.Rescigno M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011;32:256–264. doi: 10.1016/j.it.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Cummings JH, Antoine JM, Azpiroz F, Bourdet-Sicard R, Brandtzaeg P, Calder PC, Gibson GR, Guarner F, Isolauri E, Pannemans D, Shortt C, Tuijtelaars S, Watzl B. PASSCLAIM--gut health and immunity. Eur J Nutr. 2004;43 Suppl 2:II118–II173. doi: 10.1007/s00394-004-1205-4. [DOI] [PubMed] [Google Scholar]
- 52.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeitouni NE, Chotikatum S, von Köckritz-Blickwede M, Naim HY. The impact of hypoxia on intestinal epithelial cell functions: consequences for invasion by bacterial pathogens. Mol Cell Pediatr. 2016;3:14. doi: 10.1186/s40348-016-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shehade H, Oldenhove G, Moser M. Hypoxia in the intestine or solid tumors: a beneficial or deleterious alarm signal? Eur J Immunol. 2014;44:2550–2557. doi: 10.1002/eji.201444719. [DOI] [PubMed] [Google Scholar]
- 55.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glover LE, Lee JS, Colgan SP. Oxygen metabolism and barrier regulation in the intestinal mucosa. J Clin Invest. 2016;126:3680–3688. doi: 10.1172/JCI84429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu G, Xu G, Chen DW, Gao WX, Xiong JQ, Shen HY, Gao YQ. Hypoxia Exacerbates Inflammatory Acute Lung Injury via the Toll-Like Receptor 4 Signaling Pathway. Front Immunol. 2018;9:1667. doi: 10.3389/fimmu.2018.01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyagi T, Wada T, Yamaguchi K, Hata K, Shiozaki K. Plasma membrane-associated sialidase as a crucial regulator of transmembrane signalling. J Biochem. 2008;144:279–285. doi: 10.1093/jb/mvn089. [DOI] [PubMed] [Google Scholar]
- 60.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]