Abstract
Background
While evidence suggests that hydroxychloroquine (HCQ) may decrease the viral load in patients with a COVID-19 infection, a number of case reports indicate adverse dermatologic effects of this potential treatment.
Objective
To conduct a systematic review of previously reported cases of psoriasis onset, exacerbation, or relapse after HCQ treatment.
Methods
Embase and MEDLINE were comprehensively searched for original studies examining adverse effects of HCQ treatment related to psoriasis. Participant demographics and details of HCQ administration and psoriasis diagnosis were extracted from 15 articles representing 18 patients.
Results
Women accounted for a significantly larger number of cases of psoriasis compared with men and unreported sex (14 [77.8%] vs 2 [11.1%] vs 2 [11.1%], respectively). In addition, 50% (n = 9) of the patients did not have a history of psoriasis before taking HCQ. Of the 18 patients, 9 (50.0%) experienced de novo psoriasis, 5 (27.8%) experienced exacerbation of psoriatic symptoms, and 4 (22.2%) had a relapse of psoriasis after HCQ administration.
Conclusion
HCQ treatment may result in induction, exacerbation, or relapse of psoriasis. Monitoring for adverse effects of HCQ treatment is necessary, and clinical trials are essential in characterizing the safety profile of HCQ use in patients with a COVID-19 infection.
Key words: COVID-19, exacerbation, hydroxychloroquine, induction, Plaquenil, psoriasis, relapse
Capsule Summary.
-
•
Dermatologic effects of hydroxychloroquine are poorly understood.
-
•
Cases of new onset, relapse, or exacerbations of psoriasis have been reported with the use of hydroxychloroquine. It is especially important to monitor for such adverse effects during the potential use of hydroxychloroquine for treatment or prophylaxis in patients with a COVID-19 infection.
Hydroxychloroquine (HCQ) has been approved since 1955 for the prevention and treatment of malaria.1 Since then, its use has been extended to effectively treat a number of autoimmune disorders,2 such as systemic lupus erythematosus3 , 4 and rheumatoid arthritis.5 Evidence suggests that HCQ may also have potent antiviral properties. This discovery prompted recent investigations for the potential use of this drug to treat patients with COVID-19, a novel infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) initially reported in Wuhan, China, in December 20196 and resulting in more than 200,000 deaths worldwide by April 2020.7
Recent open-labeled, nonrandomized clinical trials showed that HCQ may decrease viral load and may improve outcomes in a small number of patients with COVID-19.8, 9, 10, 11 Despite the lack of strong evidence, the rapid spread of COVID-19 led the United States Food and Drug Administration to approve emergency use of HCQ in hospitalized patients who do not have alternative treatment options.12 However, the efficacy and safety profile of this drug are yet to be reported in ongoing randomized controlled trials.13
A number of case reports indicate adverse dermatologic effects of HCQ treatment, including new onset or exacerbation of psoriasis. Most recently, a 71-year-old patient with COVID-19 was reported to have an exacerbation of pre-existing psoriasis with silvery-scaled psoriatic plaques after 4 days of HCQ treatment.14
As the incidence of COVID-19 infections increase, it is important for health care providers to recognize and manage the relevant adverse effects associated with potential HCQ treatment. Therefore, this systematic review was conducted to comprehensively summarize existing literature on the new onset, exacerbations, or relapse of psoriasis after HCQ use. This will be an important step in assessing the potential dermatologic impact of HCQ.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Search strategy
The search was conducted using the Embase and MEDLINE databases in OVID on April 21, 2020. No language or date restrictions were applied. Variations of the following keywords were used for the search: “hydroxychloroquine,” “Plaquenil,” “chloroquine,” or “anti-malarial” in combination with “psoriasis,” “plaque,” “guttate,” “pustular,” “erythrodermic psoriasis,” or “psoriatic.”
Study eligibility criteria
Original articles that explored the effects of HCQ on psoriasis were included in this systematic review if they (1) involved human participants, (2) were observational (ie, case reports, case series, cross-sectional, or cohort studies) or experimental (ie, randomized controlled trials) studies, (3) involved HCQ as an intervention, (4) included patients with psoriasis, and (5) were written in the English language.
Study selection
Two reviewers (M.S and K.M.) independently screened titles, abstracts, and full texts of retrieved articles and determined study eligibility. Discrepancies or conflicts were resolved through discussion with a third reviewer (A.M.). Reference lists from all relevant articles were checked to identify additional studies not identified in the initial database search.
Data collection
Two reviewers (M.S and K.M.) independently reviewed and extracted data from each study using a structured form. Conflicts were reviewed collectively, and if consensus was not reached, a third reviewer was consulted (A.M.). Study design, patient demographic data, dose and frequency of HCQ, and details of psoriasis diagnosis and lesions were extracted and are summarized in Table I .14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Because there are no standardized response criteria for psoriatic lesions from HCQ use, we defined response as follows:
-
1.
“Exacerbation” was defined as worsening of existing psoriasis, in terms of severity or lesion count, after administration of HCQ.
-
2.
“Relapse” was defined as eruption of psoriatic lesions after administration of HCQ in individuals with a past medical history of psoriasis.
-
3.
“Induction” was defined as de novo eruption of psoriatic lesions after administration of HCQ, without a current or past medical history of psoriasis.
Table I.
The effect of hydroxychloroquine (HCQ) on psoriasis
| Study information |
Demographic information |
Information about HCQ |
Information about psoriatic lesions after HCQ administration |
Evidence level15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study type, year | Sample size | Age, sex | Comorbidities | Psoriasis history | Dose and frequency | Concurrent treatment (dose and frequency) | Latency period | Type of psoriasis | Outcome | Lesion description | Location | BSA/PASI score | |
| CR,16 2019 | 1 | 65, F | Rheumatoid arthritis | No | NR | Methylprednisolone (NR) | 1 week | Inverse psoriasis | Induction | Maculopapular, erythematous rash with silver hue and irregular borders | Scalp, face, neck, armpits, breasts, back, groin, buttocks, mouth | NR | 5 |
| CR,17 2018 | 1 | 41, F | Systemic lupus erythematosus | No | 200 mg twice daily | Prednisone (30-60 mg daily) | 2 months | Erythrodermic psoriasis | Induction | Erythroderma Toenails were yellow and showed hyperkeratosis |
Full body | BSA: 100% PASI: 61.2 |
5 |
| CR,18 2019 | 1 | 34, F | Systemic lupus erythematosus | No | 200 mg daily | Prednisolone (20 mg daily), tacrolimus (3 mg daily) | 3 weeks | Generalized pustular psoriasis | Induction | Pustular rash | Auricle, scalp, forearm 21 days after HCQ initiation | NR | 5 |
| CR,14 2020 | 1 | 71, F | COVID-19 infection | Yes | 2 × 400 mg first day, 2 × 200 mg daily | Oseltamivir (2 × 75 mg) | 4 days | NR | Relapse | Silver-scaled psoriatic plaques separated from the surrounding tissue with sharp borders | Full body | NR | 5 |
| CS,19 2014 | 2 | 40, F | Lichen planopilaris | No | 2 × 200 mg daily | None | 1 month | Pustular psoriasis of erythema centrifugum type | Induction | Erythematous papules and erythema migrans centrifugum-like skin lesions, with peripheral collarette of tiny superficial pustules | Lumbar and presternal areas, scalp | NR | 4 |
| 37, F | None | Yes | 100 mg daily | Methylprednisolone (NR) | 3 weeks | Pustular transformation of pregnancy-triggered psoriasis | Relapse | Suberythroderma with areas of extensive exfoliation and islands of healthy-looking skin partially covered by confluent superficial pustules | NR | NR | |||
| CR,20 2018 | 1 | 56, F | Crohn's disease, rheumatoid arthritis | Yes | 200 mg daily | Ustekinumab (90 mg every 2 months) | 1 year | Inverse psoriasis | Relapse | Large, well-demarcated pink plaques with minimal scale and a few satellite lesions with a collarette of scale | Vagina extending into the perineum, buttocks, and perianal area | NR | 5 |
| CR,21 1985 | 1 | 31, F | Psoriatic arthritis | Yes | 200 mg daily | None | 11 days | NR | Relapse | Generalized erythroderma with macular and popular lesions coalescing in a reticular pattern. Desquamation and bullae (0.5 cm-1cm) |
Face, arms, trunk and forearms | NR | 5 |
| CR,22 2015 | 1 | 50, F | Lichen planus pigmentosus | No | NR | NR | 4 weeks | NR | Induction | Multiple, thick, erythematous, scaly papules confluent into plaques Blue-gray ill-defined patches |
Scalp, ears, neck, back, chest, abdomen, bilateral upper and lower extremities, dorsal surfaces of hands and feet Face, neck, upper portion of chest, lower part of back, upper aspect of abdomen |
BSA: 80% | 5 |
| CR,23 1987 | 1 | 60, M | Rheumatoid arthritis | No | 200 mg twice daily | Prednisone (10 mg twice daily), naproxen (500 mg twice daily) | 3 weeks | NR | Induction | Generalized erythematous 1- to 2-mm popular and pustular eruption | Trunk, arms, hands, penis | NR | 5 |
| CR,24 1990 | 1 | 69, M | Pemphigus erythematous | No | 200 mg daily | Quinidine bisulfate (500 mg daily), isosorbide dinitrate (10 mg daily) | 2 weeks | Pustular psoriasis | Induction | Erythematous patches and multiple small pustules | Trunk and flexures | NR | 5 |
| CR,25 2015 | 1 | 57, F | Primary Sjogren syndrome (however, lack of sicca symptoms or mucosal dryness makes this diagnosis unlikely) Polyarthralgia |
No | NR | NR | 1 week | NR | Induction | Diffuse targetoid erythematous papules and plaques 15-20 hyperkeratotic 1-2 cm plaques |
Back | BSA: 80% | 5 |
| P,26 2015 | 2/114 | NR | Psoriatic arthritis | Yes | NR | NR | 3.5 years (mean) | NR | Exacerbation | Increase in psoriatic lesions | NR | NR | 4 |
| Psoriatic arthritis | Yes | NR | NR | 3.5 years (mean) | NR | Exacerbation | Increase in psoriatic lesions | NR | NR | ||||
| CS,27 1989 | 2 | 40, F | Systematic lupus erythematosus | Yes | 200 mg daily | Methotrexate (10 mg once/week) | 2 weeks | NR | Exacerbation | Plaque-like psoriatic lesions Bilateral malar patches |
Face and full body | 50% BSA | 4 |
| 25, F | Systematic lupus erythematosus | Yes | NR | NR | NR | NR | Exacerbation | Pustular lesions | Scalp, trunk, limbs | NR | |||
| CR,28 2016 | 1 | 70, F | Mixed connective tissue disorder | Yes | NR | NR | 2 weeks | Generalized pustular psoriasis | Exacerbation | Extensive erythematous patches | Full body | NR | 5 |
| CR,29 2010 | 1 | 55 F | None | No | NR | Prednisolone (0.5 mg/kg) | 3 weeks | NR | Induction | Thick, scaly psoriasiform plaques | Around eyes, upper neck, upper back | NR | 5 |
BSA, Body surface area; CR, case report; CS, case series; F, female; M, male; NR, not reported; PASI, Psoriasis Area and Severity Index; P, prospective.
Level of evidence evaluation and statistical analysis
The level of evidence for all included articles was assessed independently by 2 reviewers (M.S. and K.M.) using the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence.15 Owing to the considerable heterogeneity of the included studies, a descriptive analysis was undertaken.
Results
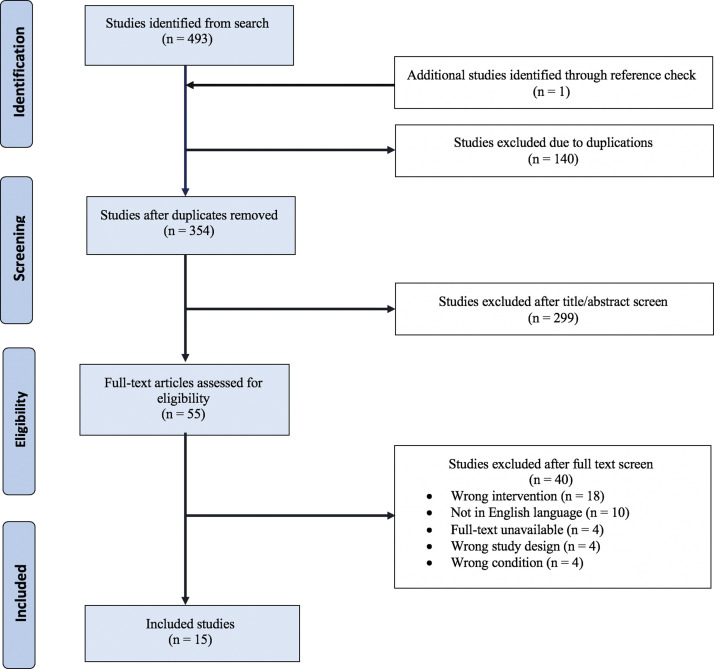
The search strategy yielded 354 records once duplicates were removed. After screening the titles and abstracts for relevance, 55 records were selected for a full-text review. In total, 15 studies met eligibility criteria and were used for data collection and analysis of 18 patients (Fig 1 , Table I).14 , 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 The analysis of the level of evidence showed that 3 studies (20.0%) had a level of evidence of 4,19 , 26 , 27 and 12 studies (80.0%) had a level of evidence of 5.14 , 16, 17, 18 , 20, 21, 22, 23, 24, 25 , 28 , 29 Overall, patients were aged between 25 and 71 years. There were 2 men (11.1%) and 14 women (77.8%), and the sex of 2 patients (11.1%) was not reported.
Fig 1.
Selection process for study inclusion in the systematic review.
Of 18 patients who reported psoriasis-related complications due to HCQ, 9 (50%) did not have a history of psoriasis before taking HCQ, and 9 (50.0%) did have a psoriasis diagnosis before HCQ treatment. Psoriasis history was not reported for 1 patient (5.6%).
Comorbidities were present in 88.9% (n = 16) of patients: 22.2% (n = 4) had systemic lupus erythematosus, 16.7% (n = 3) had rheumatoid arthritis, 16.7% (n = 3) had psoriatic arthritis, 11.1% (n = 2) had lichen planus, 5.6% (n = 1) had Crohn's disease, and 1 patient each had COVID-19, pemphigus erythematosus, mixed connective tissue disorder, polyarthralgia, and primary Sjogren syndrome.
Of the 18 patients, 50.0% (n = 9) experienced de novo psoriasis, 27.8% (n = 5) experienced exacerbation of psoriatic symptoms, and 22.2% (n = 4) had a relapse of psoriasis after HCQ administration. From the 9 de novo psoriasis cases, 33.3% (n = 3) had pustular psoriasis, 11.1% (n = 1) had inverse, 11.1% (n = 1) had erythrodermic, and the type of psoriasis was not recorded in 44.4% (n = 4). The type of psoriasis in the 9 patients experiencing exacerbation or relapse of psoriasis after HCQ treatment included 22.2% (n = 2) pustular, 11.1% (n = 1) inverse, and was not recorded in 66.7% (n = 6).
No pattern was noted in the location of the psoriatic lesions after HCQ use. Specifically, the distribution of lesions was 38.9% (n = 7), on the chest/abdomen, 33.3% (n = 6) on the limbs and digits, 27.8% (n = 5) on the scalp, 22.2% (n = 4) on the face, and 16.77% (n = 3) on the back groin/buttocks and neck, respectively. Four patients (22.2%) reported lesions covering the entire body.
Discussion
Psoriasis is an autoimmune, chronic inflammatory skin disease that may be induced or exacerbated by HCQ, a synthetic antimalarial drug commonly used for the treatment of autoimmune disorders such as systemic lupus erythematosus and rheumatoid arthritis.1 , 12 , 25 In the 15 studies identified in the literature, which included 18 patients, the data demonstrated that 50.0% of patients experienced a new diagnosis of psoriasis and that 50.0% of these patients experienced a relapse or an exacerbation of previously diagnosed psoriasis. In light of the potential for HCQ use to treat COVID-19 infections,6 , 30 it is important for health care providers to recognize the impact of HCQ on psoriasis onset, relapse, or exacerbation.
Although the exact mechanisms by which antimalarial drugs are able to induce psoriatic flares are not completely understood,19 several potential mechanisms have been implicated. An in vitro study conducted by Wolf et al31 noted hyperproliferation and irregular keratinization on skin cultures induced by HCQ. This may be due to the inhibiting effect HCQ has on epidermal transglutaminase activity, which leads to an initial break in the epidermal barrier. The resulting epidermal proliferation aimed at barrier restoration may lead to the induction or worsening of psoriasis.31 In addition, HCQ may promote the production of interleukin (IL)-17 via p38-dependant IL-23 release, resulting in increased keratinocyte growth.32 Furthermore, HCQ may interfere with the cholesterol metabolism process, which is crucial for the structural and functional integrity of the stratum corneum.33 Lastly, among the patients identified in this study, women predominated (77.8% [n = 14]). Given that autoimmune diseases are more prevalent in women, the role of sex hormones may be suggested.34
Our systematic review has several limitations that must be considered. Firstly, all summarized studies are case reports and case series. As a result, the lack of larger trials and the observational nature of the studies limit the scope of analysis and generalizability of our findings to all patients using HCQ treatment.
Additionally, attributing the development of psoriasis to HCQ use alone is difficult, because autoimmune comorbidities, such as rheumatoid arthritis and systemic lupus erythematosus, may predispose individuals to psoriasis due to dysregulation of common cytokines.17 , 18 It is important to note that 92.3% of the studied patients had comorbid conditions, with the autoimmune disorders rheumatoid arthritis (33.3%) and systemic lupus erythematosus (25.0%) being the 2 most significant. As a result, attributing causality between psoriatic development or exacerbation and HCQ use alone may be difficult, because these autoimmune comorbidities may predispose individuals to psoriasis due to dysregulation of common cytokines such as Il-17 and IL-23.35 , 36
Furthermore, 6 patients (33.3%) reported in this systematic review were also taking oral steroids. Of these 6 patients, 2 developed de novo erythrodermic psoriasis and pustular psoriasis respectively, and 1 experienced a relapse of pustular psoriasis. Given that oral steroids have been reported to induce or precipitate erythrodermic and pustular psoriasis, HCQ may not have been solely responsible for the induction or relapse of psoriasis in these cases.37 , 38
In addition to the known adverse effects of HCQ, such as QT prolongation, conduction abnormalities, and restrictive or dilated cardiomyopathy,39 , 40 it is also essential to understand the impact of HCQ on exacerbation, relapse, or new onset of psoriatic lesions. Although no robust peer-reviewed evidence exists at this time, a recent news article reported a higher mortality rate (27.8%) among patients with COVID-19 who were administered HCQ compared with those who were not (11.4%).41 This article brought attention to the significance of waiting for further evidence before extensive promotion and acceptance of this drug.
Additional studies examining the safety profile of HCQ, especially in patients with psoriasis, are desperately needed before its potential use for COVID-19 infection. Given the lack of rigorous evidence available, further studies with larger sample sizes are required to confirm the findings reported in this systematic review. Many randomized trials are actively recruiting participants to determine the safety profile of HCQ treatment in patients with COVID-19.42, 43, 44, 45, 46 The results from these studies will be key to determining whether HCQ treatment is significantly associated with exacerbation, relapse or new onset of psoriasis.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Yeung has been a speaker, consultant, and investigator for AbbVie, Allergan, Amgen, Astellas, Boehringer Ingelheim, Celgene, Centocor, Coherus, Dermira, Eli Lilly, Forward, Galderma, GSK, Janssen, Leo, MedImmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, Valeant, and Xenon. Ms Sachdeva, Mr Maliyar, and Drs Mufti and Lytvyn have no conflicts of interest to declare.
IRB approval status: Not applicable.
Reprints not available from the authors.
References
- 1.Balak D., Hajdarbegovic E. Drug-induced psoriasis: clinical perspectives. Psoriasis (Auckl) 2017;7:87–94. doi: 10.2147/PTT.S126727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rainsford K.D., Parke A.L., Clifford-Rashotte M., Kean W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 3.Ponticelli C., Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16(3):411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 4.Costedoat-Chalumeau N., Dunogué B., Morel N., Le Guern V., Guettrot-Imbert G. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med. 2014;43(6 Pt 2):e167–e180. doi: 10.1016/j.lpm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Olsen N.J., Schleich M.A., Karp D.R. Multifaceted effects of hydroxychloroquine in human disease. Semin Arthritis Rheum. 2013;43(2):264–272. doi: 10.1016/j.semarthrit.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y.-R., Cao Q.-D., Hong Z.-S., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han Y, Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective [e-pub ahead of print]. J Med Virol. doi:10.1002/jmv.25749, Accessed May 7, 2020. [DOI] [PMC free article] [PubMed]
- 8.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [e-pub ahead of print]. Int J Antimicrob Agents. doi:10.1016/j.ijantimicag.2020.105949, Accessed May 7, 2020. [DOI] [PMC free article] [PubMed] [Retracted]
- 9.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z., Hu J., Zhang Z., et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial [preprint]. Version 2. medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3. [Google Scholar]
- 11.Yan D., Zhang Z. Therapeutic effect of hydroxychloroquine on novel coronavirus pneumonia (COVID-19). Chinese Clinical Trials Registry. http://www.chictr.org.cn/showproj.aspx? proj=48880 Available at:
- 12.U.S. Food and Drug Administration Letter: Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease. 2020. https://www.fda.gov/media/136534/download Available at:
- 13.Sahraei Z., Shabani M., Shokouhi S., Saffaei A. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents. 2020;55(4):105945. doi: 10.1016/j.ijantimicag.2020.105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutlu Ö., Metin A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID-19: will cases of psoriasis increase after COVID-19 pandemic? Dermatol Ther. 2020:e13383. doi: 10.1111/dth.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centre for Evidence Based Medicine (CEBM) Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009) 2016. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009 Available at:
- 16.Ullah A., Zeb H., Khakwani Z., Murphy F.T. Hydroxychloroquine-induced inverse psoriasis. BMJ Case Rep. 2019;12(2):bcr-2018. doi: 10.1136/bcr-2018-224619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W.M., Wang K.Y., Wang T., Jin H.Z., Fang K. Hydroxychloroquine-induced psoriasis-form erythroderma in a patient with systemic lupus erythematosus. Chin Med J (Engl) 2018;131(15):1887–1888. doi: 10.4103/0366-6999.237411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shindo E., Shikano K., Kawazoe M., et al. A case of generalized pustular psoriasis caused by hydroxychloroquine in a patient with systemic lupus erythematosus. Lupus. 2019;28(8):1017–1020. doi: 10.1177/0961203319854139. [DOI] [PubMed] [Google Scholar]
- 19.Gravani A., Gaitanis G., Zioga A., Bassukas I.D. Synthetic antimalarial drugs and the triggering of psoriasis–do we need disease-specific guidelines for the management of patients with psoriasis at risk of malaria? Int J Dermatol. 2014;53(3):327–330. doi: 10.1111/ijd.12231. [DOI] [PubMed] [Google Scholar]
- 20.Darwin E., Deshpande A., Lev-Tov H. Development of drug-induced inverse psoriasis in a patient with Crohn's disease. ACG Case Rep J. 2018;5:e47. doi: 10.14309/crj.2018.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slagel G.A., James W.D. Plaquenil-induced erythroderma. J Am Acad Dermatol. 1985;12(5):857–862. doi: 10.1016/s0190-9622(85)70108-9. [DOI] [PubMed] [Google Scholar]
- 22.Seminario-Vidal L., Hughey L.S. Hydroxychloroquine de novo-induced psoriasis in a patient with lichen planus pigmentosus. Skinmed. 2015;13(6):492. [PubMed] [Google Scholar]
- 23.Friedman S.J. Pustular psoriasis associated with hydroxychloroquine. J Am Acad Dermatol. 1987;16(6):1256–1257. doi: 10.1016/s0190-9622(87)80021-x. [DOI] [PubMed] [Google Scholar]
- 24.Lotem M., Ingber A., Segal R., Sandbank M. Generalized pustular drug rash induced by hydroxychloroquine. Acta Derm Venerol. 1990;70(3):250–251. [PubMed] [Google Scholar]
- 25.McCoy S., Nagaraja V., Paviol S., Gudjonsson J.E., Kahlenberg J.M. Exacerbation of psoriasis due to hydroxychloroquine. Arch Med. 2015;7:1–3. [Google Scholar]
- 26.Aydın S., Yılmazer B., Bayındır Ö., et al. AB0827 Hydroxychloroquine does not increase psoriasis in psoriatic arthritis: time on drug analysis based on real life data. Ann Rheum Dis. 2015;74:1177. [Google Scholar]
- 27.Green L.S., Saperia D., Lowe N.J., David M. Coexistent psoriasis and lupus erythematosus: successful therapy with etretinate. J Dermatolog Treat. 1989;1(1):19–22. [Google Scholar]
- 28.Maglie R., Dini V., Romanelli M. Pustular psoriasis induced by hydroxychloroquine: a case report to confirm a rare association: 3734. J Am Acad Dermatol. 2016;74(5) [Google Scholar]
- 29.Ahmad K., Ramsay B. Evolution of signs… rethink… rebiopsy: P2208. J Am Acad Dermatol. 2010;62(3):AB79. [Google Scholar]
- 30.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf R., Schiavo A.L., Lombardi M.L., Angelis F.D., Ruocco V. The in vitro effect of hydroxychloroquine on skin morphology in psoriasis. J Dermatol. 2001;38(2):154–157. doi: 10.1046/j.1365-4362.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- 32.Said A., Bock S., Lajqi T., Müller G., Weindl G. Chloroquine promotes IL-17 production by CD4+ T cells via p38-dependent IL-23 release by monocyte-derived Langerhans-like cells. J Immunol. 2014;193(12):6135–6143. doi: 10.4049/jimmunol.1303276. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Irastorza G., Ramos-Casals M., Brito-Zeron P., Khamashta M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(01):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 34.Moroni L., Bianchi I., Lleo A. Geoepidemiology, gender and autoimmune disease. Autoimmun Rev. 2012;11(6-7):A386–A392. doi: 10.1016/j.autrev.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Ayala-Fontánez N., Soler D.C., McCormick T.S. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl) 2016;6:7. doi: 10.2147/PTT.S64950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furue K., Ito T., Tsuji G., Kadono T., Nakahara T., Furue M. Autoimmunity and autoimmune co-morbidities in psoriasis. Immunology. 2018;154(1):21–27. doi: 10.1111/imm.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elston G.E., Charles-Holmes R., Carr R.A. Precipitation of generalized pustular psoriasis by prednisolone. Clin Exp Dermatol. 2006;31(1):133–134. doi: 10.1111/j.1365-2230.2005.01910.x. [DOI] [PubMed] [Google Scholar]
- 38.Rendo M., Boster J., Dalton S.R., Yun H. An uncommon presentation of erythrodermic psoriasis in a patient without a history of psoriasis. Cureus. 2019;11(7):e5099. doi: 10.7759/cureus.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro M., Levy Y. The association between hydroxychloroquine treatment and cardiovascular morbidity among rheumatoid arthritis patients. Oncotarget. 2017;9(5):6615–6622. doi: 10.18632/oncotarget.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jallouli M. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus. JAMA Dermatol. 2013;149(8):935. doi: 10.1001/jamadermatol.2013.709. [DOI] [PubMed] [Google Scholar]
- 41.Cohen E., Nigam M. Study finds no benefit, higher death rate in patients taking hydroxychloroquine for Covid-19. April 21, 2020. CNN Health. https://www.cnn.com/2020/04/21/health/hydroxychloroquine-veterans-study/index.html Available at:
- 42.ClinicalTrials.gov Hydroxychloroquine Treatment for Severe COVID-19 Pulmonary Infection (HYDRA Trial) (HYDRA). Identifier NCT04315896. March 20, 2020. Bethesda (MD): National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04315896?id= NCT04315896 Available at:
- 43.ClinicalTrials.gov The PATCH Trial (Prevention And Treatment of COVID-19 With Hydroxychloroquine) (PATCH). Identifier NCT04329923. April 9 2020. Bethesda (MD): National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04329923?id=NCT04329923 Available at:
- 44.ClinicalTrials.gov. Hydroxychloroquine for COVID-19 (COV-HCQ). Identifier NCT04342221. April 10, 2020. Bethesda (MD): National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04342221?id=NCT04342221 Available at:
- 45.ClinicalTrials.gov A Randomized Controlled Clinical Trial: Hydroxychloroquine for the Treatment of COVID-19 in Hospitalized Patients (OAHU-COVID19). Identifier NCT04345692. April 10, 2020. Bethesda (MD): National Library of Medicine (US) https://clinicaltrials.gov/ct2/show/NCT04345692?id= NCT04345692 Available at:
- 46.ClinicalTrials.gov Hydroxychloroquine in Outpatient Adults With COVID-19). Identifier NCT04333654. April 10, 2020. Bethesda (MD): National Library of Medicine (US) 2000. https://clinicaltrials.gov/ct2/show/NCT04333654?id=NCT04333654 Available from: