Highlights
-
•
Considerable portion of COVID-19 patients presented depression and anxiety symptoms.
-
•
CRP levels correlated with the scores of PHQ-9 in patients with depression features.
-
•
The more improvement of CRP level resulted in lower level of depression.
-
•
Stigma and uncertainty of the disease were two main concerns among COVID-19 patients.
Keywords: Stress, Depression, COVID-19, Mixed-method, Peripheral inflammation
Abstract
Since the end of 2019, Corona Virus Disease 2019 (COVID-19) has been the cause of a worldwide pandemic. The mental status of patients with COVID-19 who have been quarantined and the interactions between their psychological distress and physiological levels of inflammation have yet to be analyzed. Using a mixed-method triangulation design (QUAN + QUAL), this study investigated and compared the mental status and inflammatory markers of 103 patients who, while hospitalized with mild symptoms, tested positive with COVID-19 and 103 matched controls that were COVID-19 negative. The severity of depression, anxiety, and post-traumatic stress symptoms (PTSS) was measured via an on-line survey. Using a convenience sampling technique, qualitative data were collected until the point of data saturation. In addition, a semi-structured interview was conducted among five patients with COVID-19. Peripheral inflammatory markers were also collected in patients, both at baseline and within ± three days of completing the on-line survey. Results revealed that COVID-19 patients, when compared to non-COVID controls, manifested higher levels of depression (P < 0.001), anxiety (P < 0.001), and post-traumatic stress symptoms (P < 0.001). A gender effect was observed in the score of “Perceived Helplessness”, the subscale of PSS-10, with female patients showing higher scores compared to male patients (Z = 2.56, P = 0.010), female (Z = 2.37, P = 0.018) and male controls (Z = 2.87, P = 0.004). Levels of CRP, a peripheral inflammatory indicator, correlated positively with the PHQ-9 total score (R = 0.37, P = 0.003, Spearman’s correlation) of patients who presented symptoms of depression. Moreover, the change of CRP level from baseline inversely correlated with the PHQ-9 total score (R = -0.31, P = 0.002), indicative of improvement of depression symptoms. Qualitative analysis revealed similar results with respect to patient reports of negative feelings, including fear, guilt, and helplessness. Stigma and uncertainty of viral disease progression were two main concerns expressed by COVID-19 patients. Our results indicate that significant psychological distress was experienced by hospitalized COVID-19 patients and that levels of depressive features may be related to the inflammation markers in these patients. Thus, we recommend that necessary measures should be provided to address depression and other psychiatric symptoms for COVID-19 patients and attention should be paid to patient perceived stigma and coping strategies when delivering psychological interventions.
1. Introduction
Since December 2019, a novel coronavirus with person-to-person transmission, which was named by WHO as Corona Virus Disease 2019 (COVID-19) (WHO, 2020), emerged from China and rapidly spread to many other countries becoming a worldwide pandemic (Chan et al., 2020, Cheng and Shan, 2020, Li et al., 2020a, Phan et al., 2020, Zhu et al., 2020). The escalating global morbidity and mortality of COVID-19 has raised significant public health and economic concerns. As of April 20th, 2020, the PRC officially reported that the total number of confirmed COVID-19 cases had reached more than 88,000, while the total number of international cases surged to more than 2,400,000. Previous studies of similar viral respiratory diseases, such as severe acute respiratory syndrome (SARS), have shown that the infected patients present with varying degrees of mental health problems even after being discharged from hospital, indicating that the mental status of these individuals should not be ignored (Cheng et al., 2004, Mak et al., 2009).
Based on this observation, the National Health Commission of China released several mental health guidelines for COVID-19 patients. One focused on basic principles for emergency psychological crisis interventions in pneumonia for novel coronavirus infections (National Health Commission of China, 2020). However, these documents were developed and disseminated based on evidence-based studies from non-COVID epidemics. To date, only one brief report (Bo et al., 2020) has investigated the post-traumatic stress symptoms experienced by COVID-19 patients.
According to previous studies, survivors of viral infectious diseases are prone to depression (Kuhlman et al., 2018), anxiety (Wheaton et al., 2012), adjustment disorder (van Hoek et al., 2011), acute stress-related disorder (Koopman et al., 1995), and post-traumatic disorder (Noone, 2013). Studies exploring the psychological effects during the 2002–2004 SARS outbreak in China highlighted the anxiety and depression that emerged immediately after the epidemic (Cheng et al., 2004, Wu et al., 2005). At six months post-hospital discharge, approximately 25 percent of survivors experienced significant depression, and approximately 8.3 percent of survivors had adjustment disorder or Posttraumatic Stress Disorder (PTSD) (Wing and Ho, 2004). At 30 months post-SARS, 25 percent of the patients met diagnostic criteria for PTSD, while 15.6 percent had depressive disorders (Mak et al., 2009).
Previous studies were usually conducted after the patients were discharged from hospital and thus over relied on quantitative methodology. Conversely, combining quantitative and qualitative approaches and collecting data germane to the immediate psychological distress experienced by infected patients may result in a more accurate and comprehensive understanding of a viruses’ impact on mental status (Guetterman et al., 2015, Pluye and Hong, 2014).
Additionally, the presence of mood and anxiety disorders may be highly correlated to the severity of physiological status, especially as it is reflected in blood levels of peripheral inflammation markers. Studies have consistently reported that altered levels of peripheral C-reactive protein (CRP) (Valkanova et al., 2013), white blood cell (WBC) count (Shafiee et al., 2017), and excessive cytokines (Köhler et al., 2017) correlate with symptoms of depression and anxiety. Therefore, this investigation into the interactions between mental status and inflammatory marker levels in COVID-19 patients may assist in a better understanding of the psychological impacts of this disease.
In our study, we used a mixed-methods approach to examine the immediate impact of social-psychological factors on COVID-19 patients (mild cases) who are in quarantine. We also explored the relationship between psychological distress and levels of peripheral inflammatory markers found in blood tests of COVID-19 patients.
2. Method
2.1. Design and participants
This cross-sectional study utilized a mixed-methods approach to investigate the mental status of hospitalized patients with COVID-19 and how it relates to the presence bio-markers of peripheral inflammation. Data were collected during the COVID-19 pandemic from February 10th to February 28th, 2020. Patients were recruited primarily from the Shanghai Public Health Clinical Center (Shanghai, China), except for two patients from two other provinces (Sichuan and Hubei) who were willing to participate in a telephone interview. Patients were eligible for inclusion if they volunteered, were 18 years or older, and could respond to on-line survey questions. Using diagnostic criteria found in the the 4th version of “Diagnosis and management program of novel coronavirus-infected pneumonia” released by the National Health Commission of The People's Republic of China (China, 2020), 103 patients diagnosed with mild cases of COVID-19 were included in this study. Peripheral blood samples were voluntarily obtained from hospitalized patients and all were found to contain viral nucleic acid consistent with COVID-19. None of the participants had lymphatic system disorders or malignant hematologic diseases, ensuring that the whole blood parameters were representative of typical baseline values. The 103 normal controls (NCs) were matched with the patients for age, gender, education level, and place of residence. None of the NCs had been diagnosed with COVID-19 or suspected for infection with COVID-19. NCs were recruited from local communities and volunteered to participate in our on-line survey. None of them reported a history of being diagnosed with any mental disorder, using diagnostic criteria of the International Classification of Diseases, tenth version (ICD-10).
The purpose of the on-line survey was explained in plain language to patients, “We are trying to understand how the infection of COVID-19 affects your mental status and daily-life.” The NCs were told, “We are trying to understand how the outbreak of COVID-19 affects your mental status and daily-life recently.” Verbal informed consent was provided to participants prior to the enrollment and all participants answered “Agreed” to the informed consent associated with the on-line survey. This study was approved by the Local Research Ethics Committee, Institutional Review Board, Shanghai Mental Health Center (2020–17).
2.2. Demographic data and clinical assessments
All participants completed the on-line survey with their cell phones using the Chinese professional survey website Wenjuanxing (www.sojump.com). Socio-demographic information, including the participants' gender, age, marital status (single, married, divorced, widowhood), and socio-economic background (e.g., education level, occupation), was collected. Open-ended questions with free form response fields were posed regarding current concerns and opinions regarding on-line psychological support.
The Patient Health Questionnaire, 9-item version (PHQ-9) (Kroenke et al., 2001), Generalized Anxiety Disorder Assessment 7-item version (GAD-7), Perceived Stress Scale, 10-item version (PSS-10) (Barbosa-Leiker et al., 2013), and the PTSD Checklist for DSM-5 (PCL-5) were used to assess the levels of psychological distress of all participants (Wortmann et al., 2016). All were self-report scales with clear cutoff scores determining severity of symptoms. For PHQ-9, the total scores of 5, 10, 15, and 20 represented mild, moderate, moderately severe, and severe depression, respectively. The cutoff points used for the GAD-7 for mild, moderate, and severe anxiety were 5, 10, and 15, respectively. The PSS-10 consisted of two factors, with six negatively worded items (Items 1–3, 6, 9, 10) comprising the first factor and four positively worded items (Items 4, 5, 7, 8) comprising the second factor (Barbosa-Leiker et al., 2013, Golden-Kreutz et al., 2004, Siqueira Reis et al., 2010). Specifically, the first factor represents negative feelings associated with stress, i.e., “Perceived Helplessness”, and the second factor reflects positive feelings counter to stress, “Perceived Self-Efficacy” (Roberti et al., 2006). The post-traumatic and stress-related symptoms were measured by PCL-5. The PCL-5 is a 20-item that evaluates DSM-5 PTSD symptoms caused by a currently distressing event in the past month (Wortmann et al., 2016). Four subscale scores can be calculated by summing items as follows: intrusions (Items 1–5), avoidance (Items 6–7), negative alterations in cognitions and mood (NACM) (Items 8–14), and alterations in arousal and reactivity (AR) (Items 15–20). The Chinese translated versions of the PHQ-9 (Wang et al., 2014), GAD-7 (Li et al., 2014), PSS-10 (Wang et al., 2011), and PCL-5 (Li et al., 2019) have been validated by previous studies.
The peripheral inflammatory biomarkers were collected twice in the patient group, at the first day of hospitalization and within ± three days of fulfilling the on-line survey. These biomarkers included leukocyte count, platelet count, neutrophil count, lymphocyte count, monocyte count, hypersensitive C-reactive protein (HS-CRP) level, procalcitonin (PCT), and erythrocyte sedimentation rate level (ESR). Blood specimens were collected between 6:30 am and 7:00 am by venipuncture into EDTA tubes, and were analyzed in a central laboratory within 2 hours. Blood cell counts were determined using Sysmex XT-4000i Automatic Hematology Analyzer (Sysmex Corporation, Kobe, Japan). HS-CRP was determined by nephelometry method using the Lifotronic PA-990 (Lifotronic, Shenzhen, China). PCT was measured by electrochemiluminescence immunoassay (ECLIA) using the Roche Elecsys Modular and Cobas e602 (Roche, Zurich, Switzerland). ESR was measured by the Automatic dynamic ESR analyzer, Vision-B (YHLO Biotech Co, Shenzhen, China).
Two psychologists interviewed the five patients who participated in a “semi-interview” by telephone. The semi-interviews were structured to collect insights into the perceived stress and the post-traumatic symptoms of the patients with COVID-19 (for details of the list see Supplementary List 1.). The patients were informed that all information they deemed as confidential would not be recorded in the interview. If any questions made them uncomfortable, they could refuse to answer them. The audiotaped interviews were transcribed for data analysis.
2.3. Statistical analysis
Statistical analyses of demographic, clinical data were conducted using R (Version 3.6.0), with package “psych” and package “ggplot2″ (Team, 2011). ANOVA (Analysis of Variance) models were used to compare continuous and normally distributed variables between groups. The Mann-Whitney U tests were used to analyze continuous and abnormal distributed variables. Categorical variables were described by frequencies (percent), and Chi-square tests were used to detect group differences. The relationship between patient psychological assessments and their measured levels of peripheral inflammation levels was also examined with Spearman's correlation tests.
Qualitative data were managed and analyzed using NVivo, version 11. The entire dataset, i.e., the complete responses of all participants, was coded by one author (Y. Zheng), and the content related to the aims of research was specifically noted during coding. The descriptive phenomenological approach was utilized in this study to render the results of analyzing our data accurate. The coded data corresponding to the quantitative variables in the measures, including PSS-10 and PCL-5, were extracted and compared between the two groups. The extracted narratives of patients were translated into English.
3. Results
3.1. Demographic and clinical characteristics
Demographic data did not differ in age, gender, education level, and marital status between patients with COVID-19 and NCs (Table 1 ). The mean age (SD) of patients and NCs were 42.50 (SD = 12.53) and 41.45 (SD = 13.09), respectively, with the largest proportion being between 31 and 45 years of age for both groups. Twenty-three (22.3 percent) patients had comorbidity, of which hypertension (13 patients, 10.7 percent) and diabetes (7 patients, 6.8 percent) had the highest comorbidity rates. Of note, one female patient had been diagnosed with major depression and had recovered for one year. The level of education was relatively high in our sample, as 55.3 percent (57) patients and 60.2 percent (62) NCs received a college education. Among open-ended questions, 48.5 percent (50) patients disclosed the concern about the deterioration from their disease, and 41.7 percent (43) patients worried about the physical health of their family members. About 81.6 percent (84) patients expressed the willingness to receive on-line psychological support with Relaxation Training (85.7 percent) and on-line counseling (50 percent) being most preferred. For inflammatory biomarkers, the mean counts of blood cells and the mean level of PCT and CRP was in the normal range, whereas the mean concentration of ERS was significantly elevated.
Table 1.
Demographic and clinical characteristics for patients with COVID-19 and normal controls.
| Variables | Patient group | Control group | P value |
|---|---|---|---|
| Male, n(%) | 59(57.3) | 54(52.4) | 0.212 |
| Age, (M ± SD) | 42.50 ± 12.53 | 41.45 ± 13.09 | 0.814 |
| 18–30 (%) | 17(16.5) | 17(16.5) | |
| 31–45 (%) | 52(50.5) | 50(48.5) | |
| 46–60 (%) | 22(21.4) | 24(23.3) | |
| 61–75 (%) | 12(11.7) | 12(11.7) | |
| Education, n(%) | 0.599 | ||
| Primary school | 1(1.0) | 2(1.9) | |
| High school | 22(21.4) | 18(17.5) | |
| College | 57(55.3) | 62(60.2) | |
| Postgraduate | 23(22.3) | 21(20.4) | |
| Occupation n(%) | 0.946 | ||
| Students | 0(0) | 3(2.9) | |
| Enterprise employees | 58(56.3) | 46(44.7) | |
| Government employees | 4(3.9) | 3(2.9) | |
| Teachers | 5(4.9) | 6(5.8) | |
| Medical staff | 7(6.8) | 16(15.5) | |
| Individual household | 10(9.7) | 11(10.7) | |
| Other | 19(18.4) | 18(17.5) | |
| Marriage n(%) | 0.121 | ||
| Married | 84(81.6) | 72(69.9) | |
| Unmarried | 13(12.6) | 25(24.3) | |
| Divorced or Widowhood | 6(5.8) | 6(5.8) | |
| Residential city n(%) | 0.536 | ||
| Shanghai | 73(70.9) | 75(72.8) | |
| Wuhan | 17(16.5) | 17(16.5) | |
| Hubei province (except Wuhan) | 7(6.8) | 6(5.8) | |
| Other provinces | 6(5.8) | 5(4.9) | |
| PHQ-9 (M (1st Qu, 3rd Qu)) | 5(3, 8) | 2(0, 5) | <0.001 |
| GAD-7 (M (1st Qu, 3rd Qu)) | 5(1, 7) | 0(0, 4) | <0.001 |
| PSS-10 (M (1st Qu, 3rd Qu)) | 13(10, 17) | 13(8, 16) | 0.238 |
| PCL-5 (M (1st Qu, 3rd Qu)) | 8(4, 14) | 4(1, 8.5) | <0.001 |
| Comorbidity, n(%) | NA | ||
| None | 80(77.7) | – | |
| One | 20(19.4) | – | |
| Two | 2(1.9) | – | |
| Three | 1(1.0) | – | |
| Hospitalization duration (M ± SD) | 8.16 ± 4.06 | – | NA |
| Blood tests (normal range), (M ± SD) | |||
| Leukocyte (3.50~9.50 × 10^9/L) | 5.61 ± 1.90 | – | NA |
| Platelet (125~350 × 10^9/L) | 263.24 ± 93.33 | – | NA |
| Neutrophil (1.80~6.30 × 10^9/L) | 3.28 ± 1.69 | – | NA |
| Lymphocyte (1.10~3.20 × 10^9/L) | 1.73 ± 0.56 | – | NA |
| Monocyte (0.10~0.60 × 10^9/L) | 0.49 ± 0.18 | – | NA |
| HS-CRP (0~10 mg/L) | 9.00 ± 18.69 | – | NA |
| PCT (0~0.05 ng/L) | 0.03 ± 0.19 | – | NA |
| ESR (0~15 mm/h) | 52.75 ± 29.34 | – | NA |
Key: PHQ-9, Patient Health Questionnaire, 9-item version; GAD-7, Generalized Anxiety Disorder, 7-item version; PSS-10, Perceived Stress Scale, 10-item version; PCL-5, PTSD Checklist for DSM-5; HS-CRP, Hypersensitive C-Reactive Protein; PCT, Procalcitonin; ESR, Erythrocyte Sedimentation Rate.
Note: The demographical data and psychological scales were obtained from 103 patients, while peripheral inflammatory tests were obtained from 100 patients.
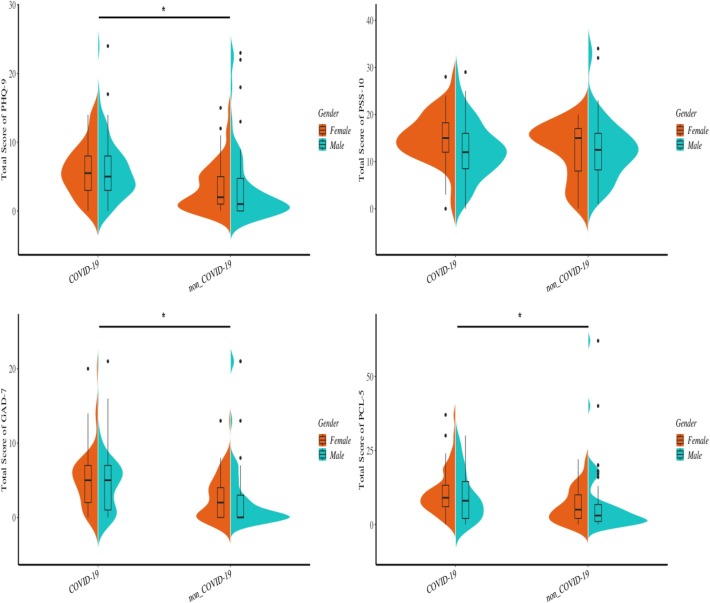
The total scores of PHQ-9, GAD-7, and PCL-5 were significantly higher in patients when compared to the NCs (Table 1 and Fig. 1 ). A considerable proportion of patients reported depression (62 [60.2 percent], the total score of PHQ-9 above 4), and anxiety (59 [55.3 percent], the total score of GAD-7 above 4) symptoms. In contrast, the proportion of normal participants who displayed depression (32 [31.1 percent]) and anxiety (23 [22.3 percent]) symptoms were significantly lower (χ2 = 17.61, P < 0.001). Given that 18 (17.5 percent) and 7 (6.8 percent) patients suffered from moderate to severe levels of depression and anxiety (Table 2 and Table 3 ), respectively, the depression symptoms (DS) appeared to be more prominent in patients with COVID-19 than anxiety symptoms. When stratified by gender, these outcomes did not differ between males and the females in each group.
Fig. 1.
The distributions presenting the total scores of PHQ-9, GAD-7, PSS-10, PCL-5, stratified by genders. The aroids represent that there are significant between-group differences.
Table 2.
Prevalence and demographics of patients with different levels of depression severity.
| Variables | Severity of depression in patients with COVID-19 (N = 103) | ||||
|---|---|---|---|---|---|
| Minimal (0–4) | Mild (5–9) | Moderate (10–14) | Moderately Severe (15–19) | Severe (20–17) | |
| N(%) | 41(39.8) | 44(42.7) | 16(15.5) | 1(1.0) | 1(1.0) |
| Gender: Male, n(%) | 25(61.0) | 23(52.3) | 9(56.3) | 1(100.0) | 1(100.0) |
| Age: n(%) | |||||
| 18–30 | 6(14.6) | 9(20.5) | 1(6.3) | 1(100.0) | 0(0.0) |
| 31–45 | 18(43.9) | 25(56.8) | 8(50.0) | 0(0.0) | 1(100.0) |
| 46–60 | 12(29.3) | 6(13.6) | 4(25.0) | 0(0.0) | 0(0.0) |
| 61–75 | 5(12.2) | 4(9.1) | 3(18.8) | 0(0.0) | 0(0.0) |
| Education: n(%) | |||||
| Primary school | 1(2.4) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) |
| High school | 11(26.8) | 7(15.9) | 4(25.0) | 0(0.0) | 0(0.0) |
| College | 19(46.3) | 26(59.1) | 11(68.8) | 1(100.0) | 0(0.0) |
| Postgraduate | 10(24.4) | 11(25.0) | 1(6.3) | 0(0.0) | 1(100.0) |
| Residential city: n(%) | |||||
| Shanghai | 28(68.3) | 33(75.0) | 11(68.8) | 0(0.0) | 1(100.0) |
| Wuhan | 7(17.1) | 8(18.2) | 1(6.3) | 1(100.0) | 0(0.0) |
| Hubei province (except Wuhan) | 4(9.8) | 0(0.0) | 3(18.8) | 0(0.0) | 0(0.0) |
| Other provinces | 2(4.9) | 3(6.8) | 1(6.3) | 0(0.0) | 0(0.0) |
| Marriage: n(%) | |||||
| Married | 32(78.0) | 36(81.8) | 14(87.5) | 1(100.0) | 1(100.0) |
| Unmarried | 5(12.2) | 7(15.9) | 1(6.3) | 0(0.0) | 0(0.0) |
| Divorced or Widowed | 4(9.8) | 1(2.23) | 1(6.3) | 0(0.0) | 0(0.0) |
Table 3.
Prevalence and demographics of patients with different levels of anxiety severity.
| Variables | Severity of anxiety in patients with COVID-19 (N = 103) | |||
|---|---|---|---|---|
| Minimal (0–4) | Mild (5–9) | Moderate (10–14) | Severe (15–21) | |
| N(%) | 46(44.7) | 50(48.5) | 4(3.9) | 3(2.9) |
| Gender: Male, n(%) | 26(56.5) | 29(58.0) | 2(50.0) | 2(66.7) |
| Age: n(%) | ||||
| 18–30 | 8(17.4) | 8(16.0) | 1(25.0) | 0(0.0) |
| 31–45 | 18(39.1) | 29(58.0) | 2(50.0) | 3(100.0) |
| 46–60 | 14(30.4) | 8(16.0) | 0(0.0) | 0(0.0) |
| 61–75 | 6(13.0) | 5(10.0) | 1(25.0) | 0(0.0) |
| Education: n(%) | ||||
| Primary school | 1(2.2) | 0(0.0) | 0(0.0) | 0(0.0) |
| High school | 12(26.1) | 9(18.0) | 1(25.0) | 0(0.0) |
| College | 21(45.7) | 32(64.0) | 3(75.0) | 1(33.3) |
| Postgraduate | 12(26.1) | 9(18.0) | 0(0.0) | 2(66.7) |
| Residential city: n(%) | ||||
| Shanghai | 28(60.9) | 39(78.0) | 3(75.0) | 3(100.0) |
| Wuhan | 10(21.7) | 6(12.0) | 1(25.0) | 0(0.0) |
| Hubei province (except Wuhan) | 5(10.9) | 2(4.0) | 0(0.0) | 0(0.0) |
| Other provinces | 3(6.5) | 3(6.0) | 0(0.0) | 0(0.0) |
| Marriage: n(%) | ||||
| Married | 35(76.1) | 43(86.0) | 4(100.0) | 2(66.7) |
| Unmarried | 8(17.4) | 5(10.0) | 0(0.0) | 0(0.0) |
| Divorced or Widowed | 3(6.5) | 2(4.0) | 0(0.0) | 1(33.3) |
Four factors of PCL-5 were also extracted and compared between groups. The patients showed significant higher scores in intrusive symptoms (Z = 4.67, P < 0.001), NACM (Z = 3.47, P = 0.001), and AR (Z = 3.17, P = 0.002) compared to normal controls. Since the PCL-5 score of 33 showed optimal efficiency for detecting PTSD cases according to PCL-5 scoring criteria, we calculate the proportions of participants who had PCL-5 score more than 33. The results revealed that only one patient (1.0 percent) and two NCs (1.9 percent) had exceeded the cutoff score. No gender effect was detected in this analysis.
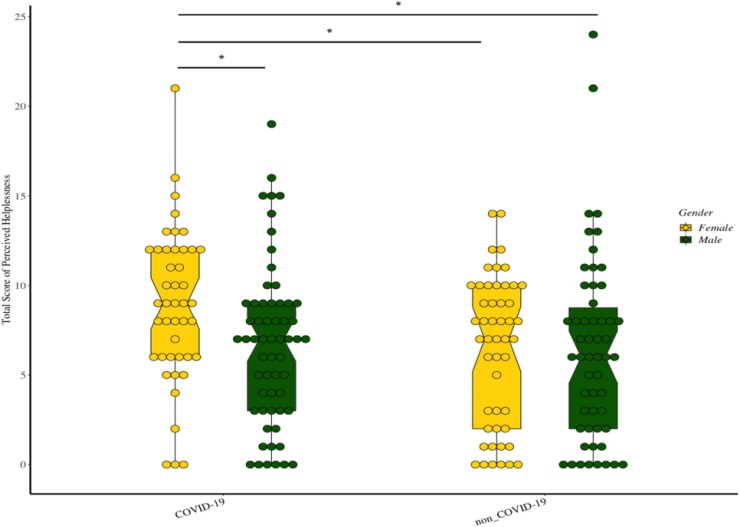
The total score of PSS-10 did not differ between the two groups. We calculated the scores of “Perceived Helplessness” and “Perceived Self-efficacy” using gender stratification. The analysis revealed that the score of “Perceived Helplessness” showed a trending difference between patients and NCs (Z = 1.85, P = 0.064), while no between-group difference was observed in the score of “Perceived Self-Efficacy”. However, when stratified by gender, the female patients exhibited a significantly higher score of “Perceived Helplessness” compared to male patients (Z = 2.56, P = 0.010), female (Z = 2.37, p = 0.018) and male controls (Z = 2.87, P = 0.004), respectively (Fig. 2 ).
Fig. 2.
The boxplot of the score of PSS-10 subscale “Perceived Helplessness” between two groups, split by gender. The aroids represent statistical difference between subgroups.
3.2. Association of psychological assessments with inflammatory biomarkers
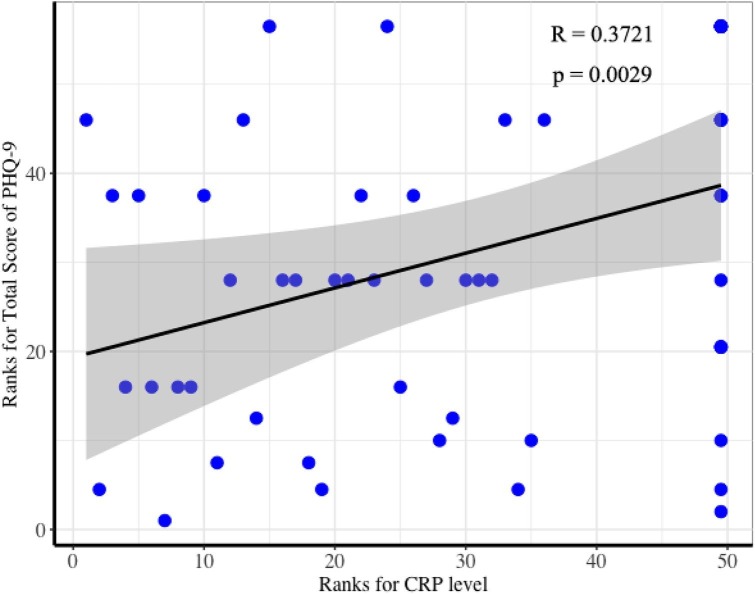
The correlation analysis revealed no significant relationship between psychological assessments and inflammatory indicators when looking at the entire patient group. However, among patients presenting depressive features (total score of PHQ-9 above 4), the level of CRP was positively correlated with the total score of PHQ-9 (Fig. 3 ) (R = 0.37, P = 0.003, Spearman's correlation)After controlling for age and duration of hospitalization, the association remained significant (R = 0.28, P = 0.028, Spearman’s correlation). The CRP level and other inflammatory markers did not show statistical difference between patients with and without DS. There was no significant relationship found between other psychological assessments and the level of CRP.
Fig. 3.
Spearman’s rank correlation coefficient analysis correlating the total score of PHQ-9 and the level of CRP among COVID-19 patients with symptoms of depression.
We further investigated the impact of inflammatory changes, from the first day of hospitalization to the point of our survey, on the outcomes of depression level among patients. As displayed in Table 4 , the patients without DS showed significant amelioration in CRP level (t = 3.76, P = 0.001), whereas the patients with DS did not show statistical change (t = 1.36, P = 0.179). Moreover, the change of CRP level from baseline reversely correlated with the total PHQ-9 score (R = -0.31, P = 0.002) at point of our survey, indicating that the improved CRP level may have resulted in less depressive symptoms at the time of our survey. Trending association between the change of CRP level and PHQ-9 total score of was also observed among patients with DS (R = -0.25, P = 0.053). Since hospitalization length of stay and age may affect the inflammatory outcomes, we compared the two variables between patients with and without DS and found no group-difference (for age: t = 1.23, P = 0.222; for hospitalization duration: t = -0.05, P = 0.957).
Table 4.
Clinical characteristics at baseline and time of fulfilling survey.
| Variables | Patients without DS | P value | Patients with DS | P value | ||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |||
| Leukocyte (10^9/L) | 5.13 (1.39) | 5.36 (1.32) | 0.321 | 5.29 (2.78) | 5.76 (2.18) | 0.137 |
| Neutrophil (10^9/L) | 3.24(1.24) | 3.00(1.03) | 0.298 | 3.50 (2.58) | 3.45 (1.97) | 0.876 |
| Lymphocyte (10^9/L) | 1.35(0.49) | 1.76(0.53) | <0.001 | 1.26(0.56) | 1.71(0.59) | <0.001 |
| Monocyte (10^9/L) | 0.49(0.22) | 0.47(0.16) | 0.323 | 0.50(0.24) | 0.50(0.18) | 0.924 |
| HS-CRP (mg/L) | 17.31 (18.69) | 6.37(13.24) | 0.001 | 14.94(20.13) | 10.61(21.29) | 0.179 |
| PCT (ng/L) | 0.04(0.02) | 0.03(0.02) | 0.009 | 0.04(0.03) | 0.03(0.02) | 0.007 |
| Platelet (10^9/L) | 182.50(59.82) | 253.39(106.05) | <0.001 | 194.06(63.86) | 269.27(84.95) | <0.001 |
| ESR (mm/h) | 50.18(35.98) | 57.45(29.80) | 0.284 | 54.68(32.81) | 49.87(28.91) | 0.166 |
Key: DS, depression symptoms; HS-CRP, Hypersensitive C-Reactive Protein; PCT, Procalcitonin; ESR, Erythrocyte Sedimentation Rate.
Note: At baseline, PCT were obtained from 98 patients, other inflammatory tests obtained from 100 patients. At follow-up, all inflammatory tests were obtained from 100 patients. The follow-up time is defined as date within ± three days of completing the on-line survey.
3.3. Mixed data: Meta-matrices of quantitative and qualitative data
The qualitative results were extracted from the narratives of patients during the semi-structured interview and were matched with the quantitative measurements (Table 5 ). The quantitative data were found to be matched with the content of items 1, 2, 6 in PSS-10, and items 6, 7, 10, 11 in PCL-5. The severities of the above items were subsequently compared between patients and NCs. The COVID-19 patients showed significant higher scores in the items 1, 6 of PSS-10 (item 1, Z = 2.02, P = 0.044; item 6, Z = 2.62, P = 0.009) and items 10, 11 of PCL-5 (item 10, Z = 3.55, P < 0.001; item 11, Z = 5.52, P < 0.001), whereas trending group differences were showed in the item 2 in in PSS-10 (Z = 1.84, P = 0.065) and items 6, 7 in PCL-5 (item 6, Z = 1.83, P = 0.068; item 7, Z = 1.78, P = 0.076).
Table 5.
Meta-matrices of frequency and percentage of quantitative data and narrations of qualitative data.
| Items | COVID-19 Patients (N = 103) (%) | Narratives (n = 5) | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| PSS-10, item 1*Upset because of something that happened unexpectedly | 23(22.3) | 27(26.2) | 45(43.7) | 7(6.8) | 1(1.0) | Patient A: “Then the ones who came here later than me were discharged, the ones who were older than me were discharged… Everyone said that I was in good condition, really good, but I was not discharged.” |
| Patient B: “At that moment, I didn't believe that I might get this disease, because I really paid attention to prevention. The other reason is, we left (Henan province) on January 23. We were afraid that the highway would be closed, so we left after eating dinner. We left during the night. We started quarantine once we arrived (Shanghai), we didn’t get out of the house… so I didn't believe that I will have this disease.” “I said that my protection was good, how could I have the disease? Because I have calculated the proportion of the disease, the proportion of individuals who were ill. (The rate) was just like winning 5 million (in the lottery) …Just very few people. Because at that time I also paid attention to the news. Because there were almost 10 million people in Wuhan, probably only ten thousand people (get the disease). I said this is about one-in-ten thousand, basically. Isn't it? So, I didn't believe it. When the test results came out and he said it was positive, I was, like, worried.” | ||||||
| Patient E: “My family members were quarantined, and some friends were quarantined, which kind of affected me. And I thought it was a viral flu before (the results came out). Although I wore a mask, I treated more than 200 patients during that time. Those patients were isolated, and I felt under pressure from this aspect. ” | ||||||
| PSS-10, item 2 Felt that you were unable to control the important things in your life | 26(25.2) | 31(30.1) | 34(33.0) | 10(9.7) | 2(1.9) | Patient A: “There are some uncertainties about the unknown.” “… Everything is unknown. You know? … So, as the extension of time, the longer the time, the more anxious (I feel). I don’t know how the progression (of disease) happened on me.” “So, the information we got wasn't enough to support my confidence, and it makes me doubt what's going on. I need to analyze it from something I have no idea with. I try to find something. The more I found, the weirder I feel. Anyway, the feeling is vague. I’m at a loss.” Patient D: ”There's a lot of uncertainty going on here. I packed up my things. I was ready to be transferred, and then there was no news. They asked me to transfer and I packed up my things, and then there was no news. It happened several times, during these days. So, the anxiety caused from this aspect is comparably obvious.““But it's a new disease. Nobody knows much about it. And nobody's ever seen what in my case, right?”Patient E: “So, would it influence, and how much influence would it make later? What kind of damage would it cause, to myself and the people around me? We're uncertain about that.”“When encountering this kind of problem, SARS and COVID-19, from the government to the hospital, and to the individual levels, I feel no one could have that defensive state once in a sudden. Of course, we have drills during peacetime, but the practice is not enough to cope with such a problem. Not enough to cope with this problem. After the whole order were all messed up at once, I felt very puzzled. At that time there was a kind of vacant feeling.” |
| PSS-10, item 6** Found that you could not cope with all the things you had to do | 21(20.4) | 32(31.1) | 39(37.9) | 11(10.7) | 0(0.0) | Patient A: [the Patient was worried about her privacy. She hid her illness from her neighbour, and she worried that the neighbour will find out the truth and their relationship will break down.] I was thinking what if one day, if I don't come home all the time, or if my condition gets worse, what could I tell them? (We have) so many years of friendship. Then they will say, you swore that you were at home, and I brought you food to your house every day. You have taken them in, and you also told me how they tasted? “Patient C: ”After being discharged, I need to find another place. To have be quarantined for a few more days. I'm afraid I won't be able to find a place to stay.“Patient D: ”I don't know if there's going to be any changes in the future, or some sort of consequence.“ ”I don't know much about the mental health problems either. How do you feel about my current mental state? Is there any mental issue or situation that might follow?“Patient E: ”One (of my worries) is fear of causing panic, the other is fear of further disclosure of privacy… There are a lot of doctors living in my residential area. If there is a panic, it's not just me (that would be influenced). Maybe the community will stop me from going back to the residential area, and the other doctors would also be expelled. So, there’s high pressure from public opinion. |
| PCL-5, item 6 Avoiding memories, thoughts, or feelings related to the stressful experience | 63(61.2) | 34(33.0) | 5(4.9) | 1(1.0) | 0(0.0) | Patient B: “Don't even think about it. The more you think about it, the worse the emotion would be.”“There’s a word called heartless. Think less about things, which might be better.”Patient C: “When you're sick, you won't get better even if you worry yourself to death. So, take it as it comes, take it as it comes!” |
| PCL-5, item 7 Avoiding external reminders of the stressful experience (for example, people, places, conversations, activities, objects, or situations | 62(60.2) | 32(31.1) | 9(8.7) | 0(0.0) | 0(0.0) | Patient B: “You see, I don't read much of the negative information on the phone… I just read the positive ones. Some (information) are about the ones who get sick and some of family members have died. So cruel. I just, I just try not to read them.”Patient C: “(I) try to fill my day a little bit, which can really help me to distract myself a little bit.” |
| PCL-5, item 10** Blaming yourself or someone else for the stressful experience or what happened after it | 52(50.5) | 39(37.9) | 7(6.8) | 5(4.9) | 0(0.0) | Patient A: “The other thing is, the doctor didn't go and ask you… He didn't have more communication with you… I really want to tell the doctor (my ideas). Things turned to be that I have said a lot, I have spoken thousands of words, and just (get) one response: “What to do? Wait for the result.” “You're not respected in this environment, you know?”Patient B: “Some of the homeowners of the community said online that, he said (he) watched me go out, and go out to buy food, and talk with others in the corridor. I said he made it up, and it’s a defamation action. My son and daughter-in-law, they have dialed 110 to call the police. ” “We can still sue him if he doesn't apologize and make this clear in the (online) group.“Patient C: ”The biggest problem is that, what is terrible is not the disease, but the human hearts.“”Then because the people around you, people all treat you, how to say,as you are the plague, you got the plague, and they give you a label. “Patient E: ”Sometimes, it's true that natural and man-made disaster, I think, they’re always combined together. Natural disaster is a man-made disaster.“ |
| PCL-5, item 11** Having strong negative feelings such as fear, horror, anger, guilt, or shame | 45(43.7) | 50(48.5) | 4(3.9) | 4(3.9) | 0(0.0) | (Fear, Horror): Patient A: “I also felt like if I follow this thought, I felt very scared.”“This horror is originated from the unknown.” “It comes from unknown. The horror is all about the unknown. Information asymmetry, yes. We can't solve it.”Patient C: “At least there's a window (in that room before confirmed infection) where we can see cars coming and going and so on. But not here, there’s nothing could be seen here… (I have the feeling of) airtight, airtight phobia. ”Patient E: “(About) pulmonary fibrosis or something like that, it's more or less about understanding whether or not it’s going to affect my life or so in the future. After all, we are people in the first place, and people could be afraid. Isn't it?” (Guilt): Patient A: “My pressure is from my neighbors and relatives, such as my sister and my parents in Wuhan, where’s my hometown. They would like to (have) video call with me these 10 s、20 s days. They want to video chat with me, a person quarantined at home [The patient lied to them. The fact is, she was hospitalized]. I can't video chat with them. Every time I need to find some reasons to reject them. I felt sad about it. The more day (passed), the more stressed I feel. And I couldn’t let them know. You have to lie to the people who care and love you.” “That kind of guilty feeling… Suddenly, I had to lie, and then I had to cover up the lie with a bunch of lies. It was really stressful.” (Helpless): Patient A: “Actually, I felt like we were abandoned. I had this kind of feeling at that moment… Really, at that time, I thought about the stories we read many years ago, about the leprosy hospital. (I) really thought pessimistic. During that time, you had no one to take care of you, and you need to survive on your own.” “The feedback from the doctors really made me feel helpless. It made me feel very helpless.” |
Key: PSS-10, Perceived Stress Scale-10; PCL-5, PTSD Checklist for DSM-5.
Note: Patient A, female, aged between 50 and 55 years old kept her infection as a secret. She did not reveal her diagnosis to her neighbors and other family members, except her husband. Patient B, male, aged between 60 and 65 years old. Patient C, male, aged between 35 and 40 years old. Patient D, male, aged between 30 and 35 years old. His family members were also infected with the virus. Patient E, female, aged between 35 and 40 years old. She was hospitalized again because of the repeat positive virus test result.
P < 0.05, **P < 0.01
The primary factors related to the patient's perception of stress were the unexpectedness, loss of control (uncertainty), and sense of powerlessness. The unexpected factors could be the infection of COVID-19 (item 1 of PSS-10, Patient B), the impact the disease has on people around them (item 1 of PSS-10, Patient C), and the length of stay of the hospitalization (item 1 of PSS-10, Patient A). The uncertainty was due to the lack of information about the patient's ongoing changes of physical status (item 2 of PSS-10, Patient A), the perceived paucity of support from health authorities (item 2 of PSS-10, Patient D, and E), and the unknown aspects of the COVID-19 disease (item 2 of PSS-10, Patient D, and E). The sense of powerlessness was engendered by the difficulties the patients could not solve, e.g., interpersonal problems (item 6 of PSS-10, Patient A), the inaccessibility to a healthcare facility during the outbreak (item 6 of PSS-10, Patient C), the side effects of treatment and morbidity and mortality associated with the disease (item 6 of PSS-10, Patient D), and the stigma associated with COVID-19 that affected themselves, their family, and friends (item 6 of PSS-10, Patient E).
The main post-traumatic symptoms found among patients were avoiding the internal and external clues of stressful experiences, blaming others, and having strong negative feelings, including fear, guilt, and helplessness. Patients reported more blaming of others, such as the doctors (item 10 of PCL-5, Patient A), neighbors (item 10 of PCL-5, Patient B, and C), and health authorities (Patient E). The feeling of fear was associated with uncertainty (item 11 of PCL-5, Patient A), the isolation associated with being confined to an enclosed space (item 11 of PCL-5, Patient C), and the worries concerning the consequence of the disease (item 6 of PSS-10, Patient E).
4. Discussion
To our knowledge, this is the first study utilizing a mixed-method triangulation design to investigate the immediate psychological impact of COVID-19 on the patients and its association with inflammatory biomarkers. Compared with normal controls, patients with COVID-19 presented higher levels of depression, anxiety, and post-traumatic stress symptoms. Though the total score of PSS-10 did not differ between patients and NCs, subgroup analysis demonstrated that female patients reported significantly more “Perceived Helplessness” than male patients and NCs. As for interactions between psychological distress and inflammatory biomarkers, the results revealed that depression levels were statistically directly related to the levels of CRP among patients with DS. Moreover, the more improvement of CRP level from baseline resulted in lower scores of PHQ-9 at the time of the survey. Finally, qualitative analysis revealed that the main factors related to a patient's perception of stress of being diagnosed with COVID-19 were the unexpectedness of the illness, loss of control (uncertainty) and sense of powerlessness, and post-traumatic symptoms. The latter included avoiding stressful experiences, blaming others, and strong negative feelings of fear, guilt, and helplessness. Stigma is also an important issue among patients with COVID-19, as it may negatively affect their daily life in the community.
Given the profoundly serious nature of the COVID-19 outbreak, it was not surprising that a significant proportion of patients studied experienced depression, anxiety, and post-traumatic symptoms. Of note, is that in 2003, at the height the SARS outbreak, severe psychological outcomes were also observed in infected patients. A survey conducted in Hong Kong at that time showed that hospitalized patients with SARS reported increased stress and negative psychological effects by the measure of PSS-10 (Chua et al., 2004), with a mean score of 20. Later, Cheng and the colleagues examined the psychological distress of SARS survivors, those who had been discharged from hospital, and found that about 35 percent of patients reported 'moderate to severe' or 'severe' ranges of anxiety and/or depressive symptoms (Cheng et al., 2004). Both studies included patients with the most severe type of SARS, with some admitted to ICU. Hence, our results may only be representative of patients who have less severe COVID-19 symptoms. A number of studies in China have investigated the mental status of COVID-19 patients (Bo et al., 2020), those COVID-19 negative (Liu et al., 2020, Qiu et al., 2020), and the healthcare workers (HCWs) (Lai et al., 2020, Li et al., 2020). These studies consistently report that citizens of Wuhan or Hubei province were prone to higher levels of post-traumatic stress symptoms (Bo et al., 2020, Liu et al., 2020) or psychological distress (Qiu et al., 2020) than other regions of China. Similar to our study, the nationwide on-line survey conducted by Qiu's group observed a gender effect on psychological outcomes (Qiu et al., 2020), with females showing significantly higher psychological distress than males. Two other investigations focusing on HCWs found a comparable proportion of participants with symptoms of depression (50.4 percent), anxiety (44.6 percent) as in our data (Lai et al., 2020). They also found that the general public and non-front-line nurses exhibited much higher vicarious traumatization than front-line nurses (Li et al., 2020).
Most importantly, we determined the positive association between the features of depression and levels of CRP. In addition, the improvement on CRP concentration strongly correlated with levels of depression at the time of our observation. Although only cross-sectional psychological distress data was collected, the levels of depression seemed to be highly influenced by previous changes of inflammatory marker. This implies that the fluctuation of inflammation can dynamically influence the depressive symptoms among COVID-19 patients. Previous research on patients with MERS-CoV and SARS-CoV validate that the coronavirus might affect the central nervous system as it can be detected in the cerebrospinal fluid (Arabi et al., 2015, Tsai et al., 2005). Recent evidence has confirmed that the COVID-19 is also neurotropic and possibly invades the brain through the olfactory tract in the early stages of infection (Wu et al., 2020). A significant number of COVID-19 patients report a sudden loss of smell or taste as an early symptom, which may indicate anosmia and dysgeusia caused by COVID-19 (Giacomelli et al., 2020). Moreover, symptoms similar to intracranial infections such as headache, epilepsy, altered consciousness, and encephalitis among COVID-19 patients (Moriguchi et al., 2020) highlight the acute neuropsychiatric symptoms due to the virus.
An initial report of 217 hospitalized patients in Wuhan, China, observed neurologic symptoms in nearly half of those with severe infection, with lower blood lymphocyte counts and elevated plasma CRP in patients with CNS-associated or muscular symptoms (Mao et al., 2020). Notably, the elevated CRP levels or neutrophil counts combined with lower lymphocyte counts were considered to be prognostic of poorer COVID-19 outcomes. Our data demonstrated increased lymphocyte counts and decreased plasma CRP in the recovery phase of the illness. However, CRP levels in patients with DS were not significantly diminished, which may lead to mild neuropsychiatric symptoms, such as those associated with depression.
This result aligned with the prior CoV-related findings in psychoneuroimmunology (Brietzke et al., 2020), in which the mood disturbance could be a direct result of the virus infection in the brain or due to the activation of immune-inflammatory response (Cheng et al., 2004). Our data, however, may be in support for the latter hypothesis. Studies have proven that COVID-19 infection triggers the overproduction of pro-inflammatory cytokines including interleukin (IL)-6, IL-8, IL-10, IL-2R and tumor necrosis factor (TNF)-alpha, which could be related to neuropsychiatric symptoms (Troyer et al., 2020). The supporting evidence that Kuhlman and colleagues have revealed is the positive linkage between the increases in IL-6 and depressed mood and cognitive symptoms after receiving seasonal influenza vaccine (Kuhlman et al., 2018). Studies of cases of influenza encephalitis also demonstrate very little evidence that the neurological complications of influenza are due to the direct effects of the virus (Whitlock, 1982). Meanwhile, studies in major depression have documented the positive association between depression levels and inflammatory biomarkers (Köhler et al., 2017, Shafiee et al., 2017, Valkanova et al., 2013), once again supporting the immune-inflammatory theory for mood disorders.
Except for the pathogen-immune–depression association, it may be meaningful to consider viral infections and subsequent immune activation as a form of stress. Dysregulation of stress could interact with the pathogenesis of depressive illness (Gold et al., 1995), most likely resulting from a dysfunction of the hypothalamic–pituitaryadrenal (HPA) axis (Arborelius et al., 1999, Marques-Deak et al., 2005). Inflammatory agents such as interleukins 6 and TNF-α can enhance HPA activation (Besedovsky et al., 1991, Wang and Dunn, 1998). Therefore, the effective anti-inflammatory medications for COVID-19 may also be expected to relive the mood disturbance of patients. Since confounders such as medication and the hospitalization length of stay varied among our sample, future studies should focus on medication-free COVID-19 patients and follow the trajectories of inflammatory biomarkers and mental status to clarify this issue.
The study is also the first utilizing mix-methods to illustrate the perceived stress and stress-related symptoms amongst COVID-19 patients. These perceptions are rarely qualitatively reported using free form responses to open-ended questions from patients. Generally, patients’ reports focused on their experiences after being diagnosed as COVID-19. Their perceptions of stress were related to the unexpectedness of the illness, loss of control (uncertainty), and a sense of powerlessness. Regarding the post-traumatic symptoms, patients reported a variety of avoidance behaviors and negative feelings, including fear, guilt, and helplessness. These results are in line with several articles about the experiences of COVID-19 patients. They, too, suggest that patients may experience fear of the consequences of infection (Xiang et al., 2020), anxiety, depression, guilt, and anger (Kim and Su, 2020). The feeling of anger was not reported directly, which might be related to social desirability bias, but could be observed when patients reported themselves blaming others. Disengagement maladaptive coping strategies were identified from avoidance behaviors and patients denying any discomfort during the interview. Due to the limitation of sample number and the study design, it is not possible to determine the entire range of coping strategies the patients used and their mediating effects of responses to stress on the association between perceived stress and stress-related symptoms. Stigma and the uncertainty about the consequence of the infection are the two main causes of the negative feelings and thoughts among COVID-19 patients. As found in SARS-related stigma studies, media miscommunications of risk mitigation strategies and inconsistent health policy have augmented uncertainty toward sudden viral epi and pandemics (Lee et al., 2005) resulting in widespread fear and anxiety. Previous scientific studies have shown that the internalized HIV stigma was a strong predictor of depression (Swendeman et al., 2018). The influence of COVID-19 stigma on the mental status of patients is worth discussing in future studies.
The results of this study generate comprehensive insights into the stress and psycho-immunological changes in COVID-19 patients with valuable evidence for clinical intervention not found in other studies. In accordance with recent commentary (Xiang et al., 2020) and guidelines, these findings highlight the importance of timely mental health care for COVID-19 patients and call for more discussion. Future research could focus on the mediating role of coping strategies and gender differences in the stress-related symptoms of patients, and the cause and consequence of the stigma associated with the psychopathology symptoms of COVID-19 patients.
5. Limitation
There are some limitations to this study. First, patients with severe and life-threatening COVID-19 were not included in this study. Second, the sample of the study was relatively small, and only five patients participated in the qualitative interview. Possible confounders, such as medication, comorbidities, and duration of hospitalization, may substantially affect the interpretation of our results. Pro-inflammatory cytokines, which may reflect the stress and mood disturbance, were not tested in our cohort of mild cases. Third, social desirability concerns may influence the responses to measurements used in this study. Finally, this is a cross-sectional study and follow-up data was not included
6. Conclusion
Depression and anxiety symptoms were more common among COVID-19 patients than in normal controls. The associations between the severity of depression symptoms and the level of CRP may indicate that the virus could affect the central nervous system and induce neuropsychiatric symptoms by activation of immune-inflammatory response. Stigma is an important issue, which may affect the daily life of patients in the community. Necessary measures should be implemented to provide treatment to COVID-19 patients with depression and other mental problems, especially those with pre-morbid risk factors. Addressing the stigma associated with having a disease with profoundly high rates of morbidity and mortality and imparting adaptive coping strategies should be included when delivering psychological interventions.
Author contribution
Concept and design: Q. Guo, Y. Zheng, J. Shi, J. Wang, T. Zhang, Y. Xu, H. Chen, Z. Wang, Z. Yang.
Acquisition, analysis, and interpretation of data: J. Shi, Z. Yang, Q. Guo, Y. Zheng, H. Xu, G. Li, C. Li, S. Yang, Y. Lu, X. Chen, X, Liu, H. Zhou, Y. Tang, X. Wang. Drafting of the manuscript: Q. Guo, Y. Zheng, Z. Yang, Q. Revision of the manuscript: Fromson JA, X. Liu, H. Hu, Z. Wang.
Statistical analysis: Q. Guo, Y. Zheng. Supervision: Z. Wang, G. Li, H. Chen.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study was supported by following grants: Medical Engineering jointed Fund of Shanghai Jiaotong University (YG2020YQ25), Funds for talents by Shanghai Mental Health Center (2019-YJ-07, 2018-FX-04), and Shanghai Mental Health Center Foundations (2019-QH-04, 2019-QH-01).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.05.038.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., Saeed B.T., Wahbi A., Saedy A., AlDabbagh T., Okaili R., Sadat M., Balkhy H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L., Owens M.J., Plotsky P.M., Nemeroff C.B. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Barbosa-Leiker C., Kostick M., Lei M., McPherson S., Roper V., Hoekstra T., Wright B. Measurement invariance of the perceived stress scale and latent mean differences across gender and time. Stress Health. 2013;29:253–260. doi: 10.1002/smi.2463. [DOI] [PubMed] [Google Scholar]
- Besedovsky H.O., Del Rey A., Klusman I., Furukawa H., Monge Arditi G., Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J. Steroid Biochem. Mol. Biol. 1991;40:613–618. doi: 10.1016/0960-0760(91)90284-C. [DOI] [PubMed] [Google Scholar]
- Bo H.X., Li W., Yang Y., Wang Y., Zhang Q., Cheung T., Wu X., Xiang Y.T. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 2020;1–7 doi: 10.1017/S0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brietzke E., Magee T., Freire R.C.R., Gomes F.A., Milev R. Three insights on psychoneuroimmunology of mood disorders to be taken from the COVID-19 pandemic. Brain Behav. Immun. Health. 2020;5 doi: 10.1016/j.bbih.2020.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J., Xing F., Liu J., Yip C.C.-Y., Poon R.W.-S., Tsoi H.-W., Lo S.K.-F., Chan K.-H., Poon V.K.-M., Chan W.-M., Ip J.D., Cai J.-P., Cheng V.C.-C., Chen H., Hui C.K.-M., Yuen K.-Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.K., Wong C.W., Tsang J., Wong K.C. Psychological distress and negative appraisals in survivors of severe acute respiratory syndrome (SARS) Psychol. Med. 2004;34:1187–1195. doi: 10.1017/S0033291704002272. [DOI] [PubMed] [Google Scholar]
- Cheng Z.J., Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China, N.H.C.o.T.P.s.R.o., 2020. Diagnosis and Management Program of Novel Coronavirus-infected Pneumonia, fourth ed. http://www.gov.cn/zhengce/zhengceku/2020-01/28/content_5472673.htm. (Accessed 8 May 2020).
- Chua S.E., Cheung V., McAlonan G.M., Cheung C., Wong J.W., Cheung E.P., Chan M.T., Wong T.K., Choy K.M., Chu C.M., Lee P.W., Tsang K.W. Stress and psychological impact on SARS patients during the outbreak. Can. J. Psychiatry. 2004;49:385–390. doi: 10.1177/070674370404900607. [DOI] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P.W., Licinio J., Wong M.L., Chrousos G.P. Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann. N. Y. Acad. Sci. 1995;771:716–729. doi: 10.1111/j.1749-6632.1995.tb44723.x. [DOI] [PubMed] [Google Scholar]
- Golden-Kreutz D.M., Browne M.W., Frierson G.M., Andersen B.L. Assessing Stress in Cancer Patients: A Second-Order Factor Analysis Model for the Perceived Stress Scale. Assessment. 2004;11:216–223. doi: 10.1177/1073191104267398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetterman T.C., Fetters M.D., Creswell J.W. Integrating Quantitative and Qualitative Results in Health Science Mixed Methods Research Through Joint Displays. Ann. Fam. Med. 2015;13:554–561. doi: 10.1370/afm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Su K.P. Using psychoneuroimmunity against COVID-19. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C.A., Freitas T.H., Maes M., de Andrade N.Q., Liu C.S., Fernandes B.S., Stubbs B., Solmi M., Veronese N., Herrmann N., Raison C.L., Miller B.J., Lanctôt K.L., Carvalho A.F. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta. Psychiatr. Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- Koopman C., Classen C., Cardeña E., Spiegel D. When disaster strikes, acute stress disorder may follow. J. Trauma. Stress. 1995;8:29–46. doi: 10.1007/BF02105405. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman K.R., Robles T.F., Dooley L.N., Boyle C.C., Haydon M.D., Bower J.E. Within-subject associations between inflammation and features of depression: Using the flu vaccine as a mild inflammatory stimulus. Brain Behav. Immun. 2018;69:540–547. doi: 10.1016/j.bbi.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Ma S., Wang Y., Cai Z., Hu J., Wei N., Wu J., Du H., Chen T., Li R., Tan H., Kang L., Yao L., Huang M., Wang H., Wang G., Liu Z., Hu S. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chan L.Y., Chau A.M., Kwok K.P., Kleinman A. The experience of SARS-related stigma at Amoy Gardens. Soc. Sci. Med. 2005;61:2038–2046. doi: 10.1016/j.socscimed.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang W., Chen W., Yuan H., Zhang S., Tian M., Qu Z. Applications of the Chinese version of the primary care PTSD screen for DSM-5 (PC-PTSD-5) for children. J. Affect. Disord. 2019;254:109–114. doi: 10.1016/j.jad.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Li Z., Ge J., Yang M., Feng J., Qiao M., Jiang R., Bi J., Zhan G., Xu X., Wang L., Zhou Q., Zhou C., Pan Y., Liu S., Zhang H., Yang J., Zhu B., Hu Y., Hashimoto K., Jia Y., Wang H., Wang R., Liu C., Yang C. Vicarious traumatization in the general public, members, and non-members of medical teams aiding in COVID-19 control. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Li M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T.K., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Lukai RongjingD., Dayi H., Sheng L. GW25-e4488 The value of Chinese version GAD-7 and PHQ-9 to screen anxiety and depression in cardiovascular outpatients. J. Am. Coll. Cardiol. 2014;64:C222. doi: 10.1016/j.jacc.2014.06.1038. [DOI] [Google Scholar]
- Liu N., Zhang F., Wei C., Jia Y., Shang Z., Sun L., Wu L., Sun Z., Zhou Y., Wang Y., Liu W. Prevalence and predictors of PTSS during COVID-19 Outbreak in China Hardest-hit Areas: Gender differences matter. Psychiatry Res. 2020;287 doi: 10.1016/j.psychres.2020.112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak I.W., Chu C.M., Pan P.C., Yiu M.G., Chan V.L. Long-term psychiatric morbidities among SARS survivors. Gen. Hosp. Psychiatry. 2009;31:318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Deak A., Cizza G., Sternberg E. Brain-immune interactions and disease susceptibility. Mol. Psychiatry. 2005;10:239–250. doi: 10.1038/sj.mp.4001643. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of China, A notice on the issuance of guidelines for emergency psychological crisis intervention in pneumonia for novel coronavirus infections (in Chinese). http://www.nhc.gov.cn/jkj/s3577/202001/6adc08b966594253b2b791be5c3b9467.shtml. (Accessed 8 May 2020).
- Noone P. Flu, Q-fever-related absence and PTSD in reservists. Occup. Med. 2013;63 doi: 10.1093/occmed/kqt022. 311–311. [DOI] [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q., Nguyen T.T., Cao T.M., Pham Q.D. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. N. Engl. J. Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluye P., Hong Q.N. Combining the power of stories and the power of numbers: mixed methods research and mixed studies reviews. Annu. Rev. Public Health. 2014;35:29–45. doi: 10.1146/annurev-publhealth-032013-182440. [DOI] [PubMed] [Google Scholar]
- Qiu J., Shen B., Zhao M., Wang Z., Xie B., Xu Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen. Psychiatr. 2020;33 doi: 10.1136/gpsych-2020-100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberti J.W., Harrington L.N., Storch E.A. Further Psychometric Support for the 10-Item Version of the Perceived Stress Scale. J. College Counsel. 2006;9:135–147. doi: 10.1002/j.2161-1882.2006.tb00100.x. [DOI] [Google Scholar]
- Shafiee M., Tayefi M., Hassanian S.M., Ghaneifar Z., Parizadeh M.R., Avan A., Rahmani F., Khorasanchi Z., Azarpajouh M.R., Safarian H., Moohebati M., Heidari-Bakavoli A., Esmaeili H., Nematy M., Safarian M., Ebrahimi M., Ferns G.A., Mokhber N., Ghayour-Mobarhan M. Depression and anxiety symptoms are associated with white blood cell count and red cell distribution width: A sex-stratified analysis in a population-based study. Psychoneuroendocrinology. 2017;84:101–108. doi: 10.1016/j.psyneuen.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Siqueira Reis R., Ferreira Hino A.A., Rodriguez Añez Romélio C. Perceived stress scale: Reliability and validity study in Brazil. J. Health Psychol. 2010 doi: 10.1177/1359105309346343. [DOI] [PubMed] [Google Scholar]
- Swendeman D., Fehrenbacher A.E., Roy S., Das R., Ray P., Sumstine S., Ghose T., Jana S. Gender disparities in depression severity and coping among people living with HIV/AIDS in Kolkata, India. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team R.D.C. the R Foundation for Statistical Computing; Vienna, Austria: 2011. R: A language and environment for statistical computing. [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.-K., Hsieh S.-T., Chang Y.-C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwan. 2005;14:113–119. doi: 10.29819/ANT.200509.0002. [DOI] [PubMed] [Google Scholar]
- Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- van Hoek A.J., Underwood A., Jit M., Miller E., Edmunds W.J. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PloS One. 2011;6 doi: 10.1371/journal.pone.0017030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Dunn A.J. Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem. Int. 1998;33:143–154. doi: 10.1016/S0197-0186(98)00016-3. [DOI] [PubMed] [Google Scholar]
- Wang W., Bian Q., Zhao Y., Li X., Wang W., Du J., Zhang G., Zhou Q., Zhao M. Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. Gen. Hosp. Psychiatry. 2014;36:539–544. doi: 10.1016/j.genhosppsych.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Wang Z., Chen J., Boyd J.E., Zhang H., Jia X., Qiu J., Xiao Z. Psychometric Properties of the Chinese Version of the Perceived Stress Scale in Policewomen. PloS One. 2011;6 doi: 10.1371/journal.pone.0028610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton M.G., Abramowitz J.S., Berman N.C., Fabricant L.E., Olatunji B.O. Psychological Predictors of Anxiety in Response to the H1N1 (Swine Flu) Pandemic. Cognit. Ther. Res. 2012;36:210–218. doi: 10.1007/s10608-011-9353-3. [DOI] [Google Scholar]
- Whitlock F.A. The neurology of affective disorder and suicide. Aust. N. Z. J. Psychiatry. 1982;16:1–12. doi: 10.3109/00048678209159465. [DOI] [PubMed] [Google Scholar]
- WHO, 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (Accessed 8 May 2020).
- Wing, Y., Ho, M., 2004. Mental health of patients infected with SARS, in: In Challenges of Severe Acute Respiratory Syndrome. Elsevier, Hong Kong, pp. 526-546.
- Wortmann J.H., Jordan A.H., Weathers F.W., Resick P.A., Dondanville K.A., Hall-Clark B., Foa E.B., Young-Mccaughan S., Yarvis J.S., Hembree E.A. Psychometric Analysis of the PTSD Checklist-5 (PCL-5) Among Treatment-Seeking Military Service Members. Psychol. Assess. 2016;28:1392–1403. doi: 10.1037/pas0000260. [DOI] [PubMed] [Google Scholar]
- Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) J. Trauma. Stress. 2005;18:39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y.T., Yang Y., Li W., Zhang L., Zhang Q., Cheung T., Ng C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., China Novel Coronavirus I., Research T. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.