Abstract
The control of colostrum quality is essential for successful calf rearing. Instruments for on-farm colostrum quality determination are mostly utilized for testing composite colostrum samples, but do not take potential variation between quarters into account. In cases of low composite colostrum quality, feeding of better quality colostrum from individual quarters might be beneficial. The objective of the present study was to identify relationships between colostrum color, colostrum quality and composition. Besides laboratory methods, a colostrometer and a Brix refractometer were used to assess colostrum quality at quarter levels. Quarter and composite colostrum samples from 17 primiparous and 11 multiparous Holstein cows were analyzed for total IgG, fat, protein and lactose content; color was measured by a spectrophotometer. In the present study, an IgG concentration below 50 g/L as determined by ELISA was found in 14.3% of the analyzed quarter samples. Concentration and mass of IgG in composite colostrum samples were greater in multiparous compared with primiparous cows. Specific gravity (SG) of colostrum of individual and composite samples was lower in primiparous compared with multiparous cows. Milk fat content was greater in quarter and composite colostrum samples of primiparous compared with multiparous dairy cows. No clear relationships between IgG content and SG, Brix, and the color space coordinates L*, a*, and b* were detected. Interestingly, results indicate that despite a similar range of the variables investigated, correlations between those parameters can differ at quarter compared to composite level. Not only for SG and Brix determination, but also for the color space coordinates measured, correlation coefficients with IgG concentration of the respective samples were greater at a composite compared with the individual quarter level. In conclusion, accuracy and limitations of on-farm instruments estimating colostrum quality apply to both quarter colostrum samples and composite evaluations. Identification of quarters with superior colostrum quality would possibly be a way to improve the immunization of newborn calves. However, the potential on-farm methods validated in the present study to estimate quarter colostrum quality are not sufficiently sensitive to distinguish between quarters. This is due to the variation of gross colostrum composition between individual quarters of a cow.
Keywords: colostrum, dairy cow, immunoglobulin G, quarter sampling, refractometer
INTRODUCTION
Since bovine calves are born agammaglobulinemic and therefore depend on passive immunization via colostrum-derived immunoglobulins (Ig), a timely supply with good quality colostrum is essential for calf health (McGuire et al., 1976; Tyler et al., 1999). However, yield and quality of colostrum obtained after parturition in dairy cows vary markedly between herds and between animals (Kehoe et al., 2007; Baumrucker et al., 2010), but also vary between quarters (Baumrucker et al., 2014; Samarütel et al., 2016). The provision of a sufficient amount of colostrum is in many cases not limiting. Thus feeding single quarter derived colostrum differing in its quality could be an alternative method to maximize Ig supply. Many studies investigated the suitability of colostrometers and refractometers to estimate colostrum quality (e.g., Chigerwe et al., 2008; Morrill et al., 2012; Quigley et al., 2013). However, predominantly composite colostrum samples were analyzed, not taking individual quarter variation into account. It is currently not known if quarter differences in IgG content within a cow can be reflected by specific gravity (SG) and Brix determination, and to which extent the outcome of measuring colostrum quality of only one quarter contributes to the overall composite colostrum obtained at first milking.
The objective of the present study was to evaluate the accuracy and precision of 2 different devices (colostrometer and optical Brix refractometer) and to identify relationships between visual appearance of colostrum, colostrum quality assessed by the 2 common on-farm instruments and composition of colostrum in primiparous and multiparous dairy cows at quarter level. In addition, individual quarters were compared to the composite colostrum results obtained to illustrate the impact of single quarters on total colostrum as commonly fed to calves.
MATERIALS AND METHODS
Animals and Colostrum Sampling
Colostrum sampling was conducted following the guidelines of the Swiss Law on Animal Protection and approved by the Veterinary Office of the Canton Fribourg, Switzerland. Cows were transferred to straw-bedded calving pens approximately 7 d before expected parturition. Dry cows were fed hay ad libitum plus 1 kg of cereal-based concentrate and 0.5 kg of mineral enriched supplement (contents per kg of dry matter: crude ash 170 g; crude fiber 112 g; crude protein 67 g; vitamin A 180,200 IE; vitamin D 14,400 IE; vitamin E 1170 IE; Ca 2 g; P 7 g) until calving. Calves were removed immediately after birth to prevent suckling.
From September to December 2012, colostrum samples of 17 primiparous and 11 multiparous Holstein dairy cows (parity: 3.5 ± 1.5; 66 ± 15 d dry) were obtained at 4 h 50 min ± 1 h 46 min, and 4 h 12 min ± 32 min after parturition (mean ± SD), respectively, by machine-milking. All cows were milked empty and colostrum of each quarter was collected into a separate container. The amount of colostrum produced per quarter was recorded and representative samples (approximately 50 mL) of each quarter as well as of all after merging individual quarters (composite sample) were stored at -20°C until further analysis.
On-farm Devices for Colostrum Quality Determination
Fresh quarter and composite colostrum samples were analyzed at 20°C using a colostrum densimeter (Kruuse A/S, Langeskov, Denmark). Temperature was measured with a universal thermometer for liquids. Approximately 250 mL of colostrum was transferred into the colostrometer measuring-cylinder provided by the manufacturer. After the colostrometer was lowered into the cylinder and allowed to float freely, SG was determined by reading the scale (SG 1.023 to 1.077) above the submerged portion of the instrument.
Additionally, all samples were measured with an optical Brix refractometer (Manual Refractometer MHRB-40 ATC, Mueller Optronic, Erfurt, Germany) with a scale ranging from 0 to 40% Brix. The refractometer was equipped with an automatic temperature compensation mechanism to ensure accurate measurements without recalibration after shifts in ambient working temperature. According to the manufacturer, the accuracy of the instrument was ± 0.2% Brix at 20°C.
Composite colostrum of different quality derived from 3 multiparous cows was diluted with distilled water to investigate the response of SG and Brix toward a reduced content of colostrum components. Figure 1 shows a linear decrease of SG (Fig. 1A) and Brix (Fig. 1B) with the addition of 100 mL of water. When merging these 3 different colostrum qualities in different ratios, the additive contribution of single proportions was reflected by the very similar results of SG and Brix determination between expected and measured values (Table 1).
Figure 1.
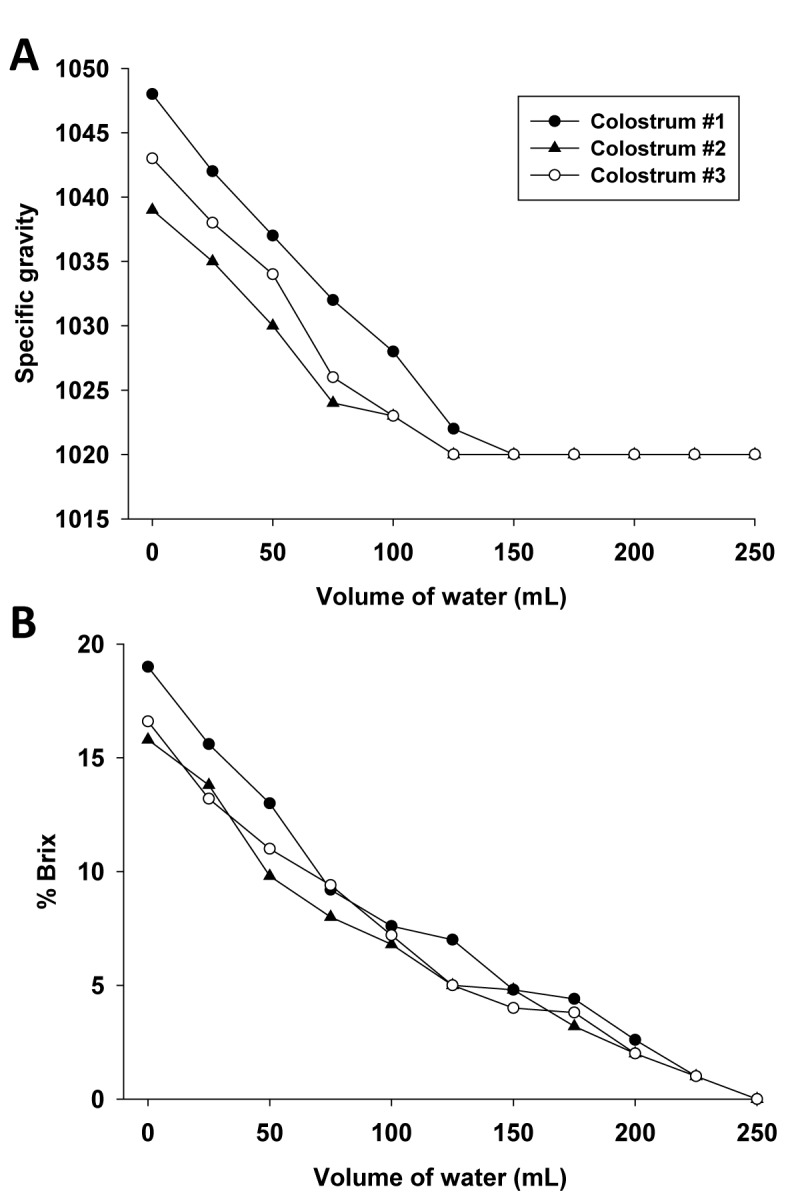
Specific gravity (Fig. 1A) and Brix-values (% Brix, Fig. 1B) of 3 fresh composite colostrum samples obtained from multiparous cows affected by adding increased volume of distilled water.
Table 1.
Comparison of different mixing ratios of 3 fresh composite colostrum samples obtained from multiparous cows
| Volume, mL of | Total volume, mL | Specific gravity | Brix, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Colostrum #1 | Colostrum #2 | Colostrum #3 | Expected1 | Measured | Deviation2, % | Expected1 | Measured | Deviation2, % | |
| 125 | 125 | 0 | 250 | 1043.5 | 1044 | 0.05 | 17.4 | 17 | −2.30 |
| 0 | 125 | 125 | 250 | 1041 | 1042 | 0.10 | 16.2 | 15.6 | −3.70 |
| 125 | 0 | 125 | 250 | 1045.5 | 1046 | 0.05 | 17.8 | 17.4 | −2.25 |
| 50 | 50 | 150 | 250 | 1043.2 | 1043 | −0.02 | 16.9 | 16.6 | −1.90 |
| 50 | 150 | 50 | 250 | 1041.6 | 1042 | 0.04 | 16.6 | 15.8 | −4.82 |
| 150 | 50 | 50 | 250 | 1045.2 | 1046 | 0.08 | 17.9 | 17.2 | −3.80 |
| 50 | 100 | 100 | 250 | 1042.4 | 1043 | 0.06 | 16.8 | 16 | −4.53 |
| 100 | 50 | 100 | 250 | 1044.2 | 1045 | 0.08 | 17.4 | 17 | −2.30 |
| 100 | 100 | 50 | 250 | 1043.4 | 1043 | −0.04 | 17.2 | 16.6 | −3.71 |
Arithmetic mean weighted by the volume of colostrum #1 (specific gravity = 1048, 19.0% Brix), colostrum #2 (specific gravity = 1039, 15.8% Brix), and colostrum #3 (specific gravity = 1043, 16.6% Brix).
Deviation of measured values from expected values calculated as follows: (expected – measured)/expected × 100 [%].
All measurements with the colostrometer (SG determination) and refractometer were performed in duplicate.
Measurement of Colostrum Color
Colostrum color was assessed as described earlier for dairy cows by Gross et al. (2014a). Color [CIE 1976 (L*, a*, b*) color space- CIELAB] was measured in thawed and homogenized (gentle shaking in a water bath at 37°C for 20 min) samples in triplicate at 25°C using a calibrated Microflash 200d spectrophotometer (Datacolor International, Dietikon, Switzerland), with the coordinates L* representing relative lightness (black to white), a* giving the relative value between green and red, and b* indicating the relative position between blue and yellow.
Colostrum IgG, Fat, Protein and Lactose Analyses
After thawing, colostral total IgG concentration was determined with a modified ELISA (Bovine IgG ELISA Quantitation Set, order no. E10–118; Bethyl Laboratories Inc. Montgomery, TX) as described recently by Lehmann et al. (2013). Samples were thawed at room temperature and serially diluted in ELISA wash buffer (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, adjusted to pH 8.0) to final dilutions of 1:400,000 and 1:800,000. Results were expressed as IgG concentration in mg/mL. Mass of IgG secreted by the individual quarters was calculated by multiplying IgG concentrations by the corresponding volume of colostrum produced.
Milk fat, protein and lactose contents in colostrum samples were determined using a FTS Infrared Milk Analyzer (Bentley Instruments Inc., Chaska, MN) in the laboratory of the Milchprüfring Baden-Württemberg e. V. (Ravensburg, Germany) as earlier described by Gross et al. (2014a,b).
Statistical Analysis
Statistical analysis was performed with SAS (Version 9.4, SAS Inst. Inc., Cary, NC). The UNIVARIATE procedure of SAS was used to check for normal distribution of data. In cases of not being normally distributed, data were log-transformed. Data presented in text and tables are means ± SEM. In agreement with earlier studies, for the present study a threshold to distinguish between high- and low-quality colostrum was set at 50 mg IgG/mL as determined by ELISA. Sensitivity, specificity, and the negative predictive value (NPV) of the Brix and SG measurements for testing IgG concentration were calculated using 2-way contingency tables according to Argüello et al. (2005) and Gross et al. (2014a). Considering IgG concentrations derived from the ELISA analysis as reference values, the Brix and SG results were classified as test values. Sensitivity was calculated by the proportion of samples identified by SG < 1045, and Brix < 22% reflecting an IgG concentration < 50 mg/mL in all samples with an IgG concentration < 50 mg/mL evaluated by ELISA. Specificity represents the probability of samples with an IgG concentration ≥ 50 mg/mL identified by the colostrometer and refractometer relative to all samples classified high-quality by ELISA. The calculation of the NPV was in agreement with Pritchett et al. (1994) and Argüello et al. (2005), and depicts the portion of truly high samples (estimated via ELISA) in all samples identified as high-quality colostrum based on data from colostrometer and refractometer.
Within individual quarter and composite samples, Pearson correlation coefficients between colostrum yield, IgG concentration, IgG mass, SG, Brix, fat, protein, lactose content and color space coordinates (L*, a*, b*) were calculated using the CORR procedure of SAS. Significant effects were considered at P < 0.05.
RESULTS AND DISCUSSION
The objective of the present study was to assess colostrum quality of primiparous and multiparous dairy cows via color measurement, specific gravity, and Brix refractometry, and to compare results at quarter and composite levels. Whereas previous studies exclusively focused on testing quality of composite colostrum samples, this is the first attempt to investigate the contribution of single quarters to the overall colostrum quality.
Colostrum Quality and Composition
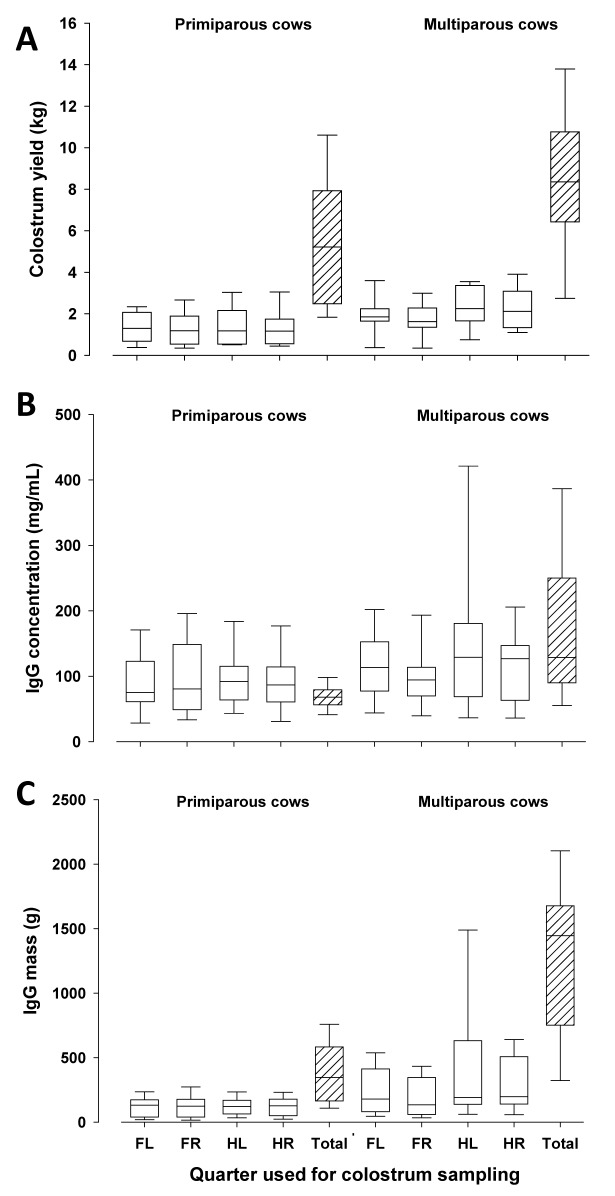
Despite a considerable variation in individual quarters, composite colostrum yield was greater in multiparous compared with primiparous dairy cows (P < 0.05, Fig. 2A). Furthermore, no differences in colostrum yield between front and rear quarters were detected. In all cows, colostrum yield was not related to IgG concentration at quarter or composite levels (Tables 2–6). Time of milking relative to calving did not affect colostral IgG concentration (P = 0.59). Concentration and total mass of IgG in composite colostrum samples were greater in multiparous compared with primiparous cows (P < 0.05), whereas no differences between quarters and number of parity were found (Fig. 2B-C). While the range of colostrum yield and IgG concentration in quarters was similar for primiparous and multiparous cows, IgG mass had a lower range in quarters of primiparous compared to multiparous cows. Variations in colostrum yield, IgG concentration and IgG mass in quarter and composite colostrum samples were similar to findings reported in the literature (Kehoe et al., 2007; Baumrucker et al., 2010, 2014; Gross et al., 2014b).
Figure 2.
Colostrum yield (Fig. 2A), IgG concentration (Fig. 2B), and IgG mass (Fig. 2C) in quarter and composite colostrum samples (FL = front left, FR = front right, HL = hind left, HR = hind right, Total = composite sample) of primiparous and multiparous dairy cows. The box represents 25th to 75th percentile of observations and the line in the box indicates the median, whiskers show fifth to 95th percentiles, and the line in the box indicates the median.
Table 2.
Front left quarter of primiparous and multiparous dairy cows: Pearson correlation coefficients between colostrum constituents and quality parameters (SG = specific gravity, color coordinates L*, a*, and b*). Significant correlation coefficients (P < 0.05) are highlighted in bold-type
| Milk yield, kg | IgG concentration, mg/mL | Brix, % | SG | Fat, % | Protein, % | L* | a* | b* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Milk yield, kg | – | 0.15 (P = 0.65) | 0.05 (P = 0.88) | 0.61 (P < 0.05) | −0.12 (P = 0.73) | −0.01 (P = 0.97) | 0.05 (P = 0.88) | −0.30 (P = 0.36) | 0.02 (P = 0.96) | Multiparous cows |
| IgG concentration, mg/mL | −0.05 (P = 0.85) | – | 0.01 (P = 0.99) | 0.10 (P = 0.78) | -0.61 (P < 0.05) | −0.46 (P = 0.15) | −0.24 (P = 0.48) | 0.18 (P = 0.60) | −0.12 (P = 0.72) | |
| Brix, % | 0.63 (P < 0.01) | 0.39 (P = 0.12) | – | 0.46 (P = 0.16) | −0.02 (P = 0.96) | 0.25 (P = 0.46) | −0.17 (P = 0.62) | 0.09 (P = 0.79) | 0.33 (P = 0.32) | |
| SG | −0.24 (P = 0.36) | 0.20 (P = 0.44) | 0.05 (P = 0.84) | – | 0.30 (P = 0.36) | 0.45 (P = 0.16) | 0.32 (P = 0.33) | −0.59 (P = 0.05) | 0.30 (P = 0.37) | |
| Fat, % | 0.43 (P = 0.12) | −0.07 (P = 0.81) | 0.26 (P = 0.36) | −0.38 (P = 0.18) | – | 0.90 (P < 0.001) | 0.27 (P = 0.42) | −0.31 (P = 0.35) | 0.29 (P = 0.39) | |
| Protein, % | 0.75 (P < 0.01) | 0.50 (P = 0.07) | 0.98 (P < 0.0001) | −0.23 (P = 0.42) | 0.19 (P = 0.52) | – | 0.00 (P = 0.99) | −0.15 (P = 0.65) | 0.29 (P = 0.39) | |
| L* | −0.48 (P = 0.05) | −0.37 (P = 0.15) | -0.69 (P < 0.01) | 0.06 (P = 0.82) | 0.21 (P = 0.48) | -0.79 (P < 0.0001) | – | -0.91 (P < 0.0001) | 0.49 (P = 0.13) | |
| a* | −0.04 (P = 0.87) | −0.08 (P = 0.76) | 0.40 (P = 0.11) | 0.48 (P = 0.05) | 0.19 (P = 0.53) | 0.44 (P = 0.11) | −0.15 (P = 0.56) | – | −0.38 (P = 0.25) | |
| b* | 0.57 (P < 0.05) | 0.14 (P = 0.60) | 0.59 (P < 0.05) | −0.14 (P = 0.60) | 0.12 (P = 0.68) | 0.70 (P < 0.01) | -0.78 (P < 0.001) | −0.05 (P = 0.86) | – | |
| Primiparous cows | ||||||||||
Table 6.
Composite colostrum samples of primiparous and multiparous dairy cows: Pearson correlation coefficients between colostrum constituents and quality parameters (SG = specific gravity, color coordinates L*, a*, and b*). Significant correlation coefficients (P < 0.05) are highlighted in bold-type
| Milk yield, kg | IgG concentration,mg/mL | Brix, % | SG | Fat, % | Protein, % | L* | a* | b* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Milk yield, kg | – | −0.42 (P = 0.20) | −0.24 (P = 0.48) | 0.41 (P = 0.21) | −0.34 (P = 0.30) | −0.19 (P = 0.57) | 0.09 (P = 0.80) | −0.29 (P = 0.39) | −0.17 (P = 0.61) | Multiparous cows |
| IgG concentration, mg/mL | 0.01 (P = 0.95) | – | 0.48 (P = 0.13) | 0.61 (P < 0.05) | 0.19 (P = 0.57) | 0.30 (P = 0.37) | -0.68 (P < 0.05) | 0.76 (P < 0.01) | −0.01 (P = 0.99) | |
| Brix, % | 0.32 (P = 0.21) | 0.18 (P = 0.48) | – | 0.17 (P = 0.62) | −0.09 (P = 0.80) | 0.95 (P < 0.0001) | −0.25 (P = 0.46) | 0.26 (P = 0.45) | 0.45 (P = 0.16) | |
| SG | −0.03 (P = 0.90) | −0.05 (P = 0.84) | 0.42 (P = 0.09) | – | −0.07 (P = 0.83) | 0.26 (P = 0.45) | 0.72 (P < 0.05) | -0.74 (P < 0.01) | 0.56 (P = 0.08) | |
| Fat, % | 0.20 (P = 0.50) | −0.30 (P = 0.29) | −0.01 (P = 0.97) | −0.36 (P = 0.20) | – | −0.27 (P = 0.43) | 0.39 (P = 0.23) | −0.16 (P = 0.64) | 0.28 (P = 0.40) | |
| Protein, % | 0.35 (P = 0.21) | 0.43 (P = 0.13) | 0.82 (P < 0.0001) | 0.35 (P = 0.22) | −0.36 (P = 0.20) | – | −0.17 (P = 0.61) | 0.13 (P = 0.76) | 0.32 (P = 0.34) | |
| L* | −0.23 (P = 0.37) | −0.24 (P = 0.35) | -0.72 (P < 0.01) | −0.24 (P = 0.35) | 0.39 (P = 0.16) | -0.68 (P < 0.01) | – | -0.93 (P < 0.0001) | 0.44 (P = 0.17) | |
| a* | 0.14 (P = 0.59) | −0.23 (P = 0.38) | 0.35 (P = 0.17) | −0.11 (P = 0.67) | 0.52 (P = 0.05) | 0.12 (P = 0.67) | −0.39 (P = 0.12) | – | −0.22 (P = 0.51) | |
| b* | 0.34 (P = 0.19) | −0.05 (P = 0.85) | 0.60 (P < 0.05) | −0.04 (P = 0.88) | 0.30 (P = 0.30) | 0.46 (P = 0.10) | -0.65 (P < 0.01) | 0.88 (P < 0.0001) | – | |
| Primiparous cows | ||||||||||
Table 3.
Front right quarter of primiparous and multiparous dairy cows: Pearson correlation coefficients between colostrum constituents and quality parameters (SG = specific gravity, color coordinates L*, a*, and b*). Significant correlation coefficients (P < 0.05) are highlighted in bold-type
| Milk yield, kg | IgG concentration, mg/mL | Brix, % | SG | Fat, % | Protein, % | L* | a* | b* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Milk yield, kg | – | 0.18 (P = 0.59) | −0.13 (P = 0.70) | 0.53 (P = 0.19) | −0.43 (P = 0.19) | 0.02 (P = 0.94) | 0.25 (P = 0.45) | −0.32 (P = 0.34) | 0.26 (P = 0.44) | Multiparous cows |
| IgG concentration, mg/mL | −0.14 (P = 0.59) | – | −0.02 (P = 0.94) | 0.14 (P = 0.68) | 0.25 (P = 0.46) | −0.05 (P = 0.88) | −0.16 (P = 0.63) | 0.19 (P = 0.58) | −0.21 (P = 0.54) | |
| Brix, % | 0.45 (P = 0.07) | 0.29 (P = 0.27) | – | 0.03 (P = 0.93) | −0.17 (P = 0.61) | 0.96 (P < 0.0001) | −0.49 (P = 0.12) | 0.51 (P = 0.11) | 0.18 (P = 0.59) | |
| SG | 0.11 (P = 0.68) | 0.17 (P = 0.50) | 0.11 (P = 0.67) | – | −0.32 (P = 0.33) | 0.28 (P = 0.40) | 0.33 (P = 0.32) | −0.48 (P = 0.13) | 0.21 (P = 0.53) | |
| Fat, % | 0.01 (P = 0.97) | −0.02 (P = 0.94) | −0.10 (P = 0.74) | −0.10 (P = 0.73) | – | −0.29 (P = 0.38) | 0.33 (P = 0.32) | −0.18 (P = 0.60) | −0.04 (P = 0.90) | |
| Protein, % | 0.27 (P = 0.35) | 0.42 (P = 0.13) | 0.95 (P < 0.0001) | −0.05 (P = 0.87) | −0.25 (P = 0.39) | – | −0.33 (P = 0.31) | 0.31 (P = 0.36) | 0.28 (P = 0.40) | |
| L* | −0.45 (P = 0.07) | −0.01 (P = 0.96) | -0.72 (P < 0.01) | 0.20 (P = 0.45) | 0.08 (P = 0.80) | -0.69 (P < 0.01) | – | -0.97 (P < 0.0001) | 0.54 (P = 0.09) | |
| a* | 0.06 (P = 0.83) | 0.30 (P = 0.24) | 0.10 (P = 0.70) | −0.20 (P = 0.44) | 0.61 (P < 0.05) | 0.10 (P = 0.73) | −0.24 (P = 0.35) | – | −0.43 (P = 0.18) | |
| b* | 0.41 (P = 0.10) | 0.16 (P = 0.54) | 0.49 (P < 0.05) | −0.26 (P = 0.31) | 0.23 (P = 0.43) | 0.51 (P = 0.06) | -0.68 (P < 0.01) | 0.79 (P < 0.001) | – | |
| Primiparous cows | ||||||||||
Table 4.
Hind left quarter of primiparous and multiparous dairy cows: Pearson correlation coefficients between colostrum constituents and quality parameters (SG = specific gravity, color coordinates L*, a*, and b*). Significant correlation coefficients (P < 0.05) are highlighted in bold-type
| Milk yield, kg | IgG concentration,mg/mL | Brix, % | SG | Fat, % | Protein, % | L* | a* | b* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Milk yield, kg | – | 0.59 (P = 0.06) | −0.16 (P = 0.63) | 0.47 (P = 0.15) | −0.05 (P = 0.89) | 0.00 (P = 0.99) | −0.02 (P = 0.95) | −0.25 (P = 0.45) | −0.09 (P = 0.79) | Multiparous cows |
| IgG concentration, mg/mL | −0.40 (P = 0.11) | – | 0.20 (P = 0.57) | 0.28 (P = 0.40) | −0.01 (P = 0.98) | 0.17 (P = 0.61) | −0.27 (P = 0.42) | 0.19 (P = 0.59) | 0.28 (P = 0.40) | |
| Brix, % | 0.10 (P = 0.70) | 0.15 (P = 0.57) | – | 0.00 (P = 0.99) | −0.04 (P = 0.90) | 0.94 (P < 0.0001) | −0.22 (P = 0.52) | 0.29 (P = 0.38) | 0.43 (P = 0.19) | |
| SG | −0.03 (P = 0.92) | 0.00 (P = 0.99) | 0.34 (P = 0.19) | – | −0.20 (P = 0.56) | 0.02 (P = 0.96) | 0.48 (P = 0.13) | -0.61 (P < 0.05) | 0.24 (P = 0.47) | |
| Fat, % | 0.46 (P = 0.10) | 0.04 (P = 0.88) | −0.19 (P = 0.53) | −0.33 (P = 0.25) | – | −0.25 (P = 0.46) | 0.35 (P = 0.29) | 0.07 (P = 0.85) | 0.52 (P = 0.10) | |
| Protein, % | 0.08 (P = 0.78) | 0.12 (P = 0.68) | 0.95 (P < 0.0001) | −0.04 (P = 0.90) | −0.30 (P = 0.30) | – | −0.28 (P = 0.40) | 0.20 (P = 0.55) | 0.17 (P = 0.62) | |
| L* | −0.26 (P = 0.32) | −0.21 (P = 0.42) | -0.82 (P < 0.0001) | −0.07 (P = 0.78) | 0.10 (P = 0.72) | -0.84 (P < 0.001) | – | -0.85 (P < 0.001) | 0.29 (P = 0.38) | |
| a* | −0.14 (P = 0.59) | 0.33 (P = 0.19) | 0.33 (P = 0.20) | −0.30 (P = 0.24) | 0.37 (P = 0.19) | 0.21 (P = 0.47) | -0.52 (P < 0.05) | – | 0.08 (P = 0.81) | |
| b* | −0.04 (P = 0.88) | 0.22 (P = 0.41) | 0.56 (P < 0.05) | −0.15 (P = 0.56) | 0.26 (P = 0.38) | 0.50 (P = 0.07) | -0.67 (P < 0.01) | 0.91 (P < 0.0001) | – | |
| Primiparous cows | ||||||||||
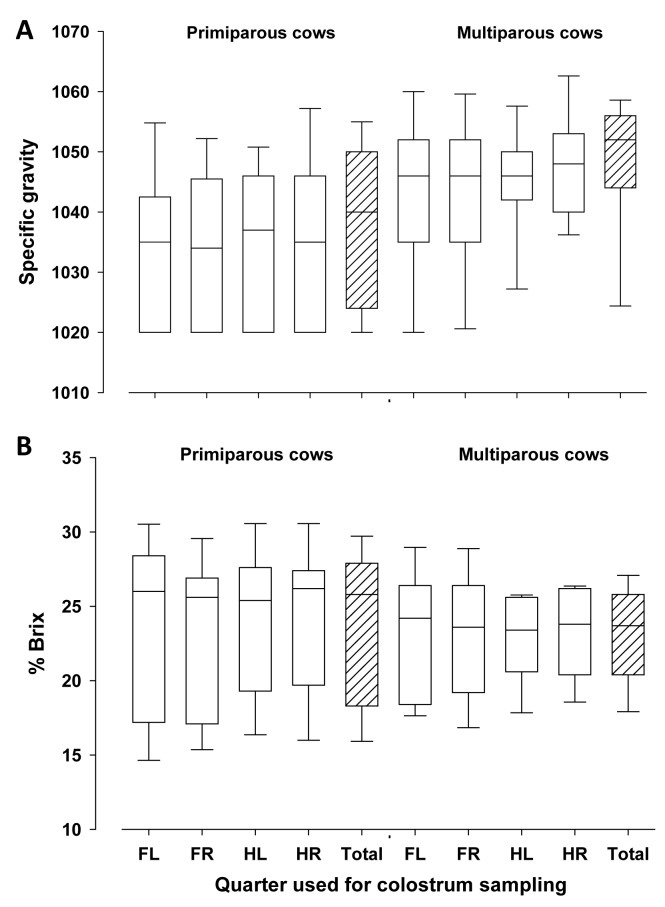
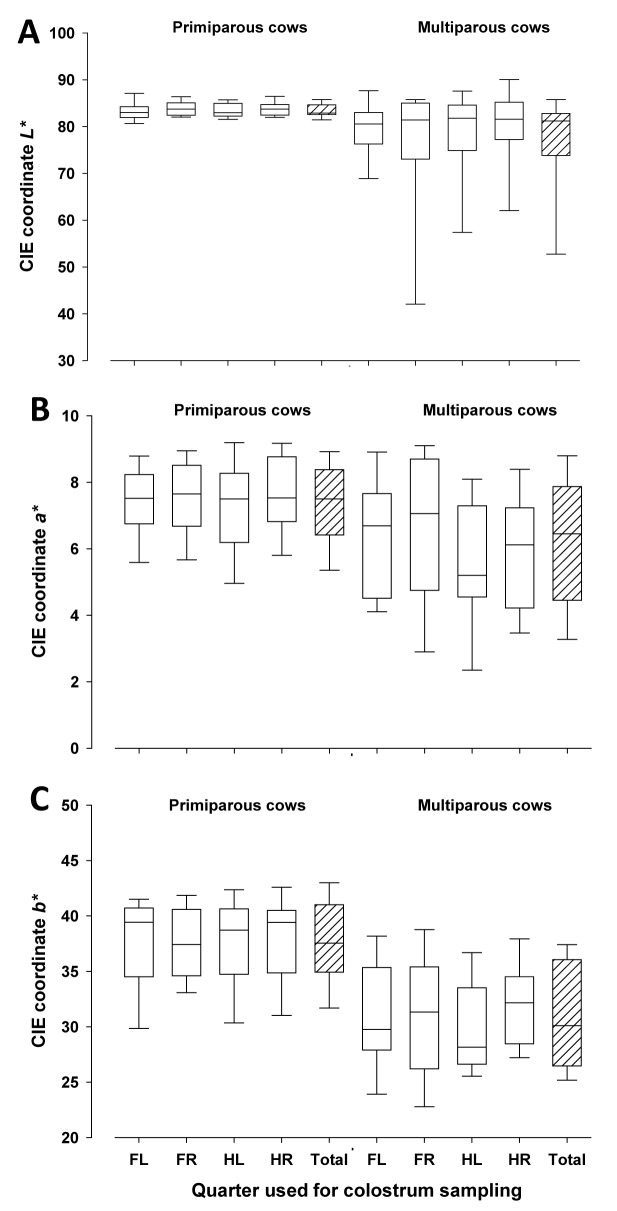
Specific gravity of colostrum from individual quarters tended to be lower (P < 0.10), and was lower in composite samples (P < 0.0001) from primiparous compared with multiparous cows (Fig. 3A) as observed earlier by Morrill et al. (2015). In contrast, Brix-values from individual quarter and composite colostrum samples did not differ between primiparous and multiparous cows (P > 0.05, Fig. 3B). Milk fat contents were greater in quarter and composite colostrum samples of primiparous compared with multiparous dairy cows (P < 0.05), whereas no differences in protein and lactose contents were found (Fig. 4A-C). Milk fat and protein contents were not related to each other (r = 0.12, P = 0.47). In primiparous cows, both individual quarter samples and composite colostrum samples had greater values for the CIE coordinates L* and b* (P < 0.05), but not for a*, compared with multiparous cows (Fig. 5A-C). The greater L* values in primiparous cows indicate the greater brightness of colostrum compared with that of multiparous cows and this may be partly due to the greater fat content (Gross et al., 2014a). As feeding conditions were the same for all cows, possible dietary effects may be excluded.
Figure 3.
Specific gravity (SG) assessed by colostrometer (Fig. 3A), and Brix-values evaluated by refractometer (%Brix; Fig. 3B) in quarters and composite colostrum samples (FL = front left, FR = front right, HL = hind left, HR = hind right, Total = composite sample) of primiparous and multiparous dairy cows. The box represents 25th to 75th percentile of observations and the line in the box indicates the median, whiskers show fifth to 95th percentiles, and the line in the box indicates the median.
Figure 4.
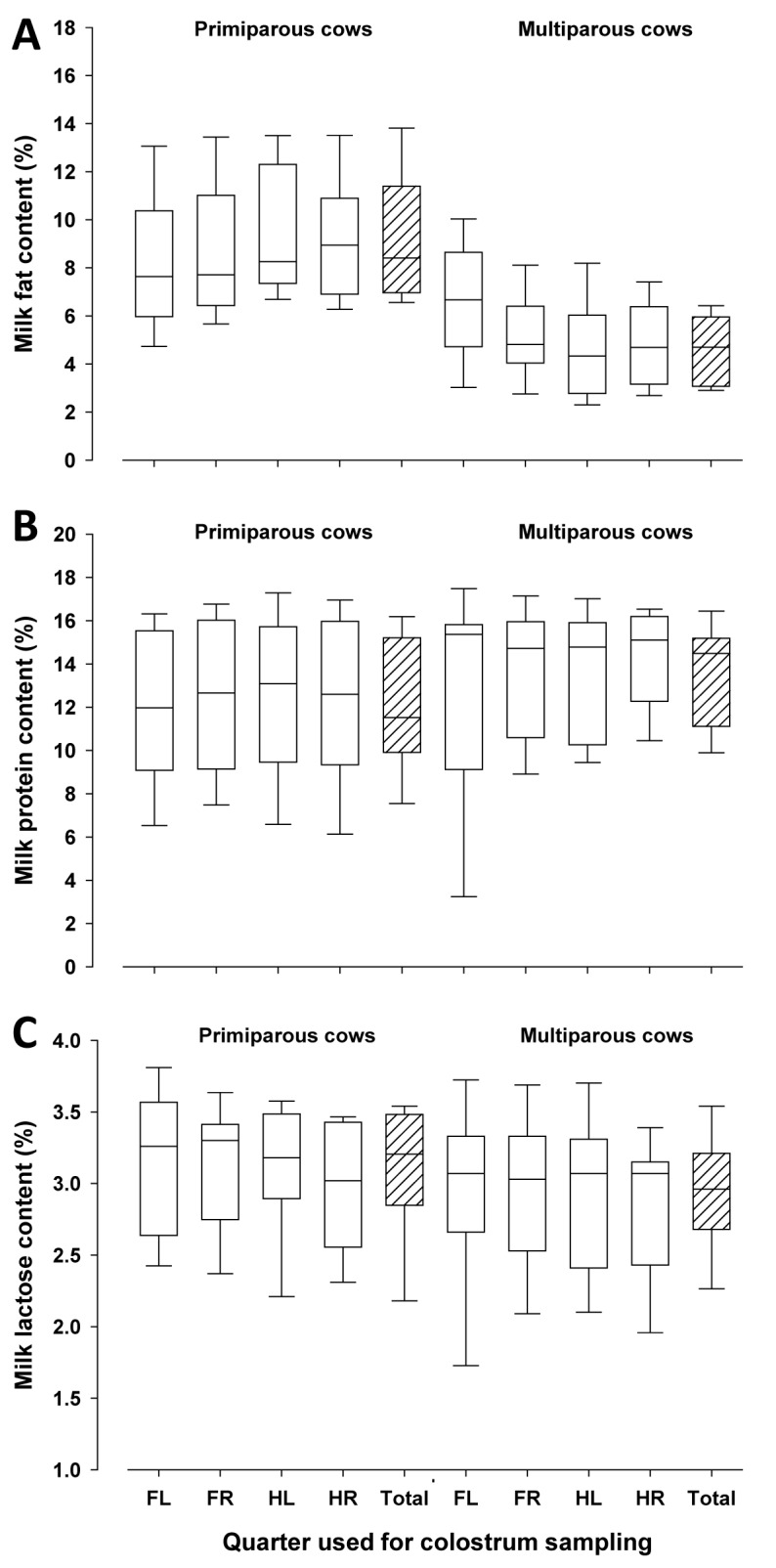
Colostrum fat (Fig. 4A), protein (Fig. 4B), and lactose contents (Fig. 4C) in quarter and composite colostrum samples (FL = front left, FR = front right, HL = hind left, HR = hind right, Total = composite sample) of primiparous and multiparous dairy cows. The box represents 25th to 75th percentile of observations and the line in the box indicates the median, whiskers show fifth to 95th percentiles, and the line in the box indicates the median.
Figure 5.
Colostrum color coordinates [CIE 1976 (L*, a*, b*) color space– CIELAB], L* (Fig. 5A), a* (Fig. 5B), and b* (Fig. 5C) in quarter and composite colostrum samples (FL = front left, FR = front right, HL = hind left, HR = hind right, Total = composite sample) of primiparous and multiparous dairy cows. The box represents 25th to 75th percentile of observations and the line in the box indicates the median, whiskers show fifth to 95th percentiles, and the line in the box indicates the median.
Relationships between Colostrum Quality Estimation and Colostrum Composition
In the present study, an IgG concentration below 50 g/L as determined by ELISA was found in 20 of the 140 samples (14.3%) analyzed. Major limitations regarding the suitability of SG determined by a colostrometer were pointed out by Morin et al. (2001). Parity, season of calving, colostrum temperature and colostral protein content, among other factors, were identified by several studies to affect accuracy and reliability of IgG estimation via SG (Mechor et al., 1992; Morin et al. 2001; Bielmann et al., 2010; Bartier et al., 2015). Therefore, the identification of sufficient quality colostrum for feeding calves requires more accurate and robust instruments, e.g., Brix refractometers (Fleenor and Stott, 1980; Morin et al., 2001; Quigley et al., 2013). When using the recommended cut-off points of 1.045 for SG and 22% for Brix (Bielmann et al., 2010), 57.1% and 36.4%, respectively, of the present samples would have been classified as poor quality colostrum by using a colostrometer or refractometer alone. Due to the low number of observations per quarter of colostrum truly classified as poor or good by either colostrometer or refractometer compared to the ELISA results, sensitivity of both on-farm instruments was between 0 and 100%. No differences between primiparous and multiparous cows were detected for sensitivity calculations. Specificity of the SG determination per quarter was lower in primiparous compared to multiparous cows (21.4 to 40.0% vs. 50.0 to 70.0%). For the refractometer, specificity was greater in quarter and composite colostrum samples of primiparous cows compared with multiparous cows (60.0 to 85.7% vs. 50.0 to 70.0%). The NPV was similar for both on-farm instruments in primiparous and multiparous cows (83.3 to 100%).
Tables 2 through 6 show the relationships between the colostrum quality estimation by SG, Brix, and color measurement, and colostrum composition (content of IgG, fat and protein) within quarter and for composite colostrum samples. In neither primiparous nor multiparous cows were clear relationships between IgG contents and SG, Brix, and the color space coordinates L*, a*, and b* detected. Except for the front left quarter in multiparous cows, the Brix-values had a high correlation coefficient with the protein content of the colostrum samples, but not with IgG concentration. This is in agreement with earlier findings (Bielmann et al., 2010; Quigley et al., 2013; Bartier et al., 2015).
Differences between Quarter and Composite Colostrum Samples
Colostrometer, refractometer and color measurements mirrored the variation in colostrum composition at both quarter and composite levels. Though earlier studies suggested that the colostrometer predicts the protein content of colostrum better than true IgG content (Morin et al., 2001), results from the present study do not show any association between SG and colostrum constituents (Tables 2 through 6). Surprisingly, in quarter colostrum samples SG did not correlate with IgG concentration (Tables 2 through 5), but after merging the quarters into a composite sample in multiparous cows, the correlation was in an acceptable range (r = 0.61, P < 0.05, Table 6). This observation can be explained by the fact that individual quarters within a cow show a marked variation in both fat and protein contents. Both fat and protein content of colostrum contribute to the overall SG and Brix-value (Mechor et al., 1992; Morin et al., 2001; Bielmann et al., 2010). Quarters within a cow do not have the same ratio of colostrum components. Merging quarters to composite colostrum samples therefore seems to compensate for the lack of single components of individual quarters contributing to SG and Brix (Table 6). In addition, the linear scale of the colostrometer and refractometer used in our study followed the gradual dilution of composite colostrum with water that concomitantly reduces all colostrum components in the same proportion (Fig. 1A,B). It is unclear if potentially further matrix effects of colostrum differ at quarter level compared to a composite colostrum sample that may affect its physical properties, e.g., density, viscosity etc. Differences in quality of mature milk at the quarter vs. composite level were previously reported (Forsbäck et al., 2009, 2011). Forsbäck et al. (2011) suggested that this discrepancy may be due to the impact of individual quarter somatic cell count (SCC) contributing to coagulation properties, and indirectly to viscosity of milk. Consequently, the measurement of SG by reading the submerged portion of a colostrometer is very likely to be affected by the overall viscous properties of colostrum. Even though SCC was not determined in colostrum samples from the current study, the first milk obtained after parturition is known to have a high content of SCC (Wall et al., 2015). It can therefore be speculated that the greater SCC in colostrum contributes to its physicochemical properties. Directly after parturition, the blood-milk-barrier is also known to be permeable so that blood-derived proteins are present in colostrum (Wall et al., 2015). These may additionally affect the matrix structure of colostrum at the quarter and composite levels. In addition, the same phenomenon was observed when determining the Brix-values even though colostrum temperature and handling were kept constant for all measurements. However, in the case of SG, Brix determination and also the measured color space coordinates, correlation coefficients with IgG concentration of the respective samples were higher at the composite compared with the individual quarter level. Besides individual quarter differences contributing to coagulation properties of the composite milk samples, differences in the structure and size of casein micelles at the quarter level were shown to be related to SCC (Frederiksen et al., 2011). When merging individual quarter colostrum samples, it can be assumed that micelles are interacting and changing their structure at the composite level and thus affecting SG and Brix measurements. However, variations in protein and fat contents between quarters of an animal are most likely responsible for the differences in SG and Brix-values at quarter and composite levels.
Table 5.
Hind right quarter of primiparous and multiparous dairy cows: Pearson correlation coefficients between colostrum constituents and quality parameters (SG = specific gravity, color coordinates L*, a*, and b*). Significant correlation coefficients (P < 0.05) are highlighted in bold-type
| Milk yield (kg) | IgG concentration (mg/mL) | Brix (%) | SG | Fat (%) | Protein (%) | L* | a* | b* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Milk yield (kg) | – | 0.27 (P = 0.42) | −0.45 (P = 0.17) | 0.05 (P = 0.88) | −0.08 (P = 0.81) | −0.43 (P = 0.19) | 0.04 (P = 0.90) | −0.21 (P = 0.53) | −0.07 (P = 0.84) | Multiparous cows |
| IgG concentration (mg/mL) | −0.20 (P = 0.45) | – | 0.02 (P = 0.95) | 0.05 (P = 0.88) | −0.12 (P = 0.72) | 0.05 (P = 0.88) | −0.50 (P = 0.12) | 0.46 (P = 0.15) | 0.12 (P = 0.73) | |
| Brix (%) | 0.10 (P = 0.69) | 0.24 (P = 0.35) | – | 0.29 (P = 0.39) | 0.00 (P = 0.99) | 0.95 (P < 0.0001) | −0.17 (P = 0.61) | 0.31 (P = 0.35) | 0.54 (P = 0.08) | |
| SG | 0.05 (P = 0.85) | 0.06 (P = 0.82) | 0.22 (P = 0.39) | – | −0.35 (P = 0.29) | 0.27 (P = 0.42) | 0.49 (P = 0.13) | −0.39 (P = 0.24) | 0.30 (P = 0.38) | |
| Fat (%) | −0.19 (P = 0.50) | −0.14 (P = 0.63) | −0.03 (P = 0.93) | −0.16 (P = 0.59) | – | −0.24 (P = 0.48) | 0.07 (P = 0.84) | 0.02 (P = 0.95) | 0.10 (P = 0.76) | |
| Protein (%) | −0.04 (P = 0.90) | 0.43 (P = 0.13) | 0.94 (P < 0.0001) | 0.05 (P = 0.87) | −0.17 (P = 0.57) | – | −0.25 (P = 0.45) | 0.30 (P = 0.36) | 0.36 (P = 0.27) | |
| L* | −0.06 (P = 0.83) | −0.07 (P = 0.80) | -0.55 (P < 0.05) | −0.13 (P = 0.62) | 0.15 (P = 0.61) | -0.54 (P < 0.05) | – | -0.91 (P < 0.0001) | 0.26 (P = 0.45) | |
| a* | −0.14 (P = 0.60) | 0.01 (P = 0.96) | 0.27 (P = 0.29) | −0.19 (P = 0.46) | 0.42 (P = 0.14) | 0.24 (P = 0.41) | −0.36 (P = 0.15) | – | −0.05 (P = 0.88) | |
| b* | 0.12 (P = 0.66) | 0.10 (P = 0.70) | 0.50 (P < 0.05) | −0.19 (P = 0.47) | 0.40 (P = 0.16) | 0.49 (P = 0.08) | −0.47 (P = 0.06) | 0.83 (P < 0.0001) | – | |
| Primiparous cows | ||||||||||
Practical Implementation of Quarter Colostrum Analysis and Collection in Dairy Farms
Up to now, quarter milking was not a topic with respect to colostrum milking in dairy practice. It is acknowledged that quarters are different in terms of IgG concentration and volume of produced colostrum (Baumrucker et al., 2014; Gross et al., 2016; Samarütel et al., 2016). Present findings revealed differences in colostrum quality between single quarters and composite colostrum samples. Technically, automatic milking systems and some devices in conventional milking parlors can separate milk (and colostrum) from single quarters. In combination with in-line devices abnormal milk, e.g., during cases of mastitis, can currently be separated (Brandt et al., 2010). If a quarter is characterized by a high colostrum quality, it would be useful to collect and feed only colostrum from this quarter. Current findings using established and previously validated instruments (i.e., refractometer, colostrometer) confirmed their potential and limitations in addressing colostrum quality. Furthermore, the present results identified possible reasons for variation at quarter level (different protein and fat content etc.) that are masked by evaluating only composite samples. Moore et al. (2005) also reported that colostrum of only one quarter is often tested although the composite colostrum is fed to calves. Considering present results, however, single quarters do not represent the overall colostrum quality. This might have detrimental effects if poor quality colostrum is given to the newborn. On the other hand, the identification of superior colostrum quality and immediate separation of particular quarters at milking could reduce the impact of low quality colostrum from other quarters and benefit calf rearing despite the known problems in terms of variation in colostrum composition at quarter and composite levels.
Conclusions
In conclusion, accuracy and limitations of on-farm instruments used to estimate colostrum quality based on composite samples also apply for quarter colostrum samples. The variations in quarter colostrum quality and composition were reflected by the outcome of refractometer and colostrometer measurements. Within individual quarters, correlation coefficients between colostral IgG concentration, SG, Brix-values, and colostrum color were poorer compared to composite samples. On the other hand, feeding high quality colostrum from individual quarters could be an option if sufficient amount of colostrum is produced.
LITERATURE CITED
- Argüello A., Castro N., Capote J.. 2005. Short Communication: Evaluation of a color method for testing immunoglobulin G concentration in goat colostrum. J. Dairy Sci. 88:1752–1754 [DOI] [PubMed] [Google Scholar]
- Bartier A. L., Windeyer M. C., Doepel L.. 2015. Evaluation of on-farm tools for colostrum quality measurement. J. Dairy Sci. 98:1878–1884. 10.3168/jds.2014-8415 [DOI] [PubMed] [Google Scholar]
- Baumrucker C. R., Burkett A. M., Magliaro-Macrina A. L., Dechow C. D.. 2010. Colostrogenesis: Mass transfer of IgG1 into colostrum. J. Dairy Sci. 93:3031–3038. doi: 10.3168/jds.2009-2963 [DOI] [PubMed] [Google Scholar]
- Baumrucker C. R., Stark A., Wellnitz O., Dechow C., Bruckmaier R. M.. 2014. Short communication: Immunoglobulin variation in quarter-milked colostrum. J. Dairy Sci. 97:3700–3706. doi: 10.3168/jds.2013-7107 [DOI] [PubMed] [Google Scholar]
- Bielmann V., Gillan J., Perkins N. R., Skidmore A. L., Godden S., Leslie K. E.. 2010. An evaluation of Brix refractometry instruments for measurement of colostrum quality in dairy cattle. J. Dairy Sci. 93:3713–3721. doi: 10.3168/jds.2009-2943 [DOI] [PubMed] [Google Scholar]
- Brandt M., Haeussermann A., Hartung E.. 2010. Invited review: Technical solutions for analysis of milk constituents and abnormal milk. J. Dairy Sci. 93:427–436. doi: 10.3168/jds.2009-2565 [DOI] [PubMed] [Google Scholar]
- Chigerwe M., Tyler J. W., Middleton J. R., Spain J. N., Dill J. S., Steevens B. J.. 2008. Comparison of four methods to assess colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 233:761–766. doi: 10.2460/javma.233.5.761 [DOI] [PubMed] [Google Scholar]
- Fleenor W. A., Stott G. H.. 1980. Hydrometer test for estimation of immunoglobulin concentration in bovine colostrum. J. Dairy Sci. 63:973–977. doi: 10.3168/jds.S0022-0302(80)83034-7 [DOI] [PubMed] [Google Scholar]
- Forsbäck L., Lindmark-Månsson H., Andrén A., Akerstedt M., Svennersten-Sjaunja K.. 2009. Udder quarter milk composition at different levels of somatic cell count in cow composite milk. Animal 3:710–717. doi: 10.1017/S1751731109004042 [DOI] [PubMed] [Google Scholar]
- Forsbäck L., Lindmark-Månsson H., Svennersten-Sjaunja K., Larsen L. B., Andrén A.. 2011. Effect of storage and separation of milk at udder quarter level on milk composition, proteolysis, and coagulation properties in relation to somatic cell count. J. Dairy Sci. 94:5341–5349. doi: 10.3168/jds.2011-4371 [DOI] [PubMed] [Google Scholar]
- Frederiksen P. D., Andersen K. K., Hammershøj M., Poulsen H. D., Sørensen J., Bakman M., Qvist K. B., Larsen L. B.. 2011. Composition and effect of blending of noncoagulating, poorly coagulating, and well-coagulating bovine milk from individual Danish Holstein cows. J. Dairy Sci. 94:4787–4799. doi: 10.3168/jds.2011-4343 [DOI] [PubMed] [Google Scholar]
- Gross J. J., Kessler E. C., Bruckmaier R. M.. 2014a. Colour measurement of colostrum for estimation of colostral IgG and colostrum composition in dairy cows. J. Dairy Res. 81:440–444. doi: 10.1017/S0022029914000466 [DOI] [PubMed] [Google Scholar]
- Gross J. J., Kessler E. C., Bjerre-Harpoth V., Dechow C., Baumrucker C. R., Bruckmaier R. M.. 2014b. Peripartal progesterone and prolactin have little effect on the rapid transport of immunoglobulin G into colostrum of dairy cows. J. Dairy Sci. 97:2923–2931. doi: 10.3168/jds.2013-7795 [DOI] [PubMed] [Google Scholar]
- Gross J. J., Schüpbach-Regula G., Bruckmaier R. M.. 2016. Rapid communication: Colostrum immunoglobulin concentration in mammary quarters is repeatable in consecutive lactations of dairy cows. J. Anim. Sci. 94:1755–1760. doi: 10.2527/jas.2016-0362 [DOI] [PubMed] [Google Scholar]
- Kehoe S. I., Jayarao B. M., Heinrichs A. J.. 2007. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J. Dairy Sci. 90:4108–4116. doi: 10.3168/jds.2007-0040 [DOI] [PubMed] [Google Scholar]
- Lehmann M., Wellnitz O., Bruckmaier R. M.. 2013. Concomitant lipopolysaccharide-induced transfer of blood-derived components including immunoglobulins into milk. J. Dairy Sci. 96:889–896. doi: 10.3168/jds.2012-5410 [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Pfeiffer N. E., Weikel J. M., Bartsch R. C.. 1976. Failure of colostral immunoglobulin transfer in calves dying from infectious disease. J. Am. Vet. Med. Assoc. 169:713–718. [PubMed] [Google Scholar]
- Mechor G. D., Gröhn Y. T., McDowell L. R., Van Saun R. J.. 1992. Specific gravity of bovine colostrum immunoglobulins as affected by temperature and colostrum components. J. Dairy Sci. 75:3131–3135. doi: 10.3168/jds.S0022-0302(92)78076-X [DOI] [PubMed] [Google Scholar]
- Moore M., Tyler J. W., Chigerwe M., Dawes M. E., Middleton J. R.. 2005. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 226:1375–1377. doi: 10.2460/javma.2005.226.1375 [DOI] [PubMed] [Google Scholar]
- Morin D. E., Constable P. D., Maunsell F. P., McCoy G. C.. 2001. Factors associated with colostral specific gravity in dairy cows. J. Dairy Sci. 84:937–943. doi: 10.3168/jds.S0022-0302(01)74551-1 [DOI] [PubMed] [Google Scholar]
- Morrill K. M., Robertson K. E., Spring M. M., Robinson A. L., Tyler H. D.. 2015. Validating a refractometer to evaluate immunoglobulin G concentration in Jersey colostrum and the effect of multiple freeze-thaw cycles on evaluating colostrum quality. J. Dairy Sci. 98:595–601. doi: 10.3168/jds.2014-8730 [DOI] [PubMed] [Google Scholar]
- Morrill K. M., Conrad E., Polo J., Lago A., Campbell J., Quigley J., Tyler H.. 2012. Estimate of colostral immunoglobulin G concentration using refractometry without or with caprylic acid fractionation. J. Dairy Sci. 95:3987–3996. doi: 10.3168/jds.2011-5104 [DOI] [PubMed] [Google Scholar]
- Pritchett L. C., Gay C. C., Hancock D. D., Besser T. E.. 1994. Evaluation of the hydrometer for testing immunoglobulin G1 concentrations in Holstein colostrum. J. Dairy Sci. 77:1761–1767. doi: 10.3168/jds.S0022-0302(94)77117-4 [DOI] [PubMed] [Google Scholar]
- Quigley J. D., Lago A., Chapman C., Erickson P., Polo J.. 2013. Evaluation of the Brix refractometer to estimate immunoglobulin G concentration in bovine colostrum. J. Dairy Sci. 96:1148–1155. doi: 10.3168/jds.2012-5823 [DOI] [PubMed] [Google Scholar]
- Samarütel J., Baumrucker C. R., Gross J. J., Dechow C. D., Bruckmaier R. M.. 2016. Quarter variation and correlations of colostrum albumin, immunoglobulin G1 and G2 in dairy cows. J. Dairy Res. 83:209–218. doi: 10.1017/S0022029916000091 [DOI] [PubMed] [Google Scholar]
- Tyler J. W., Hancock D. D., Thorne J. G., Gay C. C., Gay J. M.. 1999. Partitioning the mortality risk associated with inadequate passive transfer of colostral immunoglobulins in dairy calves. J. Vet. Intern. Med. 13:335–337. doi: 10.1111/j.1939-1676.1999.tb02191.x [DOI] [PubMed] [Google Scholar]
- Wall S. K., Gross J. J., Kessler E. C., Villez K., Bruckmaier R. M.. 2015. Blood-derived proteins in milk at start of lactation: Indicators of active or passive transfer. J. Dairy Sci. 98:7748–7756. doi: 10.3168/jds.2015-9440 [DOI] [PubMed] [Google Scholar]