Abstract
Background:
In the current study, we aimed to analyze the hypothesis that human myocardial-specific extracellular RNAs expression could be used for acute myocardial injury(AMI) diagnosis.
Methodology:
We used bioinformatics’ analysis to identify RNAs linked to ubiquitin system and specific to AMI, named, (lncRNA-RP11-175K6.1), (LOC101927740), microRNA-106b-5p (miR-106b-5p) and Anaphase, promoting complex 11 (ANapc11mRNA). We measured the serum expression of the chosen RNAs in 69 individuals with acute coronary syndromes, 31 individuals with angina pectoris without MI and non-cardiac chest pain and 31 healthy control individuals by real-time reverse-transcription PCR.
Results:
Our study revealed a significant decrease in both lncRNA-RP11-175K6.1 and ANapc11mRNA expression of in the sera samples of AMI patients compared to that of the two control groups alongside with significant upregulation of miR-106b-5p.
Conclusion:
Of note, the investigated serum RNAs decrease the false discovery rate of AMI to 3.2%.
Keywords: Myocardial infarction, miRNA, lncRNA, serum, diagnosis, extracellular RNAs
1. INTRODUCTION
Myocardial Ischemia is described as decreased blood supply to the myocardium causing lack of oxygen and decreased metabolite removal. Acute Myocardial Infarction (AMI) is the first lethal cause worldwide [1, 2]. Thus we need an earlier diagnosis in order to optimize the AMI therapy that would reduce the risk of heart failure. The electrocardiogram (ECG) has limited sensitivity (50-60%) in AMI detection. In addition, many biomarkers, such as creatine kinases (CK) cardiac troponins (cTns), and lactate dehydrogenase (LDH) are widely used in the diagnosis of acute myocardial infarction patients [3]. However, the delayed release of troponins reaching peak concentrations approximately 12-24 h after infarction remains a major problem delaying the early detection of AMI. Also, cardiac troponin is elevated in cardiac conditions other than myocardial infarction such as cardiac trauma and end stage renal disease [4]. Therefore, searching for novel biomarkers of high sensitivity as well as high accuracy for detection of AMI is a great challenge.
Several extracellular RNAs regulate the key processes linked to the pathogenesis of AMI. Many studies postulate that the expression of circulatory RNAs change over time and is affected by the duration and intensity of exposure to AMI risk factors, and may, therefore, be useful biomarkers in AMI diagnosis [5].
The ubiquitin Proteasome system (UPS) has been described as a way to remove the unwanted, or unneeded proteins [6]. It was recently found that in cardiac cells there are about nine ubiquitin ligases that act as critical players in common cardiac disease pathophysiology. Interestingly, some ubiquitin ligases, such as MDM2 and CHIP target p53 leading to proteasomal degradation and thereby protecting cardio myocytes against apoptosis in ischemia reperfusion (I.R) injury cases [7, 8]. ANPCII gene is an E3 ubiquitin-protein ligase complex (a member of the anaphase promoting complex) which regulated through the cell cycle being a part of The UPS (The ubiquitin proteasome system). ANPC/C complex functions through ubiquitination and degradation of target proteins. It also recruits the E2 ubiquitin-conjugating enzymes to the complex [9].
Many miRNAs such as miRNA-34a, miRNA-208a, miRNA-210 and miRNA-495 were studied by many researchers and were found to be important gene regulators for angiogenesis and UPS in AMI. Consequently, they are considered to be important therapeutic tools particularly for the prevention of heart failure after AMI [10, 11]. Hence, this highlights the emergence of miRNAs as sensitive biomarkers of AMI and their possible use as new biomarkers for diagnosis and therapeutic targeting of AMI being stable in plasma, serum, and urine, as well as their cell-specific physiological functions [12, 13].
On the other hand, long noncoding RNAs (lncRNAs) were suggested by other researchers to have an association with cardiac ischemia [14, 15]; e.g; myocardial infarction-associated transcript, potassium voltage-gated channel, KQT-like subfamily HOX antisense intergenic RNA and long noncoding RNA HOTAIR which was found to have a protective effect in cardiac tissue and that the extracellular HOTAIR may be a promising biomarker for AMI detection [16-18].
In this study, we hypothesize that the chosen RNAs by in silico data analysis are related to UPS system and specific to AMI might be a potential biomarker panel for the detection of AMI. We first chosen AMI specific genes and linked to UPS system and their master regulators at epigenetic level through in silico data analysis. Then, to investigate this panel, we assessed whether lncRNA-RP11-175K6, microRNA-106b-5p(miR-106b-5p) and anaphase promoting complex subunit 11 (ANAPC11) mRNA showed significantly differential expression in sera of AMI group compared to non-cardiac chest patients and healthy volunteers.
2. PATIENTS AND METHODS
The Ethical Committee at Ain Shams faculty of medicine granted this Pilot study an approval. The study includes 69 Egyptian patients with acute myocardial infarction at the Cardiovascular Department, Ain Shams University hospitals, 31 patients with angina pectoris, but without MI and non-cardiac chest pain according to the outcome of coronary angiography (non-cardiac causes due to gastrointestinal or lung or any other chest condition) and 31 healthy normal volunteers who have normal ECG, without any history of cardiovascular disease and were visiting the hospital for regular health checkups with matching sex and age to the patients’ groups after taking an informed consent. The enrolled participants were in the period from April 2017 till October 2017. AMI diagnosis was performed through evaluating the elevated serum troponin levels, CK-MB in addition to history and clinical symptoms consistent with cardiac ischemia within 6 hours of chest pain. AMI was diagnosed on the basis of a combination of several parameters: ischemic symptoms, a pathological Q wave and elevated cTnI (cardiac troponin I) with CK-MB (creatine kinase-MB) expression. All AMI patients were diagnosed for the first time and underwent primary PCI. The patients were diagnosed according to guidelines that were adopted from American College of Cardiology/American Heart Association (2018 ESC/ACC/ AHA/WHF Fourth Universal Definition) and two independent cardiologists judgment were considered. The exclusion criteria for this study were patients with a history of end-stage renal failure, hepatitis, hepatic failure, cardiomyopathy, bleeding disorders, autoimmune diseases, previous irradiation therapy to the thoracic region, arthritis or inflammatory bowel disease (IBD), chronic muscle disorders and malignancy.
Within the first 6 hours of onset of chest pain blood samples were collected for continuous assessment of cardiac markers; CK –MB and hs-cTnT. Centrifugation was done at 3,000 rpm for 15 min, separating the sera samples then it was aliquoted and eventually kept at -80°C.
2.1. Bioinformatics’ Analysis
The selection of these genes firstly, focused on the literature search that ensured the ubiquitin Proteasome system (UPS) dysregulation in acute myocardial infarction. Secondly, the Gene Expression Omnibus (GEO). Gene atlas expression, Gene card database, UniProt and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment network analyses were performed to understand the potential molecular mechanisms linking UPS dysregulation to AMI pathogenesis to select anaphase promoting complex subunit 11 (ANAPC11) due to the following causes, linked to UPS, specific to AMI, novelty, high expression in normal cardiac tissue (Supplementary Figs. 1-5 (1.7MB, pdf) ). Thirdly, miR-106b-5p has been selected as a target to ANAPC11 mRNA due to higher number of complementarity binding site, linked to UPS dysregulation, related to cardiac injury as the pathway enrichment analysis which revealed that miR-106b-5p is linked to endocytosis, TGF-beta signaling pathway, MAPK signaling pathway, Ubiquitin mediated proteolysis (Table 1, Supplementary Figs. 6-8 (1.7MB, pdf) ). Lastly, lncRNA-RP11-175K6.1 (LOC101927740) was identified to control the expression of the above genes by accessing a database of lncRNAs that act as ceRNAs (starbase database). We have selected lncRNA-RP11-175K6.1 based on novelty and presence of higher miRNA binding sites. We have also validated the alignment between lncRNA-RP11-175K6.1-miRNA 106b by using Claustal tool in European bioinformatics institute thus verifying the in silico prediction that lncRNA-RP11-175K6.1-miRNA 106b target ANapc11mRNA (Supplementary Figs. 9 (1.7MB, pdf) and 10).
Table 1. Gene ontology of ANAPC11 mRNA.
| S. No. | ANapc11mRNA (retrieved from Pathways from BioSystems available at https://www.ncbi.nlm.nih.gov/gene/51529) |
|---|---|
| 1. | APC-Cdc20 mediated degradation of Nek2A, organism-specific biosystem (from REACTOME) |
| 2. | APC/C complex, organism-specific biosystem (from KEGG) |
| 3. | APC/C complex, conserved biosystem (from KEGG) |
| 4. | APC/C-mediated degradation of cell cycle proteins, organism-specific biosystem (from REACTOME) |
| 5. | APC/C:Cdc20 mediated degradation of Cyclin B, organism-specific biosystem (from REACTOME) |
| 6. | APC/C:Cdc20 mediated degradation of Securin, organism-specific biosystem (from REACTOME) |
| 7. | APC/C:Cdc20 mediated degradation of mitotic proteins, organism-specific biosystem (from REACTOME) |
| 8. | APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1, organism-specific biosystem (from REACTOME) |
| 9. | APC:Cdc20 mediated degradation of cell cycle proteins prior to satisfation of the cell cycle checkpoint, organism-specific biosystem (from REACTOME) |
| 10. | Activation of APC/C and APC/C:Cdc20 mediated degradation of mitotic proteins, organism-specific biosystem (from REACTOME) |
| 11. | Adaptive Immune System, organism-specific biosystem (from REACTOME) |
| 12. | Antigen processing: Ubiquitination & Proteasome degradation, organism-specific biosystem (from REACTOME) |
| 13. | Autodegradation of Cdh1 by Cdh1:APC/C, organism-specific biosystem (from REACTOME) |
| 14. | Cdc20:Phospho-APC/C mediated degradation of Cyclin A, organism-specific biosystem (from REACTOME) |
| 15. | Cell Cycle, organism-specific biosystem (from REACTOME) |
| 16. | Cell Cycle Checkpoints, organism-specific biosystem (from REACTOME) |
| 17. | Cell Cycle, Mitotic, organism-specific biosystem (from REACTOME) |
| 18. | Cell cycle, organism-specific biosystem (from KEGG) |
| 19. | Cell cycle, conserved biosystem (from KEGG) |
| 20. | Cellular Senescence, organism-specific biosystem (from REACTOME) |
| 21. | Cellular responses to stress, organism-specific biosystem (from REACTOME) |
| 22. | Class I MHC mediated antigen processing & presentation, organism-specific biosystem (from REACTOME) |
| 23. | Conversion from APC/C:Cdc20 to APC/C:Cdh1 in late anaphase, organism-specific biosystem (from REACTOME) |
| 24. | HTLV-I infection, organism-specific biosystem (from KEGG) |
| 25. | HTLV-I infection, conserved biosystem (from KEGG) |
2.2. Purification of Total RNA Including miRNA and Reverse Transcription
The extraction step of RNAs from sera sample was performed using miRNEasy RNA isolation kit (Qiagen, Hilden, Germany) as reported by manufacturer. 30µl of nuclease-free water was added to every RNA samples. NanoDrop spectrophotometer (Thermo Scientific, USA) [19] was used to get the concentration and purity of RNA. cDNA for mRNAs, lncRNA and miRNA were synthesized by miScript II RT Kit (Qiagen, Germany) in Rotor gene Thermal cycler (Thermo Electron Waltham, MA).
2.3. Differential Expression of the Selected RNAs by Real Time-Qrt PCR
ANPCII mRNA and lncRNA-RP11-175K6 expressions in sera samples were quantified by using QuantiTect SYBR Green PCR Kit and RT2 SYBR Green ROX qPCR Mastermix, sequentially. RT2 lncRNA qPCR Assay for Human lncRNA-RP11-175K6.1(LOC101927740 (ENST00000499 583) and ANPCII QuantiTect Primer Assay (NM_ 001002244), Hs_ACTB_1_SG QuantiTect Primer Assay (NM_001002244) [that was used as housekeeping gene in equalization of raw data] were purchased from Qiagen, Germany.
We used miScript SYBR Green PCR Kit (Qiagen /SABiosciences Corporation, Frederick, MD) for quantification of hsa-_ miR106b-5p expression in different sera samples using either Hs_ miR106_1 miScript Primer Assay targets mature miRNA: hsa- miR106 - MIMAT0000680: 5'UAAAGUGCUGACAGUGCAGAU and snord68 that was used as housekeeping gene, Each reaction was done in duplicate. Relative quantification of RNAs expression was calculated by using the method of Leviak RQ= 2-ΔΔCt method [19].
Data analysis was performed using the Rotor Gene real time PCR (Qiagen, Hilden, Germany) which is considered negative if higher than 36 Ct value.
2.4. Statistics
The data was statistically analyzed using SPSS22. Krausakul Wallis test, chi-square test, and Spearman correlation test were used as appropriate. The receiver operating characteristic (ROC) curve was done to characterize the predictive value of the selected RNAs for AMI.
3. RESULTS
3.1. Clinical and Laboratory Data of the Study
In this study, we have not found any differences among the three investigated groups i.e., age, sex and smoker: nonsmoker ratio (p>0.05), but in serum creatinine, total Triglycerides and cardiac troponin I showed marked differences and the details are shown in (Supplementary Table 1 (1.7MB, pdf) ).
3.2. Differential Expression of Serum RNAs Among the Study Groups
We confirm our in silico data regarding the relationship between the three chosen RNAs by measuring the serum levels of the three RNAs in the study samples. We assessed the RNAs based on the fold change (RQ) values. We found that the AMI group had down regulated both lncRNA-RP11-175K6.1 and RQ-ANPCII mRNA with concomitant up regulation of RQ-miR-106b-5p in comparison with the 2 control groups (p<0.01) (Supplementary Table 2 (1.7MB, pdf) , Fig. (1 (1.7MB, pdf) ).
Comparing AMI group to non-cardiac chest pain patients and healthy control participants by ROC curve analysis shows that the best cutoff values of lncRNA-RP11-175K6.1, ANPCII mRNA and RQ-miR-106b-5p were 1.36, 0.9950 and 10.94, respectively. The measured sensitivities were found to be 97.1%, 82.6% and 100%, respectively. These results indicate that these cutoff values could be used to differentiate AMI group form non cardiac chest pain patients and enrolled healthy people as illustrated in Fig. 2(a-c) and Tables 2 and 3.
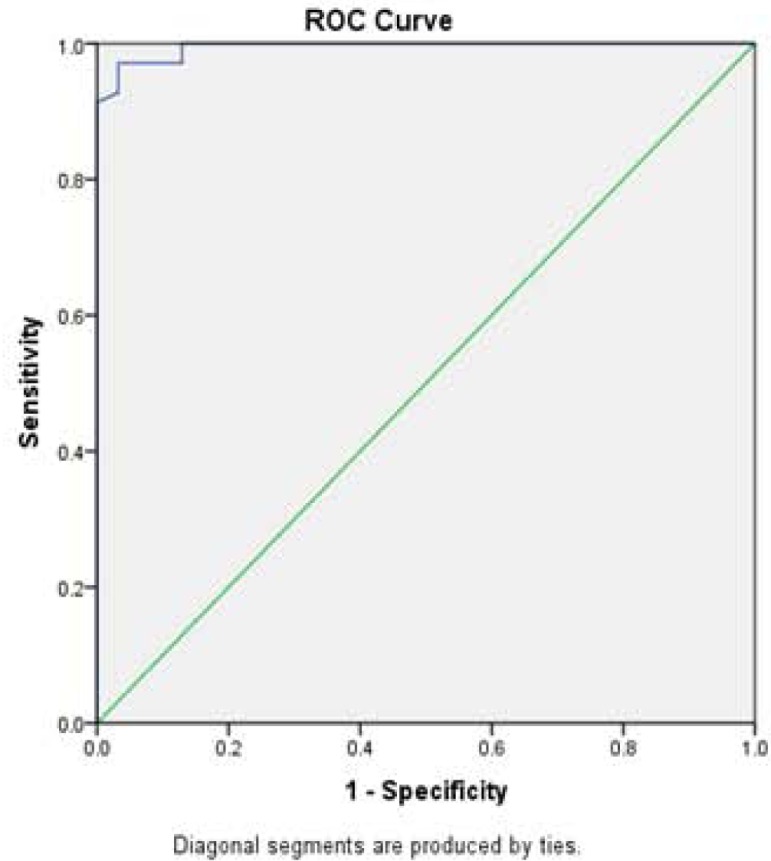
Fig. (2a).
ROC curve analysis for lncRNA-RP11-175K6 used to calculate the best cut-off point to discriminate between the AMI and healthy groups. AUC [SE]=0.995 [0.024], 95% confidence interval=0.655-0.842, (P<0.01).
Table 2. Positivity rate of serum investigated parameter based on fold change expression (RQ) among the study groups.
| - | miR106-b-5p | ANPCII mRNA | lncRNA-RP11-175K6 |
|---|---|---|---|
| Acute myocardial infarction Positive Negative |
N=69(100%) N=0(0.0%) |
N=69(100%) N=0(0%) |
N=2(2.8%) N=67(97.1%) |
| Non cardiac chest pain Positive Negative |
N=1(3.22%) N=30(96.7%) |
N=1(3.2%) N=30(96.77) |
N=1(3.2%) N=30(96.77%) |
| Control Positive Negative |
N=0(0%) N=31(100%) |
N=0(0%) N=31(100%) |
N=1(3.2%) N=30(96.77%) |
| P χ2(a) |
<.01 95.392 |
<.01 95.392 |
<.01 86.628 |
Table 3. Performance characteristics of serum investigated parameter based on fold change expression.
| Biomarker | Sensitivity | Specificity |
PPV
(Positive Predictive Value) |
NPV
(Negative Predictive Value) |
Accuracy |
|---|---|---|---|---|---|
| All the study groups (n=131) | |||||
| ANPCII mRNA | 82.6% | 96.8% | 71.4% | 98.3% | 87% |
| miR106-b-5P | 100% | 96.8% | 100% | 98.6% | 99% |
| lncRNA-RP11-175K6 | 97.1% | 96.8% | 93.8% | 98.5% | 97% |
3.3. Correlation between Serum RNAs with the Clinicopathological Factors in AMI Group
There was no significant correlation between any of the investigated serum RNAs and clinicopathological factors in the AMI group except serum ANPCII mRNA expression levels with diabetes mellitus and serum miR-106b expression levels with smoking, sex, nitrate, blocker, ACEI and statin use; lncRNA-RP11-175K6.1 expression levels with age, smoking, blockers, nitrate ACEI usage (Supplementary Table 3 (1.7MB, pdf) ).
Significant positive correlation was found between lncRNA-RP11-175K6.1 and ANPCII mRNA among the study groups (p<0.05) (Supplementary Table 4 (1.7MB, pdf) ). Moreover, there was a significant inverse correlation between hsa-miRNA-106b-5p and lncRNA-RP11-175K6.1 and ANPCII mRNA levels (r = -0.687, p<0.01 and r = -0.653, p<0.01, respectively). Remarkably, there was a significant direct correlation between hsa-miR-106b-5p and cardiac troponin I and CK-MB. There was a negative correlation between ANPCII mRNA, lncRNA-RP11-175K6.1 and cardiac Troponin I level.
Regression analysis revealed that serum miR-106b-5p was a strong independent predictor of AMI occurrence (all p<0.01) (Supplementary Table 5 (1.7MB, pdf) ).
4. DISCUSSION
AMI can lead to congestive heart failure and malignant arrhythmia causing high morbidity and mortality [19]. Therefore, in order to provide optimized benefits to current therapies, it is necessary to identify novel, highly sensitive and specific biomarkers and their regulatory targets for the early detection of AMI. Long intergeinc non coding RNAs and microRNAs have been widely investigated as potential disease biomarkers and therapeutic targets [19]. This could be attributed to their relative higher stability as they can resist the degradation process mediated by RNase enzymes [20].
By using an integrated genetic epigenetic approach, we identified the level of expression of lncRNA-RP11-175K6.1(LOC101927740), ANPCII mRNA and miR106 b-5p and were found to be strongly detected in the serum of AMI patients making them potential biomarkers in diagnosing such polygenic diseases. It is supposed that the high performance requirement of AMI diagnosis does not depend upon single gene biomarker approach, so finding a network of genes that are functionally linked to each other may raise the success percentage of AMI diagnosis in comparison to conventional single-marker approach.
Ubiquitin ligases (E3s), as part of the UPS, are the master players that direct the ubiquitin addition to the target proteins and thus changing their physical properties or decreasing their activity but most importantly mark them for degradation [21, 22]. The cardiomyocyte-specific ubiquitin ligases have been extensively studied and revealed novel functional roles for each of them [23]. ANPCII (Anaphase-promoting complex subunit 11) APC/C core complex is a cell cycle-regulated E3 ubiquitin ligase showing a high expression in heart and skeletal muscle and moreover its activators play a major role in symbiotic interactions, hormone signaling and gametogenesis [24]. Furthermore, the high expression of ANPCII mRNA is associated with chromosomal instability and residual tumor and lymphovascular invasion [25]. The overexpression of APC7 was also strongly related to primary colorectal cancer (CRC) and so it can be utilized as a potential novel biomarker of metastatic CRC [26].
Interestingly, Luo et al., found an inverse correlation between ITCH E3 ubiquitin ligase and miRNA-106b and in pancreatic cancer [27]. Moreover, miR-106b was found to have a high expression level in myocardial infarction together with an angiogenesis anti-apoptotic role in cardiomyocytes through inhibition of p21 expression [28, 29]. It is reported that microRNA-106 constitutes a cluster of 25 especially miR- 106b which is significantly increased in patients with colorectal cancer [11], hepatocellular carcinoma [30], gastric cancer [31] prostate cancer [32] and renal cancer [33].
LncRNAs are stable in plasma and show disease and tissue specificity [34]. More than 1000 lncRNAs have been linked to various crucial processes, including growth, UPS, apoptosis, and cell proliferation [35]. lncRNA-RP11-175K6 (LOC101927740) is located on the fifth chromosome with a strong relation with cardiomyocyte regeneration and angiogenesis [36]. LncRNA-RP11-714G18.1 has atheroprotective importance by suppressing vascular cell migration through RP11-714G18.1/LRP2BP/MMP1 axis expression [37].
Significantly, there was a direct correlation between lncRNA-RP11-175K6.1 and ANAPC11 mRNA (P<0.05) with inverse correlation between lncRNA-RP11-175K6.1, ANAPC11 mRNA and miR-106b-5p. We hypothesized that lncRNA-RP11-175K6.1 could act as a sponge for miR-106b-5p with subsequent modulation of ANAPC11 mRNA playing a potential role in AMI pathogenesis. miR-106b-5p seems to inhibit ANAPC11 mRNA expression at transcriptional or posttranscriptional level (Fig. 3). Of note, there was inverse correlation found between ANAPC11 mRNA and lncRNA-RP11-175K6.1 and cardiac Troponin I level.
Fig. (3).
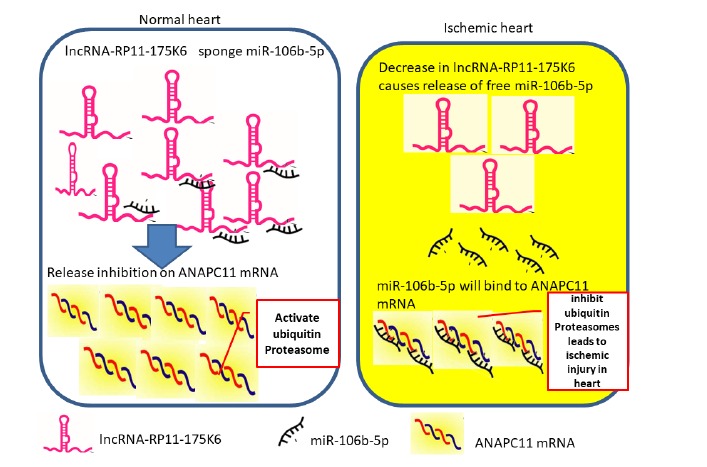
A schematic diagram to map the suggested association between the chosen genes. Schematic diagram of the study hypothesis.
The study was limited by relatively small sample size from two centers in Egypt. Moreover, in vitro and in vivo mechanistic analysis is needed to clarify the deep mechanism of RNA-RNA crosstalk in AMI which are strongly recommended.
CONCLUSION
We have identified dysregulation in the expression of serum lncRNA-RP11-175K6/ miRNA -106b -5p together with ANAPC11 mRNA in AMI patients compared to the control groups. It may be a promising strategy for the development of an RNA based biomarker panel to discriminate AMI patients and non AMI patients. These findings may provide new tools and targets for AMI therapy. Further studies are highly recommended to detect serum (lncRNA-RP11-175K6/ miRNA -106b -5p - ANAPC11 mRNA) in AMI patients’ samples using nanoassay as a non-PCR based technique with many advantages e.g. direct detection of unamplified RNA network in sera samples for the early diagnosis of AMI patients, simple, rapid and cheap assay for these nucleic acids other than PCR.
Fig. (1).
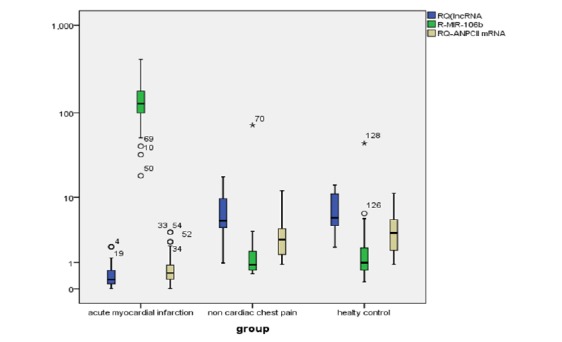
Box plot shows relative expression of the serum RNAs between AMI and control groups. The data is presented as median fold changes (P<0.05).
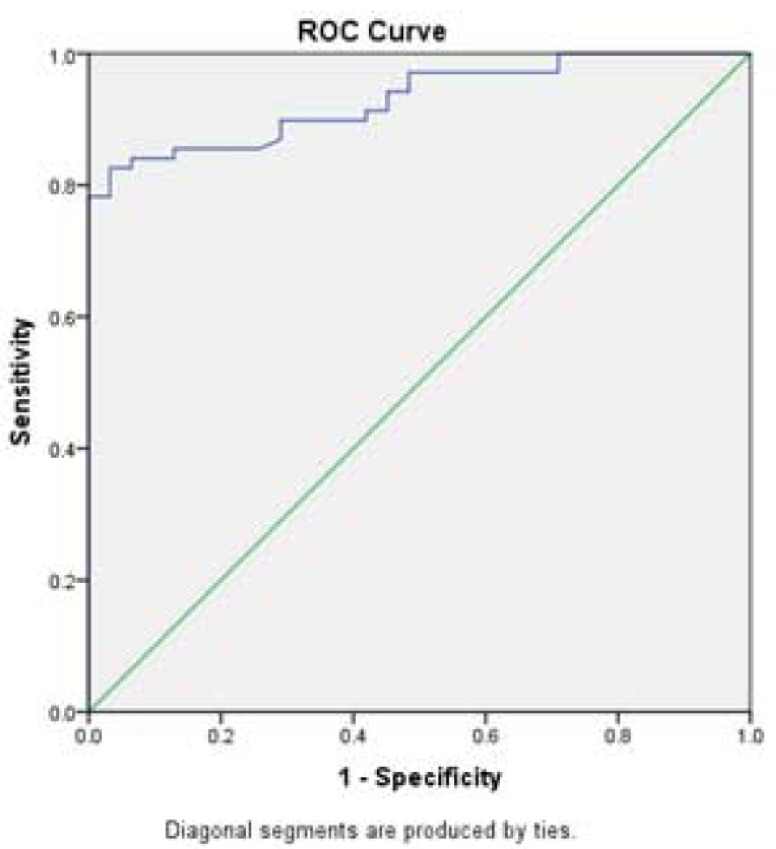
Fig. (2b).
ROC curve analysis for RQ-ANPCII mRNA used to calculate the best cut-off point to discriminate between the AMI and control groups. AUC [SE]=0.930 [0.033], 95% confidence interval=0.722-0.811, (P<0.01).
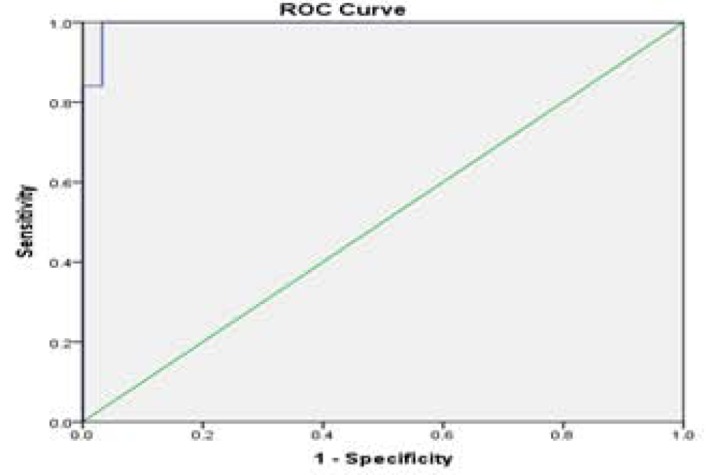
Fig. (2c).
ROC curve analysis for RQ-mIR-106b used to calculate the best cut-off point to discriminate between the AMI and control groups. AUC [SE]=0.995 [0.038], 95% confidence interval=0.845-0.931, (P<0.01).
ACKNOWLEDGEMENTS
Declared none.
AUTHOR’S CONTRIBUTIONS
Marwa Matboli has carried out in silico data analysis and designed in silico based RNA network, carried out molecular assay experiments and performed the statistical analysis. Sara H.A. Agwa has performed statistical analysis, drafting the manuscript. Ahmed K. Anwar, Amr R. Mansour, Ahmed I. Gaber, Ali E.A. Said, Paula Lwis, Marwa Hamdy participated in practical part, drafting manuscript. Sherif Sammir Elzahy has responsible for clinical samples collection with their data, and approved the final draft.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of the Faculty of Medicine, Ain Shams University, Egypt, (Approval number, FWA 000017586).
HUMAN AND ANIMAL RIGHTS
No Animals were used for studies that are the basis of this research. All the patients were diagnosed according to guidelines that were adopted from American College of Cardiology/American Heart Association (2018 ESC/ACC/AHA/ WHF Fourth Universal Definition).
CONSENT FOR PUBLICATION
Informed consent was obtained from all the patients prior to the study.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
This work was supported by the Egyptian Science and Technology Development Fund, RSTDG 25321.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Shi Q., Yang X. Circulating microRNA and long noncoding RNA as biomarkers of cardiovascular diseases. J. Cell. Physiol. 2016;231(4):751–755. doi: 10.1002/jcp.25174. [DOI] [PubMed] [Google Scholar]
- 2.Han Q.Y., Wang H.X., Liu X.H., Guo C.X., Hua Q., Yu X.H., Li N., Yang Y.Z., Du J., Xia Y.L., Li H.H. Circulating E3 ligases are novel and sensitive biomarkers for diagnosis of acute myocardial infarction. Clin. Sci. (Lond.) 2015;128(11):751–760. doi: 10.1042/CS20140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin M. Faculty of 1000 evaluation for executive summary: Heart disease and stroke statistics- 2012 update: A report from the American Heart Association. F1000 - Post-publication Peer Review of the Biomedical Literature 2013. [Google Scholar]
- 4.Shams-Vahdati S., Vand-Rajavpour Z., Paknezhad S.P., Piri R., Moghaddasi-Ghezeljeh E., Mirabolfathi S., Naghavi-Behzad M. Cost-effectiveness of cardiac biomarkers as screening test in acute chest pain. J. Cardiovasc. Thorac. Res. 2014;6(1):29–33. doi: 10.5681/jcvtr.2014.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muhlestein J.B. Biomarkers of Infection and Risk of Coronary Heart Disease. Cardiovascular Biomarkers, 319-344 . 2006.
- 6.Calise J., Powell S.R. The ubiquitin proteasome system and myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2013;304(3):H337–H349. doi: 10.1152/ajpheart.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis M.S., Bevilacqua A., Pulinilkunnil T., Kienesberger P., Tannu M., Patterson C. The role of ubiquitin ligases in cardiac disease. J. Mol. Cell. Cardiol. 2014;71:43–53. doi: 10.1016/j.yjmcc.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bader M., Steller H. Regulation of cell death by the ubiquitin-proteasome system. Curr. Opin. Cell Biol. 2009;21(6):878–884. doi: 10.1016/j.ceb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stringer D.K., Piper R.C. Terminating protein ubiquitination: Hasta la vista, ubiquitin. Cell Cycle. 2011;10(18):3067–3071. doi: 10.4161/cc.10.18.17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y., Cheng H.W., Qiu Y., Dupee D., Noonan M., Lin Y.D., Fisch S., Unno K., Sereti K.I., Liao R. MicroRNA-34a plays a key role in cardiac repair and regeneration following myocardial infarction. Circ. Res. 2015;117(5):450–459. doi: 10.1161/CIRCRESAHA.117.305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shyu K.G., Wang B.W., Cheng W.P., Lo H.M. MicroRNA-208a increases myocardial endoglin expression and myocardial fibrosis in acute myocardial infarction. Can. J. Cardiol. 2015;31(5):679–690. doi: 10.1016/j.cjca.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Fan Z.G., Qu X.L., Chu P., Gao Y.L., Gao X.F., Chen S.L., Tian N.L. MicroRNA-210 promotes angiogenesis in acute myocardial infarction. Mol. Med. Rep. 2018;17(4):5658–5665. doi: 10.3892/mmr.2018.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juni R.P., Abreu R.C., da Costa Martins P.A. Regulation of microvascularization in heart failure - an endothelial cell, non-coding RNAs and exosome liaison. Noncoding RNA Res. 2017;2(1):45–55. doi: 10.1016/j.ncrna.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchese F.P., Huarte M. Long non-coding RNAs and chromatin modifiers: Their place in the epigenetic code. Epigenetics. 2014;9(1):21–26. doi: 10.4161/epi.27472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greco S., Gorospe M., Martelli F. Noncoding RNA in age-related cardiovascular diseases. J. Mol. Cell. Cardiol. 2015;83:142–155. doi: 10.1016/j.yjmcc.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao L., Liu Y., Guo S., Yao R., Wu L., Xiao L., Wang Z., Liu Y., Zhang Y. Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cell. Physiol. Biochem. 2017;44(4):1497–1508. doi: 10.1159/000485588. [DOI] [PubMed] [Google Scholar]
- 17.Sano M., Schneider M.D. Cyclins that don’t cycle--cyclin T/cyclin-dependent kinase-9 determines cardiac muscle cell size. Cell Cycle. 2003;2(2):99–104. doi: 10.4161/cc.2.2.332. [DOI] [PubMed] [Google Scholar]
- 18.Greco S., Perfetti A., Menicanti L., Castelvecchio S., Martelli F. Heart failure modulates long noncoding RNA expression in human left ventricles. Eur. Heart J. 2013;34(Suppl. 1):P3250. doi: 10.1093/eurheartj/eht309.P3250. [DOI] [Google Scholar]
- 19.Da Sacco L., Baldassarre A., Masotti A. Bioinformatics tools and novel challenges in long non-coding RNAs (lncRNAs) functional analysis. Int. J. Mol. Sci. 2012;13(1):97–114. doi: 10.3390/ijms13010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan L., Sun L., Zhang Y., Huang Y., Hou Y., Li Q., Guo Y., Feng B., Cui L., Wang X., Wang Z., Tian Y., Yu B., Wang S., Xu C., Zhang M., Du Z., Lu Y., Yang B.F. Circulating long non-coding RNAs NRON and MHRT as novel predictive biomarkers of heart failure. J. Cell. Mol. Med. 2017;21(9):1803–1814. doi: 10.1111/jcmm.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brigant B., Metzinger-Le M.V., Rochette J., Metzinger L. TRIMming down to TRIM37: Relevance to inflammation, cardiovascular disorders, and cancer in MULIBREY nanism. Int. J. Mol. Sci. 2018;20(1):67. doi: 10.3390/ijms20010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basheer W.A., Harris B.S., Mentrup H.L., Abreha M., Thames E.L., Lea J.B., Swing D.A., Copeland N.G., Jenkins N.A., Price R.L., Matesic L.E. Cardiomyocyte-specific overexpression of the ubiquitin ligase Wwp1 contributes to reduction in Connexin 43 and arrhythmogenesis. J. Mol. Cell. Cardiol. 2015;88:1–13. doi: 10.1016/j.yjmcc.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mota R., Parry T.L., Yates C.C., Qiang Z., Eaton S.C., Mwiza J.M., Tulasi D., Schisler J.C., Patterson C., Zaglia T., Sandri M., Willis M.S. Increasing cardiomyocyte atrogin-1 reduces aging-associated fibrosis and regulates remodeling in vivo. Am. J. Pathol. 2018;188(7):1676–1692. doi: 10.1016/j.ajpath.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heyman J., De Veylder L. The anaphase-promoting complex/cyclosome in control of plant development. Mol. Plant. 2012;5(6):1182–1194. doi: 10.1093/mp/sss094. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., Wan L., Dai X., Sun Y., Wei W. Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis. Biochim. Biophys. Acta. 2014;1845(2):277–293. doi: 10.1016/j.bbcan.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim I.Y., Kwon H.Y., Park K.H., Kim D.S. Anaphase-Promoting Complex 7 is a prognostic factor in human colorectal cancer. Ann. Coloproctol. 2017;33(4):139–145. doi: 10.3393/ac.2017.33.4.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Z.L., Luo H.J., Fang C., Cheng L. Negative correlation of ITCH E3 ubiquitin ligase and miRNA-106b dic-tates metastatic progression in pancreatic cancer. Oncotarget. 2016;7(2):1477–1485. doi: 10.18632/oncotarget.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Z., Yang D., Xie P., Ren G., Sun G., Zeng X., Sun X. MiR-106b and MiR-15b modulate apoptosis and angiogenesis in myocardial infarction. Cell. Physiol. Biochem. 2012;29(5-6):851–862. doi: 10.1159/000258197. [DOI] [PubMed] [Google Scholar]
- 29.Zhu H., Fan G.C. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc. Res. 2012;94(2):284–292. doi: 10.1093/cvr/cvr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei R., Huang G.L., Zhang M.Y., Li B.K., Zhang H.Z., Shi M., Chen X.Q., Huang L., Zhou Q.M., Jia W.H., Zheng X.F., Yuan Y.F., Wang H.Y. Clinical significance and prognostic value of microRNA expression signatures in hepatocellular carcinoma. Clin. Cancer Res. 2013;19(17):4780–4791. doi: 10.1158/1078-0432.CCR-12-2728. [DOI] [PubMed] [Google Scholar]
- 31.Li F., Liu J., Li S. MicroRNA 106b ∼ 25 cluster and gastric cancer. Surg. Oncol. 2013;22(2):e7–e10. doi: 10.1016/j.suronc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Hudson R.S., Yi M., Esposito D., Glynn S.A., Starks A.M., Yang Y., Schetter A.J., Watkins S.K., Hurwitz A.A., Dorsey T.H., Stephens R.M., Croce C.M., Ambs S. MicroRNA-106b-25 cluster expression is associated with early disease recurrence and targets caspase-7 and focal adhesion in human prostate cancer. Oncogene. 2013;32(35):4139–4147. doi: 10.1038/onc.2012.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slaby O., Jancovicova J., Lakomy R., Svoboda M., Poprach A., Fabian P., Kren L., Michalek J., Vyzula R. Expression of miRNA-106b in conventional renal cell carcinoma is a potential marker for prediction of early metastasis after nephrectomy. J. Exp. Clin. Cancer Res. 2010;29(1):90. doi: 10.1186/1756-9966-29-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan Y., Zhang B., Liu N., Qi C., Xiao Y., Tian X., Li T., Liu B. Circulating long noncoding RNA UCA1 as a novel biomarker of acute myocardial infarction. BioMed Res. Int. 2016;2016:8079372. doi: 10.1155/2016/8079372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Cai Y., Wu G., Chen X., Liu Y. Plasma long non-coding RNA, CoroMarker, a novel bi-omarker for diagnosis of coronary artery disease. Clin. Sci. (Lond.) 2015;129(8):675–685. doi: 10.1042/CS20150121. [DOI] [PubMed] [Google Scholar]
- 36.Ergul A., Alhusban A., Fagan S.C. Angiogenesis: A harmonized target for recovery after stroke. Stroke. 2012;43(8):2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Zheng L., Xu B.M., Tang W.H., Ye Z.D., Huang C., Ma X., Zhao J.J., Guo F.X., Kang C.M., Lu J.B., Xiu J.C., Li P., Xu Y.J., Xiao L., Wu Q., Hu Y.W., Wang Q. LncRNA-RP11-714G18.1 suppresses vascular cell migration via directly targeting LRP2BP. Immunol. Cell Biol. 2018;96(2):175–189. doi: 10.1111/imcb.1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
Not applicable.