Highlights
-
•
Common viruses caused two-fifths of respiratory-illness-related hospitalizations, amidst a COVID-19 outbreak.
-
•
The co-infection rate between SARS-CoV-2 and other respiratory viruses was low, at 1.4%.
-
•
No increased morbidity or mortality with COVID-19 co-infections.
-
•
In-hospital mortality and intubation lower for COVID-19 compared with other respiratory viruses.
Keywords: COVID-19, Co-infections, Community-acquired, Respiratory viral infections
Abstract
Aims
During the ongoing COVID-19 outbreak, co-circulation of other common respiratory viruses can potentially result in co-infections; however, reported rates of co-infections for SARS-CoV-2 vary.
We sought to evaluate the prevalence and etiology of all community acquired viral respiratory infections requiring hospitalization during an ongoing COVID-19 outbreak, with a focus on co-infection rates and clinical outcomes.
Methods
Over a 10-week period, all admissions to our institution, the largest tertiary hospital in Singapore, were screened for respiratory symptoms, and COVID-19 as well as a panel of common respiratory viral pathogens were systematically tested for. Information was collated on clinical outcomes, including requirement for mechanical ventilation and in hospital mortality.
Results
One-fifth (19.3%, 736/3807) of hospitalized inpatients with respiratory symptoms had a PCR-proven viral respiratory infection; of which 58.5% (431/736) tested positive for SARS-CoV-2 and 42.2% (311/736) tested positive for other common respiratory viruses. The rate of co-infection with SARS-CoV-2 was 1.4% (6/431); all patients with co-infection had mild disease and stayed in communal settings. The in-hospital mortality rate and proportion of COVID-19 patients requiring invasive ventilation was low, at around 1% of patients; these rates were lower than patients with other community-acquired respiratory viruses admitted over the same period (p < 0.01).
Conclusion
Even amidst an ongoing COVID-19 outbreak, common respiratory viruses still accounted for a substantial proportion of hospitalizations. Coinfections with SARS-CoV-2 were rare, with no observed increase in morbidity or mortality.
1. Introduction
In late 2019, a novel coronavirus, SARS CoV-2, was identified as the cause of an outbreak of 2019 novel coronavirus (COVID-19) infection in Wuhan, China; since then, the situation has evolved into a global pandemic [1]. However, differentiating COVID-19 remains challenging, as common clinical manifestations of COVID-19, including fever, cough and dyspnea, are indistinguishable from those caused by other respiratory viruses [2]. Furthermore, even during outbreaks of respiratory disease caused by a novel pathogen, common respiratory viruses can still circulate and potentially cause co-infections [3]. However, early reports suggested that co-infection with SARS CoV-2 and other viral respiratory pathogens was rare. Estimates of co-infection ranged from 0-3% in some cohorts [1,4,5]; sporadic case reports of co-infection between SARS CoV-2 and other respiratory pathogens have also been reported in the literature [[6], [7], [8]]. More recent studies, however, have reported much higher rates of co-infection, with almost one-fifth of patients reported to have a concomitant viral coinfection alongside SARS CoV-2 [9]. Establishing the likelihood of co-infection is crucial, as continued testing for other respiratory pathogens was endorsed as a strategy to aid the evaluation of patients with potential COVID-19 by identifying an alternative etiology, given the low rates of co-infection that were initially reported [10,11]. However, this remains controversial given continued uncertainty over the likelihood of co-infection [9]. Furthermore, maintaining surveillance for common respiratory viruses remains challenging given the relative scarcity of studies investigating the viral etiology of pneumonia, due to limited interventions [12]. This challenge is exacerbated by resource limitations experienced during a pandemic, which can overwhelm even well-resourced hospital systems. Nevertheless, common respiratory viruses remain a major cause of morbidity and mortality amongst hospitalized inpatients with respiratory diagnoses. [13]
In Singapore, a Southeast Asian city-state, the first imported case of COVID-19 was reported in end-January 2020; followed by the first documented case of local transmission in early February 2020 [14]. By end-February 2020, the majority of cases were attributed to local transmission [15]. As part of the national strategy of containment, heightened vigilance was maintained for all hospital admissions presenting from the community with respiratory symptoms, in order to detect cases of COVID-19 through enhanced surveillance [16]. At our institution, the largest tertiary hospital in Singapore, from early February 2020 all admissions were systematically screened for respiratory symptoms, and SARS-CoV-2 was tested for along with other common respiratory viruses. Notably, surveillance for common respiratory viruses was maintained even during an ongoing COVID-19 pandemic; this allowed us to evaluate the etiology of community-acquired viral respiratory infections amongst hospitalized inpatients during an ongoing COVID-19 outbreak, with a focus on the incidence of co-infection and clinical outcomes.
2. Methodology
2.1. Institutional setting and study period
Singapore General Hospital (SGH) is the largest public tertiary hospital in Singapore, with 1785 beds. On average, almost 2000 cases of pneumonia are admitted through the emergency department (ED) each year, or around 36 patients a week [17]. Over a 10-week period from 5th February to 15th April 2020, all admissions were systematically screened for respiratory symptoms; if patients had respiratory symptoms on admission or within 72 h of admission, they were admitted to a “respiratory surveillance ward”, RSW, where COVID-19 was tested for [18]. Patients admitted into the RSW would only be transferred out if COVID-19 tests were negative on 2 consecutive occasions, done at least 24 h apart [11].
2.2. Sampling and detection of respiratory viruses
All patients with respiratory symptoms (cough, rhinorrhea, dyspnea) or radiological manifestations compatible with pneumonia on chest imaging had oropharyngeal specimens taken via Dacron-tipped swabs within 24 hours of admission to the RSW; if oropharyngeal sampling was not feasible, other respiratory specimens, such as sputum, nasopharyngeal or bronchoalveolar lavage specimens were obtained. Respiratory specimens were tested for SARS-CoV-2 RNA. This was done by qualitative real-time reverse transcription polymerase chain reaction (RT-PCR) testing. Viral RNA was first isolated from patient’s oropharyngeal swab samples and RT-PCR was performed targeting E gene and ORF1b-nsp14 for SARS-CoV-2 [19,20]. At our institution, if the first sample was negative for SARS-CoV-2 RNA, a second specimen was sent for repeated testing 24 h from the first one. If primary physicians assessed that a viral syndrome could not be excluded as a cause of the patient’s respiratory symptoms (e.g. normal procalcitonin, lymphopenia), respiratory specimens were also processed in parallel for a common panel of viral respiratory pathogens via respiratory virus (RV) multiplex PCR testing. This was performed using the Seegene Anyplex II RV16 Detection Multiplex PCR kit (Seegene, Korea). This assay simultaneously detected 16 types of human respiratory viral pathogens (influenza A and B, human parainfluenza virus (HPIV) 1/2/3/4, respiratory syncytial virus (RSV) subtypes A and B, human metapneumonvirus (hMPV), human coronavirus (HCoV) (229E/NL63/OC43), rhinovirus A/B/C, enterovirus, adenovirus and human bocavirus (HboV) 1/2/3/4.
2.3. Co-infection and clinical outcomes
Co-infection was defined as a positive result for 1 or more respiratory viral pathogens on PCR testing. At the point of admission to the RSW, basic demographic information including age, ethnicity, and gender were obtained. Basic biochemical results on admission, including leukocytosis, leukopenia, raised serum lactate dehydrogenase (LDH), and procalcitonin levels were also obtained. The following cutoffs were used for biochemical results: leukopenia, <4.0 × 109 /L; leukocytosis, >10 × 109/L; raised LDH, >245 U/L; raised procalcitonin, >0.25 ng/ml. Results of chest imaging at the point of admission were also obtained, with pneumonia being defined as the presence of a compatible clinical syndrome and presence of infiltrates on the chest radiograph. Information was collected on the following outcomes: requiring intensive care/high-dependency care during hospitalization, length of hospitalization (days), and in-hospital mortality.
2.4. Statistical analysis
Differences in proportions were compared using chi-square test. IBM SPSS Statistics for Windows, Version 21.0 was used for analysis.
2.5. Ethics approval
As this was a descriptive study based on surveillance data collected by the hospital’s Epidemic/Pandemic Readiness Taskforce and only aggregate data was collected without patient identifiers, ethics approval was not required under our hospital’s Institutional Review Board guidelines.
3. Results
3.1. Etiology of community-acquired viral respiratory infections amongst hospitalized inpatients
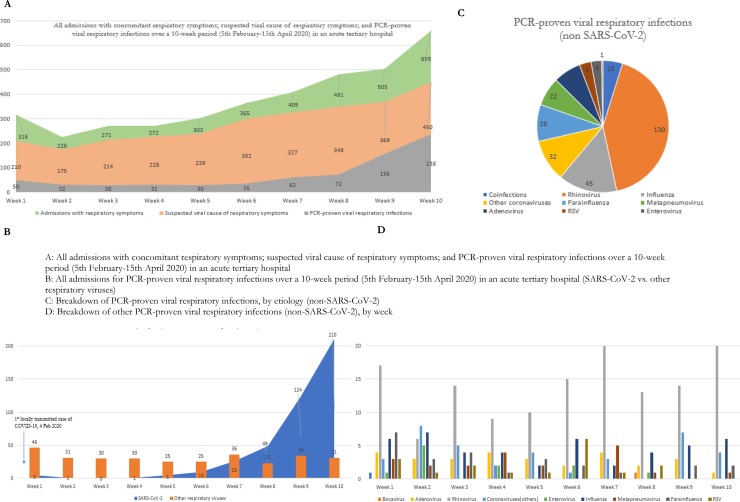
Over a 10-week period during an ongoing COVID-19 outbreak, there were a total of 3807 admissions with respiratory symptoms on admission; the admission numbers doubled over the study period, from 316 admissions in week 1 to 659 admissions in week 10 (Fig. 1 a). All patients were tested for COVID-19; almost three-quarters of admissions with respiratory symptoms (75.2%, 2862/3807) had a concurrent respiratory specimen processed for other respiratory viruses as well, given clinician suspicion of a potential viral etiology. Almost one-fifth (19.3%, 736/3807) of hospitalized inpatients with respiratory symptoms on admission were found to have a PCR-proven viral respiratory infection; of which 58.5% (431/736) tested positive for SARS-CoV-2 and 42.2% (311/736) tested positive for 1 or more other respiratory viruses. While the numbers of admissions attributed to common respiratory viruses remained fairly steady at a median of 30.5 admissions per week over the 10-week period, the number of admissions for COVID-19 increased from 5 cases in week 1 to 210 cases by week 10 (Fig. 1b). Of those who tested positive for other common respiratory viruses (Fig. 1c), the most common alternative etiology identified was rhinovirus (41.8%, 130/311), followed by influenza (14.4%, 45/311) and other coronaviruses (10.2%, 32/311). The number of admissions attributable to each common respiratory virus remained fairly stable over the 10-week period (Fig. 1d).
Fig. 1.
Epidemiology of hospitalised inpatients with community-acquired viral respiratory infections during an ongoing COVID-19 outbreak, over a 10 week period in a Singaporean tertiary hospital.
A: All admissions with concomitant respiratory symptoms; suspected viral cause of respiratory symptoms; and PCR-proven viral respiratory infections over a 10-week period (5th February-15th April 2020) in an acute tertiary hospital.
B: All admissions for PCR-proven viral respiratory infections over a 10-week period (5th February-15th April 2020) in an acute tertiary hospital (SARS-CoV-2 vs. other respiratory viruses).
C: Breakdown of PCR-proven viral respiratory infections, by etiology (non-SARS-CoV-2).
D: Breakdown of other PCR-proven viral respiratory infections (non-SARS-CoV-2), by week.
3.2. Co-infection rates and clinical outcomes
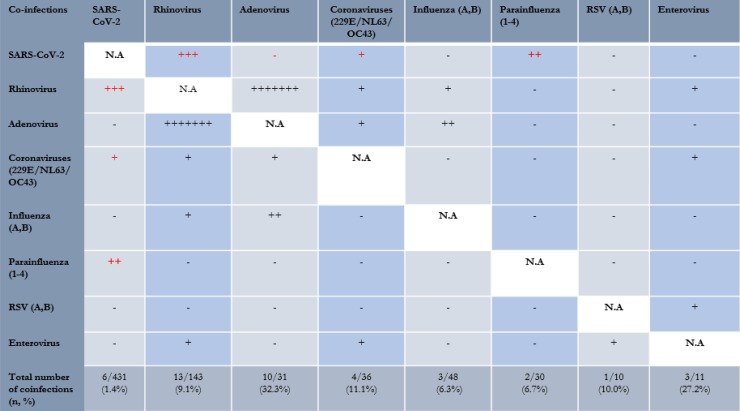
Amongst hospitalized inpatients with a PCR-proven community-acquired viral respiratory infection, 2.85% (21/736) had coinfections. A small minority (1.4%, 6/431) of patients positive for SARS-CoV-2 also tested positive for other viruses (3 rhinovirus, 2 parainfluenza, 1 other coronavirus) (Fig. 2 ). In contrast, 4.8% (15/311) of patients positive for other respiratory viruses had coinfections with more than one common respiratory virus. Patients positive for SARS-CoV-2 had lower odds of coinfection (1.4% vs. 4.8%, OR = 0.27, 95%CI = 0.11–0.72, p < 0.001) compared to those positive for other respiratory viruses. The clinical details of patients with COVID-19 and coinfections with other viral respiratory pathogens are found in Table 1 . Of note, all were foreign workers staying in a congregate setting (dormitories), and the majority (83.3%, 5/6) were contacts of a confirmed case of COVID-19 that shared accommodation or a workplace with them. All presented with upper respiratory tract infections. All cases of COVID-19 co-infections made a full recovery and did not progress to pneumonia, require supplemental oxygen or intubation and mechanical ventilation.
Fig. 2.
Distribution of co-infections between respiratory viruses.
Table 1.
COVID-19 patients coinfected with other respiratory viral pathogens (N = 6)
| Case no. | Demographic details | Epidemiology details | Cycle threshold (Ct) value for SARS-CoV-2 PCR of respiratory specimens at diagnosis | Coinfection | Presenting symptoms | Clinical syndrome (pneumonia or URTI) |
Clinical outcome |
|---|---|---|---|---|---|---|---|
| Case #1 | 31 yo Bangladeshi male | Stays in dormitory, 4 other roommates positive for COVID-19; 12 persons per room | 32.05 | Human coronavirus 229E | Fever, cough 2/7 prior to presentation | URTI | Full recovery |
| Case #2 | 31 yo Bangladeshi male | Stays in dormitory, 1 roommate positive for COVID-19; 6 persons per room | 21.23 | Rhinovirus | Cough 1/7 prior to presentation | URTI | Full recovery |
| Case #3 | 28 yo Bangladeshi male | Stays in dormitory, 1 roommate positive for COVID-19; 8 persons per room | 20.30 | Rhinovirus | Cough 2/7 prior to presentation | URTI | Full recovery |
| Case #4 | 27 yo Bangladeshi male | Stays in dormitory; 10 persons per room; 2 colleagues at construction site diagnosed with COVID-19 | 29.86 | Rhinovirus | Fever, rhinorrhea 3/7 prior to presentation | URTI | Full recovery |
| Case #5 | 28 yo Indian male | Stays in dormitory; 16 persons per room; no positive contact history | 18.00 | Parainfluenza | Fever and myalgia 2/7 prior to presentation | URTI | Full recovery |
| Case #6 | 30 yo Bangladeshi male | Stays in dormitory, 2 other roommates positive for COVID-19; 6 persons per room | 15.95 | Parainfluenza | Fever, altered taste, cough 2/7 prior to presentation | URTI | Full recovery |
Comparing clinical outcomes, amongst patients with community-acquired viral respiratory infections, there were 8 in-hospital mortalities over the period, one of which was COVID-19 related; the rest were attributable to other respiratory viruses (3 rhinovirus, 1 influenza, 1 adenovirus, 1 parainfluenza, 1 bocavirus). The in-hospital mortality rate for COVID-19 was 0.23% (1/431), compared to 1.62% (7/311) for those with other respiratory viruses; COVID-19 had lower odds of in-hospital mortality (OR = 0.10, 95%CI = 0.01–0.83, p = 0.01). Amongst patients with community-acquired viral respiratory infections, 2.17% (16/736) of patients required intubation and mechanical ventilation over the study period; five of which were COVID-19 related; the rest were attributable to other respiratory viruses (3 rhinovirus, 3 other coronaviruses, 2 influenza, 2 parainfluenza, 1 RSV). Only 1.2% (5/431) of patients with COVID-19 required intubation and mechanical ventilation over the study period, compared to 3.53% (11/311) for those with other respiratory viruses. Individuals infected with COVID-19 had lower odds of requiring mechanical ventilation compared to those with other respiratory viruses (1.2% vs. 3.53%, OR = 0.32, 95%CI = 0.11-0.93, p = 0.04).
4. Discussion
During a 10-week period of an ongoing COVID-19 outbreak with community transmission, all patients admitting with respiratory symptoms were isolated on arrival to rule out SARS-CoV-2 and the majority were concurrently tested for other common respiratory viruses. This allowed a more accurate estimate of the burden of co-infection between SARS-CoV-2 and other common respiratory viruses, particularly as testing was done in a systematic fashion over a sustained period. In line with early reports from China and elsewhere, the burden of co-infection detected in our population was low. The rate of co-infection with SARS-CoV-2 was 1.4%, similar to the rates of 0–3% reported in other cohorts [1,4,5]. While other studies cited co-infection in up to one-fifth of patients [9], this could potentially be due to spatiotemporal variation in viral epidemiology. In particular, in our cohort all cases of community-acquired viral co-infections with SARS-CoV-2 occurred amongst patients living in communal settings. Increased odds of viral respiratory infection have been associated with increased numbers of co-habitants [12]. Of note, the majority of co-infected individuals also reported ongoing outbreaks of COVID-19 in their accommodations. Previously, identification of co-circulation of human metapneumovirus and SARS-associated coronavirus during a nosocomial SARS outbreak raised the possibility of significant interaction; it was postulated that upper respiratory symptoms caused by one pathogen might enhance the dispersal of another through aerosol generation and contribute to increased infectivity [3]. However, co-infection did not appear to translate into increased morbidity or mortality. All individuals in our cohort with SARS-CoV-2 and other coinfections had mild disease; none required supplemental oxygen or intubation and mechanical ventilation. This is in keeping with other reports suggesting that patients with COVID-19 and coinfections did not have a more severe prognosis [1]. Common respiratory viruses account for a significant proportion of hospitalizations and remain a significant contributor to in-hospital mortality and morbidity [12,13], even during an ongoing COVID-19 outbreak. Notably, in our cohort, the in-hospital mortality rate and proportion of COVID-19 patients requiring invasive ventilation was low, at around 1% of patients; these rates were even lower than patients with other community-acquired respiratory viruses admitted to our institution over the same time period. While much higher rates of invasive ventilation and in-hospital mortality have been reported in other cohorts of COVID-19 patients [1,2,21], this is likely attributed to the continued functioning of our healthcare system and aggressive strategy of containment employed in Singapore over the time period of the study [[14], [15], [16]], in which all patients with COVID-19 were initially admitted to hospital, regardless of disease severity.
Reports of coinfections with SARS-CoV-2 and other respiratory pathogens have been used to argue against the utility of routine testing for non–SARS-CoV-2 respiratory pathogens during the COVID-19 pandemic, as a positive result for other respiratory pathogens does not exclude SARS-CoV-2 [9,22] and hospital resources are strained during an ongoing outbreak. While effective interventions for viral respiratory infections remain limited (eg. neuraminidase inhibitors for influenza), maintaining surveillance for common respiratory viruses may be of value in specific circumstances. In hospitals with large numbers of COVID-19 patients and limited numbers of single rooms, cohorting COVID-19 patients together may be a consideration. However, depending on the co-infection rate, it is possible that co-circulation of COVID-19 and other respiratory viruses may result in subsequent nosocomial outbreaks if such cohorting occurs [3]. Additionally, the COVID-19 outbreak has necessitated the introduction of various infection control interventions, such as enhanced cohorting, better distancing, and usage of personal protective equipment, in order to mitigate nosocomial spread [18]. These interventions likely have potential to reduce transmission of not just COVID-19, but also other respiratory viruses as well [23]. Given the in-hospital morbidity and mortality associated with viral respiratory infections, the ongoing COVID-19 outbreak affords a valuable opportunity to assess the effectiveness of these interventions, as screening for respiratory viral infection reduces risk of transmission to hospitalized inpatients [24]. However, to assess the effectiveness of interventions, surveillance is necessary to establish a baseline.
Our study has the following limitations. Being a single-centre study, differences in reported co-infection rates may be accounted for by spatiotemporal variations in viral epidemiology. To address this, we maintained surveillance for community-acquired respiratory viral infections over a 10-week period, to account for potential fluctuations that might occur in studies of shorter duration. Additionally, while seasonality is a well-known factor in outbreaks of viral respiratory infection in temperate countries, our study was conducted in a hospital in Singapore, a tropical country. Local studies have shown that while seasonality does exist, consistent seasonal variations are mainly observed only in limited numbers of pathogens [25]. This study focused on hospitalized inpatients; hospitalized inpatients may not represent the true burden of disease in the community. However, given the aggressive strategy of containment employed in Singapore [[14], [15], [16]], over the study period all patients with COVID-19 were admitted initially to hospital, regardless of disease severity. Hence, the co-infection rate reported in this study may be more representative, given that we detected coinfections solely in patients with mild disease, and exhaustive in-hospital testing was conducted for all suspected COVID-19 cases regardless of disease severity.
In conclusion, even amidst an ongoing COVID-19 outbreak, common respiratory viruses still accounted for a substantial proportion of hospitalizations over a 10-week study period. Co-infections between SARS-CoV-2 and other viral respiratory pathogens were rare and occurred mainly in individuals with mild disease staying in congregate settings; there was no increase in morbidity or mortality associated with co-infections. The in-hospital mortality rate and proportion of COVID-19 patients requiring invasive ventilation was low, at around 1% of patients; these rates were even lower compared to patients with other community-acquired respiratory viruses admitted over the same time period. Continued surveillance for other respiratory pathogens during an ongoing COVID-19 outbreak remains important, in order to identify the rate of co-infection, assess the burden of disease and evaluate the effectiveness of subsequent interventions.
Funding
This work was not grant-funded.
Conflict of interest
The authors report no conflicts of interest.
Acknowledgements
This work was not grant-funded.
References
- 1.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J. Med. Virol. 2020;(March (20)) doi: 10.1002/jmv.25781. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C.L., Wang Y.M., Li X.W. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-30185. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee N., Chan P.K., Yu I.T., Tsoi K.K., Lui G., Sung J.J., Cockram C.S. Co-circulation of human metapneumovirus and SARS-associated coronavirus during a major nosocomial SARS outbreak in Hong Kong. J. Clin. Virol. 2007;40(4):333–337. doi: 10.1016/j.jcv.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin D., Liu L., Zhang M., Hu Y., Yang Q., Guo J., Guo Y., Dai Y., Xu Y., Cai Y., Chen X., Zhang Z., Huang K. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020;(March (5)) doi: 10.1007/s11427-020-1668-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan B.E., Lim K.G.E., Chong V.C.L., Chan S.S.W., Ong K.H., Kuperan P. COVID-19 and mycoplasma pneumoniae coinfection. Am. J. Hematol. 2020;(March (15)) doi: 10.1002/ajh.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touzard-Romo F., Tapé C., Lonks J.R. Co-infection with SARS-CoV-2 and human metapneumovirus. R. I Med. J. (2013) 2020;103(2):75–76. [PubMed] [Google Scholar]
- 8.Chaung J., Chan D., Pada S., Tambyah P.A. Coinfection with COVID-19 and Coronavirus HKU1 - the critical need for repeat testing if clinically indicated. J. Med. Virol. 2020;(April (15)) doi: 10.1002/jmv.25890. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020 doi: 10.1001/jama.2020.6266. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evaluating and Testing Persons for Coronavirus Disease . 2019. (COVID-19). Centers for Disease Control and Prevention. Published March 14, 2020.https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html Accessed March 20, 2020. [Google Scholar]
- 11.Tay J.Y., Lim P.L., Marimuthu K., Sadarangani S.P., Ling L.M., Ang B.S.P., Chan M., Leo Y.S., Vasoo S. De-isolating COVID-19 suspect cases: a continuing challenge. Clin. Infect. Dis. 2020;(February (26)) doi: 10.1093/cid/ciaa179. pii: ciaa179 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh T.H., Hii K.C., Fieldhouse J.K., Ting J., Berita A., Nguyen T.T., Wong S.C., Wong T.M., Lim W.H., Ha S.J., Lau C.Z., Kong S.L., Bailey E.S., Warkentien T.E., Husain T.S., Gray G.C. High prevalence of viral infections among hospitalized pneumonia patients in equatorial Sarawak, Malaysia. Open Forum Infect. Dis. 2019;6(3) doi: 10.1093/ofid/ofz074. ofz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow E.J., Rolfes M.A., O’Halloran A., Alden N.B., Anderson E.J., Bennett N.M., Billing L., Dufort E., Kirley P.D., George A., Irizarry L., Kim S., Lynfield R., Ryan P., Schaffner W., Talbot H.K., Thomas A., Yousey-Hindes K., Reed C., Garg S. Respiratory and nonrespiratory diagnoses associated with influenza in hospitalized adults. JAMA Netw. Open. 2020;3(3) doi: 10.1001/jamanetworkopen.2020.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lum L.H., Tambyah P.A. Outbreak of COVID-19 - an urgent need for good science to silence our fears? Singapore Med. J. 2020;(February (13)) doi: 10.11622/smedj.2020018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong J.E.L., Leo Y.S., Tan C.C. COVID-19 in Singapore-current experience: critical global issues that require attention and action. JAMA. 2020;(February (20)) doi: 10.1001/jama.2020.2467. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Pung R., Chiew C.J., Young B.E., Chin S., Chen M., Clapham H.E., Cook A.R., Maurer-Stroh S., Toh M.P.H.S., Poh C.Q., Low M., Lum J., Koh V.T.J., Mak T.M., Cui L., Lin R., Heng D., Leo Y.S., Lye D., Lee V. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020 doi: 10.1016/S0140-6736(20)30528-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z.X., Yong Y., Tan W.C., Shen L., Ng H.S., Fong K.Y. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singapore Med. J. 2018;59(4):190–198. doi: 10.11622/smedj.2017079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wee L.E., Conceicao E.P., Sim X.Y.J., Aung M.K., Tan K.Y., Wong H.M. Minimising intra-hospital transmission of COVID-19: the role of social distancing. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.04.016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66(4):549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City aea. JAMA. 2020;(April (22)) doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaddour K., Sikora A., Tahir N., Nepomuceno D., Huang T. Case report: the importance of novel coronavirus disease (COVID-19) and coinfection with other respiratory pathogens in the current pandemic. Am. J. Trop. Med. Hyg. 2020;(April (17)) doi: 10.4269/ajtmh.20-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D., Lu J., Liu Y., Zhang Z., Luo L. Positive effects of COVID-19 control measures on influenza prevention. Int. J. Infect. Dis. 2020;(April (10)) doi: 10.1016/j.ijid.2020.04.009. pii: S1201-9712(20)30225-3 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mermel L.A., Jefferson J.A., Smit M.A., Auld D.B. Prevention of hospital-acquired respiratory viral infections: assessment of a multimodal intervention program. Infect. Control Hosp. Epidemiol. 2019;40(3):362–364. doi: 10.1017/ice.2018.337. [DOI] [PubMed] [Google Scholar]
- 25.Chew F.T., Doraisingham S., Ling A.E., Kumarasinghe G., Lee B.W. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol. Infect. 1998;121(August (1)):121–128. doi: 10.1017/s0950268898008905. [DOI] [PMC free article] [PubMed] [Google Scholar]