Abstract
Empathy, the capacity for shared emotional valence with others, can allow for cooperativity and social bonding between individuals. However, clinical studies indicate it is dysregulated in neuropsychiatric disorders like autism and addiction, making a translationally relevant model of empathy extremely important. The evolutionary basis of the empathic behaviors observed across numerous species can be described using the Perception Action Model (PAM), in which shared affect can promote an action that eliminates the distress of both the “Target” and, by extension, the “Observer”. Increasing evidence suggests rodents will work to reduce the distress of a conspecific, but current models of helping behavior are unable to completely parse apart whether the reported behavior is driven by empathy or social reward. The current study demonstrates, using a novel behavioral model, rats learn to aid a distressed conspecific in the absence of social reward, retain the task over time, and previous experience increases the rate of task acquisition. Further, our model suggests that empathic behavior is subject to low effort as compared to a social reward. We next validated the specificity of this model to study empathic processes, characterized the importance of both the Target’s level of distress and the impact of the Observer’s familiarity with the Target on empathic behavior. Overall, we believe this model adheres to the PAM of empathy by eliminating the influence of social interaction. Importantly, it can be used to directly evaluate the neurocircuitry of empathy and explore the interplay between blunted empathic behavior and neuropsychiatric disorders.
Subject terms: Reward, Empathy
Introduction
Empathy can be defined as the capacity for shared emotional valence, which generates shared affective states and therefore drives behaviors most appropriate to the emotional condition of others [1]. Overall, this ability is essential for cooperation towards common goals and the formation of social bonds [2, 3]. Empathy is a continuum of phenomena conceptualized as a Russian nesting doll in which the formation of increasingly more evolved and complex forms of empathy have progressively built upon, and are dependent on, more conserved empathic behaviors (as described in [3]). At the core of these empathic behaviors lies the perception action model (PAM) [2, 3] in which an “Observer” must attend to another’s distress, thereby generating a shared affective state. The Observer must regulate their emotional responsivity to effectively perform a behavior that will eliminate the distress of the “Target” and, by extension, their own. The most phylogenetically conserved manifestations of empathic behavior, yawn contagion and motor mimicry, are present in numerous animal species [3]. More cognitively driven forms of empathy, such as targeted helping and perspective-taking, were once thought to be seen exclusively in humans and non-human primates. However, a growing body of evidence suggests that many mammals, including rodents, express these more complex empathic behaviors [4–6].
Early studies [7, 8], as well as more recent experiments [1, 4–6, 9–11], have demonstrated that rats will actively work to reduce the distress of a conspecific. For example, rats will release a conspecific from restraint [10, 11] or a water-filled compartment [9]. Helping behavior is potentiated when the level of distress of the conspecific is elevated [11], and animals will forgo or delay personal reward (e.g. chocolate) to aid the conspecific [9, 11]. However, in these aforementioned tasks, distressed rats were released into the same environment as their rescuer, allowing for direct social interaction. This methodological consideration obscures whether release behavior was driven by empathic processes or the reward of social contact [12, 13].
Empathy may be directly affected by, or contribute to, several neuropsychiatric diseases, including autism spectrum disorder (ASD) and substance use disorder (SUD). Many researchers agree that empathy, broadly defined, is altered in those with ASD but the cause and consequences are poorly understood (reviewed in [14, 15]). Focusing on understanding and evaluating changes in empathy could have both diagnostic and therapeutic implications for ASD by establishing and maintaining a distinction between social and emotional impairments [16]. Empathic behaviors are also negatively impacted in SUD across several drugs of abuse [17–21] and targeting empathic processes may be a treatment-modifiable risk factor for maintaining drug-free abstinence. For example, higher levels of empathy in patients participating in 12-step recovery programs was positively correlated with enhanced involvement in such programs and prolonged abstinence periods [19]. These findings suggest restoration of empathic behaviors may improve treatment outcomes and reduce the chance of relapse [21, 22]. It is therefore imperative that a translational model for the study of empathy is available in order to discern the relationship between psychiatric disorders and empathic deficits as a potential diagnostic and therapeutic tool for clinical populations.
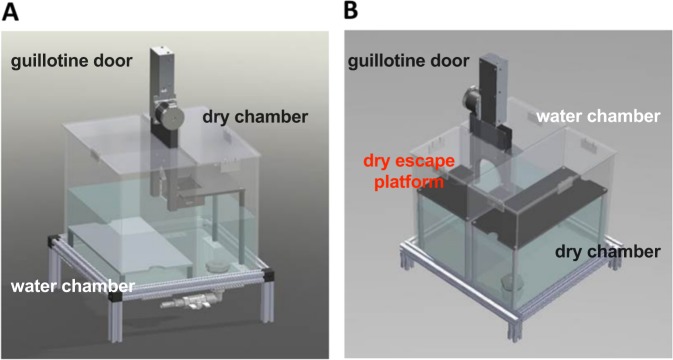
For these reasons, we have developed a novel rodent model for the study of empathic behavior independent of social interaction. In our task, a three-chamber apparatus was used in which a distressed “Target” rat was placed in a pool of water. An “Observer” rat was placed in a dry chamber with access to a chain that, when pulled, opened an automatic guillotine door and released the target into a separate dry chamber, thereby eliminating its distress (Fig. 1b). In the current report, we show that rats learned to aid a distressed conspecific in the absence of social reward, and previous experience of the distressing event significantly potentiates the rate rats learn the task. We denote tasks that include social contact after being released as “social” and the task that prevents social contact as “empathic”. We validated the specificity of this new model to empathic processes and characterized the importance of the presence of a distressed Target on the Observer’s chain pull latency. We also demonstrated that the Observer’s level of familiarity with the Target modulates helping behavior. This model may be a valuable tool to directly evaluate empathic processes in rodents and to understand the interplay between blunted empathic behavior and psychiatric disorders.
Fig. 1.
a This illustration depicts the two-chamber apparatus that allowed for social contact. Observer rats placed in the dry chamber have access to a chain that, when pulled, releases the target rat from the wet chamber onto the same side as the Observer through an automated guillotine door. b An illustration of the three-chamber empathy apparatus created to better model empathic behavior via the elimination of social reward as a confounding variable. Observer rats learn to pull a chain to release target rats from the water chamber into a separate dry chamber.
Methods
Animals
Male Sprague Dawley rats (n = 102, 51 pairs) weighing 250–275 g were pair-housed on a 12-h reversed light cycle (lights on at 1800). Animals were given food and water ad libitum until behavioral testing, when they were then switched to a daily stable intake (20 g) of rat chow (Harlan). Rats were given at least 5 days to acclimate to their cage mate. Following acclimation, one rat was randomly selected as the “Observer” and the other as the “Target”. Rats were handled and weighed for 2 days, 5 min/day before the behavioral assessment. All experimental procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and approved by the IACUC of the Medical University of South Carolina. The distribution of rats across experimental cohorts is detailed in Table 1.
Table 1.
Description of animal use throughout the manuscript.
| Experiment/Cohort | # of rats = 102 Observers/Targets | Behaviors | |||
|---|---|---|---|---|---|
| Acquisition | Reversal | Tests | Group tested | ||
| 2-chamber social | 7/7 = 14 | ✓ Increased FR | ✓ | Increased FR | None |
| 2-chamber social | 8/8 = 16 | ✓ Dry Chamber | ✓ Dry Chamber | None | None |
| 3-chamber empathy | |||||
| Cohort 1 | 8/8 = 16 | ✓ | ✓ | Fake rat, empty chamber | R-Observers |
| Cohort 2 | 8/8 = 16 | ✓ Dry Chamber | ✓ Dry Chamber | None | None |
| Cohort 3 | 8/8 = 16 | ✓ Increased FR | None | Fake rat, empty chamber, extended time course | Observers |
| Cohort 4 | 8/8 = 16 | ✓ | ✓ | Strangers | R-Observers |
| Cohort 5 | 4/4 = 8 | ✓ Water pre-exposure | None | None | None |
Apparatus and behavioral testing
Evaluation of social and empathic behaviors were performed in custom-made operant boxes by Med Associates (Fairfax, VT, USA; see Fig. 1). The social and empathy tasks occurred in a two or three-chamber apparatus, respectively. Both boxes (34.2 × 33.9 × 30.5 cm) were located in a sound-attenuated room with the lights off except for a single lamp used for the experimenter to view the test. Targets were placed in 100 mm of water in the wet compartment (19.5 × 34.2 cm two chamber and 19.5 × 19.5 cm three chamber). Observers were placed on a dry platform (19.5 × 34.2 cm, both boxes) with access to a chain that, when pulled, opened an automatic guillotine door and allowed the Target to be released into either the same dry compartment as the Observer (two chamber, Fig. 1a) or to a dry compartment (17 × 19.5 cm) separate from the Observer (three chamber, Fig. 1b). Additionally, in the three-chamber apparatus, small holes (0.25 cm radius) were drilled exclusively between the wet and the Observer’s dry compartment that allowed for improved scent detection but were small enough to prevent any physical contact between the two rats. Importantly, no holes existed between the separated dry chambers to prevent any opportunity for social interaction after the chain pull.
In both tasks, rats were transported to the experiment room and left undisturbed for 5 min. During the procedures, the Target was placed in the water-filled chamber. The Observer was placed on the dry platform chamber with access to a chain pull. In the social task, the two compartments were separated by an automated guillotine door that opened when the chain was pulled, allowing the target rat to move into the same compartment as the Observer. In the empathy task, a third compartment was separated from the wet chamber by an automated guillotine door. A chain pull opened the door allowing the Target access to a separate dry compartment. Latency to chain pull was taken as an index of helping behavior. Trials (16–20 total, labeled “Acquisition”) lasted a total of 300 s (5 min) regardless of the chain pull latency to reduce the likelihood that removal from the apparatus was a motivating factor for the behavior. If the Observer did not pull the chain within the allotted time, the experimenter ended the trial and released the Target. Following acquisition, the role of each rat in a pair was subsequently reversed (“Reversal” phase) such that a Target became the Observer (labeled “R-observer”) and the Observer becomes the target rat (“R-target”). This reversal phase was carried out for 5 days (10 trials). Two trials were conducted daily during the rats’ dark cycle. In order to evaluate the importance of the Targets’ level of distress for the Observer’s chain pull behavior in both tasks, we conducted separate experiments in which Targets were placed in the chamber without any water (i.e. a dry chamber with no distressor present) and chain pull latency was recorded. We also tested motivation to release the target by increasing the response requirement to an FR5 and then an FR10 schedule.
Characterization of empathic behavior in rats
We conducted a series of tests on the Observers and R-observers to characterize the helping behavior. First, the specificity of the empathic response was tested by replacing the live Target/R-target with a ”fake” rat (white Styrofoam of approximately equal length and width as a rat; three consecutive days) and an empty pool of water (three consecutive days). We also evaluated the time course of empathic responding by allowing rats to remain in their home cage and reintroduced them to the empathy chamber 5, 10, and 15 days after acquisition ended. We determined if helping behavior was specific to the cage mate of R-observers by replacing the R-target rat with an unfamiliar rat. Three “stranger” rats were used twice in random order for a total of six trials. Latencies in these experiments were compared to a baseline (BL) calculated by the average of the final 3 days of the empathic responding.
Data analysis
Chain pull latency was the primary dependent variable and a daily mean for each rat was calculated by averaging the two trials performed each day. No animals were excluded from the analysis in order to establish patterns of social and empathic responding. One-way repeated measures (RM) analyses of variance (ANOVAs), with day being the repeated measure, were performed to evaluate the change of chain pull latency over time for Observers and R-observers. BL latencies used for comparisons were calculated by finding the mean latency from the equivalent number of final days of the previous phase. One-way RM ANOVAs were then performed to evaluate changes in latency from BL. In addition, two-way RM ANOVAs were performed to directly compare Observers and R-observers. All post hoc comparisons were conducted using a Holm–Sidak’s correction for family wise error when appropriate, with the alpha set at 0.05. All analyses were conducted with Prism Software version 8.2. All data are expressed as the mean ± SEM.
Results
Rats readily release a conspecific from a pool of water
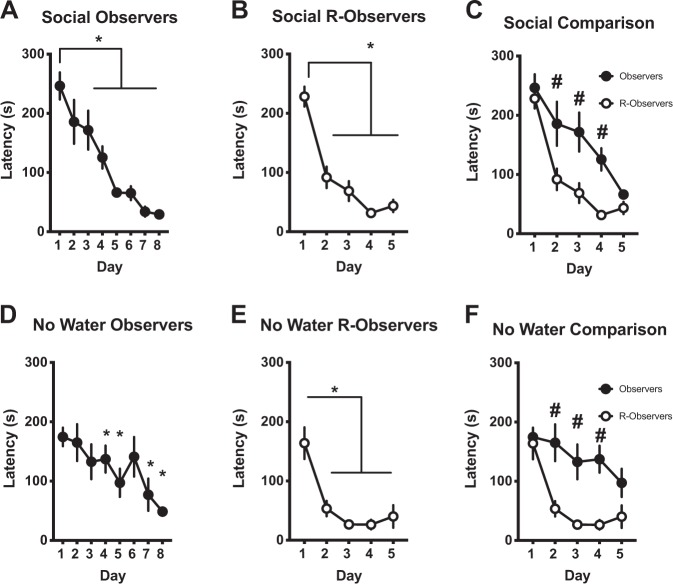
Rats performed a helping task similar to one previously described [9] (n = 7 pairs) in which release of the Target resulted in social interaction. Observers’ (Fig. 2a) chain pull latency decreased throughout the acquisition phase [F (7, 42) = 18.64, p < 0.0001], with significantly shorter latencies on days 2–8 compared to day 1 (p < 0.05). R-observers (Fig. 2b) also had decreased latencies [F (4, 24) = 36.6, p < 0.0001], with a significant reduction observed on days 2–5 compared to day 1 (p < 0.0001). A direct comparison of Observers and R-observers (Fig. 2c) over the first 5 days of the acquisition and reversal phases revealed a significant group by day interaction (F (4, 48) = 3.133, p < 0.023) in which Observers had longer latencies than R-observers on days 2–4 (p < 0.01). Consistent with Sato et al. [9], R-observers had significantly decreased latencies to release a distressed conspecific compared to Observers.
Fig. 2.
a–c Rats readily release a conspecific from a pool of water. Performance of seven pairs of male rats during the social two-chamber task. a Latency for observer rats to chain pull to release a distressed partner decreased over 8 days. Significantly shorter latencies occurred on days 2–8. b R-observer rats showed a decrease in response latency by day 2 with significantly shorter latencies occurring on days 2–5. c When the first 5 days of the acquisition and reversal phases were directly compared, R-observer rats performed the task significantly faster on days 2–4. d–f Rats will perform and operant task for social interaction. Performance of eight pairs of male rats during the social two-chamber task with the target placed in a dry compartment (i.e. no distress present). d Observer rats’ latencies to release a non-distressed target decreased over the course of 8 days with significantly shorter latencies on days 4–5 and 7–8. e R-observer rats had a decrease in the latency to release the R-target over 5 days with significantly shorter latencies on days 2–5. f When the first 5 days of the acquisition and reversal phases were directly compared, R-observer rats performed the task significantly faster on days 2–4. *Significant difference from day 1 (p < 0.05). #Significant difference from R-Observers (p < 0.05).
Rats will perform an operant task for social interaction
Target rats (n = 8 pairs) were placed in the “wet” compartment without water (i.e. no distress was present). Latency decreased over days [Fig. 2d, F (7, 49) = 10.14, p < 0.0001] such that days 4, 5, 7, and 8 were significantly less than day 1 (p < 0.05) demonstrating acquisition of the social task in the absence of a distressed Target. The R-observers’ latencies also decreased over days [Fig. 2e, F (4, 28) = 11.47, p < 0.0001] with days 2–5 significantly lower than day 1. In direct comparisons, Observers had higher latencies than R-observers [group by day interaction, [F (4, 56) = 3.602, p < 0.0111] on days 2–4 (p < 0.0001, Fig. 2f). These data suggest previous experience still plays a role in acquiring this social task in spite of the conspecific’s lack of distress.
Rats readily engage in empathic behavior independent of social interaction
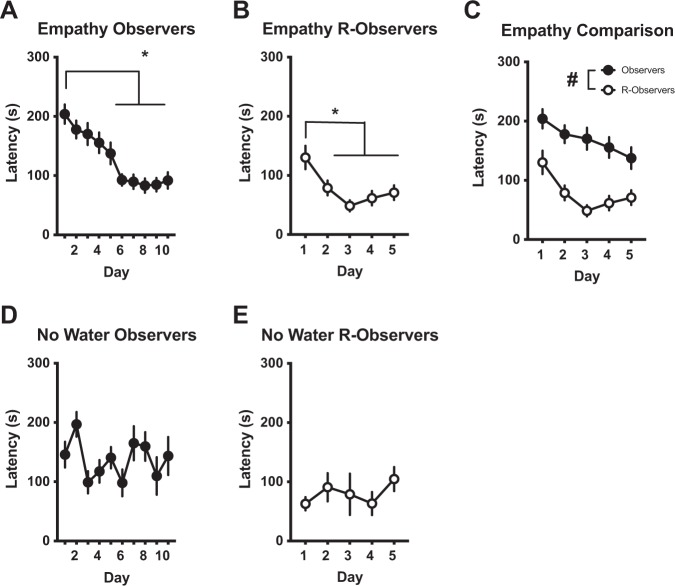
Behavior was conducted in three different cohorts of rats to provide replications of the task. Rats (n = 24 pairs) were placed in the three-chamber apparatus and latency was recorded. Observer rats (Fig. 3a) had decreased latencies over days [F (9, 207) = 14.33, p < 0.0001], with days 4–10 significantly faster compared to day 1 (p < 0.05). When the conditions were reversed, R-observers’ (Fig. 3b) latencies also decreased over days [F (4, 60) = 6.976, p = 0.0001], with days 2–5 being significantly lower than day 1 (p < 0.05). In a direct comparison, Observers had longer latencies than R-observers across all 5 days [Fig. 3c; main effect of group F (1,38) = 28.99, p < 0.0001], suggesting that previous experience to a distressing situation modulates task learning in this empathic model. In a separate cohort, target rats (n = 8 pairs) were placed in a dry compartment rather than one filled with water, thereby removing the distress of the Target. There were no differences in latency across days in Observers (Fig. 3d) or R-Observers (Fig. 3e). In the absence of a distressed conspecific and social interaction, chain pull behavior was not readily acquired and prior experience did not modulate acquisition of the task.
Fig. 3.
a–c Rats engage in empathic behavior in the absence of social interaction. Performance of 24 (acquisition: 8 pairs/cohort over 3 cohorts) or 16 (reversal: 8 pairs/cohort over 2 cohorts) of male rats during our novel empathy task. a Latency for observer rats to chain pull to release a distressed partner decreased over 10 days. Significantly shorter latencies occurred on days 4–10. b R-observer rats that previously experienced the water showed a decrease in response latency by day 2. Specifically, significantly shorter latencies occurred on days 2–5. c When the first 5 days of acquisition and reversal were compared to each other, significantly shorter latencies were found across all 5 days in the reversal compared to the acquisition phase. d, e Rats do not respond in the absence of distress or social reward. Performance of male rats (n = 8 pairs) during the novel empathy task, when the target was placed in a dry compartment (i.e. no distress present). d No clear response pattern emerged when observer rats opened the guillotine door for target rats placed in a dry compartment rather than in water. e R-observer rats also showed no difference across days. *Significant difference from day 1 (p < 0.05). #Significant difference from R-Observers (p < 0.05).
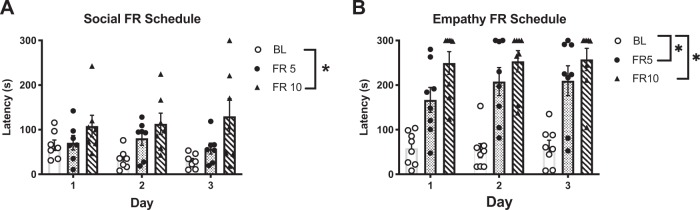
Empathic responding is subject to effort
We tested motivation to release a conspecific in both assays by increasing the FR requirement to 5 and then 10 for three consecutive days each. Social Observers (Fig. 4a) had faster latencies under the FR1 (i.e. baseline) than under an FR10 schedule but still executed the chain pull response under higher ratio schedules [main effect FR, F (2, 18) = 4.96, p = 0.019]. In contrast, Empathy Observers took significantly longer to release the Target under an FR5 and an FR10 schedule [Fig. 4b, (F (2, 21) = 25.27, p < 0.0001].
Fig. 4.
Prosocial behaviors are subject to effort. Observer rats underwent an increasing FR schedule moving from FR5 to FR10 (3 days each) to release the distressed Target following acquisition of either the two- or three-chamber task. a In the social task, latency significantly increased on an FR10 relative to an FR1. b In the empathy task, latency increased significantly on both an FR 5 and FR 10 schedule relative to BL. Removal of social interaction as a reward diminished the motivation for maintaining the chain pull behavior in the empathy task as compared to the social task. *Significant difference from baseline (p < 0.05).
Specificity of the chain pull response depends on the targets’ distress
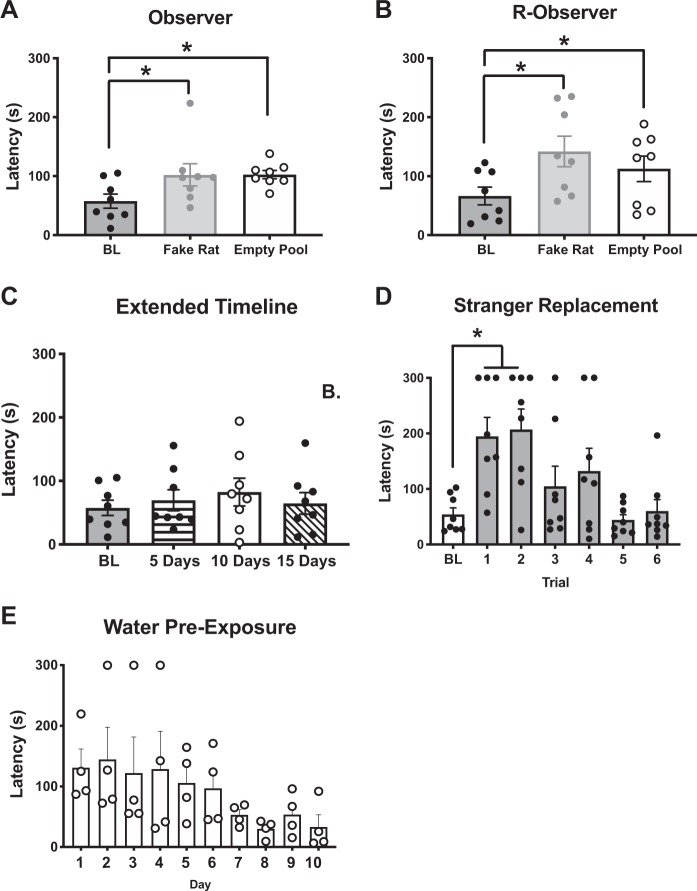
To characterize the specificity of the release behavior to the distress of the conspecific, the Target was replaced by a “fake” rat or removed, leaving an empty pool of water. For both Observers (n = 8, Fig. 5a) and R-observers (n = 8, Fig. 5b), latencies were significantly increased in both conditions relative to BL [Observers: (F (2, 14) = 6.27), p = 0.02 and Holm–Sidak’s p < 0.05; R-observers: (F (2, 14) = 7.079), p = 0.0075, and Holm–Sidak’s p < 0.05]. These data suggest that chain pull behavior in both Observer and R-observers is dependent on the presence of a distressed conspecific. We also determined that empathic behavior persisted over time because Observers (n = 8) maintained release behavior 5, 10, and 15 days following the final day of acquisition, indicated by a lack of change from BL (Fig. 5c). Additionally, we found that release behavior was sensitive to familiarity with the conspecific. Specifically, in a cohort of R-observers (n = 8 pairs), a familiar conspecific was replaced with an unfamiliar rat in the wet chamber over six trials and empathic responding was evaluated (Fig. 5d). There was a significant change in latency between trials [F (6, 42) = 8.581, p < 0.0001]. Specifically, the time to release the “stranger” was significantly slowed during the first two trials (p < 0.0005), but subsequently returned to BL levels, indicating rats were capable of learning to release an unfamiliar conspecific.
Fig. 5.
Elucidating the specificity of the chain pull response on the targets’ distress. Performance of male rats across three cohorts (8 pairs/cohort) in separate control experiments using the three-chamber empathy task. a Observer rats significantly increased their chain pull latency when the distressed conspecific was removed and replaced by either a fake rat or an empty pool of water (three consecutive days of each condition) when compared to baseline (BL, average of final 3 days of acquisition). b In a separate cohort, R-Observer rats also demosntrated an increased latency to chain pull in response to a fake rat and an empty pool relative to BL (average of final 3 days of reversal). c Following 10 days (20 trials) of acquisition, Observer rats (n = 8) maintained release behavior for 15 days. Specifically, observers were reintroduced to the three-chamber empathy task and latencies to release the distressed targets were recorded 5, 10, and 15 days following their last acquisition session. Latencies did not change over time compared to BL. d In another study, a rat that was completely unfamiliar to the R-Observer rat was introduced into the wet side of the empathy chamber (“stranger”). Latency to release the “stranger” was significantly potentiated compared to baseline during the first two trials, but subsequently returned to levels not significantly different from BL. e A cohort of observer rats (n = 4) were pre-exposed to the pool of water (yoked to time spent by Targets in Experiment 3) without being rescued prior to the start of acquisition of the empathy task. There was a significant effect across days; however, there were no differences between days 1 and 10 on post hoc analysis. *Significant difference from baseline (p < 0.05).
As a pilot study to discern if the differences in latency between Observers and R-observers resulted from a drive to reduce the distress of a conspecific or a result of experience with the associative structure of the task, a cohort of Observers (n = 4 pairs) were pre-exposed to the pool of water without being rescued before beginning the empathy task. The time spent in the pool each day was yoked to the BL acquisition curve depicted in Fig. 3a to emulate the distressing condition experienced by a cohort of Targets. Following 10 days (20 trials) of yoked pre-exposure to the water, Observers performed 10 days (20 trials) of the empathy task. An RM one-way ANOVA was significant across days [F (9, 27) = 2.5, p = 0.03]; however, there were no differences between days 1–10 on post hoc analysis (Fig. 5e).
Discussion
Our three-chamber task conforms to the PAM of empathy and may be a translational tool to understand empathy in neuropsychiatric disorders. We have expanded current models by removing social interaction as an underlying interpretation for the observed behavior, and showed that rats will still learn to release a distressed cage mate (Fig. 3a) and retain the task for an extended time period (Fig. 5c). Further, previous experience with the same distressing environment attenuates the time it takes for rats to release a distressed conspecific (Fig. 3b). Taken together, we believe that these data support and adhere to the PAM (described in [3]) as a translational evaluation of empathy.
According to the PAM, the presence of a distressed animal initially causes emotional transfer of that distress, thereby promoting a shared affect. Observer rats must regulate their own emotional state in order to respond appropriately to lower the Target’s distress. The relief of the Target will then allow for another emotional transfer between animals, and therefore reinforce the Observer’s actions. Prior exposure to the same stressor more readily enables the R-observer to state match with the R-target, perhaps by activating salient emotional memories that drive empathic behavior. When the distressed Target or R-target was either replaced or removed in the three-chamber task, the chain pull latency of the Observers and R-observers significantly increased (Fig. 5a, b). We can conclude that the rapid release latency seen in both Observers and R-observers is dependent on the presence of a distressed cage mate.
Social Observers did not maintain the chain pull response under an FR10 but did continue under an FR5 when the outcome involved direct contact with the conspecific (Fig. 4a). However, Empathy Observers did not maintain responding past an FR1 (Fig. 4b). Combined, these findings suggest that social interaction aids in maintaining this helping behavior. Along this notion, prosocial behaviors are sensitive to changes in motivation and effort required to respond. Although there is a dearth of research in rodents evaluating the motivational aspects of helping behavior, a recent study gave rats the opportunity to release a distressed conspecific or escape into a darkened cage to alleviate their own distress. Rats learned to release their cage mate, but they did so slower and less often compared to rats that did not have the choice to escape [23]. Further, clinical studies found that empathy in human subjects decreased when the effort to help increased [24] and engaging in empathy was avoided because it was considered more effortful compared to an alternative strategy [25]. These studies suggest that empathic behaviors, especially those requiring more than minimal effort, are significantly less motivating when compared to a behavior that ultimately leads to a personal reward like social interaction. Future studies could directly evaluate the motivation to perform a social task compared to an empathic task.
Familiarity between conspecifics has a role in helping behavior. For example, rats will aid a distressed stranger only if it is a strain with which the observer rat is familiar. Further, pair-housing rats with a particular strain prompt them to aid strangers of that strain, indicating prior social experience may drive helping behavior [11]. Thus, we tested the importance of familiarity for R-observers to perform the empathy task in the presence of rats with which they were completely unfamiliar. Interestingly, chain pull latency in the first two (of six) trials were significantly increased compared to BL (Fig. 5d). This finding was not due to habitation to an individual rat because three different stranger rats were used randomly over the course of the six total trials. This response pattern indicates rats quickly acclimated to the concept of aiding an unfamiliar conspecific. Prosocial behaviors in humans are modulated by the degree of affiliation, with in-group members preferentially helped as compared to unaffiliated individuals [26–28]. However, group affiliation can be overcome by changes in social experience [29], even in rats [11]. The stranger rats used in our experiment were of the same strain as the R-observers, corroborating the conclusion that the motivation to perform helping behaviors in rats is extended to strangers of familiar strains [11]. This evidence may indicate that rats form groups based on social experience, similar to humans [30]. As it pertains to the PAM, rats are less likely to generate shared affect with an out-group animal, reducing the motivation to act empathically. However, this can be more readily overcome with an unfamiliar rat of a familiar strain. Future experiments using this novel model will work to evaluate the importance of group membership via cross-fostering rats of different strains to determine if they demonstrate selectivity in empathic behavior towards strangers of the strain with which they are familiar.
It is likely that the behavior seen in our lab and other researchers’ [9–11, 31] social helping model, in which rats will aid a distressed conspecific (Fig. 2a), and previous experience with the same distressing environment directly modulates helping behavior (Fig. 2b), is driven in part by empathy. However, data from our lab and elsewhere [12, 13] suggest that these models are also driven by the opportunity for social reward. There is a wealth of evidence that demonstrates rats find various forms of social interaction, such as play fighting [32], maternal care of pups [33], and sexual behavior [34] reinforcing. Further, animals readily exhibit preference to social interaction in conditioned place preference (CPP) paradigms [35–37], and they have also been shown to value social reward above both natural rewards (palatable foods) [38] and drugs of abuse [39–41]. Venniro et al. [42] demonstrated volitional social reward reliably attenuated both methamphetamine and heroin self-administration in rats in an operant choice model. Like an operant model of social reward, Targets in the two-chamber social task were released into the same environment as the Observer. When the water was removed as a distressor, there was a delay in the acquisition of the chain pull (Fig. 2d) when compared to the task in which water is present (Fig. 2a). We posit that this delay is because a driving force of the behavior, reducing the distress of the cage mate, was removed. However, social reward was still present and thus helped to eventually promote a decrease in release latency. Moreover, it is likely that R-observers more readily acquired the task in the absence of a distressed cage mate (Fig. 2e) because they were still able to associate the chain pull with social reward from being given access to the dry chamber during the acquisition phase.
In contrast, when water was removed from the three-chamber empathy task, meaning there was neither a distressed conspecific nor the opportunity for social interaction, there was no change in chain pull response across days, indicating that Observers/R-observers did not systematically acquire a directed chain pull task (Fig. 3d, e). The absence of a distressed conspecific, as described by the PAM, prevents the motivating force of state matching in order to drive the helping behavior. Moreover, there is no prior affective experience that allows for enhanced state matching in the absence of the distressing event, which, we suggest, is why there is no difference over days in the chain pull responses in Observer or R-observer rats when water was removed during the empathy task.
In conclusion, we have expanded on the current literature to develop a method to study empathic behavior in a translationally relevant rodent paradigm based on Preston and de Waal’s PAM [2, 3]. Rats display empathic behaviors indexed as a progressive decrease in latency to release a distressed conspecific. Moreover, prior experience with the event allows for enhanced state matching and improved targeted helping. The observed empathic behavior, although subject to low effort as previously described [23–25], is specific to the presence of a distressed conspecific, and rats will also learn to aid an unfamiliar animal. Overall, we believe this model could be an excellent translational tool to better understand the underlying neurobiology of empathy as well as understanding how empathy is altered in psychiatric disorders.
Funding and disclosures
The authors cite no conflicts of interest and there are no competing financial interests with this work. The behavioral apparatus were constructed by Med Associates (Fairfax, VT, USA). Funding for the research was provided by NIH T32 GM08716 and NIDA T32 DA007288 awarded to S.S.C., and SCOR pilot project DA016511 awarded to C.M.R.
Acknowledgements
The authors would like to thank Angela Kearns, Samantha Brown, Carole Berini, and Jordan Hopkins for their assistance in collecting preliminary data for this manuscript. We would also like to thank Drs. Rachel Penrod and Brett Froeliger for comments on an earlier draft of this manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meyza KZ, Bartal IB-A, Monfils MH, Panksepp JB, Knapska E. The roots of empathy: through the lens of rodent models. Neurosci Biobehav Rev. 2017;76:216–34. doi: 10.1016/j.neubiorev.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston SD, de Waal FBM. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/S0140525X02000018. [DOI] [PubMed] [Google Scholar]
- 3.de Waal FBM, Preston SD. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci. 2017;18:498–509. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- 4.Meyza K, Knapska E. What can rodents teach us about empathy? Curr Opin Psychol. 2018;24:15–20. doi: 10.1016/j.copsyc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Sivaselvachandran S, Acland EL, Abdallah S & Martin LJ. Behavioral and mechanistic insight into rodent empathy. Neurosci Biobehav Rev. 2018;91:130–7. [DOI] [PubMed]
- 6.Panksepp J, Panksepp JB. Toward a cross-species understanding of empathy. Trends Neurosci. 2013;36:489–96. doi: 10.1016/j.tins.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52:132–4. doi: 10.1037/h0043531. [DOI] [PubMed] [Google Scholar]
- 8.Rice GE, Gainer P. ‘Altruism’ in the albino rat. J Comp Physiol Psychol. 1962;55:123–5. doi: 10.1037/h0042276. [DOI] [PubMed] [Google Scholar]
- 9.Sato N, Tan L, Tate K, Okada M. Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn. 2015;18:1039–47. doi: 10.1007/s10071-015-0872-2. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–30. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. Elife. 2014;3:e01385. doi: 10.7554/eLife.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hachiga Y, Schwartz LP, Silberberg A, Kearns DN, Gomez M, Slotnick B. Does a rat free a trapped rat due to empathy or for sociality? J Exp Anal Behav. 2018;110:267–74. doi: 10.1002/jeab.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silberberg A, Allouch C, Sandfort S, Kearns D, Karpel H, Slotnick B. Desire for social contact, not empathy, may explain “rescue” behavior in rats. Anim Cognition. 2014;17:609–18. doi: 10.1007/s10071-013-0692-1. [DOI] [PubMed] [Google Scholar]
- 14.Decety J, Moriguchi Y. The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. Biopsychosoc Med. 2007;1:22. doi: 10.1186/1751-0759-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook R, Brewer R, Shah P, Bird G. Alexithymia, not autism, predicts poor recognition of emotional facial expressions. Psychological Sci. 2013;24:723–32. doi: 10.1177/0956797612463582. [DOI] [PubMed] [Google Scholar]
- 16.Bird G, Cook R. Mixed emotions: the contribution of alexithymia to the emotional symptoms of autism. Transl Psychiatry. 2013;3:e285. doi: 10.1038/tp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurage P, et al. Dissociation between affective and cognitive empathy in alcoholism: a specific deficit for the emotional dimension. Alcohol Clin Exp Res. 2011;35:1662–8. doi: 10.1111/j.1530-0277.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 18.McCown W. The relationship between impulsivity, empathy and involvement in twelve step self-help substance abuse treatment groups. Br J Addiction. 1989;84:391–3. doi: 10.1111/j.1360-0443.1989.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 19.McCown W. The effect of impulsivity and empathy on abstinence of poly-substance abusers: a prospective study. Br J Addiction. 1990;85:635–7. doi: 10.1111/j.1360-0443.1990.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 20.Massey SH, Newmark RL, Wakschlag LS. Explicating the role of empathic processes in substance use disorders: a conceptual framework and research agenda. Drug Alcohol Rev. 2018;37:316–32. doi: 10.1111/dar.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson CSH, Fokas K, Witkiewitz K. Relationship between empathic processing and drinking behavior in project MATCH. Addict Behav. 2018;77:180–6. doi: 10.1016/j.addbeh.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Carvalheiro J, Seara-Cardoso A, Mesquita AR, de Sousa L, Oliveira P, Summavielle T, et al. Helping behavior in rats (Rattus norvegicus) when an escape alternative is present. J. Comp. Psychol. 2019;133:452–62. [DOI] [PubMed]
- 24.Lockwood PL, Hamonet M, Zhang SH, Ratnavel A, Salmony FU, Husain M, et al. Prosocial apathy for helping others when effort is required. Nat Hum Behav. 2017;1:0131. doi: 10.1038/s41562-017-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron CD, Hutcherson CA, Ferguson AM, Scheffer JA, Hadjiandreou E, Inzlicht M. Empathy is hard work: people choose to avoid empathy because of its cognitive costs. J Exp Psychol Gen. 2019;148:962–76. doi: 10.1037/xge0000595. [DOI] [PubMed] [Google Scholar]
- 26.Cialdini RB, Brown SL, Lewis BP, Luce C, Neuberg SL. Reinterpreting the empathy-altruism relationship: when one into one equals oneness. J Personal Soc Psychol. 1997;73:481–94. doi: 10.1037/0022-3514.73.3.481. [DOI] [PubMed] [Google Scholar]
- 27.Levine M, Prosser A, Evans D, Reicher S. Identity and emergency intervention: how social group membership and inclusiveness of group boundaries shape helping behavior. Personal Soc Psychol Bull. 2005;31:443–53. doi: 10.1177/0146167204271651. [DOI] [PubMed] [Google Scholar]
- 28.Echols S, Correll J. It’s more than skin deep: empathy and helping behavior across social groups. In: Decety, J, editor. Empathy: from bench to bedside. Cambridge: MIT Press; 2012. p. 55–71.
- 29.Batson CD, Lishner DA, Cook J, Sawyer S. Similarity and nurturance: two possible sources of empathy for strangers. Basic Appl Soc Psychol. 2005;27:15–25. doi: 10.1207/s15324834basp2701_2. [DOI] [Google Scholar]
- 30.Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. NeuroImage. 2010;51:1468–75. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Karakilic A, Kizildag S, Kandis S, Guvendi G, Koc B, Camsari GB, et al. The effects of acute foot shock stress on empathy levels in rats. Behavioural Brain Res. 2018;349:31–6. doi: 10.1016/j.bbr.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 32.Vanderschuren LJMJ, Achterberg EJM, Trezza V. The neurobiology of social play and its rewarding value in rats. Neurosci Biobehav Rev. 2016;70:86–105. doi: 10.1016/j.neubiorev.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: Effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behavioural Brain Res. 2000;108:215–31. doi: 10.1016/S0166-4328(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 34.Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cogn Neurosci. 2011;1:444–58. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 36.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Developmental Psychobiol. 2004;45:153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 37.Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, et al. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addiction Biol. 2011;16:273–84. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- 38.Ikemoto S, Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Developmental Psychobiol. 1992;25:261–74. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- 39.Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Developmental Psychobiol. 1990;23:75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- 40.Yates JR, Beckmann JS, Meyer AC, Bardo MT. Concurrent choice for social interaction and amphetamine using conditioned place preference in rats: effects of age and housing condition. Drug Alcohol Depend. 2013;129:240–6. doi: 10.1016/j.drugalcdep.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fritz M, Klement S, El Rawas R, Saria A, Zernig G. Sigma1 receptor antagonist BD1047 enhances reversal of conditioned place preference from cocaine to social interaction. Pharmacology. 2011;87:45–48. doi: 10.1159/000322534. [DOI] [PubMed] [Google Scholar]
- 42.Venniro M, Zhang M, Caprioli D, Hoots JK, Golden SA, Heins C, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–9. doi: 10.1038/s41593-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]