Abstract
Background
The clinical epidemiology of treated HIV infection in the United States has dramatically changed in the past 25 years. Few sources of longitudinal data exist for people with HIV (PWH) spanning that period. Cohort data enable investigating new exposure and disease associations and monitoring progress along the HIV care continuum.
Methods
We synthesized key published findings and conducted primary data analyses in the HIV Outpatient Study (HOPS), an open cohort of PWH seen at public and private HIV clinics since 1993. We assessed temporal trends in health outcomes (1993–2017) and mortality (1994–2017) for 10 566 HOPS participants.
Results
The HOPS contributed to characterizing new conditions (eg, lipodystrophy), demonstrated reduced mortality with earlier HIV treatment, uncovered associations between select antiretroviral agents and cardiovascular disease, and documented remarkable shifts in morbidity from AIDS opportunistic infections to chronic noncommunicable diseases. The median CD4 cell count of participants increased from 244 cells/mm3 to 640 cells/mm3 from 1993 to 2017. Mortality fell from 121 to 16 per 1000 person-years from 1994 to 2017 (P < .001). In 2010, 83.7% of HOPS participants had a most recent HIV viral load <200 copies/mL, compared with 92.2% in 2017.
Conclusions
Since 1993, the HOPS has been detecting emerging issues and challenges in HIV disease management. HOPS data can also be used for monitoring trends in infectious and chronic diseases, immunologic and viral suppression status, retention in care, and survival, thereby informing progress toward the Ending the HIV Epidemic initiative.
Keywords: antiretroviral therapy, clinical outcomes, HIV epidemiology, viral suppression
The US Centers for Disease Control and Prevention (CDC) estimates that ~1.1 million Americans are living with HIV infection and ~40 000 are newly diagnosed with HIV each year [1]. Given the lack of a preventive vaccine and a virally eradicative HIV cure, the initiative to end the HIV epidemic in the United States [2, 3] will require widespread deployment of well-established effective public health interventions, including HIV testing with timely linkage to HIV care and treatment and high-quality continuous care for people with HIV (PWH). PWH, similar to persons with other chronic diseases, must be able to access consistent comprehensive care, enabling the achievement and maintenance of optimal overall health, including sustained HIV virologic suppression and subsequent avoidance of excess morbidity, mortality, and transmission to others. The public health benefits of both integrated preventative care for PWH [4] and of treatment as prevention among PWH are established but not fully realized across diverse populations in the United States [5].
The HIV Outpatient Study (HOPS) cohort is a rare example of a continuously funded, well-characterized, prospectively followed, sociodemographically diverse cohort of PWH in the United States, with data collection over 25 years and nearly 11 000 patients enrolled through mid-2019. The HOPS was established in 1993 before highly effective combination therapy became available in 1995, a time when US patients experienced profound mortality and morbidity, chiefly due to AIDS-related complications. During the next 25 years, HOPS participants and physicians have both witnessed and contributed to advances and profound changes in the clinical epidemiology of treated HIV infection. The findings generated by clinical HIV research, including the HOPS, have informed the US and international guidelines and recommendations shaping clinical practice today.
The nature of the HOPS’ “real-time” longitudinal data collection has permitted prompt detection of emerging issues and challenges in HIV disease management and has allowed timely investigation of novel associations between risk factors and exposures (eg, new HIV treatments, care management strategies, behavioral risk factors) and long-term outcomes (eg, survival, disease progression, metabolic and other treatment toxicities). The HOPS also enables monitoring of disease trends over time, including components of the HIV care continuum (including antiretroviral therapy [ART] use, retention in care, and virologic suppression) as well as sociodemographic disparities in these outcomes. Whereas interventional clinical trials provide information about the efficacy and safety of HIV treatments, these typically rely on immediate clinical (eg, improvement in symptoms) and surrogate (eg, virologic suppression and immunologic recovery) end points and do not have sufficient sample sizes or the longer-term follow-up needed to ascertain rare adverse events, delayed drug-induced effects, and mortality. The populations included in observational cohorts of PWH in routine HIV care are generally more heterogeneous in terms of comorbidities, demographics, and patterns of HIV care than persons selected to participate in clinical trials [6, 7]. Observational cohorts can provide information about longer-term effectiveness, limitations, and complications of therapy in real-world clinical settings. The data from cohorts like the HOPS also offer a depth of clinical history and sociodemographic information that is not available from routine HIV surveillance in the United States [1].
In this report, we offer a perspective on the contributions of the HOPS to elucidating the epidemiology of treated HIV infection in the United States, contextualized within the broader advances in HIV research and treatment since 1993. We describe dramatic changes in PWHs’ care and health status over a 25-year period with regard to earlier HIV diagnoses, the uptake of effective ART and evolution in types of ART regimens, reduced mortality and morbidity, and improvements in immunologic control and virologic suppression. We also highlight HOPS reports documenting a remarkable shift in morbidity and mortality from AIDS opportunistic infections (OIs) to chronic noncommunicable, often age-related, conditions. We discuss how HOPS research has improved the understanding of clinical HIV epidemiology and informed national HIV treatment guidelines over the years and conclude with future opportunities and cohort research priorities for the era of Ending the HIV Epidemic in the United States.
HOPS METHODOLOGY
The HOPS is an ongoing prospective dynamic observational cohort study of HIV-infected adults receiving care in HIV clinics the United States. Fourteen HOPS clinics (university-based, public, and private) have participated since 1993, enrolling nearly 11 000 participants and >570 000 clinical encounters through mid-2019. Of these, 2346 participants were active as of December 31, 2017. A map of HOPS sites, by their current status, is shown in Figure 1.
Figure 1.
HIV Outpatient Study (HOPS) sites, 1993–2019. Eight currently active sites contributing data: Dupont Circle Physicians Group, Washington, DC (June 1993–); Denver Infectious Disease Consultants (DIDC), Colorado (May 1993–); Northwestern University Medical School, Illinois (September 1994–); State University of New York (SUNY), New York (September 1994–); Temple University School of Medicine; Pennsylvania (March 1997–); University of Illinois at Chicago (UIC), Illinois (December 2000–); APEX Family Medicine, Colorado (August 2012–); St. Joseph’s Hospital Comprehensive Research Institute, Florida (October 2018–). Six currently inactive sites that contributed data through 2017: Infectious Disease Research Institute Inc., Florida (October 1992–December 2017); Fairmont Hospital, San Leandro, California (May 1993–May 2006); Oregon Health Sciences University, Oregon (August 1991–July 1998); Adult Immunology Clinic, Oakland, California (September 1993–June 2005); Southside HealthCare, Inc., Georgia (June 1994–April 1996); National Jewish Health, Colorado (May 1993–June 2015).
Instead of structured fixed interval study visits, the HOPS relies on routine medical records data (previously in paper charts and presently in electronic medical records [EMRs]) generated during the course of routine HIV care. All HOPS data abstractors have backgrounds in nursing or other health care–related fields, are familiar with HIV disease and its manifestations, and have been trained in chart abstraction for the HOPS. Information is abstracted from outpatient records at each visit, entered electronically by trained staff, compiled centrally, and reviewed and edited before being analyzed. Abstracted data include demographic and social characteristics, risk factors for HIV infection, diagnoses (both definitive and presumptive), HIV antiretroviral and other prescribed medications (including dose and duration), all laboratory values (including HIV-related, hematologic, metabolic, liver and lipid panels, urinalysis, and immunology), hospitalizations, and deaths. Hospitalization data are abstracted from electronic medical records and discharge summaries and include diagnoses and dates of admission and discharge when available. If not already accessible in EMRs, requests for outside records of hospitalizations, diagnoses, and laboratory values are placed whenever feasible. Vital status of HOPS patients who have fallen out of care or transferred is routinely investigated by clinic site staff through Social Security Death Index searches, with cause of death information abstracted from multiple sources, including charted information provided by treating physicians, hospital discharge summaries, and death certificates. During 2000–2017, cause of death information was available for 85% of participants who died within 6 months of their last HOPS contact. HOPS data quality assurance measures include supervisory reviews of randomly selected charts to ascertain accuracy and completeness of abstracted data, centralized automated checks of data files to resolve discrepancies in diagnosis start and stop dates and in diagnosis codes vs descriptive text field information, and ongoing focused data queries for abstractors to verify information in the original record.
Since 2007, HOPS participants have been offered annually an optional (nonincentivized) brief telephone Audio-Computer Assisted Self-Interview (ACASI), which was supplemented with Web-based ACASI in 2014, that collects sociodemographic characteristics and risk behavior information. The information collected by ACASI includes age, sex at birth, use of alcohol and illicit drugs, cigarette smoking, adherence to ART, types of sexual activity, condom use, and disclosure of HIV serostatus to partners. Participants are assigned unique 4-digit numbers and asked to complete this confidential survey by dialing a toll-free number or by accessing a website from a private location in the clinic or from home. Annual ACASI offer and acceptance rates are tracked by individual sites, with a minority of patients completing the survey each year and some sites opting out of participation for administrative reasons. Among patients active in the HOPS in 2017, the last year of data featured in this paper, 62% completed the ACASI at least once.
Since its inception, the HOPS protocol has been reviewed and approved annually by the CDC’s and each local site’s institutional review board. The study protocol conforms to the guidelines of the US Department of Health and Human Services (DHHS) for the protection of human subjects in research. The HOPS’ data collection is also subject to Paperwork Reduction Act review and approval.
CHARACTERISTICS OF THE HOPS COHORT OVER TIME
The changing epidemiology of the HOPS cohort during 1993–2017 (Table 1) was characterized by shifts that reflected the changing demographics of PWH in the United States. These include greater representation of women (from 11% to 25%), older persons (represented by persons aged >55 years; from 4% to 40%), persons of black race or Hispanic/Latinx ethnicity (from 19% to 53%), persons with heterosexual risk for HIV infection (from 11% to 33%), and those with public insurance payors (from 14% to 51%). The median time since HIV diagnosis for cohort participants in 1993 vs 2017 increased from 3.9 to 15.8 years (Table 1). Moreover, with the greater ART-afforded lifespan and consequent aging of the cohort, total person-years of observation (and median duration of observation) have increased, reaching 17 171 (3.3 years) through 2000, 48 101 (7.9 years) through 2010, and 67 159 (11.5 years) through 2017 (data not shown).
Table 1.
Cohort Characteristics, the HIV Outpatient Study, 1993–2017 (n = 10 566a)
| Characteristic at Year Endb | 1993 (n = 1122) | 2000 (n = 3188) | 2010 (n = 3326) | 2017 (n = 2346) |
|---|---|---|---|---|
| Sex | ||||
| Male | 996 (88.8) | 2533 (79.5) | 2580 (77.6) | 1751 (74.6) |
| Female | 126 (11.2) | 655 (20.5) | 746 (22.4) | 595 (25.4) |
| Age, y | ||||
| <40 | 725 (64.6) | 1378 (43.2) | 684 (20.6) | 462 (19.7) |
| 40–55 | 352 (31.4) | 1560 (48.9) | 1867 (56.1) | 951 (40.5) |
| >55 | 45 (4.0) | 250 (7.8) | 775 (23.3) | 933 (39.8) |
| Median (IQR) | 36 (31–42) | 41 (36–47) | 48 (41–54) | 52 (43–59) |
| Race/ethnicity | ||||
| Non-Hispanic/Latinx white | 867 (77.3) | 1790 (56.1) | 1724 (51.8) | 994 (42.4) |
| Non-Hispanic/Latinx black | 161 (14.3) | 960 (30.1) | 1053 (31.7) | 911 (38.8) |
| Hispanic/Latinx | 48 (4.3) | 331 (10.4) | 412 (12.4) | 343 (14.6) |
| Other/unknown | 46 (4.1) | 107 (3.4) | 137 (4.1) | 98 (4.2) |
| HIV risk group | ||||
| MSM | 826 (73.6) | 1834 (57.5) | 1935 (58.2) | 1265 (53.9) |
| PWID | 105 (9.4) | 427 (13.4) | 255 (7.7) | 164 (7.0) |
| Heterosexual | 124 (11.1) | 777 (24.4) | 890 (26.8) | 763 (32.5) |
| Other/unknown | 67 (6.0) | 150 (4.7) | 246 (7.4) | 154 (6.6) |
| Payor | ||||
| Private | 434 (38.7) | 1694 (53.1) | 1759 (52.9) | 1018 (43.4) |
| Public | 160 (14.3) | 1285 (40.3) | 1217 (36.6) | 1186 (50.6) |
| Self-pay/other/unknown payor | 528 (47.1) | 209 (6.6) | 350 (10.5) | 142 (6.1) |
| Years since HIV diagnosis, median (IQR) | 3.9 (2.1–6.0) | 7.5 (4.0–11.1) | 13.2 (7.0–18.8) | 15.8 (8.9–23.0) |
| ART treatment history | ||||
| ART-naïve | 125 (11.1) | 130 (4.1) | 123 (3.7) | 26 (1.1) |
| ART-experienced | 903 (80.5) | 3015 (94.6) | 3168 (95.2) | 2315 (98.7) |
| Unknown | 94 (8.4) | 43 (1.3) | 35 (1.1) | 5 (0.2) |
| CD4+ cell count, median (IQR) | 244 (65–451) | 401 (230–620) | 535 (352–746) | 640 (448–884) |
| Log10 HIV viral load, median (IQR)c | N/A | 2.3 (1.4–3.9) | 1.4 (1.4–1.6) | 1.0 (1.0–1.0) |
| HIV viral load <200 copies/mLc | N/A | 1366 (46.3) | 2511 (83.7) | 1997 (92.2) |
| All HIV viral loads <200 copies/mLc | N/A | 876 (29.7) | 2248 (74.9) | 1852 (85.5) |
| Clinical visitd frequency, median (IQR) | 3 (2–5) | 4 (2–6) | 3 (2–4) | 2 (2–3) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; MSM, men who have sex with men; PWID, people who inject drugs; VL, viral load.
aCharacteristics of cohort participants in selected calendar years. Included participants with at least 2 HOPS clinical encounters. Patients in different years are not mutually exclusive populations; thus no statistical comparisons were made.
bReporting No. (column %) unless otherwise specified; characteristics at the end of the year, among participants with a HOPS encounter in the year shown.
cNot all persons had VL documented in the year; denominator: 2000 (n = 2949), 2010 (n = 3000), and 2017 (n = 2165).
dClinical visits included only routine, initial, return to active status, event triggered, or post-hospital follow-up.
CHARACTERISTICS OF NEW ENROLLEES OVER TIME
Over time, an increasing percentage of new HOPS enrollees have been female, non-Hispanic black, younger, and with heterosexual risk for HIV transmission (data not shown). The CD4 cell counts of ARV-naïve persons entering the HOPS have increased with calendar time [8]. Among enrollees starting observation in the HOPS in 2017 (n = 76), the median age (interquartile range [IQR]) was 35 (28–43) years, and 41% were black, 21% were Hispanic/Latinx, and 62% were gay, bisexual or other men who have sex with men (MSM); their median time since HIV diagnosis (IQR) was 1.1 (0.7–4.6) years. Therefore, recent HOPS enrollees represented demographic groups with a preponderance of new HIV diagnoses in the United States [1].
HIV TREATMENT AND ASSOCIATIONS
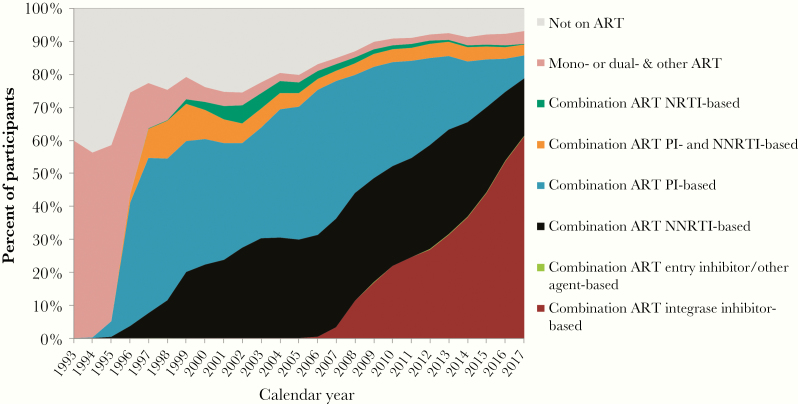
The HOPS captured the period of transition from standard use of mono- and dual-agent ART to 3-drug potent combination ART (Figure 2). The percentage of patients prescribed 3-drug ART at year-end was 5% in 1995 and 64% in 1997 (as compared with 89% in 2017). By the end of 1997, of the 64% of HOPS participants treated with ART, the majority were prescribed protease inhibitors (PIs). During the next decade, non-nucleoside reverse transcriptase inhibitor (NNRTI)–based ART came into widespread use, matching the frequency of “boosted” (pharmaco-enhanced) PI-based ART by 2010. In 2007, the first integrase-strand transfer inhibitor (INSTI) was approved and approximately two-thirds of patients treated in the HOPS were prescribed INSTI-based ART in 2017. In 2017, among 833 persons switching ART regimens, 575 (69%) persons initiated INSTI-containing ART, and among 58 persons newly starting ART, 53 (91%) began INSTI-containing ART. Moreover, as has been shown in the HOPS [9] and documented elsewhere [10], while patients on earlier ART regimens frequently changed therapy due to virologic nonsuppression, patients on more contemporary ART chiefly did so for reasons of convenience, simplification, toxicity avoidance, and minimization of drug–drug interactions.
Figure 2.
HIV treatments at the end of the year, the HIV Outpatient Study, 1993–2017, n = 10 566. Mono-, dual- & other ART: a mono-, dual, or other regimen not considered to be combination ART; combination ART NRTI-based: a combination ART regimen containing at least 3 NRTIs, without PIs or NNRTIs; combination ART PI- and NNRTI-based: a combination ART regimen that includes at least 1 PI and 1 NNRTI; combination ART PI-based: a regimen considered to be combination ART containing either just 2 PIs with no other ARVs or a combination ART regimen containing at least 1 PI and none of the following: NNRTI, integrase or entry inhibitors; combination ART NNRTI-based: a combination ART regimen containing an NNRTI and none of the following: PI, integrase or entry inhibitors; combination ART entry inhibitor/other agent-based: a combination ART regimen containing either an entry inhibitor, fusion inhibitor, or CCR with no PI or NNRTI or a combination ART regimen categorized as “other HAART”; combination ART integrase inhibitor-based: a combination ART regimen that includes an integrase inhibitor. HOPS cohort definition for whether a regimen is considered effective combination ART: (i) any 3 ARVs that include a PI, NNRTI, fusion, entry, or integrase inhibitor; (ii) any 3 NRTIs that include abacavir or tenofovir, with the exception of the following combinations: ABC + TDF + 3TC and DDI + TDF + 3TC; (iii) 2 full-dose PIs; (iv) a boosted PI and 1 of the following (an NNRTI or fusion inhibitor); and (v) an integrase inhibitor and 1 of the following (PI, NNRTI, entry inhibitor, or integrase inhibitor). If 2 of the ARVs are AZT + D4T, they are subtracted from the total ARV count. Abbreviations: ART, antiretroviral therapy; NRTIs, nucleoside reverse transcriptase inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors.
The HOPS was the first cohort to demonstrate that the use of ART with PIs was linked to reductions in mortality and opportunistic disease among PWH [11]. Subsequent HOPS analyses weighed in on the “when to start ART” question [12, 13] and demonstrated that starting ART at higher CD4 cell counts (ie, sooner after HIV diagnosis) was associated with lower all-cause mortality [14]. These results contributed to the shift in DHHS guidelines to extend treatment to persons with CD4 cell counts 200–350 and 350–500 cells/mm3, and eventually regardless of CD4 cell count [10]. The HOPS was also one of the first cohorts to identify the link between more advanced HIV disease (lower CD4 cell count) and greater risks of complications including lipodystrophy and lipoatrophy [15, 16], myocardial infarctions [17], and immune reconstitution inflammatory syndrome [18]. The HOPS also was among the first cohorts to report upon elevated rates of bone fractures in PWH [19] compared with rates in the general US population and the association between low bone mineral density and fracture risk among PWH [20]. The changes in the ART treatment guidelines regarding optimal timing of ART initiation were well reflected among HOPS participants: the median CD4 cell count of antiretroviral-naïve persons initiating ART increased from 345 cells/mm3 (n = 206) to 631 (n = 48) cells/mm3 from 2000 to 2017.
TRENDS IN MORTALITY AND IMMUNOLOGIC AND VIROLOGIC STATUS
The advent of combination ART in 1995, newer advances in treatments, and shifts in recommended practice to earlier initiation of ART have contributed to marked and durable reductions in mortality in the HOPS. Cohort-wide, the mortality rate per 1000 person-years of observation fell from 121 to 16 per 1000 person-years from 1994 to 2017 (Figure 3). The median age at death (IQR) was 39 (32–43) years for n = 159 decedents in 1994 vs 54 (50–65) years for n = 35 decedents in 2017.
Figure 3.
Mortality (95% confidence intervals) and median age at death, the HIV Outpatient Study, 1994–2017 (n = 10 566, number of deaths = 2253).
The median CD4 cell count for the population has increased from 244 cells/mm3 in 1993 to 640 cells/mm3 in 2017 (Table 1), whereas the median nadir CD4 cell count for the population has increased from 233 cells/mm3 in 1993 to 598 cells/mm3 in 2017. In 2017, 70% of HOPS participants had a CD4 cell count >500 cells/mm3, in striking contrast to 23% in 1993, 44% in 2000, and 63% in 2010. The median plasma HIV RNA (viral load [VL]) has markedly fallen from 2.3 log10 to 1.0 log10 copies/mL during the 2000–2017 period, and 92% of HOPS participants had a VL <200 copies/mL in 2017 (Table 1).
In 2010, 82% of all HOPS participants and 89% of ART-treated participants had a most recent VL <50 copies/mL, while in 2017, 91% of overall participants and 94% of ART-treated HOPS participants had a most recent VL <50 copies/mL (data not shown). We have previously reported that durable VL suppression (all VL measurements in a year <200 copies/mL) was also high [21], but disparities in virologic suppression by race/ethnicity among MSM [22] and among women [23] continued to persist in the HOPS. Finally, the HOPS found that transient low-level viremia (“HIV viral blips”) was common and did not appear to be associated with persistent subsequent viremia [24], while the risk of virologic failure was low among people with consistently suppressed viral loads [25], both studies adding to the evidence base informing clinical decision-making regarding switching ART and frequency of viral monitoring.
COMORBIDITIES AND FREQUENT DIAGNOSES
With the improved survival afforded by increasingly more effective, convenient, and tolerable ART, the principal causes of death among HOPS participants have shifted to non-AIDS causes [26], including cardiovascular, hepatic, pulmonary, renal, neoplastic, and neurologic disorders. Although the rates of hospitalizations have declined overall, an increasing fraction of hospitalizations have been associated with chronic end-organ conditions [27]. Improvements in HIV management and survival have resulted in an older cohort of PWH and an increased prevalence of age-related chronic comorbidities that are common in the general US population [28]. The rates of most malignancies mirror or exceed those observed in the general population, due to combined effects of HIV infection, behavioral risk factors such as elevated levels of tobacco use [29], and possibly overall higher rates of other risks, including oncogenic viral infections. Viral hepatitis is also highly prevalent [30–32], and increases in non-HIV sexually transmitted infections, particularly syphilis [33], chlamydia, and gonorrhea [22], have been described in our cohort. We continue to describe the rates of and to monitor risk factors for multimorbidity in our aging cohort (please see the next section).
COMPLICATIONS OF ANTIRETROVIRAL THERAPY
Observational studies in the late 1990s and early 2000s uncovered new conditions, syndromes, and toxicities present in treated PWH, variably attributed to the effects of the virus, its treatments, and traditional risk factors [34–37]. Since its inception, the HOPS has provided a platform for the study of comorbidities, clinical complications, and toxicities associated with ART use [38]. The HOPS was one of the first cohorts to reveal the increased risk of cardiovascular disease outcomes with use of PI-based therapies [39]. Alongside the results from other contemporary studies [40, 41], surveys in the HOPS have contributed to better initial characterization and understanding of the constellation of metabolic changes that represent lipodystrophy and lipoatrophy associated with use of PI-based therapies [15, 16]. As observational evidence accumulated that use of tenofovir disoproxil fumarate (TDF) was associated with decrements in renal function [42] and other adverse renal events [43], the HOPS extended these findings and corroborated recommendations for dose adjustments in patients with preexisting renal insufficiency to limit the risk of kidney end-organ disease [44, 45].
CHANGES IN MANAGEMENT OF HIV INFECTION
Analyses from the HOPS have revealed that use of genotypic resistance testing is associated with improved survival, due to the opportunities afforded by such testing to customize ART regimens in response to apparent HIV resistance patterns [46]. We have demonstrated the underperformance of several cardiovascular risk assessment equations and the FRAX (all developed for use in the general population) for prediction of myocardial infarction and bone fracture, respectively, among PWH [47, 48]. The HOPS data have contributed to guidance regarding the use and discontinuation of antimicrobial chemoprophylaxis for Mycobacterium avium complex and Pneumocystis pneumonia infection among ART-treated PWH [49, 50].
BEHAVIORAL RISK FACTORS AND DISPARITIES IN OUTCOMES
Data collection through the ACASI has enabled the HOPS to link confidential participant-provided information on risk factors (eg, sexual behavior and substance use), with diagnostic and treatment information in the medical records; the ability to combine for analysis patient-reported outcomes with abstracted data has been a strength of the HOPS. In the era of “treatment as prevention,” some HOPS participants have continued to engage in condomless sex despite having a detectable VL; for example, in our 2007–2010 analysis, ~16% of MSM who engaged in condomless anal sex with partners of HIV-negative or unknown HIV status also had HIV VL ≥400 copies/mL [51]. Our sociodemographically heterogeneous cohort has produced many analyses of outcome disparities by race/ethnicity, sex, age, insurance type, and HIV risk behavior, including, for example, lower adherence to ART among younger persons [52], delayed initiation of ART among people who inject drugs [53], higher mortality rates among publicly insured individuals [54], greater polypharmacy with receipt of contraindicated drug combinations with older age [55], poorer retention in care among publicly insured individuals [56], and greater burden of select chronic conditions among black and Hispanic/Latinx participants compared with white participants [57].
COLLABORATIONS
In addition to HOPS investigator-initiated research, since 2006, the HOPS has been contributing data to the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) for joint analyses of outcomes across HIV cohorts in North America. The demographic composition of the HOPS is similar in most respects to that of the surveillance system capturing a nationally representative sample of persons in HIV care in the United States [21], and joint analyses of data have been undertaken [58]. The HOPS has been a training ground for early career public health scientists and fellows at the CDC to build experience in longitudinal cohort analyses of PWH [9, 23, 27].
DISCUSSION
Observational databases of PWH, such as the HOPS, play an important role in characterizing and defining HIV disease, its etiology and progression [29, 59], evolving clinical presentations [18, 38], and therefore approaches to its management. The HOPS cohort continues to serve as a valuable data source for studying complications of HIV infection and therapies used in routine clinical practice. It takes advantage of the full medical record, including diagnoses, treatments, laboratory values, and clinical events (deaths and hospitalizations), with supplemental behavioral survey data. HOPS cohort methodology has permitted a range of nested surveys, case–control studies, and other designs [15, 17, 60]. Comprehensive documentation of ART prescriptions, which is also available in other large data sources [58, 61] but not in US national HIV case surveillance [1], allows the HOPS to investigate granular associations of prescribed ART use, as well as that of major types of ART regimens, with various clinical outcomes.
The HOPS cohort includes diverse privately and publicly funded HIV specialty clinics (including 3 Ryan White–funded clinics) that are more heterogeneous in their patient populations and practice patterns (and hence more likely reflective of variable standards of care across the United States) than the populations from single-payer systems (eg, health maintenance organizations or veteran and military cohorts). However, some findings (eg, prevalence of disease or use of preventative services) may not be generalizable to the overall population of PWH in the United States, PWH receiving care in rural areas, and those not engaged in HIV care. In the 2012 published comparison of PWH in the national HIV surveillance system and PWH in medical care in the United States, the HOPS cohort had a similar representation of women and MSM as these 2 large data sources; however, HOPS participants tended to be somewhat older and more frequently non-Hispanic white [21]. These findings persisted when compared with national data in 2017 [1]. Consequently, our recent recruitment efforts have prioritized younger adults (aged 18 years and over) and persons of color to ensure that the HOPS accurately represents contemporary HIV epidemiology in the United States. The HOPS includes clinics in 4 jurisdictions in Ending the HIV Epidemic target areas (Cook County, IL, USA; Philadelphia County, PA, USA; Hillsborough and Pinellas County, FL, USA; and Washington, DC, USA) [2]; however, the addition of more sites in the US South with some of the highest HIV prevalence or incidence rates in the nation [1] would further strengthen this cohort.
The HOPS findings are subject to some additional caveats and limitations due to reliance on chart-abstracted data from routine HIV patient care, with no study-specific visits or biological specimen repository. Such data involve variability in the timing of participant health care contact screenings, including HIV and other laboratory measurements, and some variability in the depth of pre-enrollment clinical history. HOPS physicians may order testing at their discretion, and the diagnoses are not validated or standardized across sites. Although the HOPS has multiple data quality control measures in place, information may be missing if not elicited by clinicians, not charted, or not reported back to the site (eg, gender identity, reasons for medication discontinuation, dates of hospitalization admission, and discharge diagnoses). Although the HOPS already collects data on insurance/payer at every clinic visit and education and employment at enrollment, strengthening and expanding data collection on social determinants of health are future goals.
In conclusion, the HOPS cohort has had a seminal and ongoing impact on the understanding of the importance of early ART for reducing morbidity and mortality and characterization of the growing role of noninfectious chronic complications of aging with HIV infection in the last 2 decades. The cohort remains well poised for investigating novel associations between HIV and non-HIV treatments and clinical outcomes among PWH, as well as studying trends in comorbidities, survival, preventative care practices, and measures on the HIV care continuum (eg, CD4 cell count at ART start, virologic suppression, and retention in care). Current and future investigative directions for the HOPS include (1) generating data to inform and help optimize long-term care management and outcomes among women and patients of black race and Hispanic/Latinx ethnicity who are aging with HIV and represent an increasing proportion of PWH in the United States; (2) monitoring disparities in HIV care and outcomes by sex, age, race/ethnicity, and HIV risk group to identify gaps that must be narrowed to end the HIV epidemic; and (3) studying the long-term effectiveness and safety of therapies and disease management strategies for 1.1 million (and growing) Americans living with HIV.
Acknowledgments
The authors thank HOPS study participants and site research staff, without whose time and dedication this research would not have been possible. We thank Kalliope Chagaris for technical assistance during manuscript revisions. The authors also acknowledge the longstanding contributions to the HOPS of the following individuals: Scott Holmberg, Anne Moorman, John T. Brooks, Kathy Wood, Kenneth Lichtenstein, Benjamin Young, and Bienvenido G. Yangco.
HOPS Investigators during 2019. Jun Li, Kate Buchacz, Marcus D. Durham, Division of HIV/AIDS Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, GA; Cheryl Akridge, Stacey Purinton, Nabil Rayeed, Selom Agbobil-Nuwoaty, Kalliope Chagaris, Kimberly Carlson, Carl Armon, Linda Battalora, Jonathan Mahnken, Cerner Corporation, Kansas City, MO; Frank J. Palella, Saira Jahangir, Conor Daniel Flaherty, Patricia Bustamante, Feinberg School of Medicine, Northwestern University, Chicago, IL; John Hammer, Kenneth S. Greenberg, Barbara Widick, Rosa Franklin, Rocky Mountain Cares, Denver, CO; Douglas J. Ward, Troy Thomas, Cheryl Stewart, Dupont Circle Physicians Group, Washington, DC; Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, Jane Esteves, State University of New York (SUNY), Stony Brook, NY; Ellen M. Tedaldi, Ramona A. Christian, Faye Ruley, Dania Beadle, Princess Davenport, Lewis Katz School of Medicine at Temple University, Philadelphia, PA; Richard M. Novak, Andrea Wendrow, Stockton Mayer, University of Illinois at Chicago, Chicago, IL; Mia Scott, Billie Thomas, Loraine Van Slyke, APEX Family Medicine, Denver, CO; Cynthia Mayer, Terry Beitler, Karen Maroney, Denise Franklin, SJH Comprehensive Research Institute, Tampa, FL.
Financial support. This work was supported by the Centers for Disease Control and Prevention (contract Nos. 200-2001-00133, 200-2006-18797, and 200-2011-41872).
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the HIV Outpatient Study (HOPS) Investigators:
Jun Li, Kate Buchacz, Marcus D Durham, Cheryl Akridge, Stacey Purinton, Nabil Rayeed, Selom Agbobil-Nuwoaty, Kalliope Chagaris, Kimberly Carlson, Carl Armon, Linda Battalora, Jonathan Mahnken, Cerner Corporation, Kansas City, Frank J Palella, Saira Jahangir, Conor Daniel Flaherty, Patricia Bustamante, John Hammer, Kenneth S Greenberg, Barbara Widick, Rosa Franklin, Douglas J Ward, Troy Thomas, Cheryl Stewart, Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, Jane Esteves, Stony Brook, Ellen M Tedaldi, Ramona A Christian, Faye Ruley, Dania Beadle, Princess Davenport, Richard M Novak, Andrea Wendrow, Stockton Mayer, Mia Scott, Billie Thomas, Loraine VanSlyke, Cynthia Mayer, Terry Beitler, Karen Maroney, and Denise Franklin
References
- 1. Centers for Disease Control and Prevention. HIV surveillance report, 2018; vol. 30. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed December 20, 2019.
- 2. Department of Health and Human Services. What is “ending the HIV epidemic: a plan for America?” Available at: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview. Accessed October 2, 2019.
- 3. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321(9):844–5. [DOI] [PubMed] [Google Scholar]
- 4. Aberg JA, Gallant JE, Ghanem KG, et al. ; Infectious Diseases Society of America Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:e1–34. [DOI] [PubMed] [Google Scholar]
- 5. El-Sadr WM, Mayer KH, Rabkin M, Hodder SL. AIDS in America - back in the headlines at long last. N Engl J Med 2019; 380:1985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–510. [DOI] [PubMed] [Google Scholar]
- 7. Shafran SD. HIV coinfected have similar SVR rates as HCV monoinfected with DAAs: it’s time to end segregation and integrate HIV patients into HCV trials. Clin Infect Dis 2015; 61:1127–34. [DOI] [PubMed] [Google Scholar]
- 8. Buchacz K, Armon C, Palella FJ, et al. CD4 cell counts at HIV diagnosis among HIV outpatient study participants, 2000-2009. AIDS Res Treat 2012; 2012:869841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheth AN, Ofotokun I, Buchacz K, et al. Antiretroviral regimen durability and success in treatment-naive and treatment-experienced patients by year of treatment initiation, United States, 1996–2011. J Acquir Immune Defic Syndr 2016; 71:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed October 2, 2019.
- 11. Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 12. Opravil M, Ledergerber B, Furrer H, et al. ; Swiss HIV Cohort Study Clinical efficacy of early initiation of HAART in patients with asymptomatic HIV infection and CD4 cell count > 350 x 10(6) /l. AIDS 2002; 16:1371–81. [DOI] [PubMed] [Google Scholar]
- 13. Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 2001; 286:2568–77. [DOI] [PubMed] [Google Scholar]
- 14. Palella FJ Jr, Deloria-Knoll M, Chmiel JS, et al. ; HIV Outpatient Study Investigators Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med 2003; 138:620–6. [DOI] [PubMed] [Google Scholar]
- 15. Lichtenstein KA, Ward DJ, Moorman AC, et al. ; HIV Outpatient Study Investigators Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS 2001; 15:1389–98. [DOI] [PubMed] [Google Scholar]
- 16. Lichtenstein KA, Delaney KM, Armon C, et al. ; HIV Outpatient Study Investigators Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr 2003; 32:48–56. [DOI] [PubMed] [Google Scholar]
- 17. Lichtenstein KA, Armon C, Buchacz K, et al. ; HIV Outpatient Study (HOPS) Investigators Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010; 51:435–47. [DOI] [PubMed] [Google Scholar]
- 18. Novak RM, Richardson JT, Buchacz K, et al. ; HIV Outpatient Study (HOPS) Investigators Immune reconstitution inflammatory syndrome: incidence and implications for mortality. AIDS 2012; 26:721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young B, Dao CN, Buchacz K, et al. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis 2011; 52:1061–8. [DOI] [PubMed] [Google Scholar]
- 20. Battalora L, Buchacz K, Armon C, et al. ; HIV Outpatient Study (HOPS) and SUN Study Investigators Low bone mineral density and risk of incident fracture in HIV-infected adults. Antivir Ther 2016; 21:45–54. [DOI] [PubMed] [Google Scholar]
- 21. Buchacz K, Frazier EL, Hall HI, et al. A matter of perspective: comparison of the characteristics of persons with HIV infection in the United States from the HIV outpatient study, medical monitoring project, and national HIV surveillance system. Open AIDS J 2015; 9:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buchacz K, Armon C, Tedaldi E, et al. Disparities in HIV viral load suppression by race/ethnicity among men who have sex with men in the HIV Outpatient Study. AIDS Res Hum Retroviruses 2018. doi: 10.1089/AID.2017.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geter A, Sutton MY, Armon C, et al. Trends of racial and ethnic disparities in virologic suppression among women in the HIV Outpatient Study, USA, 2010–2015. PLoS One 2018; 13:e0189973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sklar PA, Ward DJ, Baker RK, et al. ; HIV Outpatient Study (HOPS) Investigators Prevalence and clinical correlates of HIV viremia (‘blips’) in patients with previous suppression below the limits of quantification. AIDS 2002; 16:2035–41. [DOI] [PubMed] [Google Scholar]
- 25. Young B, Hart RL, Buchacz K, et al. ; HIV Outpatient Study (HOPS) HIV viral load monitoring frequency and risk of treatment failure among immunologically stable HIV-infected patients prescribed combination antiretroviral therapy. J Int Assoc Provid AIDS Care 2015; 14:536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palella FJ Jr, Baker RK, Moorman AC, et al. ; HIV Outpatient Study Investigators Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 27. Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS 2008; 22:1345–54. [DOI] [PubMed] [Google Scholar]
- 28. Buchacz K, Baker RK, Palella FJ Jr, et al. ; HIV Outpatient Study Investigators Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther 2013; 18:65–75. [DOI] [PubMed] [Google Scholar]
- 29. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148:728–36. [DOI] [PubMed] [Google Scholar]
- 30. Spradling PR, Richardson JT, Buchacz K, et al. Prevalence of chronic hepatitis B virus infection among patients in the HIV Outpatient Study, 1996–2007. J Viral Hepat 2010; 17:879–86. [DOI] [PubMed] [Google Scholar]
- 31. Spradling PR, Richardson JT, Buchacz K, et al. Trends in hepatitis C virus infection among patients in the HIV Outpatient Study, 1996–2007. J Acquir Immune Defic Syndr 2010; 53:388–96. [DOI] [PubMed] [Google Scholar]
- 32. Samandari T, Tedaldi E, Armon C, et al. Incidence of hepatitis C virus infection in the Human Immunodeficiency Virus Outpatient Study cohort, 2000–2013. Open Forum Infect Dis 2017; 4:ofx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novak RM, Ghanem A, Hart R, et al. Risk factors and incidence of syphilis in human immunodeficiency virus (HIV)-infected persons: the HIV Outpatient Study, 1999–2015. Clin Infect Dis 2018; 67:1750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sobieszczyk ME, Hoover DR, Anastos K, et al. ; Women’s Interagency HIV Study Prevalence and predictors of metabolic syndrome among HIV-infected and HIV-uninfected women in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr 2008; 48:272–80. [DOI] [PubMed] [Google Scholar]
- 35. Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 2007; 45:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hooshyar D, Hanson DL, Wolfe M, et al. Trends in perimortal conditions and mortality rates among HIV-infected patients. AIDS 2007; 21:2093–100. [DOI] [PubMed] [Google Scholar]
- 37. Friis-Møller N, Sabin CA, Weber R, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003.14627784 [Google Scholar]
- 38. Moorman AC, Holmberg SD, Marlowe SI, et al. Changing conditions and treatments in a dynamic cohort of ambulatory HIV patients: the HIV Outpatient Study (HOPS). Ann Epidemiol 1999; 9:349–57. [DOI] [PubMed] [Google Scholar]
- 39. Holmberg SD, Moorman AC, Williamson JM, et al. ; HIV Outpatient Study (HOPS) investigators Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet 2002; 360:1747–8. [DOI] [PubMed] [Google Scholar]
- 40. Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998; 12:F51–8. [DOI] [PubMed] [Google Scholar]
- 41. Schindler JT, Spooner KM, Decker CF. “Buffalo humps” associated with protease inhibitors. Ann Intern Med 1998; 129:164. [DOI] [PubMed] [Google Scholar]
- 42. Gallant JE, Parish MA, Keruly JC, Moore RD. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis 2005; 40:1194–8. [DOI] [PubMed] [Google Scholar]
- 43. Izzedine H, Isnard-Bagnis C, Hulot JS, et al. Renal safety of tenofovir in HIV treatment-experienced patients. AIDS 2004; 18:1074–6. [DOI] [PubMed] [Google Scholar]
- 44. Young B, Buchacz K, Baker RK, et al. ; HIV Outpatient Study Investigators Renal function in tenofovir-exposed and tenofovir-unexposed patients receiving highly active antiretroviral therapy in the HIV Outpatient Study. J Int Assoc Physicians AIDS Care (Chic) 2007; 6:178–87. [DOI] [PubMed] [Google Scholar]
- 45. Young B, Buchacz K, Moorman A, et al. ; HIV Outpatient Study (HOPS) Investigators Renal function in patients with preexisting renal disease receiving tenofovir-containing highly active antiretroviral therapy in the HIV outpatient study. AIDS Patient Care STDS 2009; 23:589–92. [DOI] [PubMed] [Google Scholar]
- 46. Palella FJ Jr, Armon C, Buchacz K, et al. ; HOPS (HIV Outpatient Study) Investigators The association of HIV susceptibility testing with survival among HIV-infected patients receiving antiretroviral therapy: a cohort study. Ann Intern Med 2009; 151:73–84. [DOI] [PubMed] [Google Scholar]
- 47. Thompson-Paul AM, Lichtenstein KA, Armon C, et al. Cardiovascular disease risk prediction in the HIV Outpatient Study. Clin Infect Dis 2016; 63:1508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Battalora L, Buchacz K, Armon C, et al. New fracture risk and FRAX 10-year probability of fracture in HIV-infected adults. Paper presented at: 21th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston, MA. [Google Scholar]
- 49. Yangco BG, Buchacz K, Baker R, et al. ; HIV Outpatient Study Investigators Is primary Mycobacterium avium complex prophylaxis necessary in patients with CD4 <50 cells/μL who are virologically suppressed on cART? AIDS Patient Care STDS 2014; 28:280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yangco BG, Von Bargen JC, Moorman AC, Holmberg SD. Discontinuation of chemoprophylaxis against Pneumocystis carinii pneumonia in patients with HIV infection. HIV Outpatient Study (HOPS) Investigators. Ann Intern Med 2000; 132:201–5. [DOI] [PubMed] [Google Scholar]
- 51. Durham MD, Buchacz K, Richardson J, et al. Sexual risk behavior and viremia among men who have sex with men in the HIV Outpatient Study, United States, 2007–2010. J Acquir Immune Defic Syndr 2013; 63:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Durham MD, Hart R, Buchacz K, et al. Antiretroviral nonadherence and condomless sex in the HIV Outpatient Study, USA, 2007–2014. Int J STD AIDS 2018; 29:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Novak RM, Hart RL, Chmiel JS, et al. Disparities in initiation of combination antiretroviral treatment and in virologic suppression among patients in the HIV Outpatient Study, 2000–2013. J Acquir Immune Defic Syndr 2015; 70:23–32. [DOI] [PubMed] [Google Scholar]
- 54. Palella FJ Jr, Baker RK, Buchacz K, et al. ; HOPS Investigators Increased mortality among publicly insured participants in the HIV Outpatient Study despite HAART treatment. AIDS 2011; 25:1865–76. [DOI] [PubMed] [Google Scholar]
- 55. Holtzman C, Armon C, Tedaldi E, et al. ; and the HOPS Investigators Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. J Gen Intern Med 2013; 28:1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tedaldi EM, Richardson JT, Debes R, et al. ; HOPS Investigators Retention in care within 1 year of initial HIV care visit in a multisite US cohort: who’s in and who’s out? J Int Assoc Provid AIDS Care 2014; 13:232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Palella FJ, Hart R, Armon C, et al. ; HIV Outpatient Study (HOPS) Non-AIDS comorbidity burden differs by sex, race, and insurance type in aging adults in HIV care. AIDS 2019; 33:2327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Althoff KN, Buchacz K, Hall HI, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med 2012; 157:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tedaldi EM, Baker RK, Moorman AC, et al. ; HIV Outpatient Study (HOPS) Investigators Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis 2003; 36:363–7. [DOI] [PubMed] [Google Scholar]
- 60. Buchacz K, Wiegand R, Armon C, et al. Long-term immunologic and virologic responses on raltegravir-containing regimens among ART-experienced participants in the HIV Outpatient Study. HIV Clin Trials 2015; 16:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vu QM, Shouse RL, Brady K, et al. Changes in HIV antiretroviral prescribing practices in the United States. Int J STD AIDS 2020; 31:22–9. [DOI] [PubMed] [Google Scholar]