Abstract
To determine the effects of poor maternal nutrition on offspring body and organ growth during gestation, pregnant Western White-faced ewes (n = 82) were randomly assigned into a 3 × 4 factorial treatment structure at d 30.2 ± 0.2 of gestation (n = 5 to 7 ewes per treatment). Ewes were individually fed 100% (control), 60% (restricted) or 140% (over) of NRC requirements for TDN. Ewes were euthanized at d 45, 90 or 135 of gestation or underwent parturition (birth) and tissues were collected from the offspring (n = 10 to 15 offspring per treatment). Offspring from control, restricted and overfed ewes are referred to as CON, RES and OVER, respectively. Ewe data were analyzed as a completely randomized design and offspring data were analyzed as a split-plot design using PROC MIXED. Ewe BW did not differ at d 30 (P ≥ 0.43), however restricted ewes weighed less than overfed and overfed were heavier than controls at d 45, and restricted weighed less and overfed were heavier than controls at d 90 and 135 and birth (P ≤ 0.05). Ewe BCS was similar at d 30, 45 and 90 (P ≤ 0.07), however restricted ewes scored lower than control at d 135 and birth (P ≤ 0.05) and over ewes scored higher than control at d 135 (P ≤ 0.05) but not at birth (P = 0.06). A maternal diet by day of gestation interaction indicated that at birth the body weight (BW) of RES offspring was less than CON and OVER (P ≤ 0.04) and heart girth of RES was smaller than CON and OVER (P ≤ 0.004). There was no interaction of maternal diet and day of gestation on crown-rump, fetal, or nose occipital length, or orbit or umbilical diam. (P ≥ 0.31). A main effect of maternal diet indicated that the RES crown-rump length was shorter than CON and OVER (P ≤ 0.05). An interaction was observed for liver, kidney and renal fat (P ≤ 0.02). At d 45 the liver of RES offspring was larger than CON and OVER (P ≤ 0.002), but no differences observed at d 90, 135 or birth (P ≥ 0.07). At d 45, the kidneys of OVER offspring were larger than CON and RES (P ≤ 0.04), but no differences observed at d 90, 135 or birth (P ≥ 0.60). At d 135, OVER had more perirenal fat than CON and RES (P ≤ 0.03), and at birth RES had more perirenal fat than CON and OVER (P ≤ 0.04). There was no interaction observed for offspring heart weight, length or width, kidney length, adrenal gland weight, loin eye area or rib width (P ≥ 0.09). In conclusion, poor maternal nutrition differentially alters offspring body size and organ growth depending on the stage of gestation.
Keywords: fetus, gestation, maternal nutrition, organs, sheep
INTRODUCTION
Variations in feed and forage quality and availability can result in periods of suboptimal nutrition for livestock species. This is problematic as poor maternal nutrition during gestation has immediate and long-lasting consequences on production efficiency and health of offspring, including reductions in birth weight, pre-weaning survival, postnatal growth rate, feed utilization, carcass quality, and lifespan (Godfrey and Barker, 2001; Wu et al., 2006; Caton and Hess, 2010; Ford and Long, 2011; Du et al., 2013; Reed et al., 2014). Maternal programming is defined as alterations to the intrauterine environment that affect the growth and development of the fetus, resulting in changes in offspring growth, metabolism and organogenesis (Wu et al., 2006). Organogenesis primarily occurs during gestation, making it especially vulnerable to the effects poor maternal nutrition. As a result, multiple organ systems can potentially be affected; thus, predisposing offspring to metabolic and endocrine disorders (Hyatt et al., 2008; Benz and Amann, 2010; Tarry-Adkins et al., 2013).
We previously demonstrated that both restricted- or over-feeding during gestation can alter lamb growth rates, muscle and adipose composition, and organ size at early postnatal time points (Godfrey and Barker, 2001; Hoffman et al., 2014; Reed et al., 2014; Raja et al., 2016; Hoffman et al., 2016a). Although others have evaluated the effects of maternal diet at specific time points of gestation, most were targeted at 1 to 2 time points and 1 dietary treatment (Godfrey and Barker, 2001; Vonnahme et al., 2003; Wu et al., 2006). Therefore, to better understand when poor maternal nutrition affects growth and organ development during gestation, our objective was to develop a model that would evaluate the effects of poor maternal nutrition on fetal and organ growth at 3 gestational and 1 early postnatal time point, with the ability to compare control, restricted, and over feeding in 1 experiment. We hypothesized that maternal restricted- and over-feeding during gestation would negatively impact offspring growth and organ development at specific stages of gestation and at birth.
MATERIALS AND METHODS
All animal procedures were reviewed and approved by the University of Connecticut Institutional Animal Care and Use Committee (A13-059).
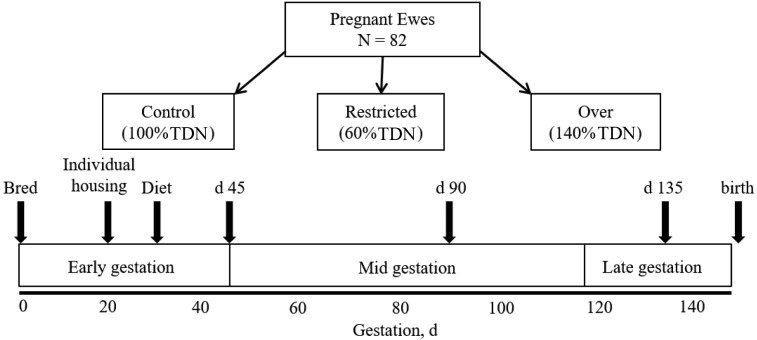
Multiparous Western White-faced ewes (n = 82), 3 yr or older were estrus synchronized and bred as previously described (Jones et al., 2016). Briefly, a controlled intravaginal drug release device (CIDR; Easi-Breed CIDR Sheep Insert, v, Inc., Parsippany, NJ) was inserted intravaginally into each ewe, removed after 12 d and then ewes received a single injection of PGF2a i.m. [Lutalyse, 5 mg/mL; Zoetis, Inc.; (Knights et al., 2001)]. Ewes were group housed with 1 of 4 related Dorset rams, obtained from a closed flock, for breeding. Day 0 of pregnancy was considered the d that the ewe received a rump mark with no subsequent remarking. Twenty d after mating, ewes were moved to individual pens (3 × 1 m; with visual but not physical contact with other ewes) and each ewe was fed individually for the duration of the experiment. At d 20, over a 7 d period, ewes were transitioned onto a complete pelleted feed at 100% NRC. Eighty-two ewes were confirmed pregnant by trans-abdominal ultrasound on d 28.5 ± 0.4 (Jones et al., 2016) and were randomly assigned into a 3 × 4 factorial arrangement of treatment structure at d 30 of gestation with main effects of diet (3) and time point of gestation (4; n = 5 to 7 ewes per treatment combination; Fig. 1).
Figure 1.
Experimental design. To evaluate the effects of restricted- or over-feeding on offspring development during gestation, pregnant ewes (n = 82) were housed in individual pens on d 20 of gestation (Individual housing) and randomly assigned into a 3 × 4 factorial arrangement of treatments which began at d 30 of gestation (Diet). The treatment structure included 3 dietary levels (Control: 100% TDN; Restricted: 60% TDN; Over: 140% TDN) and 4 time points during gestation (n = 5 to 7 ewes per treatment combination). At d 45, 90, or 135 of gestation, ewes were euthanized and the fetus(es) were removed for necropsy and tissue analysis. A fourth group of ewes was allowed to undergo parturition (birth), after which lambs were necropsied within 24 h of parturition.
At d 30.2 ± 0.2 of gestation, pregnant ewes were fed either a control (100%; n = 27), restricted (60%; n = 28) or over (140%; n = 27) diet based on the NRC requirement for TDN (National Research Council, 1985). Treatment diets (Central Connecticut Farmer's COOP, Manchester, CT; Table 1) of 60, 100, and 140% NRC were achieved by offering different quantities of the complete pelleted feed based on ewe BW as previously described (Hoffman et al., 2014; Reed et al., 2014; Hoffman et al., 2016a; 2016b). Nutrient analyses were completed by Dairy One, Inc. (Ithaca, NY; Table 1) for each new grain delivery (n = 3). Fresh water and blocks containing sodium chloride only were available to ewes ad libitum. Ewes were weighed and BCS (Russel, 1984) was independently evaluated by 2 trained observers at the beginning of dietary treatment, and weekly thereafter. Ewes remained on their diets until they were euthanized at 1 of the 3 gestational time points or until parturition.
Table 1.
Formulation and chemical composition of ewe diet (as-fed basis)
| Item | Diet composition |
|---|---|
| Ingredient1, % | |
| Soybean hulls | 42.86 |
| Alfalfa meal, 17% | 20.00 |
| Ground beet pulp | 16.67 |
| Corn meal, fine | 16.20 |
| Molasses | 3.00 |
| Monoamonium phosphate | 0.65 |
| Sheep premix | 0.42 |
| Salt | 0.21 |
| Nutrient Analysis2 | |
| DE, Mcal/kg | 3.28 |
| ME, Mcal/kg | 2.86 |
| Moisture, % | 10.23 |
| DM, % | 89.80 |
| CP, % | 11.57 |
| Adjusted CP, % | 11.57 |
| ADF, % | 28.07 |
| NDF, % | 40.40 |
| TDN, % | 65.67 |
| NEL, Mcal/kg | 1.53 |
| NEM, Mcal/kg | 1.53 |
| NEG, Mcal/kg | 0.98 |
| Ca, % | 0.89 |
| P, % | 0.34 |
| Mg, % | 0.21 |
| K, % | 1.09 |
| Na, % | 0.11 |
| Fe, mg/kg | 381.00 |
| Zn, mg/kg | 64.00 |
| Cu, mg/kg | 7.00 |
| Mn, mg/kg | 56.33 |
| Mo, mg/kg | 1.33 |
| S, % | 0.21 |
Feed was formulated by Central Connecticut Co-Op (Manchester, CT).
Nutrient analyses were performed by Dairy One, Inc. (Ithaca, NY; average analysis of 3 separate grain deliveries for entire experiment).
At d 45, d 90, or d 135 of gestation, ewes (n = 5 to 7 per treatment combination) were weighed and euthanized with an i.v. injection of Beuthanasia-D Special (Merck Animal Health; Summit, NJ) containing 390 mg/mL sodium pentobarbital and 50 mg/mL phenytoin based on BW, followed by exsanguination as previously described (Reed et al., 2014). Subsequently, a hysterectomy was performed to remove the uterus and fetuses for tissue collection. A fourth group of ewes was allowed to undergo parturition (birth; n = 5 to 7 per dietary treatment). Lambs at birth were allowed to nurse for up to 24 h before euthanasia to ensure adequate colostrum intake. If a ewe did not produce colostrum, lambs were offered manufactured colostrum replacer (4 ewes total: 2 twin control litters and 2 twin restricted litters; DuMor Blue Ribbon Multi-Species Colostrum Supplement; Distributed by Tractor Supply Company, Brentwood, TN). Within 24 h of parturition, lambs were weighed and euthanized as described above for tissue collection. Offspring born to control-, restricted-, or over-fed ewes are referred to as CON, RES and OVER, respectively, hereafter. Four ewes did not complete the study due to reasons unrelated to the experiment; thus 78 ewes and their respective offspring were available for fetal and organ measurements. Five offspring were not viable (2 mummified and 3 still born) and were not included for analysis, for a total of 147 offspring from the 78 ewes that were included in the analysis. There were missing data at d 45 for offspring BW (CON Singleton: n = 1 fetus) and fetal morphometric measurements (CON Twin: n = 2 fetuses). Also, data were missing at d 90 for offspring BW (CON Singleton: n = 1 fetus; RES Triplet: n = 1 fetus) and fetal morphometric measurements (CON Singleton: n = 1 fetus).
At d 45, identification of fetuses as male or female by visual inspection was not possible. Therefore, male fetuses were identified as those individuals that expressed the sex-determining region Y (SRY) gene using endpoint PCR. Briefly, tails were collected from d 45 fetuses at necropsy, snap frozen in liquid nitrogen, and stored at -20°C. DNA was isolated as previously described (Truett et al., 2000). Primers for the sex determining region Y (SRY) gene (Forward: 5′-AACGAAGACGAAAGGTGGCT-3′ Reverse: 3′–TCCTCAAAGAATGGGCGCTT-5′) were designed using the ovine reference sequence (AY604733.1), NCBI-Primer blast, and UCSC In-silico PCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Forward: 5′-GGCGTGAACCACGAGAAGTATAA-3′ and Reverse: 3′-CCTCCACGATGCCAAAGTG-5′) was used as the positive control (Buza et al., 2009). Endpoint PCR was performed as previously described (Hoffman et al., 2014). The sex of offspring and litter size distribution for all animals included for these analyses are reported in Table 2.
Table 2.
Number of ewes assigned per diet and day of gestation that completed study1 and the resulting number of offspring acquired for analysis
| Treatment2 | ||||
|---|---|---|---|---|
| Item | Control | Restricted | Over | Sum |
| Pregnant ewes | 25 | 27 | 26 | 78 |
| d 45 | 7 | 7 | 7 | 21 |
| d 90 | 7 | 7 | 6 | 20 |
| d 135 | 6 | 6 | 7 | 19 |
| Birth | 5 | 7 | 6 | 18 |
| Total offspring | 47 | 51 | 49 | 147 |
| d 45 | 12 | 12 | 15 | 39 |
| d 90 | 14 | 14 | 11 | 39 |
| d 135 | 11 | 12 | 11 | 34 |
| Birth | 10 | 13 | 12 | 35 |
| Singleton offspring | 7 | 6 | 6 | 19 |
| d 45 | 3 | 3 | 1 | 7 |
| d 90 | 2 | 2 | 2 | 6 |
| d 135 | 1 | 1 | 2 | 4 |
| Birth | 1 | 0 | 1 | 2 |
| Twin offspring | 23 | 32 | 31 | 86 |
| d 45 | 6 | 6 | 8 | 20 |
| d 90 | 6 | 6 | 6 | 18 |
| d 135 | 7 | 8 | 9 | 24 |
| Birth | 4 | 12 | 8 | 24 |
| Triplet offspring | 17 | 13 | 12 | 42 |
| d 45 | 3 | 3 | 6 | 12 |
| d 90 | 6 | 6 | 3 | 15 |
| d 135 | 3 | 3 | 0 | 6 |
| Birth | 5 | 1 | 3 | 9 |
| Male:Female offspring ratio | 31:16 | 28:23 | 21:28 | 80:67 |
| d 45 | 12:0 | 9:3 | 11:4 | 32:7 |
| d 90 | 8:6 | 8:6 | 3:8 | 19:20 |
| d 135 | 7:4 | 8:4 | 3:8 | 18:16 |
| Birth | 4:6 | 3:10 | 4:8 | 11:24 |
Four ewes did not complete study due to reasons unrelated to the experiment. These ewes and their offspring are not included in this table or any of the data analyses (Control, Birth n = 2; Restricted, d 135 n = 1; Over-fed, Birth n = 1). Beginning at d 30 of gestation, pregnant ewes were randomly assigned to a control-fed (100%), restricted-fed (60%) or over-fed (140%) diet based on NRC TDN requirements. Ewes were euthanized at d 45, 90, or 135 of gestation and a hysterectomy was performed to acquire the fetus(es), or ewes were allowed to undergo parturition in which lambs were necropsied within 24 h of birth.
LSMeans are reported.
Before sample collection, offspring BW was recorded and fetal morphometric measurements were obtained using handheld electronic calipers or a soft tape measure for all lambs. Measurements included crown-rump length (forehead to taildock), fetal length (longitudinally from nose to rump), heart girth (thoracic circumference behind the elbow, at the level of the heart), nose occipital length (longitudinally from the nose to occipital bone), orbit diameter (from the medial to lateral point of the eye) and umbilical diameter (averaged from 3 points of the umbilicus). Umbilical diameter could not be measured from lambs at the birth time point.
To obtain fetal organs, a mid-ventral incision extending from the thoracic cavity to the lower abdominal cavity was made. Organs [liver, heart, kidneys (both combined), adrenal glands (both combined) and perirenal fat (both sides)] were excised from each offspring and weighed. Organ measurements [heart length and width, kidney length, and rib width (averaged for the fifth, sixth and seventh rib)] were obtained using a handheld electronic digital caliper at all time points. Loin eye area (LEA) of the LM was measured between the 12th and 13th rib using a grid specific to pork and lamb (Iowa State University Extension and Outreach, Ames). At d 45 of gestation, the adrenal glands and perirenal fat were not detectable. The LEA was only measurable at d 135 and birth due to the small size at earlier time points.
All data were analyzed using PROC MIXED (SAS Inst. Inc., Cary, NC; version 9.4). Ewe BW and BCS data were analyzed as a completely randomized design, with fixed effects of diet, d of gestation, litter size, and their interactions. Sire had no effect on the fetal variables presented (P ≥ 0.12) and therefore was removed from the model. Offspring data were analyzed as a split-plot design. In the whole plot, ewes were randomly assigned into the 3 × 4 factorial arrangement of treatments of diet (3) and d of gestation (4). In the sub plot, offspring were assigned to a 3 × 4 × 3 × 2 factorial arrangement of treatments, including maternal diet (3), d of gestation (4), litter size (3) and sex (2), respectively and listed as the fixed effects with all interactions. A random statement of ewe by diet by d of gestation was included to define the whole plot. Organ data were expressed as a percent of BW to understand organ growth relative to BW, and all data are presented as least square means ± SE. Significance was considered at P ≤ 0.05. Comparisons of pairwise incidence for the maternal diet by d of gestation were conducted if significant using the LSMEANS statement with PDIFF option. In the absence of a maternal diet by d of gestation interaction, main effects are discussed.
RESULTS
Significant interactions of diet by d of gestation were observed for ewe BW (P < 0.0001) and BCS (P < 0.0001; Table 3). Body weights of pregnant ewes did not differ at the start of feeding the experimental diet (P ≥ 0.43; Table 3). At d 45 of gestation (15 d on experimental diets) over-fed weighed 10% more than restricted-fed ewes (P = 0.001) with control ewes intermediate (P ≥ 0.09). The divergence in over-fed and restricted-fed ewe BW was consistently observed at d 90 and d 135 of gestation, and at birth (P < 0.0001; Table 3). At d 90, d 135, and birth, restricted-fed ewes weighed less than control-fed and over-fed ewes, and over-fed ewes weighed more than control-fed and restricted-fed ewes (P ≤ 0.03; Table 3). No difference in ewe BCS was observed at d 30, d 45, or d 90 of gestation between experimental diets (P ≥ 0.07; Table 3). At d 135 of gestation, BCS was greater in over-fed than restricted-fed and control-fed ewes, whereas the BCS of restricted-fed ewes was less compared with control-fed and over-fed ewes (P ≤ 0.05, Table 3). At parturition, the over-fed and control-fed ewes had similar BCS (P = 0.06) but BCS of restricted-fed ewes was less than control-fed and over-fed ewes (P ≤ 0.002; Table 3). Maternal diet had no effect on the length of gestation for ewes that gave birth (P = 0.26). Together, these data demonstrate the effectiveness of experimental diets fed to the ewes during gestation.
Table 3.
Effect of diet and d of gestation on ewe BW and BCS1
| Treatment3 | P-value | |||||
|---|---|---|---|---|---|---|
| Item | n2 | Control | Restricted | Over | SEM | Diet by d of gestation |
| BW, kg | < 0.0001 | |||||
| d 30 | 78 | 83.2ace | 81.7abe | 83.6ace | 1.9 | |
| d 45 | 78 | 82.4abce | 78.5be | 86.3ac | 1.9 | |
| d 90 | 57 | 87.4c | 78.7e | 95.7dg | 2.2 | |
| d 135 | 37 | 97.9d | 83.2ce | 109.6f | 2.8 | |
| Birth | 18 | 99.0d | 87.2ceg | 110.1f | 4.0 | |
| BCS4 | < 0.0001 | |||||
| d 30 | 78 | 3.08ad | 3.04ab | 3.06acd | 0.026 | |
| d 45 | 78 | 3.02ab | 2.98b | 3.03acd | 0.024 | |
| d 90 | 57 | 3.00ab | 2.99bc | 3.06acd | 0.028 | |
| d 135 | 37 | 3.03ab | 2.87e | 3.11d | 0.034 | |
| Birth | 18 | 3.07abd | 2.82e | 3.16d | 0.046 | |
Denotes Ismean differences for the diet by d of gestation interaction (P ≤ 0.05), within each variable.
Beginning at d 30 of gestation, 82 pregnant ewes were randomly assigned to a control-fed (100%), restricted-fed (60%) or over-fed (140%) diet based on NRC TDN requirements during gestation. Four ewes did not complete the experiment due to reasons unrelated to the experiment. Ewes were euthanized at d 45, 90, or 135 of gestation, or allowed to undergo parturition (n = 5 to 7 ewes per diet per d of gestation).
Number of live ewes that BW and BCS were recorded at each d of gestation. Ewes were weighed immediately before euthanasia or within 7 d before parturition.
LSMeans are reported.
Ewe BCS was individually recorded by 2 observers using a scale of 1 to 5 (Russel, 1984).
Significant 3-way interactions of litter size by maternal diet by d of gestation were observed for offspring BW (P = 0.001) and perirenal fat expressed as a percent of BW (P = 0.02; Supplementary Table 1). No 3-way interactions of litter size by maternal diet by d of gestation were observed for any other fetal morphometric measurements or weights of organs expressed as a percent of BW (P ≥ 0.09). Thus, in the interest of how maternal diet affects the offspring at specific stages of gestation, only the main effects and interaction of maternal diet and d of gestation are reported herein.
A significant interaction of maternal diet by d of gestation was observed for offspring BW (P = 0.03; Table 4) and heart girth circumference (P = 0.009; Table 4). No differences between maternal diets were observed for fetal BW at d 45, 90, or 135 of gestation (P ≥ 0.24). However, at birth, RES offspring weighed 18.4% less than CON and 13.1% less than OVER (Table 4; P ≤ 0.04). Similarly, at birth, the heart girth of RES offspring was 9.2% smaller than CON and 8.0% smaller than OVER (P ≤ 0.004), with no differences (P ≥ 0.18) in fetal heart girth observed between treatments at d 45, 90, or 135 of gestation. An interaction of maternal diet by d of gestation was not observed for offspring crown-rump length, fetal length, nose-occipital length, orbit diameter, or umbilical diameter (P ≥ 0.31; Table 4). A main effect of maternal diet was observed for the crown-rump length with RES offspring measuring 3.4% shorter than CON and 2.9% shorter than OVER (360.3 ± 28.3, 348.6 ± 25.3, 358.6 ± 28.9 g; CON, RES, OVER, respectively; P = 0.05) regardless of stage of gestation, but not for other variables. A main effect of gestation was observed for all fetal morphometric variables in that the crown-rump, fetal, and nose-occipital length of offspring were longer, the heart girth of offspring was larger, and the orbit and umbilicus of offspring were wider as gestation advanced (P < 0.0001; Table 4).
Table 4.
Effect of maternal diet and d of gestation on fetal size variables
| Treatment1,2 | P-value | ||||
|---|---|---|---|---|---|
| Fetal variable | CON | RES | OVER | SEM | Maternal diet by d of gestation |
| BW, g | 0.0310 | ||||
| d 45 | 11.1a | 10.3a | 9.2a | 0.55 | |
| d 90 | 604.8b | 595.8b | 634.7b | 23.36 | |
| d 135 | 4,882.9c | 4,531.2c | 4,633.9c | 213.52 | |
| Birth | 4,878.7c | 3,982.6d | 4,583.7c | 234.94 | |
| Crown-rump length, mm | 0.3142 | ||||
| d 45 | 79.4 | 78.8 | 75.3 | 5.20 | |
| d 90 | 287.1 | 286.2 | 289.1 | 11.56 | |
| d 135 | 527.2 | 509.5 | 526.7 | 31.25 | |
| Birth | 549.6 | 519.9 | 542.2 | 28.11 | |
| Fetal length, mm | 0.6587 | ||||
| d 45 | 60.6 | 62.0 | 57.2 | 4.86 | |
| d 90 | 254.5 | 254.7 | 263.9 | 16.03 | |
| d 135 | 478.8 | 455.2 | 474.1 | 22.07 | |
| Birth | 502.0 | 483.9 | 490.9 | 28.03 | |
| Heart girth, mm | 0.0089 | ||||
| d 45 | nd3 | nd | nd | nd | |
| d 90 | 176.2a | 172.1a | 176.8a | 9.63 | |
| d 135 | 334.1bc | 320.1b | 332.7bc | 23.00 | |
| Birth | 381.9d | 346.6c | 376.7d | 21.50 | |
| Nose occipital length, mm | 0.9852 | ||||
| d 45 | 21.6 | 21.9 | 20.8 | 1.91 | |
| d 90 | 77.4 | 79.2 | 76.4 | 4.90 | |
| d 135 | 125.0 | 123.1 | 124.4 | 5.98 | |
| Birth | 131.8 | 124.2 | 129.8 | 9.30 | |
| Orbit diam., mm | 0.3506 | ||||
| d 45 | 5.1 | 5.7 | 4.0 | 0.76 | |
| d 90 | 17.4 | 18.4 | 21.7 | 3.50 | |
| d 135 | 23.9 | 26.1 | 28.5 | 3.53 | |
| Birth | 29.8 | 27.3 | 29.4 | 2.61 | |
| Umbilical diam., mm | 0.3499 | ||||
| d 45 | 5.7 | 5.7 | 4.5 | 1.00 | |
| d 90 | 14.7 | 14.6 | 15.7 | 2.59 | |
| d 135 | 16.5 | 18.8 | 15.9 | 4.07 | |
| Birth | nd | nd | nd | nd | |
Denotes Ismean differences for the maternal diet by d of gestation interaction (P ≤ 0.05) for each variable.
Offspring from ewes fed a control (100% NRC), restricted (60% NRC), or over-fed (140% NRC) diets are referred to as CON, RES and OVER, respectively. Ewe diet was based on the NRC TDN requirement for pregnant ewes bearing twins, and began at d 30 of gestation. At d 45, 90, or 135 of gestation, ewes were euthanized and a hysterectomy was performed to acquire the fetus(es). Lambs were collected within 24 h of birth from ewes allowed to undergo parturition (n = 10 to 15 fetuses or lambs from 5 to 7 ewes per diet per d of gestation).
LSMeans are reported.
nd = not detectable.
Due to the differences observed in offspring BW, all organs were expressed as a percent of BW (actual organ weight data are provided in Supplementary Table 2). When expressed as percent of BW, an interaction of maternal diet by d of gestation was observed for weight of offspring kidneys (P = 0.02), liver (P = 0.01), and perirenal fat (P = 0.002; Table 5). At d 45 of gestation, the kidneys of OVER offspring were 35.6% larger than CON and 26.0% larger than RES (P < 0.04). However, at the d 90, d 135, and birth time points no differences in fetal kidney weights were observed between maternal diets (P ≥ 0.60; Table 5). At d 45 of gestation, the liver of RES offspring was 13.0% larger than CON and 15.6% larger than OVER offspring (P ≤ 0.002). However, these differences were not maintained in the liver between CON, RES and OVER at d 90, d 135 or birth time points (P ≥ 0.07). Perirenal fat was not detectable at d 45 of gestation, and no differences in perirenal fat were observed in offspring at d 90 of gestation (P ≥ 0.51). However, at d 135 of gestation, OVER offspring had 26.3% more perirenal fat than CON and RES lambs (P < 0.03). At birth, RES offspring had 26.4% more perirenal fat than CON and 40% more perirenal fat than OVER lambs (P < 0.04). No interactions of maternal diet by d of gestation were observed for weight of the adrenal glands or heart as a percent BW, or heart length, heart width, kidney length, rib width, or loin eye area (P ≥ 0.23; Table 5). A main effect of maternal diet was observed for heart length, such that the hearts of RES and OVER offspring were 8.1% and 11.1% shorter than CON, respectively, regardless of the stage of gestation (55.9 ± 3.6, 51.7 ± 2.7, 50.3 ± 2.8, mm; CON, RES, OVER, respectively; P ≤ 0.05). A main effect of gestation was observed for all organ variables (P < 0.0001), such that organ weight as a percent of BW decreased (Table 4) but average organ size increased as gestation advanced (P < 0.0001; Supplementary Table 2). The width of the heart and ribs of offspring, and length of the heart and kidneys of offspring increased as gestation advanced (P < 0.0001). The loin eye area was measurable in offspring only at d 135 and birth, and no main effect of gestation, maternal diet, or interaction was observed (P ≥ 0.32; Table 5).
Table 5.
Effects of maternal diet and d of gestation on fetal organ variables
| Treatment1,2 | P-value | ||||
|---|---|---|---|---|---|
| Fetal variable3 | CON | RES | OVER | SEM | Maternal diet by d of gestation |
| Adrenal gland wt,4 % BW | 0.0991 | ||||
| d 45 | nd5 | nd | nd | nd | |
| d 90 | 0.028 | 0.030 | 0.031 | 0.0004 | |
| d 135 | 0.012 | 0.010 | 0.011 | 0.0009 | |
| Birth | 0.020 | 0.024 | 0.021 | 0.0017 | |
| Heart wt, % BW | 0.2309 | ||||
| d 45 | 1.06 | 1.22 | 1.31 | 0.07 | |
| d 90 | 0.85 | 0.79 | 0.83 | 0.02 | |
| d 135 | 0.68 | 0.50 | 0.66 | 0.02 | |
| Birth | 0.77 | 0.87 | 0.82 | 0.02 | |
| Heart length, mm | 0.5226 | ||||
| d 45 | nd | nd | nd | nd | |
| d 90 | 31.6 | 30.4 | 30.9 | 3.20 | |
| d 135 | 68.3 | 56.1 | 60.2 | 11.03 | |
| Birth | 67.7 | 64.2 | 63.9 | 6.24 | |
| Heart width, mm | 0.9839 | ||||
| d 45 | 6.3 | 5.8 | 6.2 | 0.60 | |
| d 90 | 23.2 | 22.5 | 22.2 | 2.77 | |
| d 135 | 49.9 | 43.8 | 42.9 | 4.79 | |
| Birth | 49.6 | 47.9 | 45.9 | 4.03 | |
| Kidney wt,6 % BW | 0.0213 | ||||
| d 45 | 0.67ad | 0.77ace | 1.04b | 0.07 | |
| d 90 | 0.96bc | 0.97bc | 0.99b | 0.02 | |
| d 135 | 0.54ad | 0.47d | 0.59de | 0.02 | |
| Birth | 0.59d | 0.57de | 0.55de | 0.02 | |
| Kidney length, mm | 0.9667 | ||||
| d 45 | nd | nd | nd | nd | |
| d 90 | 22.3 | 22.0 | 23.4 | 1.45 | |
| d 135 | 43.2 | 38.6 | 39.7 | 6.35 | |
| Birth | 40.1 | 37.7 | 39.0 | 3.63 | |
| Loin eye area,7 mm2 | 0.9813 | ||||
| d 45 | nd | nd | nd | nd | |
| d 90 | nd | nd | nd | nd | |
| d 135 | 266.5 | 282.8 | 274.8 | 24.6 | |
| Birth | 316.7 | 254.8 | 280.7 | 25.2 | |
| Liver wt, % BW | 0.0114 | ||||
| d 45 | 6.7a | 7.7b | 6.5ad | 0.21 | |
| d 90 | 5.5c | 5.4c | 5.8cd | 0.09 | |
| d 135 | 2.8e | 2.1e | 2.8 e | 0.12 | |
| Birth | 2.2e | 2.3e | 2.3 e | 0.06 | |
| Perirenal fat wt,8 % BW | 0.0017 | ||||
| d 45 | nd | nd | nd | nd | |
| d 90 | 0.44ab | 0.40a | 0.44ab | 0.02 | |
| d 135 | 0.42ac | 0.42ac | 0.57b | 0.02 | |
| Birth | 0.39a | 0.53bc | 0.35a | 0.03 | |
| Rib width, mm | 0.4387 | ||||
| d 45 | 0.88 | 0.83 | 1.00 | 0.17 | |
| d 90 | 2.30 | 2.38 | 2.89 | 0.70 | |
| d 135 | 4.51 | 4.18 | 4.33 | 0.69 | |
| Birth | 4.54 | 4.56 | 4.37 | 0.75 | |
Denotes Ismean differences for the diet by d of gestation interaction (P ≤ 0.05), within a variable.
Offspring from ewes fed a control (100% NRC), restricted (60% NRC), or over-fed (140% NRC) diet are referred to as CON, RES and OVER, respectively. Ewe diet was based on the NRC TDN requirement for pregnant ewes bearing twins and began at d 30 of gestation. At d 45, 90, or 135 of gestation, ewes were euthanized and a hysterectomy was performed to acquire the fetus(es). Lambs were collected within 24 h of birth from ewes allowed to undergo parturition (n = 10 to 15 fetuses or lambs from 5 to 7 ewes per diet and d of gestation combination).
LSMeans are reported.
Organ weights are expressed as a percent of offspring BW.
Adrenal gland weight is reported for sum of the pair of adrenal glands.
nd = not detectable.
Kidney weight is reported for the sum of the pair of kidneys.
Loin eye area was estimated using a plastic grid specific to pork and lamb (Iowa State University Extension and Outreach).
Perirenal fat weight is reported for the combined weight of perirenal fat collected from around both kidneys.
DISCUSSION
To maximize production efficiency, animals need to grow quickly and produce a carcass that yields more muscle than fat. However, the growth and development of the offspring can be negatively influenced by environmental stressors to which the dam is exposed during gestation, such as poor maternal nutrition. In livestock species, both restricted- and over-feeding during gestation results in reduced growth rates, reduced muscle fiber size, and increased adiposity of the offspring (Redmer et al., 2004; Wu et al., 2006; Du et al., 2013). Although both under- and over-feeding often lead to similar phenotypic changes in offspring, many studies only focus on 1 model of poor maternal nutrition such as under- (Vonnahme et al., 2003; Daniel et al., 2007; Ford et al., 2007; Ge et al., 2013) or over-feeding (Zhu et al., 2008; Tong et al., 2009; Caton et al., 2009a; Long et al., 2010; Yan et al., 2011). We have developed a unique model that includes both restricted- and over-feeding of the ewe beginning at early gestation (Hoffman et al., 2014; Reed et al., 2014; Jones et al., 2016; Raja et al., 2016; Hoffman et al., 2016a; 2016b). This allows for direct comparisons between offspring from control-, restricted- and over-fed mothers. These comparisons are important because as we previously reported, although negative effects on muscle and metabolism are similar, mechanisms contributing to these phenotypes are different in offspring from restricted and over-fed mothers (Hoffman et al., 2016b). Proper organ and tissue growth during fetal development is essential for optimal health and growth during postnatal life. In sheep, fetal organogenesis is largely complete during early gestation, yet only 10% of fetal growth has occurred by d 90 of gestation (Vonnahme et al., 2003; Redmer et al., 2004; Caton and Hess, 2010). Any delay or altered growth during gestation can have negative effects on production. The current model includes key time points during early (d 45), mid (d 90), and late (d 135) gestation and immediately following parturition, allowing us to target specific stages of development. By developing a model of both maternal under- and over-feeding and 4 developmental time points for offspring analysis in 1 study, we have the ability to make treatment by time comparisons to further understand the impact of maternal nutrition on offspring growth and development over time.
The BW and BCS of control-fed ewes was maintained through mid-gestation and increased during late gestation. In the restricted-fed ewes, this BW gain was limited throughout gestation and concurrently demonstrated gradual depletion of nutrient reserves. Alternately, BW gain was more rapid and exaggerated in ewes fed 140% of the control diet, who also exhibited greater adiposity, as demonstrated by greater BCS as gestation advanced compared with control-fed ewes. Such depleted or excessive maternal nutrient reserves can alter nutrient availability to the developing fetus (Wu et al., 2006) and subsequently compromise fetal growth (McGovern et al., 2015). Furthermore, maternal nutrition can affect BW gain and BCS during gestation and this, in turn, can proportionately affect fetal and organ development (Costello et al., 2013).
In ruminants, the majority of fetal growth occurs during the last third of gestation while organogenesis occurs during the first third of gestation (Redmer et al., 2004; Vonnahme, 2007; Caton et al., 2009b). Therefore, the effects of maternal nutrition on fetal size and organ development may be time dependent. In the current study, differences in offspring body size were observed at birth whereas differences in fetal organ mass were primarily observed at d 45 and 90 of gestation. The lack of BW difference observed during early- and mid-gestation is consistent with previous studies in which reduced offspring BW in offspring of both restricted- (Wallace et al., 2015; Hoffman et al., 2016a) and over-fed (Caton et al., 2009b; Hoffman et al., 2014) ewes were observed at d 130 of gestation, but not at d 50 or 90. It should be noted that offspring BW in response to maternal diet is variable with many reporting no effect on BW (Long et al., 2009; Peel et al., 2012; Kleemann et al., 2015), but significant changes in body composition, growth or metabolism.
Although BW differences were not observed until birth, changes in body composition occur earlier, which can affect overall performance and health of offspring later in life. In support of the thrifty phenotype hypothesis (Godfrey and Barker, 2001), increased perirenal fat was observed in both RES and OVER offspring by d 135 of gestation. The increase in perirenal fat may be indicative of offspring preparing for a suboptimal postnatal environment (Godfrey and Barker, 2001). In particular, the RES offspring are programmed to survive on minimal nutrients at birth. However, in the OVER, this may be due to excess nutrients during gestation and altered glucose and/or insulin metabolism. Perirenal fat during early prenatal life is vital for thermogenesis and therefore has a key role in offspring survival. Therefore, it is likely that accretion of perirenal fat is particularly sensitive to maternal nutrient intake before or during mid-gestation, with the resulting phenotype observed in late-gestation or postnatally. In support of this, when ewes were under-fed between d 28 and d 78 of gestation, lambs reared until 250 d of age exhibited increased BW gain, adiposity, and fat to lean ratio (Ford et al., 2007) indicating that maternal under-feeding from early to mid-gestation contributed to postnatal adiposity. Similarly, maternal obesity can lead to increased fat accumulation in adult offspring (Long et al., 2015). Increased adiposity can negatively affect the metabolic health of the animal and compromise carcass quality later in life (Ford et al., 2007; Du et al., 2013; 2015).
After only 15 d of dietary intervention, both restricted- and over-feeding of ewes altered the growth patterns of the fetal liver and kidney, respectively during the first third of gestation. Ovine hepatogenesis occurs as early as d 30 of gestation, and typically the liver occupies less space in the abdomen with advancing gestation (Douart et al., 2015). Although the liver size increased for all treatment groups over time and were similar during late gestation, the liver of RES offspring was larger at d 45. Similarly, Meyer et al. reported increased liver size, when adjusted for BW, in offspring of restricted-fed mothers (Meyer et al., 2013). Altered development of the liver as a result of poor maternal nutrition can result in hepatic triglyceride accumulation, inflammation and insulin resistance leading to increased visceral adiposity (Kabir et al., 2005; Segovia et al., 2015). Therefore, early changes in liver development may be linked to the increased perirenal fat observed at birth, indicative that maternal nutrition affects multiple organ systems in an inter-dependent manner. Further studies are needed in the sheep model to determine if the changes in liver development are linked to changes in metabolism and/or postnatal growth of the offspring.
Maternal over-feeding caused early acceleration of kidney growth in offspring at d 45 of gestation. This is consistent with Jackson et al. (2012), who reported that over-feeding during gestation in rats altered development and functions of kidney in offspring exposed to over- nutrition during gestation (Jackson et al., 2012). In addition, in humans and sheep exposure of the fetus to suboptimal maternal diet during gestation has been linked to poor postnatal development and pathological conditions of kidneys (Moritz and Wintour, 1999; Luyckx and Brenner, 2015). For example, decreased protein nutrition in rats reduces the number of nephrons in kidneys and affects renin-angiotensin system, which may predispose offspring to adult diseases, such as hypertension (Woods et al., 2001). The absence of an effect of maternal diet observed after d 45 of gestation is likely due to nutrient partitioning toward the liver and kidney, because ruminants rely on the liver for gluconeogenesis and regulation of the somatotropic axis, and the kidneys for phosphate and calcium balance (Hyatt et al., 2007; 2008; Benz and Amann, 2010; Douart et al., 2015; Luyckx and Brenner, 2015).
Conclusion
There is a need to increase the efficiency of production to meet increasing global demand for food, including protein sources, while developing and maintaining a sustainable production system. Nutrient availability to the dam has direct effects on fetal development and permanent effects on offspring health and physiology later in life. The effects of poor maternal nutrition can also impact meat quality and quantity. By developing a model to test the effects of poor maternal nutrition, including both restricted- and over-feeding models, during gestation on offspring growth and health, it will be possible to identify methods to manage the negative outcomes and improve production efficiency. This is important based on recent findings that although changes in muscle were similar in RES and OVER offspring, changes in gene expression were different, suggesting the involvement of different mechanisms (Hoffman et al., 2016b). The current study has developed a model to evaluate key stages of fetal development in poorly fed ewes that identified that poor maternal diet can have negative effects within 2 wk of dietary changes and as early as d 45 of gestation with effects persisting until birth. In conclusion, we determined that both maternal restricted- and over-feeding during gestation differentially alter organogenesis of the liver and kidneys during early gestation, and body size and composition during late gestation and at parturition. Further investigation into the tissue structure and molecular regulation of each organ at each stage of gestation is necessary to identify mechanisms by which poor maternal nutrition predisposes postnatal organ dysfunction, even in the absence of alterations in mass.
Supplementary Material
Footnotes
The authors report no competing conflicts of interest.
The authors thank V. Delaire and the UConn Livestock staff for their technical assistance, and T. Hoagland and the University of Connecticut Animal Science undergraduate students for animal care during the duration of the experiment. The authors thank Zoetis for their kind donation of the controlled intravaginal drug release devices (CIDR; Florham Park, NJ) used for estrus synchronization of ewes.
This material is based on work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2013–01919.
LITERATURE CITED
- Benz K., Amann K.. 2010. Maternal nutrition, low nephron number and arterial hypertension in later life. Biochim. Biophys. Acta. 1802(12):1309–1317. doi: 10.1016/j.bbadis.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Buza T. J., Kumar R., Gresham C. R., Burgess S. C., McCarthy F. M.. 2009. Facilitating functional annotation of chicken microarray data. BMC Bioinformatics. 10Suppl 11: S2–2105-10-S11-S2. doi:10.1186/1471-2105-10-S11-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton J. S., Hess B. A.. 2010. Maternal plane of nutrition: Impacts on fetal outcomes and postnatal offspring responses. Proc. 4th grazing livestock nutrition conference; West. Sect. Am. Soc. Anim. Sci, Champaign, IL. [Google Scholar]
- Caton A. R., Bell E. M., Druschel C. M., Werler M. M., Lin A. E., Browne M. L., McNutt L. A., Romitti P. A., Mitchell A. A., Olney R. S., Correa A. National Birth Defects Prevention Study . 2009a. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension 54(1):63–70doi: 10.1161/HYPERTENSIONAHA.109.129098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton J. S., Reed J. J., Aitken R. P., Milne J. S., Borowicz P. P., Reynolds L. P., Redmer D. A., Wallace J. M.. 2009b. Effects of maternal nutrition and stage of gestation on body weight, visceral organ mass, and indices of jejunal cellularity, proliferation, and vascularity in pregnant ewe lambs. J. Anim. Sci. 87(1):222–235. doi: 10.2527/jas.2008-1043 [DOI] [PubMed] [Google Scholar]
- Costello P. M., Hollis L. J., Cripps R. L., Bearpark N., Patel H. P., Sayer A. A., Cooper C., Hanson M. A., Ozanne S. E., Green L. R.. 2013. Lower maternal body condition during pregnancy affects skeletal muscle structure and glut-4 protein levels but not glucose tolerance in mature adult sheep. Reprod. Sci. 20(10):1144–1155. doi: 10.1177/1933719113477494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel Z. C., Brameld J. M., Craigon J., Scollan N. D., Buttery P. J.. 2007. Effect of maternal dietary restriction during pregnancy on lamb carcass characteristics and muscle fiber composition. J. Anim. Sci. 85(6):1565–1576. doi: 10.2527/jas.2006-743 [DOI] [PubMed] [Google Scholar]
- Douart C., Briand L., Betti E., Bencharif D., Tainturier D.. 2015. Temporal evolution of hepatic anatomy during gestation and growth in the sheep. Anat. Histol. Embryol. 44(1):22–36. doi: 10.1111/ahe.12104 [DOI] [PubMed] [Google Scholar]
- Du M., Huang Y., Das A. K., Yang Q., Duarte M. S., Dodson M. V., Zhu M. J.. 2013. Meat science and muscle biology symposium: Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 91(3):1419–1427. doi: 10.2527/jas.2012-5670 [DOI] [PubMed] [Google Scholar]
- Du M., Wang B., Fu X., Yang Q., Zhu M.. 2015. Fetal programming in meat production. Meat Sci.doi: 10.1016/j.meatsci.2015.04.010 [DOI] [PubMed]
- Ford S. P., Hess B. W., Schwope M. M., Nijland M. J., Gilbert J. S., Vonnahme K. A., Means W. J., Han H., Nathanielsz P. W.. 2007. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J. Anim. Sci. 85(5):1285–1294. doi: 10.2527/jas.2005-624 [DOI] [PubMed] [Google Scholar]
- Ford S. P., Long N. M.. 2011. Evidence for similar changes in offspring phenotype following either maternal undernutrition or overnutrition: Potential impact on fetal epigenetic mechanisms. Reprod. Fertil. Dev. 24(1):105–111. doi: 10.1071/RD11911 [DOI] [PubMed] [Google Scholar]
- Ge W., Hu N., George L. A., Ford S. P., Nathanielsz P. W., Wang X. M., Ren J.. 2013. Maternal nutrient restriction predisposes ventricular remodeling in adult sheep offspring. J. Nutr. Biochem. 24(7):1258–1265. doi: 10.1016/j.jnutbio.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Godfrey K. M., Barker D. J.. 2001. Fetal programming and adult health. Public Health Nutr. 4(2B):611–624. doi: 10.1079/PHN2001145 [DOI] [PubMed] [Google Scholar]
- Hoffman M. L., Peck K. N., Forella M. E., Fox A. R., Govoni K. E., Zinn S. A.. 2016a. The effects of poor maternal nutrition on postnatal growth and development of lambs. J. Anim. Sci. 94(2):789–799. doi: 10.2527/jas.2015-9933 [DOI] [PubMed] [Google Scholar]
- Hoffman M. L., Peck K. N., Wegrzyn J. L., Reed S. A., Zinn S. A., Govoni K. E.. 2016b. Poor maternal nutrition during gestation alters the expression of genes involved in muscle development and metabolism in lambs. J. Anim. Sci. 94(7):3093–3099. doi: 10.2527/jas.2016-0570 [DOI] [PubMed] [Google Scholar]
- Hoffman M. L., Rokosa M. A., Zinn S. A., Hoagland T. A., Govoni K. E.. 2014. Poor maternal nutrition during gestation in sheep reduces circulating concentrations of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in offspring. Domest. Anim. Endocrinol. 49:39–48. doi: 10.1016/j.domaniend.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Hyatt M. A., Budge H., Symonds M. E.. 2008. Early developmental influences on hepatic organogenesis. Organogenesis 4(3):170–175. doi: 10.4161/org.4.3.6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt M. A., Gopalakrishnan G. S., Bispham J., Gentili S., McMillen I. C., Rhind S. M., Rae M. T., Kyle C. E., Brooks A. N., Jones C., Budge H., Walker D., Stephenson T., Symonds M. E.. 2007. Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J. Endocrinol. 192(1):87–97. doi: 10.1677/joe.1.06801 [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Alexander B. T., Roach L., Haggerty D., Marbury D. C., Hutchens Z. M., Flynn E. R., Maric-Bilkan C.. 2012. Exposure to maternal overnutrition and a high-fat diet during early postnatal development increases susceptibility to renal and metabolic injury later in life. Am. J. Physiol. Renal Physiol. 302(6):F774–F783. doi: 10.1152/ajprenal.00491.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. K., Gately R. E., McFadden K. K., Zinn S. A., Govoni K. E., Reed S. A.. 2016. Transabdominal ultrasound for detection of pregnancy, fetal and placental landmarks, and fetal age before day 45 of gestation in the sheep. Theriogenology. 85(5):939–945. doi: 10.1016/j.theriogenology.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Kabir M., Catalano K. J., Ananthnarayan S., Kim S. P., Van Citters G. W., Dea M. K., Bergman R. N.. 2005. Molecular evidence supporting the portal theory: A causative link between visceral adiposity and hepatic insulin resistance. Am. J. Physiol. Endocrinol. Metab. 288(2):E454–61. doi: 10.1152/ajpendo.00203.2004 [DOI] [PubMed] [Google Scholar]
- Kleemann D. O., Kelly J. M., Rudiger S. R., McMillen I. C., Morrison J. L., Zhang S., MacLaughlin S. M., Smith D. H., Grimson R. J., Jaensch K. S., Brien F. D., Plush K. J., Hiendleder S., Walker S. K.. 2015. Effect of periconceptional nutrition on the growth, behaviour and survival of the neonatal lamb. Anim. Reprod. Sci. 160:12–22. doi: 10.1016/j.anireprosci.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Knights M., Hoehn T., Lewis P. E., Inskeep E. K.. 2001. Effectiveness of intravaginal progesterone inserts and FSH for inducing synchronized estrus and increasing lambing rate in anestrous ewes. J. Anim. Sci. 79(5):1120–1131. doi: 10.2527/2001.7951120x [DOI] [PubMed] [Google Scholar]
- Long N. M., George L. A., Uthlaut A. B., Smith D. T., Nijland M. J., Nathanielsz P. W., Ford S. P.. 2010. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J. Anim. Sci. 88(11):3546–3553. doi: 10.2527/jas.2010-3083 [DOI] [PubMed] [Google Scholar]
- Long N. M., Rule D. C., Tuersunjiang N., Nathanielsz P. W., Ford S. P.. 2015. Maternal obesity in sheep increases fatty acid synthesis, upregulates nutrient transporters, and increases adiposity in adult male offspring after a feeding challenge. PLoS One 10(4):e0122152. doi: 10.1371/journal.pone.0122152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long N. M., Vonnahme K. A., Hess B. W., Nathanielsz P. W., Ford S. P.. 2009. Effects of early gestational undernutrition on fetal growth, organ development, and placentomal composition in the bovine. J. Anim. Sci. 87(6):1950–1959. doi: 10.2527/jas.2008-1672 [DOI] [PubMed] [Google Scholar]
- Luyckx V. A., Brenner B. M.. 2015. Birth weight, malnutrition and kidney-associated outcomes–a global concern. Nat. Rev. Nephrol. 11(3):135–149. doi: 10.1038/nrneph.2014.251 [DOI] [PubMed] [Google Scholar]
- McGovern F. M., Campion F. P., Lott S., Boland T. M.. 2015. Altering ewe nutrition in late gestation: I. the impact on pre- and postpartum ewe performance. J. Anim. Sci. 93(10):4860–4872. doi: 10.2527/jas.2015-9019 [DOI] [PubMed] [Google Scholar]
- Meyer A. M., Neville T. L., Reed J. J., Taylor J. B., Reynolds L. P., Redmer D. A., Hammer C. J., Vonnahme K. A., Caton J. S.. 2013. Maternal nutritional plane and selenium supply during gestation impact visceral organ mass and intestinal growth and vascularity of neonatal lamb offspring. J. Anim. Sci. 91(6):2628–2639. doi: 10.2527/jas.2012-5953 [DOI] [PubMed] [Google Scholar]
- Moritz K. M., Wintour E. M.. 1999. Functional development of the meso- and metanephros. Pediatr. Nephrol. 13(2):171–178. doi: 10.1007/s004670050587 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) 1985. Nutrient requirements of sheep. Subcommittee on Sheep Nutrition, ed. Washington, DC, National Academic Press. [Google Scholar]
- Peel R. K., Eckerle G. J., Anthony R. V.. 2012. Effects of overfeeding naturally-mated adolescent ewes on maternal, fetal, and postnatal lamb growth. J. Anim. Sci. 90(11):3698–3708. doi: 10.2527/jas.2012-5140 [DOI] [PubMed] [Google Scholar]
- Raja J. S., Hoffman M. L., Govoni K. E., Zinn S. A., Reed S. A.. 2016. Restricted maternal nutrition alters myogenic regulatory factor expression in satellite cells of ovine offspring. Anim.:1–4. doi: 10.1017/S1751731116000070 [DOI] [PubMed]
- Redmer D. A., Wallace J. M., Reynolds L. P.. 2004. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest. Anim. Endocrinol. 27(3):199–217. doi: 10.1016/j.domaniend.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Reed S. A., Raja J. S., Hoffman M. L., Zinn S. A., Govoni K. E.. 2014. Poor maternal nutrition inhibits muscle development in ovine offspring. J. Anim. Sci. Biotechnol. 5(1):43-1891-5-43 eCollection 2014. doi: 10.1186/2049-1891-5-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel A. 1984. Farm practice: Body condition scoring of sheep. In Pract. 6:91–93. doi: 10.1136/inpract.6.3.91 [DOI] [PubMed] [Google Scholar]
- Segovia S. A., Vickers M. H., Zhang X. D., Gray C., Reynolds C. M.. 2015. Maternal supplementation with conjugated linoleic acid in the setting of diet-induced obesity normalises the inflammatory phenotype in mothers and reverses metabolic dysfunction and impaired insulin sensitivity in offspring. J. Nutr. Biochem. 26(12):1448–1457. doi: 10.1016/j.jnutbio.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Tarry-Adkins J. L., Martin-Gronert M. S., Fernandez-Twinn D. S., Hargreaves I., Alfaradhi M. Z., Land J. M., Aiken C. E., Ozanne S. E.. 2013. Poor maternal nutrition followed by accelerated postnatal growth leads to alterations in DNA damage and repair, oxidative and nitrosative stress, and oxidative defense capacity in rat heart. FASEB J. 27(1):379–390. doi: 10.1096/fj.12-218685 [DOI] [PubMed] [Google Scholar]
- Tong J. F., Yan X., Zhu M. J., Ford S. P., Nathanielsz P. W., Du M.. 2009. Maternal obesity downregulates myogenesis and beta-catenin signaling in fetal skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 296(4):E917–E924. doi: 10.1152/ajpendo.90924.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., Warman M. L.. 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). BioTechniques. 29(1):52, 54. [DOI] [PubMed] [Google Scholar]
- Vonnahme K. A. 2007. Nutrition during gestation and fetal programming. In: the range beef cow symposium, Fort Collins, CO. [Google Scholar]
- Vonnahme K. A., Hess B. W., Hansen T. R., McCormick R. J., Rule D. C., Moss G. E., Murdoch W. J., Nijland M. J., Skinner D. C., Nathanielsz P. W., Ford S. P.. 2003. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol. Reprod. 69(1):133–140. doi: 10.1095/biolreprod.102.012120 [DOI] [PubMed] [Google Scholar]
- Wallace J. M., Milne J. S., Aitken R. P., Redmer D. A., Reynolds L. P., Luther J. S., Horgan G. W., Adam C. L.. 2015. Undernutrition and stage of gestation influence fetal adipose tissue gene expression. J. Mol. Endocrinol. 54(3):263–275. doi: 10.1530/JME-15-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L. L., Ingelfinger J. R., Nyengaard J. R., Rasch R.. 2001. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr. Res. 49(4):460–467. doi: 10.1203/00006450-200104000-00005 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Wallace J. M., Spencer T. E.. 2006. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 84(9):2316–2337. doi: 10.2527/jas.2006-156 [DOI] [PubMed] [Google Scholar]
- Yan X., Huang Y., Zhao J. X., Long N. M., Uthlaut A. B., Zhu M. J., Ford S. P., Nathanielsz P. W., Du M.. 2011. Maternal obesity-impaired insulin signaling in sheep and induced lipid accumulation and fibrosis in skeletal muscle of offspring. Biol. Reprod. 85(1):172–178. doi: 10.1095/biolreprod.110.089649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. J., Han B., Tong J., Ma C., Kimzey J. M., Underwood K. R., Xiao Y., Hess B. W., Ford S. P., Nathanielsz P. W., Du M.. 2008. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J. Physiol. 586(10):2651–2664. doi: 10.1113/jphysiol.2007.149633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.