To the Editor:
The first case of SARS-CoV-2 infection in India was reported on January 30, 2020, from Kerala.1 The disease since then has increased manifold to reach figures of over 45,000 infections across the country, making a significant impact on healthcare with drastic changes in clinical practice. Multiple society guidelines have been published since the outbreak of the virus, with a major focus on screening and precautions for patients undergoing endoscopy. Continuing hospital services in a smooth and effective manner while taking care of patients’ and caregivers’ safety remains a priority. COVID-19 has had its economic impact; hospitals have cut down on elective procedures, affecting patient care and also the revenue generated. One question that has remained largely unanswered in all guidelines is whether we should routinely test for COVID-19 before elective and semiurgent endoscopies.
A recent study from Duval County in Florida by Corral et al2 tried to analyze the economic and health effects of interventions, namely, endoscopies for urgent indications alone, with testing done beforehand without awaiting the results, versus testing before semiurgent procedure, versus testing all patients and performing semiurgent and elective endoscopies. The authors had been performing only 12.8% of the endoscopy volume done before COVID-19. On the other hand, by using testing in patients with semiurgent indications, they would have been able to do 19% of procedures with an additional cost of $22 U.S. dollars (USD) for polymerase chain reaction (PCR) testing per patient. On the other hand, the third strategy of testing all patients would help increase the caseload back to 95%, but with an additional cost of $105 USD per patient for PCR testing. The model they designed tried to factor, based on the prevalence of disease in the community, what the costs would be, also accounting for standard endoscopy costs across the United States. Considering the low prevalence of disease, with low rates of false negative results, the rates of healthcare professionals infected per week would be low (<1), if breaches of personal protective equipment (PPE) do not occur. The weekly costs incurred according to each strategy would be $6 million, $13 million, and $64 million USD, respectively. The net gain for providers factoring endoscopy costs would be $75 million, $165 million, and $767 million USD, respectively. The authors concluded that testing before endoscopy remains a reasonable strategy for prevention over a 3-month period, considering the rate of infection, helping to generate significant revenue despite the money spent on testing.
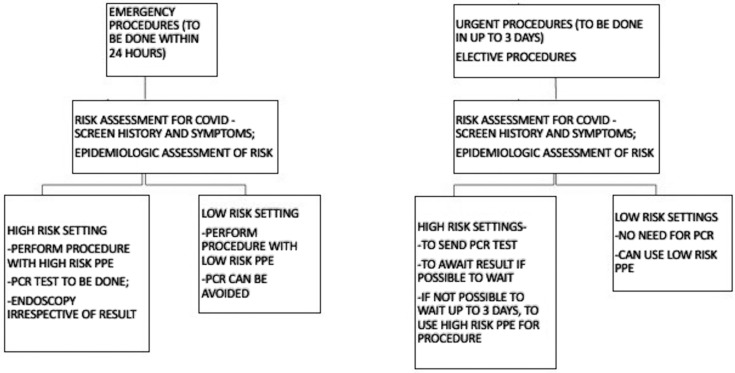
India is a unique healthcare setting; the costs of healthcare interventions are very low.3 The average upper GI endoscopy cost in India is between rupees (Rs) 2000 and Rs 4000 (∼$30 to $60 USD). Colonoscopy costs are between Rs 7000 and Rs 10,000 (∼$100 to $140 USD). Meanwhile, the average testing cost for novel coronavirus PCR is Rs 4500 (∼$60 to $65 USD) in private laboratories. Also, the report may not be available right away, considering that pool testing is done at various centers. The low endoscopy costs and higher PCR test costs may not justify PCR testing for all patients. The population prevalence of the disease in India is approximately 3.6 per 100,000 as compared with 122 per 100,000 in the study by Corral et al.2 The chances of disease detection fall significantly, considering the low prevalence. The disease prevalence in high-risk pockets like Mumbai are close to 70 per 100,000 population, which is much less than the prevalence in the study previously quoted. However, an important consideration becomes the likelihood that disease prevalence will increase further. Hence, testing for all may not be the right strategy at the moment. However, constant appraisal of the situation will guide us better for further decisions on testing. Adequate screening before patient assessment and endoscopy remains the cornerstone for prevention. Clinical judgement should take precedence over laboratory investigations to decide the necessity of investigations. The prudent use of PPE appropriate to the risk setting remains imperative and cannot be overemphasized. We designed an algorithm for restarting semiurgent and elective procedures once there is de-escalation of isolation measures (Fig. 1 ).
Figure 1.
Algorithm for testing before endoscopic procedures. High-risk clinical setting includes symptomatic patient with severe acute respiratory syndrome or influenza-like illness or asymptomatic contact with COVID-positive patient. High-risk epidemiologic setting includes hailing from a high-prevalence area/hotspot/containment zone; areas where no cases are reported for 14 days can be classified as low-risk epidemiologic setting. PCR, Polymerase chain reaction; PPE, personal protective equipment.
Our reliance on PCR makes it difficult to test all individuals, considering the logistic and financial difficulties. Serologic tests with antibody testing may be the solution, where tests can be offered for all individuals. However, current first-generation enzyme-linked immunoassays for COVID-19 IgM and IgG are still in the stages of evolution and require validation in our setting.4 The caveat is also that early stages of the disease may not be detected, leading to increased infections in the hospital. The American Enterprise Institute has provided a roadmap to reopening after the coronavirus pandemic.5 India is likely to go from phase 1 to phase 2 after lockdown measures are relaxed. Despite our slogan being “Go Corona Go,” I guess that the virus is here to stay. What remains crucial is to build our disease surveillance, testing, and treatment capacity to smooth the transition. To conclude, we may still not be ready for prime time with PCR testing for all patients, largely because we may not need it in the first place at the moment.
Disclosure
The author disclosed no financial relationships.
References
- 1.Yadav P.D., Potdar V.A., Choudhary M.L. Full-genome sequences of the first two SARS-CoV-2 viruses from India. Indian J Med Res. 2020;151:200–209. doi: 10.4103/ijmr.IJMR_663_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corral JE, Hoogenboom SA, Kröner PT, et al. COVID-19 polymerase chain reaction testing before endoscopy: an economic analysis. Gastrointest Endosc. Epub 2020 Apr 28. [DOI] [PMC free article] [PubMed]
- 3.Chatterjee S., Levin C., Laxminarayan R. Unit cost of medical services at different hospitals in India. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA. Epub 2020 Apr 17. [DOI] [PubMed]
- 5.National coronavirus response: a road map to reopening. American Enterprise Institute; 2020. https://www.aei.org/research-products/report/national-coronavirus-response-a-road-map-to-reopening/ Available at: [Google Scholar]