Abstract
Background
Without breastfeeding and maternal antiretroviral therapy (ART), HIV-exposed uninfected (HEU) infants experience greater infectious morbidity than HIV-unexposed (HU) infants. We hypothesised that with universal maternal ART, breastfed HEU and HU infants experience similar morbidity.
Methods
We recruited HIV-negative, and HIV-positive women initiating ART, at first antenatal visit in Cape Town, South Africa (March 2013-August 2015). Women were followed through delivery, and postpartum with breastfeeding infants for ≥12 months, through March 2017. Infection-related hospitalisation data abstracted from routine health records were analysed using incidence rate ratios (IRR) from Poisson regression (variance corrected for clustering).
Findings
Mother-infant pairs (n=410 HU, n=459 HEU; pre-ART median CD4 count, 354 cells/μL; HIV viral load, HIV-VL 4·0 log10 copies/mL; gestation, 22 weeks) were followed for median 12 months. HEU (vs HU) infants experienced more infection-related hospitalisations between 7 days and 3 months (incidence/100 child-years, cy: 34·2 [95% CI 24·4–47·9] vs 9·8 [95% CI 5·1–18·8]; IRR 3·50 [95% CI 1·68–7·30]), but rates were similar at other ages. Rates for HEU infants with healthier mothers (n=84; ART initiation <24 weeks’ gestation, CD4 count>350 cells/μL, HIV-VL<4·0 log10 copies/mL: 15·88/100cy [95% CI 5·12–49·23]) approximated those of HU infants (IRR vs HU, 1·62 [95% CI 0·44–6·00]); HEU infants of mothers with late ART initiation and advanced disease had the highest rates (n=44; ART≥24 weeks’ gestation, CD4 count≤350 cells/μL, HIV-VL≥4·0 log10 copies/mL: 40·44/100cy [95% CI 15·18–107·74]; IRR vs HU, 4·14 [95% CI 1·27–13·44]). Reduced rates were seen among optimally breastfed, timely-vaccinated HEU infants (n=90; 9·63/100cy [95% CI 2·41–38·49].
Interpretation
Despite ART in pregnancy, breastfed HEU versus HU infants had transiently increased infectious morbidity risks in early infancy. However, differences were driven by factors potentially amenable to intervention including delayed diagnosis and ART initiation among HIV-positive mothers alongside suboptimal breastfeeding and vaccination of their HEU infants.
Funding
NICHD, EGPAF, SA-MRC, Fogarty Foundation
Keywords: HIV-exposed uninfected infants, Africa, prevention of perinatal HIV transmission, antiretroviral therapy, breastfeeding, diarrhoea, pneumonia
INTRODUCTION
An estimated one million HIV-exposed uninfected (HEU) infants are born in sub-Saharan Africa annually.1 Historically, HEU infants have had higher risks of infectious morbidity than HIV-unexposed (HU) infants, hypothesized to be driven by a range of biological and socio-economic mechanisms.2–5 These may include (1) an altered fetal immunological milieu, the result of maternal immune dysregulation directly caused by replicating HIV virus and/or maternal co-infections, (2) reduced transplacental transfer of maternal antibodies, (3) increased household infectious burden due to high maternal susceptibility to infectious illness, (4) reduced household socio-economic circumstances in turn associated with higher risks of childhood malnutrition and suboptimal child care, and (5) avoidance of breastfeeding to minimize perinatal HIV transmission.2–4
However, evidence for these associations and mechanisms are based predominantly on data predating the current standard of care for HIV-infected women and their infants in sub-Saharan Africa.5 The past decade has seen a dramatic shift towards universal maternal ART and the promotion of early, exclusive breastfeeding extended with appropriate complementary feeds through at least one year of age.6,7 These policy changes have the potential to ameliorate several of the factors hypothesised to drive the infectious morbidity risk differential between HEU and HU infants. Specifically, maternal ART substantially improves maternal health and survival,8 in turn addressing factors (1–4) above, while the protective benefits of breastfeeding against infectious morbidity are well established.9 We therefore hypothesised that, under currently promoted policies of universal maternal ART with breastfeeding, the infectious morbidity risks of HEU infants may approximate those of otherwise similar HU infants. To test this hypothesis, we examined rates of infection-related hospitalisations and longitudinal prevalence of infectious illness, comparing breastfed HEU infants born to women initiating universal ART in pregnancy versus breastfed HU infants in Cape Town, South Africa.
METHODS
We prospectively enrolled HIV-positive pregnant women initiating universal ART and a parallel cohort of HIV-negative pregnant women from the same community.10 Study activities were based at the Gugulethu Midwife Obstetric Unit (MOU), which provides primary-level obstetric care (including prevention of mother-to-child HIV transmission services) to a predominantly low-income, urban population of roughly 350 000 (30% antenatal HIV prevalence).11 Local clinics provide child health care (vaccinations include Bacille Calmette-Guerin (birth); rotavirus (6 and 14 weeks); and pneumococcal conjugate vaccines (6, 10 and 14 weeks)).12 The area has a high infant mortality rate (15·6 per 1000 live births at the time of the study).13
At first antenatal care (ANC) visit, eligible pregnant women (≥18 years of age, planning to deliver in Cape Town, any gestation) were screened for enrolment into the Maternal Child Health Antiretroviral Therapy (MCH-ART; HIV-infected women, enrolment March 2013-June 2014), and HIV-Unexposed Uninfected studies (HU2; a sub-study of MCH-ART for HIV-negative women, enrolment September 2014-August 2015).10 All HIV-infected women initiated lifelong ART at first ANC visit without CD4 or gestational age restrictions. The delivery unit and referral hospitals are certified baby-friendly; breastfeeding is promoted as infant feeding of choice for HIV-positive mothers on ART. The HU2 study was conceived and designed specifically to complement MCH-ART, by providing a control cohort of HIV-negative mothers and breastfeeding HU infants sampled from the same community. Both studies utilised the same staff, study approaches and, apart from HIV-specific measures, measurement tools and procedures, as described elsewhere.14 Women were followed through pregnancy to delivery. At the neonatal study visit (scheduled within seven days after birth), breastfeeding mother-child pairs were eligible for further postnatal follow-up (visits at six weeks, and three-monthly from three to 12 months; a subset of MCH-ART participants returned for an additional visit at 18 months).
All women provided written informed consent. Both the MCH-ART and HU2 studies were approved by the Human Research Ethics Committee of the University of Cape Town. In addition, MCH-ART was approved by the Columbia University Medical Centre Institutional Review Board and is registered on Clinicaltrials.gov (NCT01933477).
Measurements
We used standardised questionnaires, administered at study visits by trained field workers, to measure maternal health, psychosocial and behavioural factors; the same questionnaires were administered to both groups of women.14 Feeding was primarily assessed with 24-hour maternal recall; last study visit with report of breastfeeding was used as date of breastfeeding cessation.15 Duration of exclusive breastfeeding (EBF, defined as consistently receiving only breastmilk and prescribed medicine) was calculated using (1) date of last study visit with report of EBF and (2) maternal report of the age at which non-breastmilk liquids or solids were introduced. Prevalence of infant infectious illness in the preceding two weeks was based on maternal self-report, using questions based on Demographic & Health Survey (DHS) questionnaires. Presumed lower respiratory tract infection (LRTI) was defined as maternal report of cough plus both fever and difficulty in breathing; diarrhoeal illness, as increased or loose stools. Immunisation data were abstracted from patient-held records (Road to Health booklets); delayed vaccination was defined as > two weeks after recommended age.16 In the event of a child death, study staff compiled all available information on possible causes including, where possible, copies of death certificates, medical records and autopsy reports, and administered the World Health Organization verbal autopsy tool to willing mothers.17
Hospitalisation data were obtained from a centralised provincial health database, with diagnoses based on ICD-10 coding from hospital discharge summaries.18 Admissions with same-day discharge were excluded from analysis as ambulatory events; interhospital transfers counted as a single admission. Where multiple infectious diagnoses were listed, the most life-threatening or severe infection was allocated as primary infectious cause.19 An independent paediatrician, blinded to HIV exposure status, reviewed all hospital diagnoses to adjudicate whether the primary cause for admission was infection-related vs non-infection-related; the same pediatrician reviewed all available mortality information to allocate a final cause of death for study purposes.
HIV infection was excluded among HEU infants at six and 48 weeks of age (HIV-PCR: Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 qualitative assay; Roche Molecular systems, Branchburg, NJ.10 Final HIV status for infants censored before 48 weeks reflected 6 weeks’ results. Previously HIV-negative women received regular HIV counselling and testing throughout follow-up; where seroconversion had occurred, HIV infection was excluded in the infants. Infants who acquired HIV infection at any time during follow-up were excluded from this analysis.
Statistical methodology
Sample size was calculated for differences in prevalence of infectious illness. Assuming an underlying risk of diarrhoea among HU infants of 0·15,13 a sample size of 880 (440:440) would provide 84% power to detect ≥0·08 absolute difference in risk, and 80% power to detect a relative difference of at least 1·5 at α=0·05.
We evaluated the effect of HIV exposure on two primary outcomes: hospitalisation (all-cause, and infection-related) and prevalence of child infectious illness. Analysis was restricted to singleton or first-born twins. Hospitalisation rates (admissions per 100 child-years, cy) were compared with crude and adjusted incidence rate ratios (IRR and aIRR, respectively) from Poisson regression (sandwich variance estimator to account for clustering).20 Overall person-time was from date of birth, until censoring at (1) date of death; (2) final study visit, if no hospitalisations within 3 months thereafter; or (3) day of hospital discharge if admitted within 3 months of final study visit. Following a prespecified analysis plan, we generated age-stratified rates in 3-monthly intervals. To differentiate early neonatal from later admissions, we further divided the 0–3 month interval into 0–7 days and >7 days to 3 months. Prevalence of infectious illness was compared with crude and adjusted prevalence ratios (PR and aPR, respectively), from modified Poisson models (population-averaged models with sandwich variance estimator).21 Third variables were chosen a priori based on a directed acyclic graph (supplemental figure 1). “Optimal” breastfeeding was defined has (1) early initiation of breastfeeding (< hour of birth), with (2) EBF at all attended visits before age five months and (3) breastfeeding continued throughout follow-up. Undernutrition was accounted for using time-varying weight-for-age Z-scores (underweight: WAZ<−2); lack of robust birth length measures precluded adjustment for stunting and wasting. Potential effect modifiers included maternal HIV disease severity, timing of ART initiation, breastfeeding, vaccination and birth outcomes (gestation and size at birth). Where multiple measures were available for a single construct (e.g. socio-economic measures), variable choice followed best model fit (Akaike’s Information Criterion). We conducted sensitivity analyses to assess robustness of findings within strata of child characteristics known to be associated with infectious morbidity. Missing data were assumed to be missing at random; we used a missing indicator variable for variables with >10% missing data and complete case analysis otherwise. Analyses used Stata 14·0 (Statacorp, College Station, TX, USA). Results are presented with 95% confidence intervals (CI), and p-values are two-sided.
Role of the funding source
The study sponsors had no role in study design, data collection, analysis, interpretation or writing of the report. The corresponding author had full access to all study data and final responsibility for the decision to submit for publication.
RESULTS
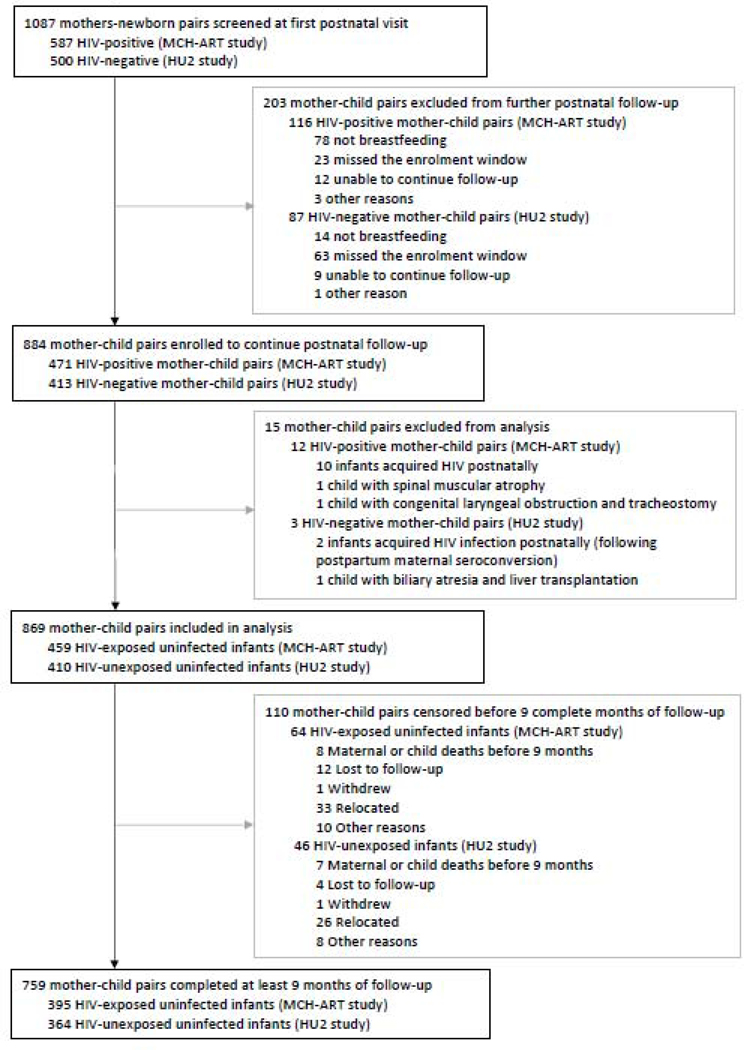
Of 1087 mother-newborn pairs screened at the neonatal visit (median age 5 days, interquartile range, IQR 4–8), 884 (471 born to HIV-positive, 413 to HIV-negative, mothers) were enrolled for postnatal follow-up (figure 1) Fifteen infants were excluded from analysis, including 12 who acquired HIV and three with underlying congenital diseases predisposing to recurrent admissions and infections (figure 1). The median follow-up was 12 months [IQR 9–16]; 395 [86%] of 459 HEU and 364 (89%) of 410 HU infants completed ≥ nine months of follow-up. There were minor differences between these infants and those censored before nine months (appendix, page 3).
FIGURE 1.
Study flow
Maternal and child characteristics are shown in table 1. In general, HIV-positive women (at ART initiation, median [IQR] log10 HIV viral load, HIV-VL 4·0 [3·3–4·5]; CD4 cells/uL, 354 [248–528]; gestation, 22 weeks [17–28]) experienced more economic, psychosocial and behavioural risk factors than HIV-negative women. Proportions of preterm (HEU 12% vs HU 9%, p=0·17) and small-for-gestational age (11% vs 10%, p=0·45) births were similar. Most HEU infants received co-trimoxazole preventive therapy (CPT, 238 [69%] of 347 with data on usage). HEU (vs HU) infants had shorter overall durations of breastfeeding (median 3·9 vs 9·0 months, p=0·001) but were more likely to ever exclusively breastfeed (91% vs 81%, p<0·0001). Overall, only 122 [12%] of 869 children had received optimal breastfeeding (table 1). Time-varying child characteristics are shown per visit in the appendix, page 5. By 12 months, only 144 (41%) of 355 HEU and 174 (50%) of 346 HU infants were still breastfeeding; 171 (52%) of 328 HEU and 107 (37%) of 286 HU infants with known vaccination status had had all vaccinations at the correct ages.
TABLE 1.
Maternal and child characteristics by HIV infection and exposure status
| Total (N=869) | HIV-positive mothers and HEU infants (n=459) | HIV-negative mothers and HU infants (n=410) | p-value | |
|---|---|---|---|---|
| Maternal/household characteristics at first antenatal visit | ||||
| Age in years | 28 (24; 32) | 28 (24; 32) | 27 (23; 32) | 0·11 |
| Married/cohabiting | 372 (43%) | 188 (41%) | 184 (45%) | 0·24 |
| Completed secondary education | 297 (34%) | 113 (25%) | 184 (45%) | <0·0001 |
| Employed | 376 (43%) | 182 (40%) | 194 (47%) | 0·023 |
| Formal housing | 432 (50%) | 218 (47%) | 214 (52%) | 0·17 |
| Flush toilet inside home | 290 (33%) | 125 (27%) | 165 (40%) | <0·0001 |
| Running water inside home | 402 (46%) | 188 (41%) | 214 (52%) | 0·001 |
| Household crowding (≥10 people) | 40 (5%) | 28 (6%) | 12 (3%) | 0·026 |
| Poverty category1 | 0·036 | |||
| Least disadvantaged | 259 (30%) | 145 (32%) | 114 (28%) | |
| Moderate disadvantage | 334 (38%) | 158 (34%) | 176 (43%) | |
| Most disadvantaged | 276 (32%) | 156 (34%) | 120 (29%) | |
| Risky drinking2 | 146 (17%) | 116 (25%) | 30 (7%) | <0·0001 |
| Intimate partner violence 3 | 133 (15%) | 101 (22%) | 32 (8%) | <0·0001 |
| Depression4 | 74 (9%) | 46 (10%) | 28 (7%) | 0·089 |
| Season/time of year5 | 0·12 | |||
| Warm months (Spring/Summer) | 457 (53%) | 230 (50%) | 227 (55%) | |
| Cold months (Autumn/Winter) | 412 (47%) | 229 (50%) | 183 (45%) | |
| Timing of HIV diagnosis | - | |||
| Prior to current pregnancy | - | 197 (43%) | - | |
| During current pregnancy | - | 262 (57%) | - | |
| HIV viral load at ART initiation (log10 copies/mL) | - | 4·0 (3·3–4·5) | - | - |
| CD4 cell count at ART initiation (cells/mm3)6 | - | 354 (248–528) | - | - |
| CD4 cell count > 350 cells/mm3 | - | 231 (52%) | - | - |
| Gestational age at ART initiation (weeks)7 | - | 22 (17–28) | - | - |
| ART initiated < 24 weeks | - | 277 (61%) | - | - |
| Birth and infant characteristics | ||||
| Duration of ART use in pregnancy (weeks) 7 | - | 16·7 (11·1–21·7) | - | - |
| HIV viral load at time of delivery (log10 copies/mL) | - | 1·59 (1·59–1·64) | - | - |
| Maternal viral suppression achieved by time of delivery (HIV viral load <50 copies/mL) | - | 108 (23%) | - | - |
| Season/time of year, at birth5 | <0·0001 | |||
| Warm months (Spring/Summer) | 426 (49%) | 255 (56%) | 171 (42%) | |
| Cold months (Autumn/Winter) | 443 (51%) | 204 (44%) | 239 (58%) | |
| Place of delivery | 0·016 | |||
| Primary care | 344 (39%) | 181 (39%) | 163 (40%) | |
| Hospital care | 512 (59%) | 266 (58%) | 246 (60%) | |
| Born before arrival | 13 (2%) | 12 (3%) | 1 (<1%) | |
| Gestational age at delivery (weeks) | 39 (38–40) | 39 (38–40) | 39 (38–40) | 0·42 |
| Preterm birth (<37) | 93 (11%) | 55 (12%) | 38 (9%) | 0·20 |
| Weight-for-age Z-score at birth | −0·13 (−0·84; 0·50) | −0·21 (−0·94; 0·37) | −0·05 (−0·71; 0·64) | 0·001 |
| Small-for-gestational-age8 (SGA, birthweight <10th centile) | 90 (10%) | 51 (11%) | 39 (10%) | 0·44 |
| Categories of preterm and SGA | ||||
| AGA, term | 695 (80%) | 358 (78%) | 337 (82%) | 0·47 |
| AGA, preterm | 84 (10%) | 50 (11%) | 34 (8%) | |
| SGA, term | 81 (9%) | 46 (10%) | 35 (9%) | |
| SGA, preterm | 9 (1%) | 5 (1%) | 4 (1%) | |
| Weight at birth (kg) | 3·18 (2·82–3·46) | 3·13 (2·76–3·40) | 3·22 (2·86–3·51) | 0·0003 |
| Low birth weight (<2500g) | 97 (11%) | 61 (13%) | 36 (9%) | 0·035 |
| Very low birth weight (<1500g) | 9 (1%) | 5 (1%) | 4 (1%) | 0·87 |
| Male sex | 426 (49%) | 230 (50%) | 196 (48%) | 0·50 |
| Early initiation of breastfeeding (EIBF, within 1 hour of birth) | 786 (91%) | 399 (87%) | 387 (95%) | <0·0001 |
| Ever exclusively breastfed (EBF)9 | 781 (86%) | 419 (91%) | 332 (81%) | <0·0001 |
| Duration of EBF (months)9 | 1·4 (0·2–3·1) | 1·5 (0·3–5·3) | 1·4 (0·2–3·0) | 0·0066 |
| Duration of any breastfeeding (months) | 6·0 (1·5–12·0) | 3·9 (1·4–12·0) | 9·0 (3·0–12·0) | 0·0001 |
| Optimal breastfeeding: Early initiation, exclusively breastfed for ≥5 months and breastfed throughout follow-up9 | 122 (14%) | 80 (17%) | 42 (10%) | 0·002 |
| Postnatal characteristics | ||||
| Maternal depression at 6 weeks’ postnatal visit10 | 30 (4%) | 19 (4%) | 11 (3%) | 0·24 |
| Ever received co-trimoxazole preventive therapy11 | - | 237 (69%) | - | - |
| Any cigarette smoking in the home (maternal or others) 12 |
296 (36%) | 187 (43%) | 109 (27%) | <0·0001 |
| Household energy sources ever included biomass fuel or paraffin13 | 208 (25%) | 180 (42%) | 28 (7%) | <0·0001 |
| Season/ time of year, 3 months of age5 | 0·83 | |||
| Warm months (Spring/Summer) | 406 (47%) | 216 (47%) | 190 (46%) | |
| Cold months (Autumn/Winter) | 463 (53%) | 243 (53%) | 220 (54%) | |
HEU, all HIV-exposed uninfected infants followed postnatally (n=2 infants not included in further analysis); HU, all HIV-unexposed uninfected infants followed postnatally (n=1 child excluded from further analysis); CD, cluster of differentiation; SD, standard deviation; Results are n (column %) with p-value from chi2 test; median (interquartile range, IQR) with p-value from Kruskal-Wallis
Composite indicator based on standardized asset score (includes running water and flush toilet as assets) and employment
Risky drinking, defined as Alcohol use disorders identification test (AUDIT-C) score ≥3 as reported at first antenatal visit (missing data, n=2)
Any physical, sexual or psychological violence as measured with World Health Organization violence against women questionnaire at first antenatal visit (missing data, n=4)
Maternal depression, EPDS (Edinburgh postnatal depression scale) score of ≥ 13 at first antenatal visit (missing data, n=2)
Seasons defined as: Spring, 01 September to 30 November; Summer, 01 December to 28/29 February; Autumn, 01 March to 31 May; Winter, 01 June to 31 August
Missing data, n=12
Missing data, n=3
Birth weight percentile based on Intergrowth-21st reference standards, AGA defined as ≥10th centile
Maternal report (24 hour recall); exclusive breastfeeding defined as only breastmilk and prescribed medicine; duration of EBF excludes those who never exclusively breastfed; for calculation of “optimal breastfeeding” practices, exclusive breastfeeding for 5 months presumed if EBF reported at last attended follow-up visit prior to 5 months
Maternal depression, EPDS (Edinburgh postnatal depression scale) score of ≥ 13 at 6 weeks’ postnatal visit (missing data, n=51)
Maternal report at 6 weeks’ and/or 3 months’ study visit: infant receipt of co-trimoxazole preventive therapy (missing data, n=114)
Maternal report at 6 weeks, 6 or 12 months’ study visit: any household members smoking
Maternal report at 6 weeks, 6 or 12 months’ study visit: biomass fuel defined as burning of wood or charcoal in the home (missing data, n=38)
Mortality
Fourteen (1·6% of 872) infants died in the first 12 months (2·51 deaths per child-year), with no difference between HEU (8/461, 1·7% or 2·48 per child-year) and HU infants (6/411, 1·5% or 2·54 per child-year; mortality IRR 0·98, 95% CI 0·30–3·41). There were four deaths due to infectious causes: two were due to diarrhoea (both HEU, 5 and 10 months old respectively), one bacterial sepsis (HEU, 1 month old) and one multi-drug resistant tuberculosis (HU, 11 months old), appendix page 8.
Incidence of all-cause hospitalisation
Among 869 infants (933 child-years of follow-up), 378 experienced 475 hospital admissions (table 2); 310 infants had one, while 68 infants had multiple admissions (HEU: 43/459, 9% vs HU: 25/410, 6%). In both groups, the commonest diagnosis was non-infectious neonatal illness (245/475, 52%; appendix page 4). Average all-cause hospitalisation rates were similar for HEU and HU infants (IRR 0·91, 95% CI 0·76–1·10, table 2). Peak all-cause hospitalisation rates were in the first 7 days of life (similar rates for HEU and HU infants). Between 7 days and 3 months of life, rates decreased more slowly among HEU than HU infants, resulting in higher incidence among the former (IRR 2·85, 95% CI 1·56–5·20). After 3 months of age, all-cause hospitalisation rates remained low for both HEU and HU infants. Sixteen (3%) of 475 all-cause hospitalisations resulted in an intensive care unit admission or in-hospital child death (appendix, page 10). This proportion was slightly higher among HEU (12 [5%] of 261 admissions) than HU infants (4 [2%] of 214; crude OR 2·53, 95% CI 0·81–7·95), but associated with similar causes (appendix page 12).
TABLE 2.
All-cause and infectious-cause hospitalizations comparing HIV-exposed uninfected to HIV-unexposed children: crude incidence rates and incidence rate ratios per age interval
| ALL-CAUSE 1 | INFECTIOUS ILLNESS 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| All children N=869 | HEU children N=459 | HU children N=410 | IRR (95% CI) HEU vs HU children3 | All children N=869 | HEU children N=459 | HU children N=410 | IRR (95% CI) HEU vs HU children3 | |
| Overall, n | 869 | 459 | 410 | 0·91 (0·76–1·10) | 869 | 459 | 410 | 1·40 (0·97–2·02) |
| Admissions | 475 | 261 | 214 | 155 | 101 | 54 | ||
| Person-time (cy) | 932·6 | 533·2 | 399·4 | 932·6 | 533·2 | 399·4 | ||
| Incidence/100 cy (95% CI) | 50·9 (46·5–55·3) | 48·9 (43·3–55·3) | 53·6 (46·9–61·3) | 16·6 (14·2–19·4) | 18·9 (15·6–23·0) | 13·5 (10·3–17·7) | ||
| 0 to 7 days, n | 869 | 459 | 410 | 0·82 (0·67–0·99) | 869 | 459 | 410 | 1·16 (0·43–3·11) |
| Admissions | 286 | 136 | 150 | 16 | 9 | 7 | ||
| Person-time (cy) | 16·5 | 8·7 | 7·8 | 16·5 | 8·7 | 7·8 | ||
| Incidence/100cy (95% CI) | 1734·5 (1544·7–1947·6) | 1566·8 (1324·4–1853·6) | 1920·8 (1636·8–2254·2) | 97·0 (59·4–158·4) | 103·7 (53·9–199·3) | 89·6 (42·7–188·0) | ||
| >7 days to 3 months, n | 849 | 444 | 405 | 2·85 (1·56–5·20) | 849 | 444 | 405 | 3·50 (1·68–7·30) |
| Admissions | 57 | 43 | 14 | 43 | 34 | 9 | ||
| Person-time (cy) | 191·5 | 99·4 | 92·1 | 191·5 | 99·4 | 92·1 | ||
| Incidence/100 cy (95% CI) | 29·8 (23·0–38·6) | 43·4 (32·1–58·3) | 15·2 (9·0–25·7) | 22·4 (16·6–30·3) | 34·2 (24·4–47·9) | 9·8 (5·1–18·8) | ||
| >3 to 6 months, n | 808 | 415 | 393 | 1·34 (0·68–2·63) | 808 | 415 | 393 | 1·21 (0·63–2·32) |
| Admissions | 49 | 29 | 20 | 37 | 21 | 16 | ||
| Person-time (cy) | 198·3 | 103·1 | 95·2 | 198·3 | 103·1 | 95·2 | ||
| Incidence/100cy (95% CI) | 24·7 (18·7–32·7) | 28·1 (19·5–40·5) | 21·0 (13·6–32·6) | 18·7 (13·5–25·8) | 20·4 (13·3–31·2) | 16·8 (10·3–27·4) | ||
| > 6 to 9 months, n | 782 | 405 | 377 | 1·01 (0·45–2·26) | 782 | 405 | 377 | 1·16 (0·46–2·93) |
| Admissions | 23 | 12 | 11 | 18 | 10 | 8 | ||
| Person-time (cy) | 191·1 | 99·2 | 91·9 | 191·1 | 99·2 | 91·9 | ||
| Incidence/100cy (95% CI) | 12·0 (8·0–18·1) | 12·1 (6·9–21·3) | 12·0 (6·6–21·6) | 9·4 (5·9–15·0) | 10·1 (5·4–18·7) | 8·7 (4·3–17·4) | ||
| >9 to 12 months, n | 745 | 384 | 361 | 1·22 (0·56–2·65) | 745 | 384 | 361 | 1·15 (0·49–2·65) |
| Admissions | 25 | 14 | 11 | 22 | 12 | 10 | ||
| Person-time (cy) | 179·8 | 91·9 | 87·9 | 179·8 | 91·9 | 87·9 | ||
| Incidence/100 cy (95% CI) | 13·9 (9·4–20·6) | 15·2 (9·0–25·7) | 12·5 (6·9–22·6) | 12·2 (8·1–18·6) | 13·1 (7·4–23·0) | 11·4 (6·1–21·2) | ||
| > 12 months, n | 664 | 335 | 329 | 0·64 (0·27–1·49) | 664 | 335 | 329 | 0·71 (0·23–2·13) |
| Admissions | 35 | 28 | 8 | 19 | 15 | 4 | ||
| Person-time (cy) | 155·5 | 130·9 | 24·6 | 155·5 | 130·9 | 24·6 | ||
| Incidence/100cy (95% CI) | 22·5 (16·3–31·3) | 20·6 (14·1–30·1) | 32·5 (16·2–64·9) | 12·2 (7·8–19·1) | 11·5 (6·9–19·0) | 16·2 (6·1–43·3) | ||
Abbreviations: IRR, incidence rate ratio; CI, confidence interval; cy, child-years
Hospitalized for at least 2 days, all primary diagnoses
Hospitalization for at least 2 days, primary diagnosis infectious in origin
Overall IRR from crude Poisson regression analysis with variance corrected for clustering by child; IRR per age category from crude regression analysis restricted by age intervals without variance correction
Incidence of infection-related hospitalisation
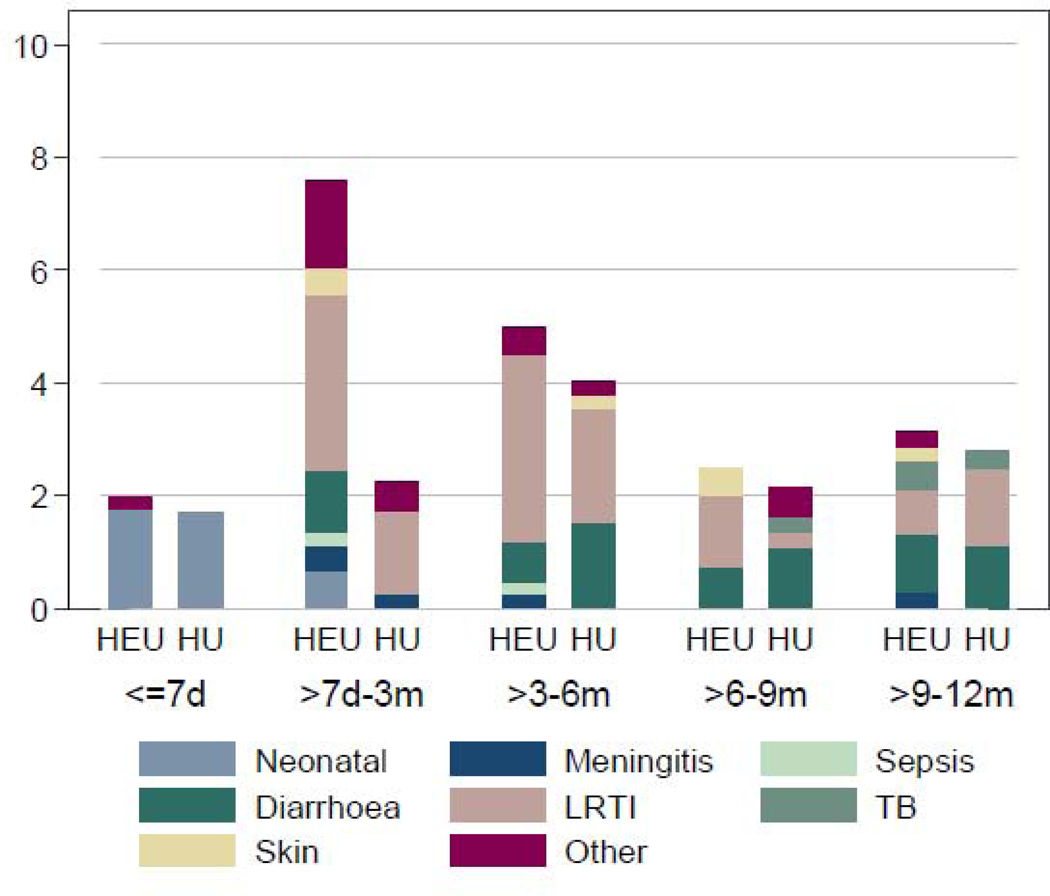
Approximately one-third of all hospital admissions were primarily infectious (appendix, page 9). The proportion of admissions due to infection was higher among HEU (101 [39%] of 261) than HU infants (54 [25%] of 214, p=0·002). The most common infectious causes were LRTI (pneumonia, pulmonary tuberculosis or bronchiolitis, 67 [43%] of 155 infectious admissions), followed by diarrhoea (37 [24%] of 155), Figure 1A and appendix, page 9.
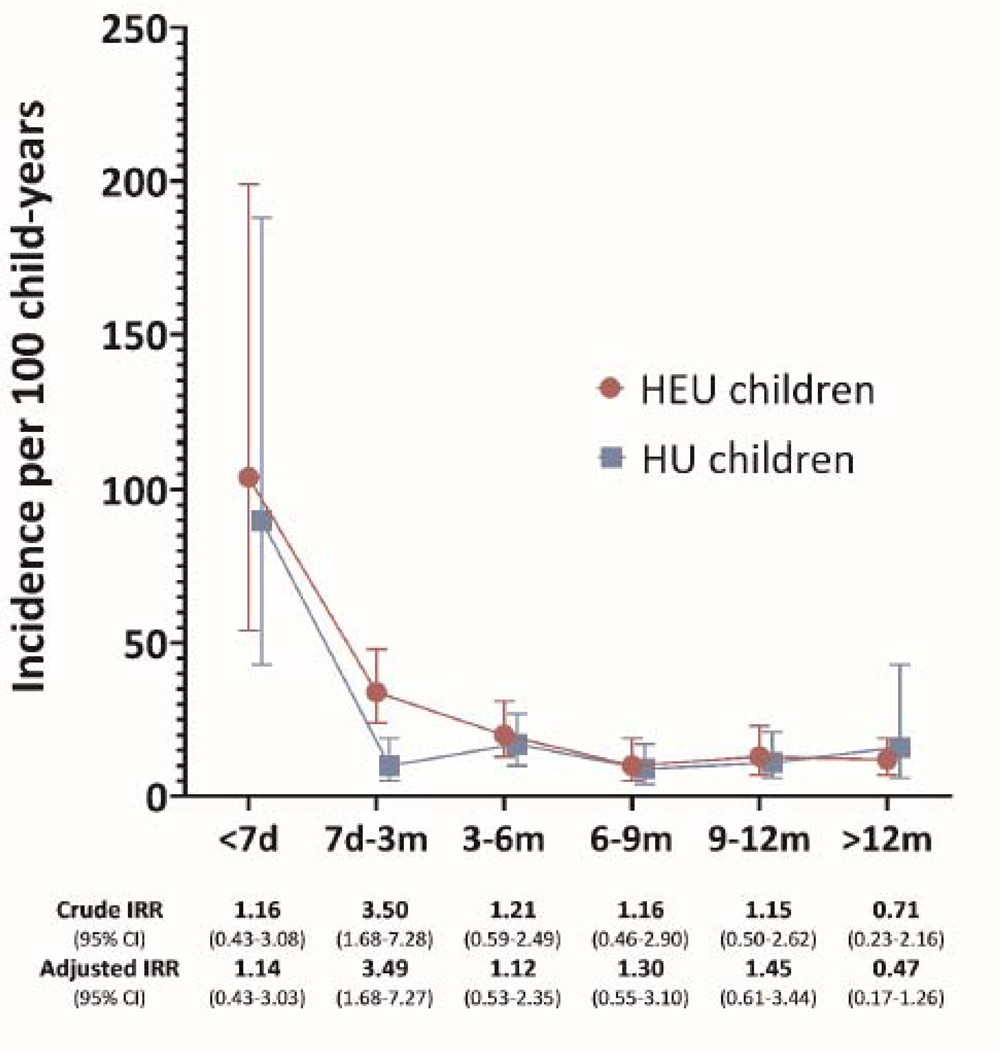
The overall incidence of infection-related hospitalisation was 16·6/100cy (95% CI 14·2–19·4), slightly higher among HEU than HU infants (IRR 1·40, 95% CI 0·97–2·02), in crude and adjusted models (table 2, appendix page 13). The highest rates were in the first 7 days (overall, 97·0/100cy, 95% CI 59·4158·4), and similar for HEU and HU infants. Rates decreased in the 7 days to 3 months interval, with greater reduction among HU than HEU infants (figure 1B). In this age group, the crude incidence was 34·2/100cy (95% CI 24·4–47·9) among HEU compared to 9·8/100cy (95% CI 5·1–18·8) among HU infants, an absolute excess of roughly 20 admissions for every 100 infants. In relative terms, HEU infants were more than 3 times as likely to experience an infection-related hospitalisation than HU infants (IRR 3·50, 95% CI 1·64–8·30), tables 2 & 3, figure 1B. This discrepancy disappeared after the age of 3 months, where-after incidence rates remained low, and similar for HEU and HU infants (table 2, figure 1B).
TABLE 3.
Predictors and incidence of infectious-cause hospitalization comparing HIV-exposed uninfected to HIV-unexposed children between 7 days and 3 months of age: crude and adjusted incidence rate ratios from Poisson regression analysis
| Crude IRR (95% CI)1 | Adjusted IRR (95% CI)1 Model A2 | Adjusted IRR (95% CI)1 Model B3 | |
|---|---|---|---|
| HIV exposure (HEU vs HU) | 3·50 (1·68–7·30) | 3·49 (1·68–7·27) | 3·39 (1·64–7·02) |
| Maternal education4 | 0·51 (0·25–1·07) | - | - |
| Running water and flush toilet in home5 | 0·39 (0·18–0·88) | 0·40 (0·18–0·87) | 0·43 (0·19–0·94) |
| Poverty category6 | |||
| Most disadvantaged | 1·00 | - | - |
| Moderate disadvantage | 0·80 (0·41–1·56) | - | - |
| Least disadvantaged | 0·48 (0·21–1·11) | - | - |
| Cigarette smoking in home7 | 0·51 (0·24–1·06) | - | - |
| Biomass fuel or paraffin use in home8 | 1·22 (0·62–2·40) | - | - |
| Intimate partner violence, antenatal9 | 1·49 (0·71–3·11) | - | - |
| Risky drinking, antenatal10 | 0·80 (0·34–1·91) | - | - |
| Postpartum depression11 | 2·15 (0·66–6·95) | 2·53 (0·85–7·51) | 2·45 (0·82–7·30) |
| Preterm <37 weeks | 1·64 (0·73–3·69) | 0·79 (0·27–2·34) | - |
| Small-for-gestational age12 | 1·94 (0·90–4·18) | - | - |
| Male vs female | 0·81 (0·44–1·47) | - | - |
| Season of birth: warm vs cold months13 | 1·31 (0·71–2·43) | 1·16 (0·65–2·08) | 1·26 (0·70–2·27) |
| Early initiation of breastfeeding14 | 0·44 (0·20–1·00) | - | - |
| Ever exclusively breastfed (EBF)14 | 0·58 (0·28–1·21) | - | - |
| Optimal breastfeeding until 3 months14 | 0·42 (0·18–0·99) | 0·36 (0·14–0·92) | - |
| WAZ <−2 in previous age interval15 | 3·73 (1·73–8·03) | 2·87 (1·41–5·85) | - |
| Vaccination status, per age interval16 | |||
| Complete and timely | 1·00 | 1·00 | 1·00 |
| Delayed or incomplete | 5·47 (2·13–14·07) | 8·01 (2·18–29·43) | 9·25 (3·76–22·79) |
| Data not available | 0·68 (0·30–1·55) | 0·64 (0·27–1·49) | 0·66 (0·28–1·59) |
Abbreviations: IRR, incidence rate ratios; CI, confidence interval; HEU, HIV-exposed uninfected children; HU, HIV-unexposed children
Admission defined as 2 or more days; estimates from Poisson regression with robust variance estimates, clustered on mother-child pairs
Model A includes potential confounders and mediators of the HIV-exposure – hospitalization relationship (access to flush toilet and running water, maternal postpartum depression, preterm birth, breastfeeding, underweight, season of birth and vaccination status)
Model B only includes confounders (access to flush toilet and running water, postpartum depression, season of birth and vaccination status)
Completed vs did not complete secondary education
vs does not have both
Categories based on poverty “score” (combination of employment and a standardized asset score which includes running water and flush toilet)
Maternal report of own or other household members smoking, at 6 weeks’, 6 and/or 12 months’ study visits (vs never)
Maternal report of energy sources at 6 weeks’, 6 and/or 12 months’ study visits (vs. never)
Any physical, sexual or psychological violence as measured with World Health Organization violence against women questionnaire at first antenatal visit (vs none)
Risky drinking, defined as Alcohol use disorders identification test (AUDIT-C) score ≥3 vs <3, as reported at first antenatal visit
Edinburgh postnatal depression score ≥13 vs <13 at 6 weeks’ study visit
Birthweight for gestational age, percentile <10th, based on Intergrowth-21st growth reference standards (vs appropriate for gestational age)
Seasons defined as: Spring, 01 September to 30 November; Summer, 01 December to 28/29 February; Autumn, 01 March to 31 May; Winter, 01 June to 31 August
Based on 24 hour recall, maternal report; exclusive defined as only breastmilk with prescribed medicine; non-time-varying; early initiation defined as within 1 hour of birth; “optimal” defined for time period of 0–3 months, ie early initiation and exclusive breastfeeding for at least 3 months
Time-varying: weight-for-age Z-score < 2 (underweight) at any point during the preceding age interval
Time varying: based on Road to Health Booklet information; delayed vaccination defined as receipt >2 weeks after recommended age
Effect measure modification tests (interaction terms using adjusted model A): HEU status and infant sex, p=0·36; HEU status and optimal breastfeeding, p=0·80; HEU and vaccination status, p=0·17; HEU status and preterm birth, p=0·18; HEU status and SGA, p=0·52
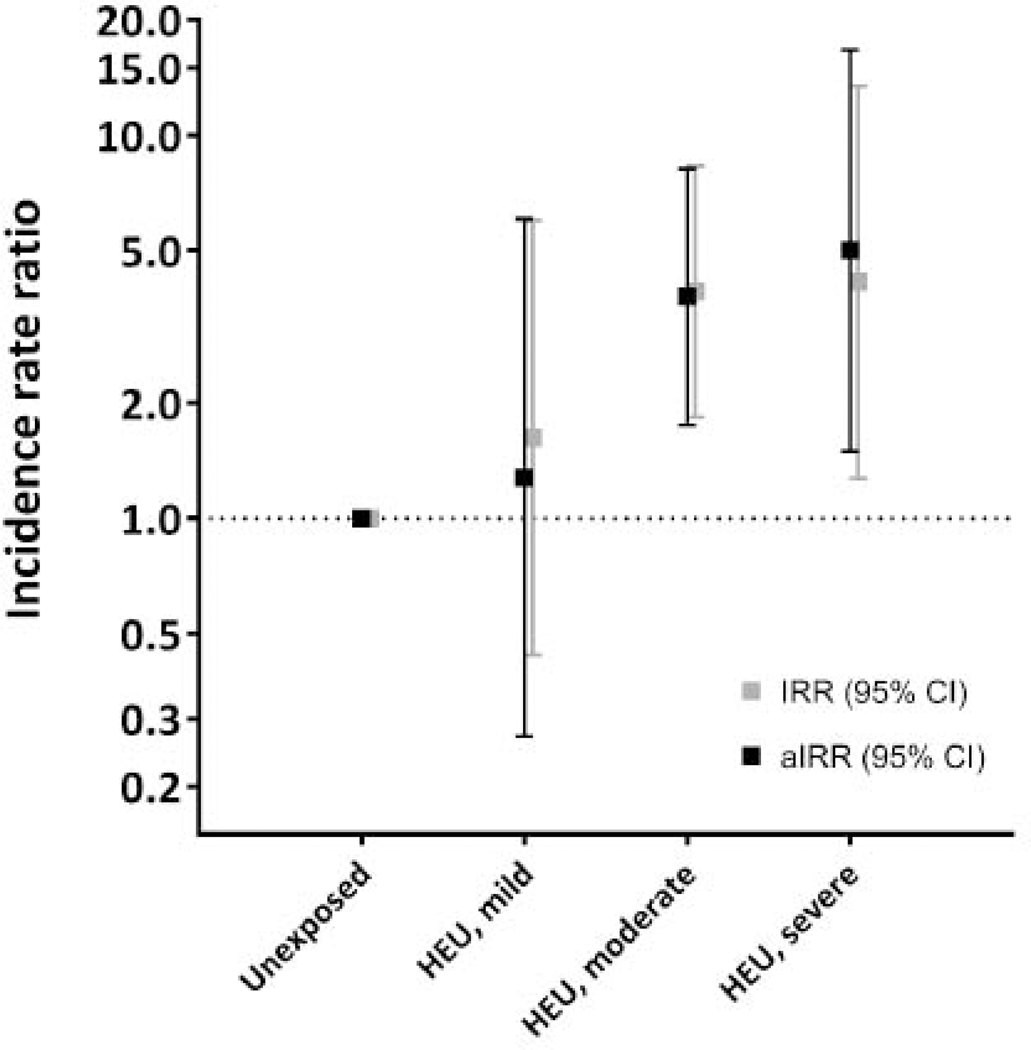
We explored the effects of potentially modifiable factors on the HIV exposure-hospitalisation relationship between 7 days and 3 months of age (appendix page 15). Maternal disease severity and gestation at ART initiation modified the HIV-exposure effects on infection-related hospitalisation (Fig 2A; appendix, page 15): HEU infants of women with advanced HIV disease and late ART initiation were at the highest relative risk compared to HU infants (aIRR 5·01; 95% CI 1·50–16·71). By contrast, the incidence rates among HEU infants of women with early disease stages and timely ART initiation (15·88/100cy, 95% CI 5·12–49·23) approximated those of HU infants (9·77/100cy, 95% CI 5·08–18·78; aIRR 1·28, 95% CI 0·27–6·05). A similar dose-response relationship was seen with breastfeeding and vaccination (figure 2B; appendix, page 15). Notably, there were no infection-related hospitalisations among HEU infants (n=15) with early maternal ART initiation at less severe disease stages, who subsequently received optimal breastfeeding and timely vaccinations (appendix, page 15). Compared to term HU infants, preterm HU infants had similar hospitalisation rates, whereas term HEU infants had 3-fold higher rates (aIRR 3·33, 95% CI 1·52–7·31), and preterm HEU infants, 5-fold higher rates (aIRR 5·38, 95% CI 1·86–15·49). A similar pattern was evident for HIV exposure and size at birth (appendix, page 15). Despite some loss of precision, inferences were unchanged in sensitivity analysis (appendix, page 19). Increased risks of infectious-cause hospitalization were seen among HEU compared to HU infants in analyses restricted to (1) term infants born appropriate-forgestational age, (2) infants with optimal breastfeeding, (3) infants from homes with running water and flush toilet, or (4) infants born in the same season. Results were unchanged in analysis excluding HEU infants who did not receive CPT (Appendix, page 19). Factors associated with infection-related hospitalisation specifically among HEU infants are shown in the appendix, page 20.
FIGURE 2.
Infection-related hospitalizations comparing HIV-exposed uninfected and HIV-unexposed children over time: distribution of diagnoses and incidence over time
2A. Infection-related causes for hospitalization: distribution by HIV exposure and age category
Legend: HEU, HIV-exposed uninfected; HU, HIV-unexposed uninfected; d, days; m, months; LRTI, lower respiratory tract infection (includes pneumonia and bronchiolitis); TB, pulmonary tuberculosis; Percentages based on number of admissions divided by number of children in category
2B.Infection-related hospitalization: incidence rates and rate ratios comoaring HIV-exposed uninfected to HIV-unexposed children over time
Legend: HEU, HIV-exposed uninfected children; HU, HIV-unexposed chidren; d, days; m, months; IRR, incidence rate ratio from Poisson regression models adjusted for clustering; Cl, confidence internal. Muitivariable models adjusted for socio-economic factors (access to flush toilet and running water inside the home). maternal depression, preterm birth, child underweight, vaccination status. quality of breastfeeding and season at birth
Sampie size per age internal: <7 days, N=869; 7 days-3 months, N=849; 3–6 months, N=808; 6–9 months, N=782; 9–12 months, N=745; >12 months, N=664
Longitudinal prevalence of infectious illness
Prevalence of LRTI and diarrhoeal illness increased over time in both HEU and HU infants (appendix, page 21). On average, HEU infants had significantly higher prevalence of LRTI than HU infants (PR 3·23, 95% CI 2·20–4·74; aPR 2·54, 95% CI 1·72–3·76), and slightly higher prevalence of diarrhoeal illness in crude (PR 1·25, 95% CI 1·03–1·52) but not adjusted (aPR 1·07, 95% CI 0·87–1·31) models (data not shown). Child age modified these associations: among infants ≤6 months, HIV-exposure was associated with an almost 5-fold increased risk of LRTI (aPR 4·69, 95% CI 2·40–9·17), and a 3-fold increased risk of diarrhoeal illness (aPR 2·93, 95% CI 1·70–5·07; appendix, page 22). After 6 months of age, the risk differential between HEU and HU infants was substantially smaller for LRTI, and absent for diarrhoeal illness (appendix, page 24). Variations in the HIV exposure-infectious illness relationship by maternal HIV disease severity, timing of ART initiation, breastfeeding and vaccination status approximated those of infection-related hospitalization, and were most marked in the first 6 months (appendix, page 26).
DISCUSSION
In this urban South African cohort, HIV exposure was associated with transiently increased infectious morbidity risks in early infancy, even in the context of universal maternal ART and breastfeeding. However, excess risks appeared to be predominantly driven by advanced maternal HIV disease with late ART initiation in pregnancy, alongside suboptimal vaccination and inadequate breastfeeding practices. Mortality rates were similar for HEU and HU infants, and approximated the background risk of infant mortality in this setting.13
Although direct comparison with other studies is complicated by heterogeneity of morbidity measures, our overall estimates of hospitalisation and prevalence data for HIV-unexposed infants were in keeping with previous reports from South Africa.13,22,23 Reassuringly, our estimates of HEU infectious morbidity largely approximated those of HU infants in late infancy. The transiently increased risk we observed in early infancy aligns with findings from African studies predating universal maternal ART, hypothesised to reflect the residual effects of HIV-driven maternal-fetal immune dysregulation including reduced transplacental transfer of maternal antibodies.4 Our data suggest that this early peak in vulnerability among HEU infants may not be fully addressed by universal maternal ART when only initiated later in pregnancy, or at more advanced disease stages. In keeping with these observations, a convincing risk gradient by timing of maternal ART initiation was recently described in Europe.24 Belgian HEU infants with pre-pregnancy maternal ART initiation experienced substantially less immune dysregulation and infection-related hospitalisation than those with maternal ART initiation in pregnancy, and in turn had hospitalisation rates which approximated those of HU controls. Taken together, the data suggest that optimising maternal HIV-related health prior to conception may have direct child health benefits extending beyond HIV-free survival. Indeed, pre-conception maternal health is increasingly recognised as a strong determinant of subsequent child health generally, and deserves greater attention in the context of maternal HIV.25
Optimal breastfeeding and timely childhood vaccinations are foundational components of global strategies to prevent morbidity and mortality from LRTI and diarrhoea.26 Not surprisingly, most infectious episodes in our cohort could be ascribed to these common childhood illnesses.4 Although all mother-child pairs in our study were initially breastfeeding, delayed initiation and early cessation of both exclusive and non-exclusive breastfeeding was common, and corresponded to increasing risks of illness. Strong evidence exists for strategies to improve breastfeeding practices27,28 but adaptation and implementation has been slow across settings including South Africa.29 Our data also reveal concerningly high rates of delayed and incomplete vaccination among most infants, highlighting the urgent need for improvement of basic, primary health care in our setting, which would benefit both HEU and HU infants. Indeed, the reduction in infectious morbidity among those HIV-exposed infants in our cohort who were exclusively breastfed and adequately vaccinated underscores the benefits of optimising well-established, scalable interventions.
Our study has limitations. Although we aimed to address potential confounding through rigorous study design and analysis, unmeasured confounding remains a possibility. Measurement error due to maternal recall bias may have influenced our estimates of childhood illness, particularly as some of the DHS questions can be non-specific, and positive predictive value is reduced in areas with low background prevalence of disease.30 Simultaneously, our hospitalisation rates may be underestimations, as admissions in other provinces could have been missed. Nonetheless, the alignment of our prevalence and hospitalisation findings are reassuring and suggest that a relationship does exist between HIV exposure and infectious illness in early infancy. Our study lacked detailed immunological measures, which should ideally be incorporated into future studies evaluating drivers of excess HEU child morbidity in resource-limited settings. While one of our study strengths is the homogenous use of maternal ART in pregnancy, there remained substantial variation in the severity of maternal disease at time of ART initiation. Accordingly, several of our subgroup analyses lacked precision. Our findings cannot be generalised to women who initiate treatment prior to pregnancy; however, given the persistently high incidence of HIV among young women in Africa,1 many women will continue to receive their first diagnosis and initiate ART during rather than before pregnancy. For these HEU infants, it may be particularly important to promote breastfeeding and timely completed vaccination. Our findings may also not extend to rural areas with poor access to health care and even higher infectious disease burdens, including malaria. Despite these limitations, this study provides one of the first reports on infectious morbidity among uniformly breastfed HEU infants born under current ART policies in Africa.
CONCLUSION
Even with universal ART and breastfeeding, HEU infants, on average, experienced more infectious morbidity in early infancy than their HU counterparts. Reassuringly, our data suggest that these excess risks may potentially be addressed through earlier diagnosis and ART initiation for HIV-infected women, alongside optimal breastfeeding and timely vaccination of their HEU infants. Improved implementation and scaling up of available interventions to optimize breastfeeding and vaccination should be public health priorities.
Supplementary Material
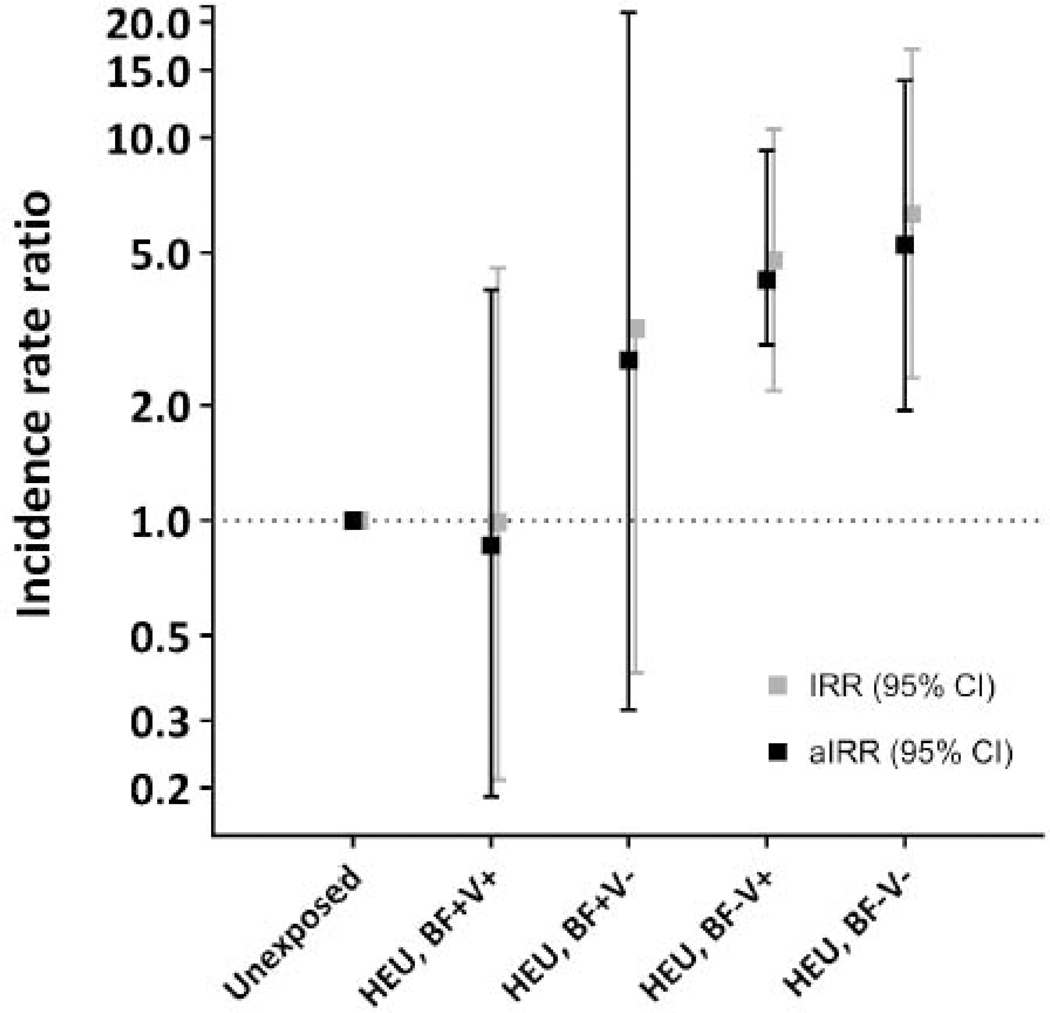
FIGURE 3.
Incidence rate ratios of infection-related hospitalization between 7 days and 3 months of age comparing HIV-exposed uninfected to HIV-unexposed children
3A. Variation by maternal HIV disease severity and gestation at ART initiation in pregnancy
Legend:
IRR, crude incidence rate ratio from Poisson regression analiysis corrected for clustering; aIRR, incidence rate ratios adjusted for access to flush toilet and running water, postpartum depression, preterm birth, season at birth, being underweight in a previous age interval, quality of breastfeeding (early initiation and Exclusivity in the first 3 months) and vaccination status Reference group includes all HU children (N=405)
HEU categories (CD4 tell count and HIV viral load category boundaries set at group median value):
“Mild” (N=84): pre-ART maternal CD4 cell count > 350 cells/mm3 AND HIV viraI load <4·0 log10 copies/mL AND ART initiated prior to 24 weeks’ gestation in pregnancy (vs HU, aIRR 1–28, 95% CI 0·27-6·05)
“Moderate” (N=320): pre-ART maternal CD4 cell court ≤ 350 cells/mm3 OR HIV viral load ≥4·0 log10 copies/mL but ART initiated prior to 24 weeks’ gestation in pregnancy, OR, maternal CD4 cell count > 350 cells/mm3 OR HIV viral load <4·0 log10 copies/mL but ART initiation at or after 24 weeks’ gestation in pregnancy (vs HU, aIRR 3.79, 95% CI 1·75–8.18]
“Severe: (N=44): pre-ART maternal CD cell count ≤350 AND HIV viral load ≥4·0 log10 copies/mL AND ART initiation at or after 24 weeks’ gestation in pregnancy (vs HU, aIRR 5·01,95% CI 1·50–16·71)
3B: Variation by quality of breastfeeding and timelines of vaccination in the first 3 months of life
Legend:
IRR, crude incidence rate ratio from Poisson regression analysis corrected for clustering; aIRR, incidence rate ratios adjusted for access to flush toilet and running water, postpartum depression, preterm birth, season at birth and being underweight in a previous age interval; “Optimal breastfeedng” defined as initiation within 1 hour of birth and exclusive breastfeeding through 3 months of age; vaccinations considered timely if all required doses received within 2 weeks of indicated age (based on 6 and 10 weeks’ South African schedule}
Reference category includes all HU children (N=405)
HEU categories:
BF+ V+: Optimal breastfeeding and timely vaccination (N=90); vs HU, aIRR 0.86, 95% CI 0.19–4.00
BF+ V−: Optimal breastfeeding but delayed or incomplete vaccination (N=14); vs HU, aIRR 2.62, 95% CI 0.32–21.26
BF− V+: Suboptimal breastfeeding but timely vaccination (N=187); vs HU, aIRR 4.15, 95% CI 1.88–9.15
BF− V−: Suboptimal breastfeeding and delayed or incomplete vaccination (N=50); vs HU, aIRR 5.24, 95% CI 1.94–14.14
RESEARCH IN CONTEX.
Evidence before this study
We previously published a systematic review of clinical health outcomes among HIV-exposed uninfected (HEU) compared to HIV-unexposed (HU) children in sub-Saharan Africa, including publications through 1 October 2015. Using the same search strategy (combinations of terms for “HIV”, “pregnancy” and “child”), we performed a supplemental search (1 October 2015–1 August 2019) on MEDLINE for original papers reporting clinical infectious morbidity or hospitalisations. Eligible studies were longitudinal with sampling based on HIV exposure. Eight additional articles from 7 studies were identified for review. From settings across the continent, increased risks of hospitalisation, lower respiratory tract infection (LRTI) and diarrhoea were reported among HEU compared to HU children. Maternal HIV disease severity, use of antiretroviral therapy and breastfeeding emerged as key determinants of infectious risks alongside poverty and malnutrition, although few studies accounted for all these factors. Studies generally predated current HIV policies, so that most HIV-infected mothers did not receive ART, or initiated ART due to low CD4 cell counts; only one study included a small subgroup of women who initiated ART in pregnancy without CD4 cell count restrictions. There were no published reports directly comparing infectious morbidity of breastfed HEU and HU children within the context of universal maternal ART.
Added value of this study
To our knowledge, we present the first report of infectious morbidity among HEU children (uniformly breastfed and born to women who initiated universal ART in pregnancy) compared to a well-controlled comparison group of breastfed HU children sampled from the same community. Our findings suggest that universal maternal ART, initiated later in pregnancy or at more advanced disease stages, does not fully correct the excess infectious morbidity of young HEU children. However, the infectious risks of optimally breastfed, fully vaccinated HEU children approximate those of HU children when mothers initiate ART early in pregnancy and at less advanced disease stages.
Implications of all the available evidence
HEU (vs HU) children are at increased risk of hospitalisation, LRTI and diarrhoea in early infancy, driven by maternal HIV disease severity, lack of ART and suboptimal infant feeding. While maternal use of ART improves the outcomes of HEU children, initiation in late pregnancy or at more advanced disease stages does not fully address the risk differential between HEU and HU children, especially when breastfeeding is suboptimal and childhood vaccinations are delayed or missed. The full child health benefits of universal maternal ART policies will not be realised unless mothers and mothers-to-be are diagnosed and treated at early disease stages, with support to provide optimal breastfeeding to their HEU infants. Efforts to improve early diagnosis and initiation of ART among women of child-bearing age may also benefit future generations of HEU children. Data are required on the health outcomes of HEU children whose mothers conceived on ART initiated at early disease stages. Simultaneously, there is an urgent need for improved breastfeeding and vaccination practices to promote the health of both HEU and HU children.
Acknowledgements
We are grateful to the Provincial Health Data Centre for the many hours of work involved in cleaning and identifying hospitalisation and laboratory data for our study participants. The authors also thank the research and clinical staff who supported this work, and the families who participated in the study.
Funding
This study was supported by PEPFAR through NICHD under Cooperative Agreement 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council (Clinician-Researcher PhD Scholarship), the Fogarty Foundation (NIH Fogarty International Center Grant #5R25TW009340) and the Office of AIDS Research. The authors have no conflict of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Start Free Stay Free AIDS Free. UNAIDS report 2019. https://www.unaids.org/sites/default/files/media_asset/20190722_UNAIDS_SFSFAF_2019_en.pdf (accessed 7 August 2019).
- 2.le Roux SM, Abrams EJ, Nguyen K, Myer L. Clinical outcomes of HIV-exposed, HIV-uninfected children in sub-Saharan Africa. Trop Med Int Health 2016; 21(7): 829–45. [DOI] [PubMed] [Google Scholar]
- 3.Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infectious Diseases, 2016. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(16)00055-4/fulltext (accessed 31 August 2019). [DOI] [PubMed] [Google Scholar]
- 4.Slogrove AL, Goetghebuer T, Cotton MF, Singer J, Bettinger JA. Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Front Immunol, 2016. 10.3389/fimmu.2016.00164 (accessed 31 August 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan AT, Bonawitz R, Gill CJ, et al. A Meta-analysis Assessing Diarrhea and Pneumonia in HIV-Exposed Uninfected Compared With HIV-Unexposed Uninfected Infants and Children. J Acquir Immune Defic Syndr 2019; 82(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO guidelines on HIV and infant feeding 2010. An updated framework for priority action. (accessed. [Google Scholar]
- 7.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Second edition.2016. http://apps.who.int/iris/bitstream/10665/246200/1/9789241511124-eng.pdf (accessed 28 July 2016). [PubMed]
- 8.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev, 2011. 10.1002/14651858.CD003510.pub3 (accessed 31 August 2019). [DOI] [PubMed] [Google Scholar]
- 9.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016; 387(10017): 475–90. [DOI] [PubMed] [Google Scholar]
- 10.Myer L, Phillips TK, Zerbe A, et al. Optimizing Antiretroviral Therapy (ART) for Maternal and Child Health (MCH): Rationale and Design of the MCH-ART Study. J Acquir Immune Defic Syndr 2016; 72 Suppl 2: S189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myer L, Phillips T, Manuelli V, McIntyre J, Bekker LG, Abrams EJ. Evolution of antiretroviral therapy services for HIV-infected pregnant women in Cape Town, South Africa. J Acquir Immune Defic Syndr 2015; 69(2): e57–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Expanded Programme on Immunization - EPI (SA) Revised Childhood Immunisation Schedule from December 2015. https://www.westerncape.gov.za/assets/departments/health/2016_schedule.pdf (accessed 7 August 2019).
- 13.Hendricks M K, Linley L, Westwood A, Hawkridge A. A Situation Analysis of Neonatal and Child Health Status in the Metro West Geographic Service Area of the Western Cape. Cape Town, Western Cape Department of Health; 2012. [Google Scholar]
- 14.le Roux SM, Abrams EJ, Donald KA, et al. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: a prospective study. Lancet Child Adolesc Health 2019; 3(4): 234–44. [DOI] [PubMed] [Google Scholar]
- 15.WHO, UNICEF, USAID, AED, UCDAVIS, IFPRI. Indicators for assessing infant and young child feeding practices. Part I: Definitions.2008. http://www.who.int/nutrition/publications/infantfeeding/9789241596664/en/ (accessed 19 July 2015).
- 16.Carter ED, Tam Y, Walker N. Impact of vaccination delay on deaths averted by pneumococcal conjugate vaccine: Modeled effects in 8 country scenarios. Vaccine 2019; 37(36): 5242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Verbal autopsy standards: The 2012 WHO verbal autopsy instrument. Release Candidate 1. https://www.who.int/healthinfo/statistics/verbalautopsystandards/en/index1.html (accessed 26 May 2019).
- 18.Provincial Health Data Centre, Department of Health, Western Cape Government.2019. https://www.westerncape.gov.za/general-publication/provincial-health-data-centre (accessed 26 May 2019).
- 19.Mussi-Pinhata MM, Freimanis L, Yamamoto AY, et al. Infectious disease morbidity among young HIV-1-exposed but uninfected infants in Latin American and Caribbean countries: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Pediatrics 2007; 119(3): e694–704. [DOI] [PubMed] [Google Scholar]
- 20.Rogers WH. Regression standard errors in clustered samples. Stata Technical Bulletin 1993. https://www.stata.com/support/faqs/statistics/stb13_rogers.pdf (accessed 7 August 2019). [Google Scholar]
- 21.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013; 22(6): 661–70. [DOI] [PubMed] [Google Scholar]
- 22.le Roux DM, Nicol MP, Myer L, et al. Lower Respiratory Tract Infections in Children in a Wellvaccinated South African Birth Cohort: Spectrum of Disease and Risk Factors. Clin Infect Dis, 2019. 10.1093/cid/ciz017 (accessed 31 August 2019). [DOI] [PubMed] [Google Scholar]
- 23.Doherty T, Jackson D, Swanevelder S, et al. Severe events in the first 6 months of life in a cohort of HIV-unexposed infants from South Africa: effects of low birthweight and breastfeeding status. Tropical Medicine & International Health 2014; 19(10): 1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetghebuer T, Smolen KK, Adler C, et al. Initiation of Antiretroviral Therapy Before Pregnancy Reduces the Risk of Infection-related Hospitalization in Human Immunodeficiency Virus-exposed Uninfected Infants Born in a High-income Country. Clin Infect Dis 2019; 68(7): 1193–203. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson J, Heslehurst N, Hall J, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018; 391(10132): 1830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025 The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). WHO/UNICEF 2013. https://apps.who.int/iris/bitstream/handle/10665/79200/9789241505239_eng.pdf?sequence=1 (accessed 7 August 2019). [DOI] [PubMed] [Google Scholar]
- 27.Rollins N, Doherty T. Improving breastfeeding practices at scale. Lancet Glob Health, 2019. 10.1016/S2214-109X(18)30557-6 (accessed 31 August 2019). [DOI] [PubMed] [Google Scholar]
- 28.Rollins NC, Bhandari N, Hajeebhoy N, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016; 387(10017): 491–504. [DOI] [PubMed] [Google Scholar]
- 29.Doherty T, Sanders D, Jackson D, et al. Early cessation of breastfeeding amongst women in South Africa: an area needing urgent attention to improve child health. BMC Pediatr, 2012. https://bmcpediatr.biomedcentral.com/articles/10.1186/1471-2431-12-105 (accessed 20 September 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer Walker CL, Fontaine O, Black RE. Measuring coverage in MNCH: current indicators for measuring coverage of diarrhea treatment interventions and opportunities for improvement. PLoS Med, 2013. 10.1371/journal.pmed.1001385 (accessed 11 August 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.