Dear Editor,
A substantial portion of patients with coronavirus disease (COVID-19) developed rapidly progressive pneumonia leading to acute respiratory distress syndrome (ARDS) and multiple organ dysfunctions, conditions associated with high mortality [1]. Adjuvant corticosteroid therapy of such patients is common in clinical practice, but evidence is scarce regarding the efficacy of adjuvant corticosteroids in patients who are critically ill with COVID-19.
We retrospectively reviewed medical records of adult patients with COVID-19 who were admitted to Tongji Hospital (Wuhan, China) from January 25 to February 25, 2020. Two hundred forty-four eligible patients who had complete records and were critically ill and treated with antiviral agents were enrolled. Critically ill patients were defined as those with ARDS (PaO2/FiO2 ≤ 300 mmHg; when PaO2 is not available, SpO2/FiO2 ≤ 315 suggests ARDS) or sepsis with acute organ dysfunction [2]. We converted all preparations to hydrocortisone-equivalent doses (methylprednisolone 1:5, dexamethasone 1:25) [3]. The clinical outcome was 28-day mortality after admission.
We adjusted for differences in baseline characteristics by propensity score, using multivariate logistic regression without regard to outcomes [4]. Potential confounders considered in propensity score matching (PSM) were variables included in the final model by step-wise backward elimination with P < 0.20 [5]. Corticosteroid treatment effect on outcome was analyzed by multivariate logistic regression with adjustment for major variables (age, SpO2/FiO2, and lymphocytes) associated with mortality; individual propensity score was incorporated as a covariable to calculate the propensity-adjusted odds ratio (OR) [5]. PSM generated propensity score-matched pairs without replacement, and survival probability was compared by the Kaplan-Meier curve and analyzed with the log-rank test. Cox regression was used to estimate hazard ratio (HR) with 95% CI. For unadjusted comparisons, a two-sided P < 0.05 was considered statistically significant.
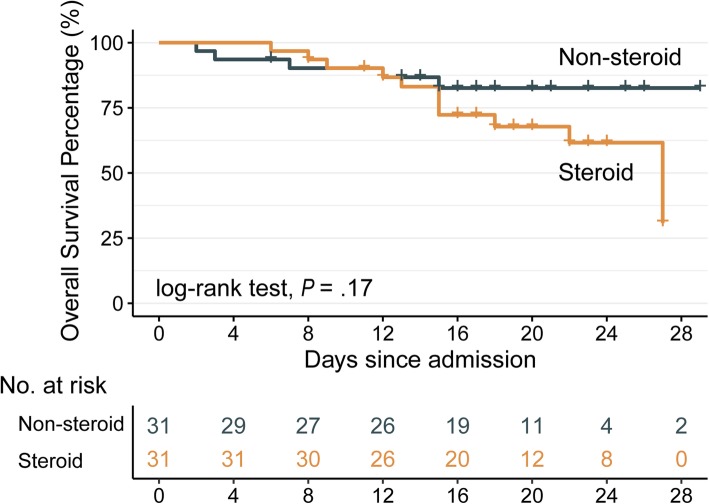
Of the 244 critically ill patients with COVID-19, the median age was 62 (50–71) years, and 52% were male. All patients were given antiviral therapy (e.g., oseltamivir, arbidol, lopinavir/ritonavir, ganciclovir, interferon-α), and 151 (62%) were given adjuvant corticosteroid treatment (median hydrocortisone-equivalent dosage 200 [range 100–800] mg/day). Five (5.4%) and 79 (52.3%) patients died in non-steroid and steroid groups, respectively. The median (IQR) administration duration of corticosteroid treatment was 8 (4–12) days. Multiple organ dysfunctions were more common in the steroid group than in the non-steroid group. Multivariate analysis that adjusted for major mortality-associated variables and propensity score indicated that corticosteroid treatment was independent from overall mortality (adjusted OR 1.05; 95% CI 0.15–7.46). One hundred forty-seven (60%) had dyspnea and 87 (36%) had ARDS, and subgroup analyses revealed corticosteroid treatment was not associated with 28-day mortality (both, P > 0.3). Sixty-two patients in 31 pairs were matched (Table 1), and 28-day mortality rate was 39% in case subjects and 16% in control subjects (P = 0.09). Likewise, addition of adjuvant corticosteroid therapy to standard antiviral treatment was not associated with 28-day mortality (P = 0.17; Fig. 1). However, increased corticosteroid dosage was significantly associated with elevated mortality risk after adjustment for administration duration (P = 0.003); every 10-mg increase in dosage was associated with additional 4% mortality risk (adjusted HR 1.04, 95% CI 1.01–1.07).
Table 1.
Baseline characteristics for steroid treatment and non-steroid treatment groups comprising critically ill patients with COVID-19 before and after propensity score matching
| Cohort study | Case-control study (PSM) | |||||
|---|---|---|---|---|---|---|
| Steroid (151) | Non-steroid (93) | P | Steroid (31) | Non-steroid (31) | P | |
| Age, years | 64 (53–71) | 59 (47–69) | .09 | 57 (51–69) | 58 (50–67) | .98 |
| Gender, male | 83 (55) | 45 (48) | .39 | 16 (52) | 16 (52) | 1 |
| Signs and symptoms | ||||||
| Fever | 136 (90) | 81 (87) | .61 | 30 (97) | 26 (84) | .2 |
| Dry cough | 112 (74) | 57 (61) | .05 | 21 (68) | 21 (68) | 1 |
| Dyspnea | 94 (62) | 53 (57) | .5 | 20 (65) | 19 (61) | 1 |
| Fatigue | 70 (46) | 48 (52) | .51 | 12 (39) | 13 (42) | 1 |
| Expectoration | 69 (46) | 30 (32) | .05 | 12 (39) | 12 (39) | 1 |
| Diarrhea | 45 (30) | 22 (24) | .37 | 10 (32) | 7 (23) | .57 |
| Anorexia | 42 (28) | 25 (27) | .99 | 5 (16) | 8 (26) | .53 |
| Original comorbidities | ||||||
| Hypertension | 61 (40) | 34 (37) | .64 | 16 (52) | 12 (39) | .44 |
| Diabetes | 34 (23) | 10 (11) | .03 | 4 (13) | 7 (23) | .51 |
| CVD | 15 (10) | 13 (14) | .45 | 2 (7) | 2 (7) | 1 |
| COPD | 9 (6) | 3 (3) | .51 | 0 | 1 (3) | 1 |
| Vital signs | ||||||
| T, °C | 37.0 (36.2–38) | 36.7 (36.4–37.3) | .02 | 37 (36.5–37.6) | 37 (36.5–37.3) | .93 |
| Breathing, rpm | 22 (20–25) | 20 (20–22) | < .01 | 21 (20–24) | 20 (20–22) | .08 |
| Pulse, bpm | 92 (82–105) | 88 (78–98) | .03 | 95 (78–106) | 93 (82–100) | .51 |
| SpO2/FiO2 | 259 (121–303) | 297 (279–388) | < .01 | 291 (212–452) | 294 (246–396) | .57 |
| Laboratory findings (WBCs, lymphocytes, neutrophils, platelets, × 109/L) | ||||||
| WBCs | 6.7 (4.9–8.9) | 5.0 (4.0–6.5) | < .01 | 6.6 (4.0–8.6) | 5.1 (3.5–6.8) | .12 |
| Lymphocytes | 0.7 (0.5–1.0) | 1.2 (0.9–1.6) | < .01 | 0.9 (0.5–1.3) | 1.1 (0.6–1.2) | .64 |
| Neutrophils | 5.4 (3.6–7.6) | 3.2 (2.4–4.2) | < .01 | 5.2 (2.6–7.4) | 3.5 (2.3–4.7) | .09 |
| Platelets | 181 (138–248) | 224 (170–298) | < .01 | 168 (138–214) | 206 (155–230) | .23 |
| HGB, g/L | 130 (117–141) | 127 (117–139) | .42 | 128 (118–138) | 125 (117–133) | .56 |
| Organ function damage | ||||||
| ARDS | 81 (54) | 6 (7) | < .01 | 12 (39) | 6 (19) | .16 |
| Septic shock | 69 (46) | 2 (2) | < .01 | 8 (26) | 2 (7) | .08 |
| Myocardial infarction | 64 (42) | 3 (3) | < .01 | 10 (32) | 3 (10) | .06 |
| AKI | 46 (31) | 5 (5) | < .01 | 8 (26) | 3 (10) | .18 |
| DIC | 39 (26) | 2 (2) | < .01 | 6 (19) | 2 (7) | .26 |
| Liver injury | 28 (19) | 6 (7) | < .01 | 7 (23) | 3 (10) | .3 |
| Treatment | ||||||
| Anti-bacteria | 142 (94) | 42 (45) | < .01 | 25 (81) | 26 (84) | 1 |
| Gamma globulin | 84 (56) | 8 (9) | < .01 | 11 (36) | 8 (26) | .58 |
| MV | 78 (52) | 4 (4) | < .01 | 11 (36) | 4 (13) | .08 |
| Muscle relaxant | 25 (17) | 0 | < .01 | 4 (13) | 0 | .12 |
| HFNC | 21 (14) | 1 (1) | < .01 | 6 (19) | 1 (3) | .11 |
Abbreviations: CVD cardiovascular disease, COPD chronic obstructive pulmonary disease, WBCs white blood cells, ARDS acute respiratory distress syndrome, AKI acute kidney injury, DIC disseminated intravascular coagulation, MV mechanical ventilation, HFNC high flow nasal cannula
Continuous variables were described as the median (IQR) while categorical variables were expressed as frequencies (%). Hypothesis testing using Fisher’s exact test for categorical data and Mann-Whitney test for continuous data
Fig. 1.
Survival curves stratified by adjuvant corticosteroid treatment. Thirty-one critically ill patients with COVID-19 who received corticosteroid treatment (yellow line) are compared with 31 matched control subjects (green line) who did not receive corticosteroid treatment
We acknowledged limitations. First, we did not distinguish patients who received corticosteroids for underlying disease (e.g., COPD), the number of which was however small. Second, PSM is limited by adjusting for observed covariables only; randomized placebo-controlled trials are therefore warranted. Altogether, our investigation indicated limited effect of corticosteroid therapy could pose to overall survival of critically ill patients with COVID-19. Given the adverse effects, corticosteroid therapy must be commenced with caution, and prudent dosage should be promoted under certain circumstances.
Acknowledgements
We would like to thank all the hospital staff members for their efforts in collecting the information used in this study and all the patients who consented to donate their data for the analysis and the medical staff members who are on the front line of caring for patients.
Authors’ contributions
Conceptualization: X. Lu, T. Chen. Acquisition, analysis, or interpretation of the data: J. Wang, Y. Wang, X. Lu, T. Chen. Statistical analysis: X. Lu, F. Yan.
Investigation: X. Lu, T. Chen, Y. Wang. Drafting of the manuscript and editing: X. Lu, T. Chen, Y. Wang. Funding acquisition: J. Wang, Y. Wang, F. Yan. Supervision: J. Wang, F. Yan. The author(s) read and approved the final manuscript.
Funding
This work was supported by the National Key R&D Program of China (2019YFC1711000), the National Natural Science Foundation of China (81973145), the “Double First-Class” University Project (CPU2018GY09), the China Postdoctoral Science Foundation (2019 M651805), the Science Foundation of Jiangsu Commission of Health (H2018117), and the Emergency Project for the Prevention and Control of the Novel Coronavirus Outbreak in Suzhou (SYS2020012).
Availability of data and materials
Dr. J. Wang had full access to all of the data in the study. After publication, the data will be made available to others on reasonable requests after approval from the corresponding author (J.W, dr_wangjun@suda.edu.cn) and Wuhan Tongji Hospital.
Ethics approval and consent to participate
Ethical approval was waived by the Ethics Committee of Tongji Hospital (Wuhan, China) in view of the retrospective and observational nature of the study and all the procedures being performed were part of the routine care.
Consent for publication
The informed consents of patients were waived by the Ethics Commission of Tongji Hospital (Wuhan, China) for the rapid emergence of this epidemic.
Competing interests
The authors declared no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaofan Lu, Taige Chen, Yang Wang and Jun Wang contributed equally to this work.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736(20):30566–73. [DOI] [PMC free article] [PubMed]
- 2.Organization WH: Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. In: World Health Organization; 2020.
- 3.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 5.Kim S-H, Hong S-B, Yun S-C, Choi W-I, Ahn J-J, Lee YJ, Lee H-B, Lim C-M, Koh Y. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011;183(9):1207–1214. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dr. J. Wang had full access to all of the data in the study. After publication, the data will be made available to others on reasonable requests after approval from the corresponding author (J.W, dr_wangjun@suda.edu.cn) and Wuhan Tongji Hospital.