To the Editor: Biologic agents have revolutionized psoriasis treatment.1 However, they are considered “immunosuppressive,” and thus, safety assessments focus on infection, particularly those that are serious or opportunistic, or both. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has focused attention on respiratory track infections (RTIs).2 The conceptual model of COVID-19 is that immunosuppression early in disease may be harmful, yet may be helpful in “late” severe COVID-19 illness; which may be mediated by a dysregulated hyperimmune response characterized by proinflammatory cytokines including interleukin 17 (IL-17).3
The effect of IL-17 inhibitors on COVID-19 is unknown, neither the risk of initial infection nor the risk of progression to worse disease. Current understanding of viral immunology suggests that IL-17 is not a dominant cytokine in viral immunity; however, IL-17 is important to mucosal immunity, raising the hypothesis that biologics targeting IL-17 could potentially increase RTI risk.4
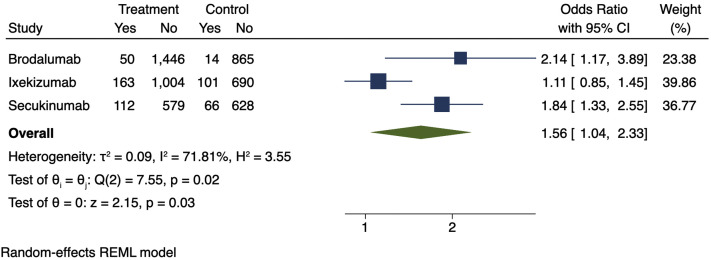
To test this hypothesis, we calculated a meta-estimate from the placebo-controlled period of phase 3 pivotal IL-17 trials of terms consistent with RTI of secukinumab, ixekizumab, and brodalumab abstracted from United States Food and Drug Administration prescribing information. RTI is a broad term classified by clinical judgment. The Medical Dictionary for Regulatory Activities (MedDRA), used to classify adverse events (AEs), has multiple terms for RTIs. To assess for RTIs, we summed the number of AEs that are associated with RTIs, divided by the total number of subjects in each study, and then calculated a meta-estimate. We found an increased risk of RTIs in the groups receiving IL-17 inhibitors compared with placebo (odds ratio, 1.56; 95% confidence interval, 1.04-2.33; Fig 1 ).
Fig 1.
Meta-estimate of respiratory tract infections (includes “upper respiratory tract infections,” “nasopharyngitis,” “rhinorrhea,” “influenza,” “oropharyngitis,” “pharyngitis,” and “pharyngolaryngeal pain”) from prescribing information adverse events tables. Doses used in this meta-estimate: secukinumab, 300 mg; brodalumab, 210 mg; and ixekizumab, 80 mg every 2 weeks, because these doses are indicated for moderate to severe psoriasis. The size of the square corresponds to the relative weight assigned in the pooled analysis, and the horizontal lines indicate the confidence interval (CI). The diamond denotes the overall effect size, and the lateral tips of the diamond indicate the associated CI. REML, Restricted maximum likelihood.
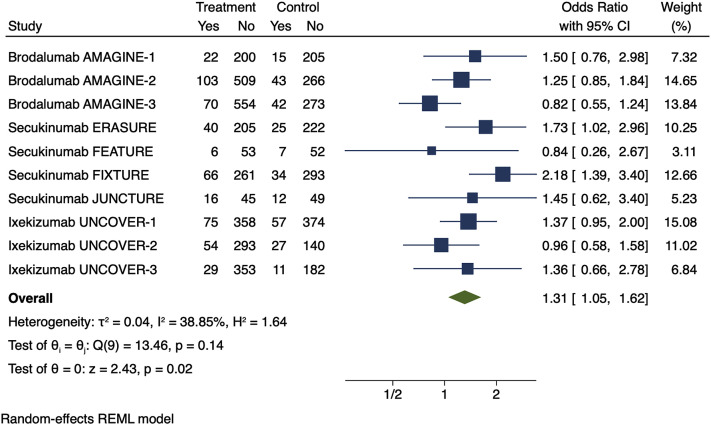
Because prescribing information is not inclusive of all respiratory AEs from the pivotal trials that supported approval of IL-17 inhibitors, we conducted a summary risk estimate using data from the placebo-controlled period of these studies obtained from clinicaltrials.gov. This more detailed analysis yielded similar findings to our meta-estimate of prescribing information data (odds ratio, 1.31; 95% confidence interval, 1.05-1.62; Fig 2 ). Sensitivity analyses varying the terms analyzed yielded similar findings but with loss of statistical significance.
Fig 2.
Meta-estimate of respiratory tract infections (includes “upper respiratory tract infections,” “viral respiratory tract infections,” “influenza,” “influenza-like illness,” “sinusitis,” “pharyngitis,” “bronchitis,” “cough,” “nasopharyngitis,” “oropharyngeal pain,” and “pneumonia”) from clinicaltrials.gov in the phase 3 randomized control trials that were submitted for United States Food and Drug Administration approval. Doses used in this meta-estimate: secukinumab, 300 mg; brodalumab, 210 mg; and ixekizumab, 80 mg every 2 weeks, because these doses are indicated for moderate to severe psoriasis. The size of the square corresponds to the relative weight assigned in the pooled analysis, and the horizontal lines indicate the confidence interval (CI). The diamond denotes the overall effect size, and the lateral tips of the diamond indicate the associated CI. REML, Restricted maximum likelihood.
Evaluating the risk of RTI in clinical trials is difficult because the diagnosis is made clinically without objective testing, and therefore, the etiology of these symptoms, be they viral, bacterial, fungal, or allergic, is unknown. Furthermore, there is substantial variation in the rates of RTIs in the placebo groups across the trials, demonstrating a lack of precision in measuring this outcome. For example, rates of “upper RTI” ranged from 0.0% to 7.44% in the placebo groups evaluated. In addition, owing to variation in reporting of MedDRA terms, the events were unevenly pooled because terms are reported inconsistently. It is also possible that patients may have had more than one RTI event, which could impact our estimates.
These findings highlight the need for more meticulous evaluation of the impact of IL-17 inhibitors on RTIs in the setting of the novel coronavirus pandemic. Nevertheless, our meta-estimate demonstrates a potential safety signal for RTI associated with IL-17 inhibition and supports guidance issued by American Academy of Dermatology that clinicians should use their clinical judgment to continue or discontinue patients on these drugs in patients who have not tested positive or exhibited symptoms of COVID-19 and to discontinue these agents in patients who test positive for COVID-19 symptoms.5
Footnotes
Funding sources: Supported in part by a grant (P30-AR0-69589-03) from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases to Dr Gelfand.
Conflicts of interest: Dr Gelfand served as a consultant for Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Janssen Biologics, Novartis Corp, Regeneron, UCB (Data Safety and Monitoring Board), Sanofi, and Pfizer Inc, receiving honoraria; receives research grants (to the Trustees of the University of Pennsylvania) from AbbVie, Janssen, Novartis Corp, Sanofi, Celgene, Ortho Dermatologics, and Pfizer Inc, and has received payment for continuing medical education work related to psoriasis that was supported indirectly by Eli Lilly and Company and Ortho Dermatologics. In addition, Dr Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma, and is a deputy editor for the Journal of Investigative Dermatology, receiving honoraria from the Society for Investigative Dermatology. Dr Wan is supported in part by a grant from Pfizer. Dr Winthrop receives grants from Bristol-Myers Squibb and Pfizer, and is a consultant for UCB, AbbVie, Eli Lilly and Company, Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, and Roche.
IRB approval status: Not applicable.
Reprints not available from the authors.
References
- 1.Menter A., Strober B.E., Kaplan D.H., et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 2.Lebwohl M., Rivera-Oyola R., Murrell D.F. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82(5):1217–1218. doi: 10.1016/j.jaad.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schett G., Sticherling M., Neurath M.F. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20(5):271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das S., Khader S. Yin and yang of interleukin-17 in host immunity to infection. F1000Res. 2017;6:741. doi: 10.12688/f1000research.10862.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Dermatology Association Guidance on the use of immunosuppressive agents. https://www.aad.org/member/practice/coronavirus/clinical-guidance/biologics Available at: