Abstract
Major depressive disorder with psychotic features (psychotic depression) is a severe disorder. Compared with other psychotic disorders such as schizophrenia, relatively few studies on the neurobiology of psychotic depression have been pursued. Neuroimaging studies investigating psychotic depression have provided evidence for distributed structural brain abnormalities implicating the insular cortex and limbic system. We examined structural brain networks in participants (N = 245) using magnetic resonance imaging. This sample included healthy controls (n = 159) and the largest cross-sectional sample of patients with remitted psychotic depression (n = 86) collected to date. All patients participated in the Study of Pharmacotherapy of Psychotic Depression II randomized controlled trial. We used a novel, whole-brain, data-driven parcellation technique—non-negative matrix factorization—and applied it to cortical thickness data to derive structural covariance networks. We compared patients with remitted psychotic depression to healthy controls and found that patients had significantly thinner cortex in five structural covariance networks (insular-limbic, occipito-temporal, temporal, parahippocampal-limbic, and inferior fronto-temporal), confirming our hypothesis that affected brain networks would incorporate cortico-limbic regions. We also found that cross-sectional depression and severity scores at the time of scanning were associated with the insular-limbic network. Furthermore, the insular-limbic network predicted future severity scores that were collected at the time of recurrence of psychotic depression or sustained remission. Overall, decreased cortical thickness was found in five structural brain networks in patients with remitted psychotic depression and brain-behavior relationships were observed, particularly between the insular-limbic network and illness severity.
Subject terms: Diagnostic markers, Translational research
Introduction
Major depressive disorder (MDD) with psychotic features (psychotic depression) is a severe disorder with a lifetime prevalence of 0.35–1% [1]. Psychotic features emerge during the onset of a depressive episode in these patients and resolve as the depressive episode remits. This contrasts with schizophrenia and schizoaffective disorder in which psychotic symptoms remain after depression remits, or bipolar I disorder, where psychosis may also emerge during mania [2]. Psychotic depression commonly involves mood-congruent somatic, nihilistic, or guilty delusions [3]. Compared to patients with non-psychotic depression, patients with psychotic depression often demonstrate higher total scores on the Hamilton Rating Scale for Depression (HAM-D), suggesting psychotic depression may be a more severe form of depression. In particular, patients with psychotic depression have more robust associations with psychomotor agitation and retardation [4–9] as well as guilt when compared to patients with non-psychotic depression [4–7, 9]. Importantly, without effective treatment, psychotic depression is associated with long recovery times, disability, mortality, and suicidality [10–15].
The pathogenesis of psychotic depression is unknown, although several lines of neurobiological inquiry have been pursued. Hypothalamic-pituitary-adrenal axis dysregulation [16–20], decreased plasma levels of dopamine-β-hydroxylase [21–23], abnormalities in rapid eye movement sleep [24], and cognitive impairment [3] have all been observed. Brain imaging with computed tomography has also demonstrated increased ventricular volume [25]. However the development of magnetic resonance imaging (MRI) has allowed for more advanced functional and structural imaging [26, 27].
Functional MRI (fMRI) studies of psychotic depression have examined several aspects of task-related cognition. For example, partially remitted patients have less language lateralization compared to healthy controls [28]. In patients with an active episode of psychotic depression, a study of verbal memory encoding found reductions in hippocampal and insular activation when compared with healthy controls [29]. Moreover, a study of working memory found overactivation of the right parahippocampal gyrus in patients relative to healthy controls [30]. Resting state fMRI (R-fMRI) studies have also included patients in remission and demonstrated abnormalities between the somatosensory/insular cortices and the default mode network (DMN) [31]. R-fMRI studies in patients with active psychotic depression have reported abnormalities in hypothalamic and subgenual cingulate cortex (SCC) [32] and fronto-parietal [33] functional connectivity.
Structural MRI (sMRI) studies of specific brain regions have reported reductions in gray matter volume in brain regions including the amygdala and SCC [34–39]. A brain network based on the regions implicated by previous sMRI and fMRI studies could be constructed and examined for structure–function relationships. However, these regional analyses may fail to capture the distributed abnormalities found in psychotic disorders [40, 41]. A network-level approach may better capture these distributed abnormalities while more directly relating to the functional abnormalities observed in psychotic depression. Structural covariance networks have been successfully employed in investigations of schizophrenia and bipolar disorder [42–44]. However, no such investigation has been undertaken in patients with psychotic depression.
Non-negative matrix factorization (NMF) is a multivariate method that differs from independent component analysis (ICA) and principal component analysis (PCA) in that NMF factorizes data under non-negativity constraints. This leads to a parts-based representation of data in which compact networks capture different parts of the brain and are combined in an additive way to form a whole (Fig. 1). The derived non-negative networks have improved specificity and reproducibility compared to commonly used techniques such as ICA and PCA [45, 46] and relate to neurodevelopment [46] and neuropsychiatric symptoms [47–50]. More recently, NMF-derived structural covariance networks have demonstrated relationships with functional brain networks [51]. Overall, NMF allows for the examination of distributed structural abnormalities that may relate to functional connectivity abnormalities observed in patients with psychotic depression [31–33, 52].
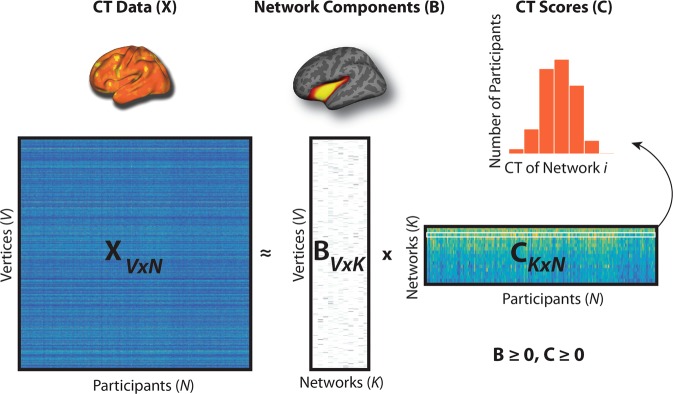
Fig. 1. Non-negative matrix factorization (NMF).
In this figure, X represents the original data matrix as the product of two matrices, B and C. X contains the cortical thickness (CT) data (visualized above the X matrix) for each vertex (rows) and for all participants (columns). B is a matrix that contains, in each column, the loading for each vertex on one of the K networks derived by NMF (an example is visualized above the B matrix). C is a matrix that contains, in each row, the participant-specific coefficients (CT Scores) for each network derived from NMF. The histogram above the C matrix illustrates a sample row of the C matrix with scores for all participants in one network. Both B and C are greater than or equal to 0, thus elements of the factorization are non-negative. Matrices are shown with the following dimensions: V = number of vertices, N = number of participants; K = number of networks.
This study addresses the lack of structural brain network studies of psychotic depression, examines cortical thickness in the largest sample of patients with remitted psychotic depression to date, and compares patients to healthy controls. The remitted status of all patients affords a unique observational window as the networks observed may be less influenced by the states of the illness and more influenced by the traits of the illness. Remitted status also provides a reference point from which structural brain networks may predict whether patients remain well or become unwell. Given the neuroimaging literature on psychotic depression and cortical thickness in depression [53], and our R-fMRI findings implicating the insula [31], we hypothesized that NMF would identify cortico-limbic (including insular-limbic) abnormalities in patients with remitted psychotic depression when compared with healthy controls. In addition, we aimed to explore clinically relevant brain-behavior associations with structural covariance networks. We hypothesized that cortico-limbic networks would be associated with symptom scores at the time of scanning and predict future scores.
Materials and methods
Participants
A total of 245 (psychotic depression n = 86; healthy controls n = 159) participants were included in this analysis. The Toronto site included patients with psychotic depression recruited at the University Health Network and scanned at the Center for Addiction and Mental Health (CAMH). To increase statistical power, the Toronto site control sample was augmented with controls across the adult lifespan from another CAMH study with identical imaging parameters. The majority of participants were recruited at the Toronto site (psychotic depression n = 33; controls n = 91), followed by the University of Massachusetts (psychotic depression n = 22; controls n = 20), Cornell University/Nathan Kline Institute for Psychiatric Research (psychotic depression n = 17; controls n = 23), and the University of Pittsburgh Medical Center (psychotic depression n = 14; controls n = 25).
All patients were enrolled in the Study of the Pharmacotherapy of Psychotic Depression (STOP-PD) II randomized controlled trial (RCT). This RCT examined the benefits and risks of continuing olanzapine versus placebo, in combination with sertraline, in the continuation treatment of remitted psychotic depression [54]. The design of STOP-PD II, including the eligibility criteria, has been described elsewhere [54, 55]. Briefly, patients were men and women aged 18–85 years and who met the criteria for MDD with psychotic features based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Axis I Disorders (SCID-IV) administered by a trained research associate. Exclusion criteria included current or lifetime DSM-IV-TR criteria for: any other psychotic disorder or bipolar disorder, substance abuse or dependence within 3 months preceding enrollment, and dementia preceding the index episode of depression or a 26-item IQCODE [56] mean score ≥4 at acute phase baseline.
STOP-PD II included three consecutive phases. First, during the acute phase, participants received open-label treatment with sertraline (target dose: 150 − 200 mg/day) and olanzapine (15–20 mg/day) for 4–12 weeks to attain remission or “near remission.” Second, during an 8-week stabilization phase, open-label treatment with sertraline and olanzapine continued to ensure that remission was sustained. Third, a 36-week RCT phase compared the efficacy and tolerability of sertraline plus olanzapine with sertraline plus placebo in preventing recurrence of psychotic depression. In line with the goal of the present study to differentiate remitted psychotic depression from healthy controls, all participants in the current analysis were scanned at the end of the second phase of the study.
Healthy controls were aged 18–85 years, did not meet the criteria for any psychiatric disorders on the SCID-IV (except for adjustment disorder or phobic disorder), and did not have a neurological disorder (including dementia or head trauma with loss of consciousness). A urine toxicology screen was obtained and controls with a positive screen, current substance abuse, or history of substance dependence within the past 6 months were excluded. Global cognitive impairment and comorbid physical illness burden were assessed in patients and controls using the Mini-Mental State Examination (MMSE) [57] and Clinical Illness Rating Scale-Geriatrics (CIRS-G) [58], respectively. Patients with MMSE scores <24 were excluded.
Exploratory analyses were pursued to determine the specificity of our findings. Age-matched (>60 years of age) subsets of participants with remitted psychotic depression (n = 35) and healthy controls (n = 43) from our primary analysis were compared with scans of participants with remitted non-psychotic depression (n = 42) obtained from the Prevention of Alzheimer's dementia with Cognitive remediation plus Transcranial direct current stimulation in Mild cognitive impairment and Depression (PACt-MD) RCT (NCT02386670) (Supplementary Materials and methods). Using procedures approved by the local institutional review boards, written informed consent was obtained from all participants or their legal representative prior to the initiation of any research assessment or treatment.
Scanning and analysis of MRI data
All participants completed 3-Tesla MRI scans. Scanner models varied by site and, prior to study start, efforts were made to harmonize acquisition protocols with other studies at local sites and across sites on key parameters (Supplementary Table 1).
sMRI data were preprocessed with FreeSurfer version 6.0 (Martinos Center for Biomedical Imaging). Image quality may be reduced by motion. The mris_euler_number function in FreeSurfer was used to derive the Euler number to quantify image quality [59]. Registration to a template was followed by intensity normalization, gray and white matter segmentation, and tessellation of the boundaries between gray and white matter, as well as gray matter and cerebrospinal fluid [60]. A cortical surface model was calculated for each participant. Cortical thickness was measured as the minimal distance between the tessellated pial and white matter surfaces across the entire cortical mantle [61]. Cortical surfaces were inflated and normalized to the fsaverage5 template using spherical registration [62]. The spatially normalized cortical thickness maps were smoothed using a 20 mm full-width at half-maximum isotropic Gaussian kernel.
Non-negative matrix factorization
Details about the formalization of NMF are provided in the Supplementary Materials and methods. Code for NMF (https://github.com/asotiras/brainparts) adopts orthonormality constraints for the estimated structural covariance networks and projective constraints for their respective participant-specific coefficients [63].
Primary analysis
All statistical analyses were completed using RStudio version 1.1.453 (R Development Core Team, 2018) and included age, age2, sex, and site as covariates. For the primary analysis comparing patients with remitted psychotic depression and controls, a linear model was employed as follows:
CT = intercept + age + age2 + sex + site + group.
CT here represents the average cortical thickness in an NMF-derived structural covariance network and age includes linear and nonlinear effects given our lifespan sample. The false discovery rate (pFDR < 0.05) was used to correct for multiple comparisons.
Sensitivity analyses
A sensitivity analysis was performed to enhance age matching by limiting patients with psychotic depression and controls to those older than 30 years; this improved age matching between patients (n = 79; mean (SD) age: 57.1 (12.9) years) and controls (n = 105; 55.5 (13.0) years) (t = 0.861, p = 0.39). Sensitivity analyses for education and physical illness burden were pursued in the full sample by including years of education and total CIRS-G scores as covariates in separate linear models. Additional aspects of illness burden related to depression were examined in the psychotic depression sample by including the related SCID-IV item as a continuous (years of illness, months of current episode) or categorical (past suicide attempt) covariate in a linear model.
Exploratory analyses in patients with remitted psychotic depression
Exploratory analyses were also pursued solely in the remitted psychotic depression group. Since all of these patients were taking sertraline and olanzapine at the time scanning, sertraline and olanzapine dosages were both included as covariates in a linear model. Two additional exploratory analyses examined associations between structural covariance networks and total HAM-D scores (for depressive symptomatology) and CGI (clinical global impression) severity scores (for severity).
Symptom scores at the time of scanning may predict future scores and can serve as a null model. For the structural covariance network significantly associated with symptom measures, we applied the following models without (null model) and with the average cortical thickness values in the network:
Y = intercept + age + age2 + sex+ site + symptom score at time of scanning.
Y = intercept + age + age2 + sex + site + CT + symptom score at time of scanning.
Y here represents the final available symptom score (total HAM-D or CGI severity at the time of recurrence or completion of the RCT). An F test was then performed to test the predictive ability of the structural covariance network over and above the symptom score at the time of scanning.
Results
Table 1 presents characteristics of patients with psychotic depression at initiation of the acute phase of open-label treatment with olanzapine and sertraline and at the end of the second phase (i.e., at the time of scanning). The dosages were at or near target for both sertraline (mean (SD): 164.5 (35.0) mg/day; median: 150 mg/day; mode: 150 mg/day) and olanzapine (14.9 (4.4) mg/day; median: 15 mg/day; mode = 15 mg/day). Patients with psychotic depression had nearly two decades of illness (mean years of illness = 17.7, SD = 17.5) and their current episode lasted a year (mean months of episode 12.0, SD = 20.7). Over a third (n = 34) of our sample previously attempted suicide. In terms of image quality assurance, the Euler number for all participants was 2 (indicating no defects in image quality), irrespective of whether they were in the remitted psychotic depression, remitted non-psychotic depression, or healthy control group.
Table 1.
(a) Characteristics of patients and healthy controlsa and (b) comparison of key patient variables at the acute phase and time of scanningb.
| (a) | Patients (n = 86) | Controls (n = 159) | χ2 | t (df = 243) | p Value |
|---|---|---|---|---|---|
| Sex (n) | M 34, F 52 | M 72, F 87 | 0.751 | 0.386 | |
| Age (years) | 54.5 (15.2) | 44.7 (18.5) | 1.970 | <0.001 | |
| Education (years) | 13.9 (3.5) | 15.6 (2.4) | −4.516 | <0.001 | |
| Total MMSE | 28.0 (2.0) | 29.3 (1.0) | −6.778 | <0.001 | |
| Total CIRS-G | 3.6 (3.6) | 2.0 (2.0) | 4.651 | <0.001 |
| (b) | Acute | Time of scanning | t (df = 85) | p Value |
|---|---|---|---|---|
| HAM-D | 28.3 (4.6) | 5.8 (3.7) | 38.245 | <0.001 |
| CGI-S | 5.1 (0.9) | 1.4 (0.7) | 31.608 | <0.001 |
| Weight (kg) | 73.7 (17.9) | 81.7 (17.8) | −11.213 | <0.001 |
| BMI (kg/m2) | 26.5 (5.8) | 29.3 (5.5) | −11.165 | <0.001 |
Mean (SD) unless indicated otherwise.
F female, M male, Total MMSE Mini-Mental State Examination, Total CIRS-G Cumulative Illness Rating Scale-Geriatrics, HAM-D 17 Item Hamilton Depression Rating Scale, CGI-S Clinical Global Impression Severity, BMI body mass index.
aSignificance is reported for two-sample, two-tailed t tests, assuming equal variance.
bSignificance for the comparison of variables at the acute phase to time of scanning is reported for paired, one-tailed t tests, assuming equal variance.
Primary analysis
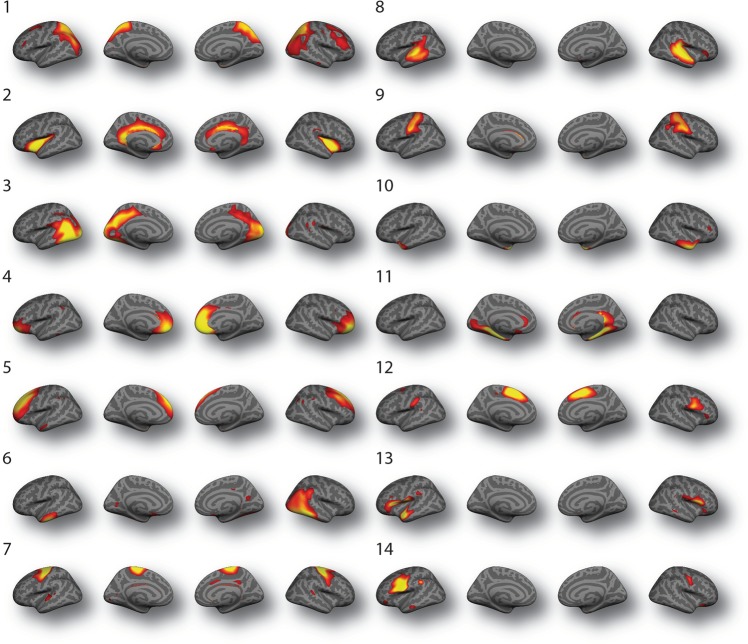
Structural covariance networks were defined using NMF at multiple resolutions in steps of 2 up to 30. The final 14-network solution was chosen on the basis of two considerations. We evaluated the gradient of reconstruction error (Supplementary Fig. 1a), which showed only nominal decrements in error beyond 14 networks. We further checked the split-half reproducibility at this resolution and quantified the overlap between the independently estimated structural covariance networks for the two subsamples using the adjusted rand index (ARI) (Supplementary Figure 1b). The ARI was 0.39 for the 14-network solution, suggesting that this solution is reproducible. Accordingly, the 14-network solution was used for all subsequent analyses (Fig. 2).
Fig. 2. Structural covariance networks derived using non-negative matrix factorization (NMF).
Structural covariance networks are shown for the 14-network NMF solution. The spatial distribution of each network is indicated by loadings at each vertex in arbitrary units. Structural covariance networks include: (1) fronto-parietal; (2) insular-limbic; (3) occipital; (4) fronto-polar; (5) dorso-lateral prefrontal; (6) occipito-temporal; (7) pre-central; (8) temporal; (9) post-central; (10) temporo-polar; (11) parahippocampal-limbic; (12) cingular-post-central; (13) inferior fronto-temporal; and (14) inferior frontal networks.
Patients with remitted psychotic depression consistently demonstrated thinner cortex when compared with controls in five networks with medium effect sizes: insular-limbic (Network 2; t = 3.658, p < 0.001, pFDR = 0.004; d = 0.65), occipito-temporal (Network 6; t = 2.434, p = 0.016, pFDR = 0.044; d = 0.69), temporal (Network 8; t = 3.160, p = 0.002, pFDR = 0.008; d = 0.60), parahippocampal-limbic (Network 11; t = 3.290, p = 0.001, pFDR = 0.008; d = 0.60), and inferior fronto-temporal networks (Network 13; t = 2.684, p = 0.008, pFDR = 0.027; d = 0.63) (Table 2 and Fig. 3).
Table 2.
Structural covariance networks comparing cortical thickness in the full sample of patients with remitted psychotic depression and healthy controls.
| Network | Mpt (SD) | Mct (SD) | β | SE | t | p | pFDR | d |
|---|---|---|---|---|---|---|---|---|
| 1. Fronto-parietal | 2.205 (0.113) | 2.253 (0.112) | 0.020 | 0.013 | 1.563 | 0.119 | 0.139 | 0.43 |
| 2. Insular-limbic | 2.591 (0.142) | 2.678 (0.125) | 0.048 | 0.013 | 3.658 | <0.001 | 0.004 | 0.65 |
| 3. Occipital | 2.236 (0.098) | 2.276 (0.096) | 0.025 | 0.011 | 2.175 | 0.031 | 0.070 | 0.41 |
| 4. Fronto-polar | 2.484 (0.133) | 2.548 (0.127) | 0.025 | 0.014 | 1.790 | 0.075 | 0.105 | 0.49 |
| 5. Dorso-lateral prefrontal | 2.446 (0.130) | 2.527 (0.151) | 0.019 | 0.015 | 1.272 | 0.205 | 0.220 | 0.57 |
| 6. Occipito-temporal | 2.539 (0.102) | 2.614 (0.116) | 0.031 | 0.013 | 2.434 | 0.016 | 0.044 | 0.69 |
| 7. Pre-central | 2.396 (0.136) | 2.447 (0.144) | 0.002 | 0.016 | 0.143 | 0.886 | 0.886 | 0.36 |
| 8. Temporal | 2.528 (0.125) | 2.607 (0.137) | 0.045 | 0.014 | 3.160 | 0.002 | 0.008 | 0.60 |
| 9. Post-central | 2.115 (0.119) | 2.175 (0.117) | 0.024 | 0.014 | 1.780 | 0.076 | 0.105 | 0.51 |
| 10. Temporo-polar | 3.003 (0.134) | 3.073 (0.143) | 0.038 | 0.018 | 2.120 | 0.035 | 0.070 | 0.51 |
| 11. Parahippocampal-limbic | 2.542 (0.132) | 2.614 (0.107) | 0.050 | 0.015 | 3.290 | 0.001 | 0.008 | 0.60 |
| 12. Cingular-post-central | 2.537 (0.132) | 2.613 (0.143) | 0.028 | 0.015 | 1.878 | 0.062 | 0.105 | 0.55 |
| 13. Inferior fronto-temporal | 2.556 (0.126) | 2.638 (0.134) | 0.037 | 0.014 | 2.684 | 0.008 | 0.027 | 0.63 |
| 14. Inferior frontal | 2.497 (0.123) | 2.571 (0.131) | 0.025 | 0.014 | 1.742 | 0.083 | 0.105 | 0.58 |
Mpt mean cortical thickness (in mm) in a given network for patients with remitted psychotic depression (n = 86), Mct mean cortical thickness (in mm) in a given network for healthy controls (n = 159), SD standard deviation, β regression coefficient for patients with remitted psychotic depression versus healthy controls, SE standard error for regression coefficient, t value for testing against a mean difference of zero, p values and FDR-corrected p values are obtained from separate linear models run for each network, d effect size expressed as Cohen’s d.
Fig. 3. Patients demonstrated thinner cortex in five structural covariance networks.
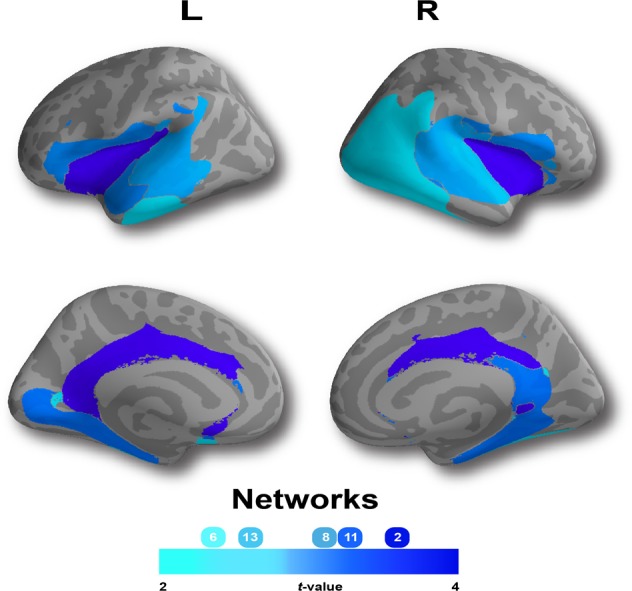
When compared with healthy control participants, patients with remitted psychotic depression demonstrated significant (p < 0.05, False Discovery Rate corrected) reductions in cortical thickness (CT) in five structural networks, including: insular-limbic (Network 2); occipito-temporal (Network 6); temporal (Network 8); parahippocampal-limbic (Network 11); and inferior fronto-temporal (Network 13) networks. Composite network boundaries were obtained by assigning each vertex to the network with the highest loading for that vertex (from the B matrix) across all 14 networks.
Sensitivity analyses
When limited to participants older than 30 years of age, cortical thickness differences between patients with remitted psychotic depression and controls remained significant in the insular-limbic (Network 2; t = 3.653, p < 0.001, pFDR = 0.005), temporal (Network 8; t = 3.009, p = 0.003, pFDR = 0.014), parahippocampal-limbic (Network 11; t = 3.269, p = 0.001, pFDR = 0.009), and inferior fronto-temporal networks (Network 13; t = 2.716, p = 0.007, pFDR = 0.025), but not in the occipito-temporal network (Network 6; t = 1.985, p = 0.049, pFDR = 0.088).
There was no significant association between years of education and any of the 14 networks; when controlling for years of education, patients with remitted psychotic depression and controls continued to show differences in the insular-limbic (Network 2; t = 3.352, p < 0.001, pFDR = 0.013), temporal (Network 8; t = 2.818, p = 0.005, pFDR = 0.024), and parahippocampal-limbic networks (Network 11; t = 3.040, p = 0.003, pFDR = 0.018). There was no significant association between total CIRS-G scores and any of the 14 networks; when controlling for total CIRS-G scores, patients with remitted psychotic depression and controls continued to show differences in the insular-limbic (Network 2; t = 3.595, p < 0.001, pFDR = 0.005), occipito-temporal (Network 6; t = 2.490, p = 0.014, pFDR = 0.038), temporal (Network 8; t = 2.996, p = 0.003, pFDR = 0.014), parahippocampal-limbic (Network 11; t = 3.408, p = 0.001, pFDR = 0.005), and inferior fronto-temporal networks (Network 13; t = 2.543, p = 0.012, pFDR = 0.038).
Sensitivity analyses were additionally pursued in a sample limited to patients with psychotic depression. There were no significant FDR-corrected associations between any of the 14 networks and years of illness, months of current episode, or history of suicide attempts.
Exploratory analyses of remitted psychotic depression, remitted non-psychotic depression, and healthy controls
Exploratory analyses were pursued to determine the specificity of our findings. Subsets of participants with remitted psychotic depression (69.1 (5.8) years), remitted non-psychotic depression (69.7 (5.1)), and healthy controls (68.8 (5.1)) were successfully matched on age (F = 0.282, p = 0.76) and group-wise differences were examined (Supplementary Results).
Exploratory analyses in patients with remitted psychotic depression
Exploratory analyses were also pursued exclusively in patients with remitted psychotic depression and examined associations between networks and medication dosages. There was no significant association between sertraline or olanzapine dosages and any of the 14 networks. Exploratory analyses were also pursued to examine the association between all 14 networks and symptom measures. The only significant association was between the insular-limbic network and total HAM-D scores (t = −2.067, p = 0.042) and CGI severity scores (t = −2.326, p = 0.023). The correlation between CGI severity and total HAM-D scores at the time of scanning (r = 0.29) increased when measured again at the time of relapse or sustained remission (r = 0.86). We then tested whether the insular-limbic network predicted final symptom scores, over and above symptom scores at the time of scanning. A linear model that included the insular-limbic network failed to predict final HAM-D scores better than the null model (F = 3.724, p = 0.058) without this network, however a linear model that included the insular-limbic network predicted final CGI severity scores better than the null model (F = 5.363, p = 0.024).
Discussion
Our primary aim was to compare structural covariance networks in patients with remitted psychotic depression and healthy controls. We found that patients with remitted psychotic depression had significantly thinner cortex in five networks (insular-limbic, occipito-temporal, temporal, parahippocampal-limbic, and inferior fronto-temporal) and confirmed our hypothesis that affected networks incorporated cortico-limbic regions. Our second aim was to explore associations between networks and symptom scores. We confirmed our hypothesis and found that total HAM-D and CGI severity scores at the time of scanning were associated with the insular-limbic network. This network also predicted final CGI severity scores better than the CGI severity scores at the time of scanning, providing evidence for a clinically relevant brain-behavior relationship.
Our exploratory results across depression groups suggest trait-level abnormalities in networks specific to remitted psychotic depression, rather than remitted depression in general. The five networks in our primary analysis demonstrated significantly thinner cortex in patients with remitted psychotic depression when compared to remitted non-psychotic depression. With reference to the literature, gray matter volume reductions in the dorsal anterior cingulate and bilateral insula have been observed in a large, transdiagnostic meta-analysis [64]. In contrast, our networks were derived from cortical thickness, not gray matter volume, and identified networks with regions beyond the dorsal anterior cingulate and insula. A recent ENIGMA study examined cortical thickness in a sample that combined remitted and unremitted depression and healthy controls [65]. Significantly thinner cortex was observed in the orbito-frontal, cingulate, insula, and temporal cortex in this study. These results stand in partial contrast to our lack of differences between remitted non-psychotic depression patients and controls. However, the ENIGMA study included actively ill patients and analyses were regional rather than network-based. It remains possible that a much larger sample of patients with non-psychotic depression across the lifespan may have revealed differences compared to controls using the NMF approach.
Our results are based on the largest sample of patients with remitted psychotic depression collected to date. The size of this sample and ability of NMF to parcellate cortical thickness data into networks allowed for robust analyses to be pursued. Our results partly fit with previous structural findings, although it is noteworthy that the smaller sample size and region of interest-based approaches of previous studies have rendered inconsistent results. For example, the insular cortex and hippocampi have been implicated in psychotic depression [26]. In unremitted psychotic depression, decreases in hippocampal and subcallosal cingulate volume [66], decreases in amygdalar (and not hippocampal) volume [36], and increases in amygdalar volume and decreases in SCC volume (without any significant change in hippocampal volume) [37] have all been reported. When viewed more broadly in terms of frontal, temporal, and posterior gray matter, no differences have been noted in patients with unremitted psychotic depression [35]. Differences between the literature and our results may be due to our cortical thickness network approach, the remitted status of our patients, or our larger sample.
NMF has been used to identify networks implicated in brain-behavior relationships [47–50]. These brain-behavior relationships support the pertinence of the associations we found between the insular-limbic network and depressive symptom and severity scores. NMF networks have also been found to relate to functional brain networks [51]. This structure–function relationship is also present in our analyses, with some overlap between our structural brain networks (particularly the insular-limbic network) and previously reported abnormalities in R-fMRI brain networks [31, 32].
Our findings can be viewed through a limbic system or central autonomic network (CAN) lens. The limbic system has been implicated in depression across the lifespan [67–69]. Evolutionarily, the limbic system rings the base of the cortex and forms a component of all cortical systems [70]. Consistent with our findings, there is evidence for connectivity between the insular cortex and the limbic system [71]. The growing literature on prediction error [72, 73] and its association with interoception in depression [74] and the insular cortex [75] provides evidence for the behavioral manifestation of insular-limbic dysfunction, as do theories of delusion formation in psychotic depression [34]. We have previously reported R-fMRI abnormalities between the DMN and insula in psychotic depression [31]. Medial regions of the DMN correspond to dorsal aspects of the limbic system and are connected via the dorsal cingulum [76]. Thus, there may be an interplay between our current structural findings and previous functional observations.
The CAN regulates the central and autonomic nervous system and includes cortical and subcortical regions [77–80]. The cortical insular-limbic and parahippocampal-limbic networks we identified include core CAN regions [80]; however, they do not include subcortical regions. Alternatively, our findings may better relate to cortically based general networks of cognition which support conscious, emotional feelings [81]. These cortical networks are held to give rise to higher-order representations of lower-order information and thereby provide a non-subcortical substrate for feelings.
Nodes of cortico-limbic networks have translational potential. Intermittent theta-burst TMS has demonstrated modulation of prefrontal-insular functional connectivity [82]. Engaging cortico-limbic circuits with novel pharmacological treatments may prove tractable and targeting the dynamics of monoamine systems and cortico-limbic networks is supported by animal studies [83]. Human studies have examined the interplay between oxytocin and serotonin and the impact on cortico-limbic networks [84]. Thus, the insular-limbic network may hold translational potential for brain stimulation, pharmacotherapy, or even as a treatment-independent biomarker for symptomatic improvement specific to psychotic depression.
Our findings should be interpreted with some additional considerations. Although scanners were different across sites, we controlled for site in our statistical models and selected structural covariance networks that demonstrated split-half reproducibility with halves that were balanced for site. We have also previously shown that the analysis of gray matter structure from harmonized T1-weighted scans had limited susceptibility to inter-site effects [85]. In addition to the multi-site nature of this study, all patients were receiving sertraline and olanzapine at the time of scanning. Nevertheless, the unique context of patients receiving the same medications permitted exploratory analyses of sertraline and olanzapine dosages and no significant association between any of the networks and dosage were observed.
In summary, we compared structural covariance networks in patients with remitted psychotic depression and healthy controls using a novel, data-driven approach. By applying NMF to cortical thickness data, we isolated structural covariance networks that implicated cortico-limbic networks, and in particular, found an insular-limbic network related to symptom scores that also predicted final severity scores. Future studies should examine longitudinal changes in these networks and further examine their usefulness in predicting treatment outcomes.
Funding and disclosure
Grant support for this study was provided by the National Institute of Mental Health (NIMH) under the following grant numbers: BSM (U01MH062518), AJR (U01MH062624), EMW (U01MH062565), AJF (U01MH062446), and ANV (R01MH099167). Eli Lilly provided olanzapine and matching placebo pills and Pfizer provided sertraline; neither company provided funding for the study, participated in data analysis, or participated in the preparation of this manuscript. In addition to support for this study, NHN reported grants from the Canadian Institutes of Health Research (CIHR), University of Toronto, and Physicians' Services Incorporated Foundation. ANK reported grants from the NIMH and the Brain and Behavior Research Foundation (BBRF). AS reported no conflict of interest. BHM reported grants from Brain Canada, Centre for Addiction and Mental Health (CAMH) Foundation, CIHR, and NIMH; nonfinancial support from Pfizer, Eli Lilly, Capital Solution Design LLC, HAPPYneuron, and General Electric. EWD reported grants from the NIMH and BBRF. AJF reported grants from NIMH, National Institutes of Health (NIH), Patient-Centered Outcomes Research Institute, CIHR, Brain Canada, Ontario Brain Institute, and Alzheimer's Association; nonfinancial support from Pfizer and Eli Lilly. BSM reported grants from the NIMH; nonfinancial support from Pfizer and Eli Lilly. GSA reported grants from the NIMH under grant number P50MH113838; nonfinancial support from Pfizer and Eli Lilly; personal fees from Takeda, Lundbeck, Otsuka, Allergan, Astra Zeneca, and Sunovion. AJR reported grants from the NIMH, the Irving S. and Betty Brudnick Endowed Chair in Psychiatry, Allergan, Janssen, and Takeda; nonfinancial support from Pfizer and Eli Lilly; personal fees from Alkermes, GlaxoSmithKline, Sage Therapeutics, Sanofi-Aventis, UMass Medical School, the American Psychiatric Press, and UpToDate. EMW reported grants from the NIMH, NIH, and Health Resources and Services Administration; nonfinancial support from Pfizer and Eli Lilly. LM reported grants from the Alzheimer's Society of Canada, Brain Canada, Centre for Aging and Brain Health Innovation, and Ontario Ministry of Health and Long-Term Care; nonfinancial support from Brainsway Ltd. JN reported nonfinancial support from Alkermes. MJH reported grants from the American Foundation for Suicide Prevention and University of Toronto/NIMH; salary from the New York State Office of Mental Health; consulting fees from Kessler Research Foundation. CD reported grant support under grant numbers R01MH112070 and RF1AG054409. TDS reported grant support under grant numbers R01MH120482, R01MH107703, R01MH112847, and R01MH113550. ANV reported grants from the NIMH, CIHR, Canadian Foundation for Innovation, CAMH Foundation, BBRF, and the University of Toronto.
Supplementary information
Author contributions
Conceptualization: NHN, ANK, AS, BHM, EWD, AJF, BSM, GSA, AJR, EMW, LM, JN, MJH, CD, TDS, and ANV; methodology: NHN, ANK, AS, EWD, CD, TDS, and ANV; formal analysis: NHN and ANK; resources: TDS and ANV; writing—original draft: NHN; writing—review and editing: NHN, ANK, AS, BHM, EWD, AJF, BSM, GSA, AJR, EMW, LM, JN, MJH, CD, TDS, and ANV; visualizations: NHN, ANK, and TDS; supervision: TDS and ANV.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-0646-7).
References
- 1.Jääskeläinen E, Juola T, Korpela H, Lehtiniemi H, Nietola M, Korkeila J, et al. Epidemiology of psychotic depression—systematic review and meta-analysis. Psychol Med. 2018;48:905–18. doi: 10.1017/S0033291717002501. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington: American Psychiatric Publishing; 2013.
- 3.Fleming SK, Blasey C, Schatzberg AF. Neuropsychological correlates of psychotic features in major depressive disorders: a review and meta-analysis. J Psychiatr Res. 2004;38:27–35. doi: 10.1016/s0022-3956(03)00100-6. [DOI] [PubMed] [Google Scholar]
- 4.Coryell W, Pfohl B, Zimmerman M. The clinical and neuroendocrine features of psychotic depression. J Nerv Ment Dis. 1984;172:521–8. doi: 10.1097/00005053-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lykouras E, Malliaras D, Christodouiou GN, Moussas G, Christodoulou D. Delusional depression: phenomenology and response to treatment. Psychopathology. 1986;19:157–64. doi: 10.1159/000284441. [DOI] [PubMed] [Google Scholar]
- 6.Frances A, Brown RP, Kocsis JH, Mann JJ. Psychotic depression: a separate entity? Am J Psychiatry. 1981;138:831–3. doi: 10.1176/ajp.138.6.831. [DOI] [PubMed] [Google Scholar]
- 7.Glassman AH, Roose SP. Delusional depression. A distinct clinical entity? Arch Gen Psychiatry. 1981;38:424–7. doi: 10.1001/archpsyc.1981.01780290058006. [DOI] [PubMed] [Google Scholar]
- 8.Nelson JC, Bowers MB., Jr Delusional unipolar depression: description and drug response. Arch Gen Psychiatry. 1978;35:1321–8. doi: 10.1001/archpsyc.1978.01770350047004. [DOI] [PubMed] [Google Scholar]
- 9.Charney DS, Nelson JC. Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Am J Psychiatry. 1981;138:328–33. doi: 10.1176/ajp.138.3.328. [DOI] [PubMed] [Google Scholar]
- 10.Coryell W, Leon A, Winokur G, Endicott J, Keller M, Akiskal H, et al. Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry. 1996;153:483–9. doi: 10.1176/ajp.153.4.483. [DOI] [PubMed] [Google Scholar]
- 11.Maj M, Pirozzi R, Magliano L, Fiorillo A, Bartoli L. Phenomenology and prognostic significance of delusions in major depressive disorder: a 10-year prospective follow-up study. J Clin Psychiatry. 2007;68:1411–7. doi: 10.4088/jcp.v68n0913. [DOI] [PubMed] [Google Scholar]
- 12.Ohayon MM, Schatzberg AF. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry. 2002;159:1855–61. doi: 10.1176/appi.ajp.159.11.1855. [DOI] [PubMed] [Google Scholar]
- 13.Rothschild AJ. Psychotic depression and suicide. Acta Psychiatr Scand. 2018;137:364–5. doi: 10.1111/acps.12864. [DOI] [PubMed] [Google Scholar]
- 14.Zalpuri I, Rothschild AJ. Does psychosis increase the risk of suicide in patients with major depression? A systematic review. J Affect Disord. 2016;198:23–31. doi: 10.1016/j.jad.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Gournellis R, Tournikioti K, Touloumi G, Thomadakis C, Michalopoulou PG, Christodoulou C, et al. Psychotic (delusional) depression and suicidal attempts: a systematic review and meta-analysis. Acta Psychiatr Scand. 2018;137:18–29. doi: 10.1111/acps.12826. [DOI] [PubMed] [Google Scholar]
- 16.Rothschild AJ. Clinical manual for diagnosis and treatment of psychotic depression. Arlington: American Psychiatric Publishing; 2009.
- 17.Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154:1497–503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- 18.Anton RF. Urinary free cortisol in psychotic depression. Biol Psychiatry. 1987;22:24–34. doi: 10.1016/0006-3223(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 19.Owashi T, Otsubo T, Oshima A, Nakagome K, Higuchi T, Kamijima K. Longitudinal neuroendocrine changes assessed by dexamethasone/CRH and growth hormone releasing hormone tests in psychotic depression. Psychoneuroendocrinology. 2008;33:152–61. doi: 10.1016/j.psyneuen.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Coryell W, Fiedorowicz J, Zimmerman M, Young E. HPA-axis hyperactivity and mortality in psychotic depressive disorder: preliminary findings. Psychoneuroendocrinology. 2008;33:654–8. doi: 10.1016/j.psyneuen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meltzer HY, Cho HW, Carroll BJ. Serum dopamine-β-hydroxylase activity in the affective psychoses and schizophrenia: decreased activity in unipolar psychotically depressed patients. Arch Gen Psychiatry. 1976;33:585–91. doi: 10.1001/archpsyc.1976.01770050047007. [DOI] [PubMed] [Google Scholar]
- 22.Mód L, Rihmer Z, Magyar I, Arató M, Alföldi A, Bagdy G. Serum DBH activity in psychotic vs. nonpsychotic unipolar and bipolar depression. Psychiatry Res. 1986;19:331–3. doi: 10.1016/0165-1781(86)90127-7. [DOI] [PubMed] [Google Scholar]
- 23.Cubells JF, Price LH, Meyers BS, Anderson GM, Zabetian CP, Alexopoulos GS, et al. Genotype-controlled analysis of plasma dopamine β-hydroxylase activity in psychotic unipolar major depression. Biol Psychiatry. 2002;51:358–64. doi: 10.1016/s0006-3223(01)01349-x. [DOI] [PubMed] [Google Scholar]
- 24.Thase ME, Kupfer DJ, Ulrich RF. Electroencephalographic sleep in psychotic depression. A valid subtype? Arch Gen Psychiatry. 1986;43:886–93. doi: 10.1001/archpsyc.1986.01800090076010. [DOI] [PubMed] [Google Scholar]
- 25.Rothschild AJ, Benes F, Hebben N, Woods B, Luciana M, Bakanas E, et al. Relationships between brain CT scan findings and cortisol in psychotic and nonpsychotic depressed patients. Biol Psychiatry. 1989;26:565–75. doi: 10.1016/0006-3223(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 26.Busatto GF. Structural and functional neuroimaging studies in major depressive disorder with psychotic features: a critical review. Schizophr Bull. 2013;39:776–86. doi: 10.1093/schbul/sbt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Connor S, Agius M. A systematic review of structural and functional MRI differences between psychotic and nonpsychotic depression. Psychiatr Danub. 2015;27:S235–S239. [PubMed] [Google Scholar]
- 28.Sommer IEC, Vd Veer AJ, Wijkstra J, Boks MPM, Kahn RS. Comparing language lateralization in psychotic mania and psychotic depression to schizophrenia; a functional MRI study. Schizophr Res. 2007;89:364–5. doi: 10.1016/j.schres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Kelley R, Garrett A, Cohen J, Gomez R, Lembke A, Keller J, et al. Altered brain function underlying verbal memory encoding and retrieval in psychotic major depression. Psychiatry Res. 2013;211:119–26. doi: 10.1016/j.pscychresns.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry. 2011;168:173–82. doi: 10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- 31.Neufeld NH, Mulsant BH, Dickie EW, Meyers BS, Alexopoulos GS, Rothschild AJ, et al. Resting state functional connectivity in patients with remitted psychotic depression: a multi-centre STOP-PD study. EBioMedicine. 2018;36:446–53. doi: 10.1016/j.ebiom.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudheimer K, Keller J, Gomez R, Tennakoon L, Reiss A, Garrett A, et al. Decreased hypothalamic functional connectivity with subgenual cortex in psychotic major depression. Neuropsychopharmacology. 2015;40:849–60. doi: 10.1038/npp.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oudega ML, van der Werf YD, Dols A, Wattjes MP, Barkhof F, Bouckaert F, et al. Exploring resting state connectivity in patients with psychotic depression. PLoS ONE. 2019;14:e0209908. doi: 10.1371/journal.pone.0209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson S, Baldwin RC, Jackson A, Burns A. The differentiation of DSM-III-R psychotic depression in later life from nonpsychotic depression: comparisons of brain changes measured by multispectral analysis of magnetic resonance brain images, neuropsychological findings, and clinical features. Biol Psychiatry. 1999;45:193–204. doi: 10.1016/s0006-3223(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 35.Salokangas RKR, Cannon T, Van Erp T, Ilonen T, Taiminen T, Karlsson H, et al. Structural magnetic resonance imaging in patients with first-episode schizophrenia, psychotic and severe non-psychotic depression and healthy controls. Br J Psychiatry. 2002;181:s58–s65.. doi: 10.1192/bjp.181.43.s58. [DOI] [PubMed] [Google Scholar]
- 36.Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss A, et al. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165:872–80. doi: 10.1176/appi.ajp.2008.07081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vassilopoulou K, Papathanasiou M, Michopoulos I, Boufidou F, Oulis P, Kelekis N, et al. A magnetic resonance imaging study of hippocampal, amygdala and subgenual prefrontal cortex volumes in major depression subtypes: melancholic versus psychotic depression. J Affect Disord. 2013;146:197–204. doi: 10.1016/j.jad.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Kim DK, Kim BL, Sohn SE, Lim SW, Na DG, Paik CH, et al. Candidate neuroanatomic substrates of psychosis in old-aged depression. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:793–807. doi: 10.1016/s0278-5846(99)00041-x. [DOI] [PubMed] [Google Scholar]
- 39.Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry. 2005;162:1706–12. doi: 10.1176/appi.ajp.162.9.1706. [DOI] [PubMed] [Google Scholar]
- 40.Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum Neurosci. 2014;8:1–18. [DOI] [PMC free article] [PubMed]
- 41.Behdinan T, Foussias G, Wheeler AL, Stefanik L, Felsky D, Remington G, et al. Neuroimaging predictors of functional outcomes in schizophrenia at baseline and 6-month follow-up. Schizophr Res. 2015;169:69–75. doi: 10.1016/j.schres.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler AL, Wessa M, Szeszko PR, Foussias G, Chakravarty MM, Lerch JP, et al. Further neuroimaging evidence for the deficit subtype of schizophrenia: a cortical connectomics analysis. JAMA Psychiatry. 2015;72:446–55. doi: 10.1001/jamapsychiatry.2014.3020. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp. 2009;30:711–24. doi: 10.1002/hbm.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wannan CMJ, Cropley VL, Chakravarty MM, Bousman C, Ganella EP, Bruggemann JM, et al. Evidence for network-based cortical thickness reductions in schizophrenia. Am J Psychiatry. 2019;176:552–63. doi: 10.1176/appi.ajp.2019.18040380. [DOI] [PubMed] [Google Scholar]
- 45.Sotiras A, Resnick SM, Davatzikos C. Finding imaging patterns of structural covariance via non-negative matrix factorization. Neuroimage. 2015;108:1–16. doi: 10.1016/j.neuroimage.2014.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sotiras A, Toledo JB, Gur RE, Gur RC, Satterthwaite TD, Davatzikos C. Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc Natl Acad Sci USA. 2017;114:3527–32. doi: 10.1073/pnas.1620928114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pehlivanova M, Wolf DH, Sotiras A, Kaczkurkin A, Moore TM, Ciric R, et al. Diminished cortical thickness is associated with impulsive choice in adolescence. J Neurosci. 2018;38:2471–81. doi: 10.1523/JNEUROSCI.2200-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, et al. Evidence for dissociable linkage of dimensions of psychopathology to brain structure in youths. Am J Psychiatry. 2019;176:1000–9. doi: 10.1176/appi.ajp.2019.18070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jirsaraie RJ, Kaczkurkin AN, Rush S, Piiwia K, Adebimpe A, Bassett DS, et al. Accelerated cortical thinning within structural brain networks is associated with irritability in youth. Neuropsychopharmacology. 2019;44:2254–62. doi: 10.1038/s41386-019-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaczkurkin AN, Sotiras A, Baller EB, Barzilay R, Calkins ME, Chand GB, et al. Neurostructural heterogeneity in youth with internalizing symptoms. Biol Psychiatry. 2020;87:473–82. [DOI] [PMC free article] [PubMed]
- 51.Cui Z, Li H, Xia CH, Larsen B, Adebimpe A, Baum GL, et al. Individual variation in functional topography of association networks in youth. Neuron. 2020;106:1–14. [DOI] [PMC free article] [PubMed]
- 52.Croarkin PE. Indexing the neurobiology of psychotic depression with resting state connectivity: Insights from the STOP-PD study. EBioMedicine. 2018;37:32–33. doi: 10.1016/j.ebiom.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suh JS, Schneider MA, Minuzzi L, MacQueen GM, Strother SC, Kennedy SH, et al. Cortical thickness in major depressive disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:287–302. doi: 10.1016/j.pnpbp.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, Alexopoulos GS, Rudorfer MV, et al. Effect of continuing olanzapine vs placebo on relapse among patients with psychotic depression in remission: the STOP-PD II Randomized Clinical Trial. JAMA. 2019;322:622–31. doi: 10.1001/jama.2019.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, Mulsant BH, Rudorfer MV, et al. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:1–12. [DOI] [PMC free article] [PubMed]
- 56.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 57.Folstein MF, Folstein SE, McHugh PR. Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 58.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 59.Rosen AFG, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa LP, et al. Quantitative assessment of structural image quality. Neuroimage. 2018;169:407–18. doi: 10.1016/j.neuroimage.2017.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 61.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z, Oja E. Linear and nonlinear projective nonnegative matrix factorization. IEEE Trans Neural Netw. 2010;21:734–49. doi: 10.1109/TNN.2010.2041361. [DOI] [PubMed] [Google Scholar]
- 64.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bijanki KR, Hodis B, Brumm MC, Harlynn EL, McCormick LM. Hippocampal and left subcallosal anterior cingulate atrophy in psychotic depression. PLoS ONE. 2014;9:e110770. doi: 10.1371/journal.pone.0110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Redlich R, Opel N, Bürger C, Dohm K, Grotegerd D, Förster K, et al. The limbic system in youth depression: brain structural and functional alterations in adolescent in-patients with severe depression. Neuropsychopharmacology. 2018;43:546–54. doi: 10.1038/npp.2017.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stickel S, Wagels L, Wudarczyk O, Jaffee S, Habel U, Schneider F, et al. Neural correlates of depression in women across the reproductive lifespan—an fMRI review. J Affect Disord. 2019;246:556–70. doi: 10.1016/j.jad.2018.12.133. [DOI] [PubMed] [Google Scholar]
- 69.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–95. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 70.García-Cabezas MÁ, Zikopoulos B, Barbas H. The Structural Model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex. Brain Struct Funct. 2019;224:985–1008. doi: 10.1007/s00429-019-01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghaziri J, Tucholka A, Girard G, Houde J-C, Boucher O, Gilbert G, et al. The corticocortical structural connectivity of the human insula. Cereb Cortex. 2017;27:1216–28. doi: 10.1093/cercor/bhv308. [DOI] [PubMed] [Google Scholar]
- 72.Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:1–26. [DOI] [PMC free article] [PubMed]
- 73.Sterzer P, Adams RA, Fletcher P, Frith C, Lawrie SM, Muckli L, et al. The predictive coding account of psychosis. Biol Psychiatry. 2018;84:634–43. doi: 10.1016/j.biopsych.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eggart M, Lange A, Binser MJ, Queri S, Müller-Oerlinghausen B. Major depressive disorder is associated with impaired interoceptive accuracy: a systematic review. Brain Sci. 2019;9:1–17. [DOI] [PMC free article] [PubMed]
- 75.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Catani M, Dell’acqua F, Thiebaut, de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37:1724–37. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 78.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 79.Hagemann D, Waldstein SR, Thayer JF. Central and autonomic nervous system integration in emotion. Brain Cogn. 2003;52:79–87. doi: 10.1016/s0278-2626(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 80.Beissner F, Meissner K, Bär K-J, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–11. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LeDoux JE, Brown R. A higher-order theory of emotional consciousness. Proc Natl Acad Sci USA. 2017;114:E2016–E2025. doi: 10.1073/pnas.1619316114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iwabuchi SJ, Raschke F, Auer DP, Liddle PF, Lankappa ST, Palaniyappan L. Targeted transcranial theta-burst stimulation alters fronto-insular network and prefrontal GABA. Neuroimage. 2017;146:395–403. doi: 10.1016/j.neuroimage.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 83.Lee E-H, Han P-L. Reciprocal interactions across and within multiple levels of monoamine and cortico-limbic systems in stress-induced depression: a systematic review. Neurosci Biobehav Rev. 2019;101:13–31. doi: 10.1016/j.neubiorev.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 84.Mottolese R, Redouté J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proc Natl Acad Sci USA. 2014;111:8637–42. doi: 10.1073/pnas.1319810111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hawco C, Viviano JD, Chavez S, Dickie EW, Calarco N, Kochunov P, et al. A longitudinal human phantom reliability study of multi-center T1-weighted, DTI, and resting state fMRI data. Psychiatry Res Neuroimaging. 2018;282:134–42. doi: 10.1016/j.pscychresns.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.