Highlights
-
•
TMS was used to map the cortical representation of seven resting lower-limb muscles.
-
•
TMS was delivered through a 110-mm double-cone coil.
-
•
The topography of each muscle was complex and variable across individuals.
-
•
There was no evidence for discrete cortical representations of different muscles.
Keywords: Motor cortex, Cortical mapping, Lower limb, Double-cone coil, Transcranial magnetic stimulation, Leg
Abstract
Objective
To evaluate the extent to which transcranial magnetic stimulation (TMS) can identify discrete cortical representation of lower-limb muscles in healthy individuals.
Methods
Motor evoked potentials were recorded from resting vastus medialis, rectus femoris, vastus lateralis, medial and lateral hamstring, and medial and lateral gastrocnemius muscles on the right leg of 16 young healthy adults using bipolar surface electrodes. TMS was delivered through a 110-mm double-cone coil at 63 sites over the left hemisphere. Location and size of cortical representation and number of discrete peaks were quantified.
Results
Within the quadriceps group there was a main effect of muscle on anterior-posterior centre of gravity (p = 0.010), but the magnitude of the difference was small. There was also a main effect of muscle on medial–lateral hotspot (p = 0.027) and map volume (p = 0.047), but no post-hoc tests were significant. The topography of each lower-limb muscle was complex and variable across individuals.
Conclusions
TMS delivered with a 110-mm double-cone coil could not reliably identify discrete cortical representations of resting lower-limb muscles when responses were measured using bipolar surface electromyography.
Significance
The characteristics of the cortical representation provide a basis against which to evaluate cortical reorganisation in clinical populations.
1. Introduction
Transcranial magnetic stimulation (TMS) can be used to study the representation of muscles within the primary motor cortex. Although the extent of somatotopy within the primary motor cortex is debated (Donoghue et al., 1992, Schellekens et al., 2018, Schieber, 2001), TMS has revealed alterations in the cortical representation of muscles in several clinical conditions (Liepert et al., 1995, Schabrun et al., 2009, Schabrun et al., 2015, Schwenkreis et al., 2001, Schwenkreis et al., 2003, Te et al., 2017, Tsao et al., 2008, Tsao et al., 2011b). This suggests that TMS can identify clinically meaningful differences in cortical representation between groups of individuals.
The majority of work on the representation of muscles within the primary motor cortex has been conducted for muscles of the hand and upper limb. For the lower limb, TMS has been used to quantify the size and the amplitude-weighted centre (centre of gravity [CoG]) of the cortical representation of the resting tibialis anterior muscle (Liepert et al., 1995, Lotze et al., 2003), the resting quadriceps femoris muscle (Schwenkreis et al., 2003), the resting vastus lateralis muscle (Al Sawah et al., 2014), and the active rectus femoris (Te et al., 2017, Ward et al., 2016a, Ward et al., 2016b) and vastii (Te et al., 2017) muscles. No studies have reported the representation of the hamstring or gastrocnemius muscles. Understanding the cortical representation of lower-limb muscles involved in control of the knee joint is relevant not only to common clinical knee conditions such as osteoarthritis and patellofemoral pain, but also to other conditions that may involve altered control of walking such as stroke and Parkinson’s disease. Obtaining data on the representation of these muscles in healthy individuals is important to inform future studies on cortical reorganisation in clinical populations.
Although TMS has revealed discrete cortical representation of the deep and superficial fascicles of the paraspinal muscles (Tsao et al., 2011a), the extent to which TMS can be used to identify discrete cortical representation of lower-limb muscles is unclear. Only one study has quantified the representation of multiple lower-limb muscles in the same individuals. In statistical analysis there was no main effect of muscle, suggesting similar cortical representation of the active rectus femoris and vastii muscles (Te et al., 2017). However, the separation between the representation of the three muscles was smaller in individuals with patellofemoral pain than in healthy controls, suggesting that separation between muscles might be a measure of interest (Te et al., 2017). This supports findings in other chronic musculoskeletal pain conditions, where there was reduced distinction in the TMS-evaluated cortical representation of two back muscles in individuals with low-back pain (Tsao et al., 2011b) and two wrist extensor muscles in individuals with elbow pain (Schabrun et al., 2015), and functional magnetic resonance imaging findings suggested altered organisation of the motor cortex in individuals with knee osteoarthritis (Shanahan et al., 2015). However, despite its potential clinical relevance to knee conditions (Shanahan et al., 2015, Te et al., 2017), the extent to which TMS can be used to identify discrete cortical representation of lower-limb muscles involved in control of the knee joint is not clear. In addition, recent studies have identified multiple discrete peaks in the cortical representation of a muscle (Schabrun et al., 2015, Te et al., 2017), but no normative data exists on this measure for lower-limb muscles involved in control of the knee joint.
The relative paucity of TMS studies on the cortical representation of lower-limb muscles is likely due in part to the fact that it is more difficult to evoke responses in lower-limb muscles than it is in upper-limb muscles (Groppa et al., 2012). One reason for this is the location of the cortical representation within the intercerebral fissure, at greater depth from the scalp surface than the representation of upper-limb muscles, making it difficult to reach with TMS. The depth of stimulation can be increased by using a circular or double-cone coil instead of a figure-of-eight coil, as recommended by the International Federation of Clinical Neurophysiology for the study of lower-limb muscles (Groppa et al., 2012). A recent comparison of coil types recommends standard use of the double-cone coil for lower-limb studies (Dharmadasa et al., 2019); however, there are no normative data on the cortical representation of lower-limb muscles evaluated using this coil.
The aim of this study was to evaluate the extent to which TMS delivered with a double-cone coil can identify discrete cortical representation of lower-limb muscles involved in the control of the knee joint in healthy individuals. Cortical representation was mapped for seven resting lower-limb muscles (rectus femoris, vastus lateralis, vastus medialis, medial hamstring, lateral hamstring, medial gastrocnemius, and lateral gastrocnemius) and was quantified using size, CoG, hotspot and number of discrete peaks. These measures were compared between muscles from the same group (quadricep, hamstring, plantar flexor) to evaluate the extent to which TMS can identify discrete cortical representation of lower-limb muscles. These data describe the characteristics of the cortical representation of lower-limb muscles in healthy individuals and provide a basis against which to evaluate reorganisation in clinical populations.
2. Methods
This study was carried out at the Cardiff University Brain Research Imaging Centre and was approved by the Cardiff University School of Psychology Ethics Committee.
2.1. Participants
A convenience sample of 18 young healthy adults (13 women, five men; mean [SD] age 23.0 [2.5] years) was recruited from an existing participant database and advertisements placed around Cardiff University. All participants were screened for contraindications to TMS (including history of seizures, neurological injury or head injury) and to ensure that they met the following inclusion criteria: No recent, recurring or chronic pain in any part of the body, no history of surgery in the lower limbs, and not taking any psychiatric or neuroactive medications. The full screening questionnaire is available on the Open Science Framework (https://osf.io/npvwu/). All participants reported that they were right-leg dominant, defined by the leg they would use to kick a ball. All participants attended a single testing session between January and March 2018, and provided written informed consent prior to the start of the experiment. Participants were instructed to have a good night’s sleep the night prior to the experiment, not to consume recreational drugs or more than three units of alcohol on the day of or night prior to the experiment, and not to consume more than two caffeinated drinks in the two hours prior to the experiment.
2.2. Electromyography
Surface electrodes (Kendall 230 series; Covidien, MA) were placed on the following muscles of the right leg according to the SENIAM project guidelines (http://seniam.org/): Rectus femoris, vastus lateralis, vastus medialis, medial hamstring (semitendinosus), lateral hamstring (biceps femoris), medial gastrocnemius, and lateral gastrocnemius. Prior to electrode placement the skin was prepared with exfoliant and alcohol swabs. Data were passed through a HumBug Noise Eliminator (Digitimer, Hertfordshire, UK) and a D440/4 amplifier (Digitimer) where they were amplified x1000 and bandpass filtered (1–2000 Hz; Groppa et al., 2012) before being sampled at 6024 samples per second in Signal software (version 6; Cambridge Electronic Designs, Cambridge, UK). Electromyography (EMG) data were stored for offline analysis and viewed in real time using Signal software.
2.3. TMS
TMS was delivered through a 110-mm double-cone coil (Magstim, Whitland, UK) using a single-pulse monophasic stimulator (2002, Magstim). The coil was oriented such that current in the coil at the intersection of the two windings flowed from anterior to posterior. Throughout the experiment, a neuronavigation system (Brainsight TMS navigation, Rogue Resolutions, Cardiff, UK) was used to track the position of the coil relative to the participant’s head. The vertex was identified as the intersection of the interaural line and the line connecting the nasion and inion.
2.4. Experimental protocol
Participants were seated and chair height and arm rests were adjusted to optimise comfort. The coil was placed slightly lateral to the vertex, over the left hemisphere. Stimuli were delivered and stimulus intensity was gradually increased until motor evoked potentials (MEPs) were observed in the EMG data. Participants were instructed to stay relaxed, and this was confirmed by visual inspection of the EMG data in real time. For each participant, a stimulus intensity was selected that elicited consistent MEPs in all muscles, but that would be tolerable for the remainder of the experiment. In two participants (one woman, one man) it was not possible to elicit MEPs on resting muscles with a tolerable stimulus intensity, and these participants did not participate further. In the remaining 16 participants, the mean (SD) selected stimulation intensity was 52 (9.3)% maximum stimulator output (range, 38–65% maximum stimulator output).
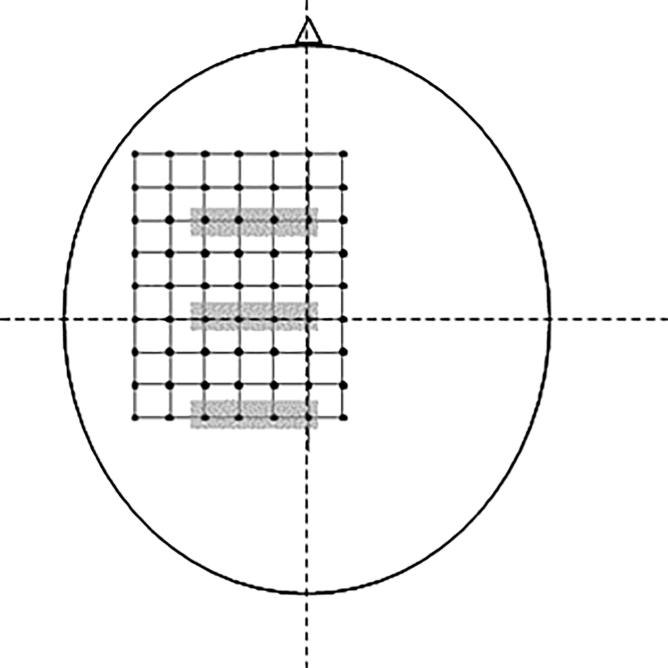
The neuronavigation software was used to project a 9 × 7 cm grid with 1-cm spacings over the left hemisphere of a representation of the skull that was visible to the experimenter on a monitor. The front-rightmost corner of the grid was positioned 1 cm to the right and 5 cm anterior to the vertex, and the back-leftmost corner was 5 cm to the left and 3 cm posterior to the vertex (Fig. 1). This resulted in a total of 63 grid sites. The target grid was centred slightly anterior to the vertex based on previous reports that the CoG of lower-limb muscles is anterior to the vertex (Al Sawah et al., 2014, Schwenkreis et al., 2003, Te et al., 2017, Ward et al., 2016a, Ward et al., 2016b). The target grid was designed to cover a large area to capture the boundaries of the cortical representations. The order of the targets was randomised and each target was stimulated once at the predetermined stimulus intensity. The inter-stimulus interval was at least 5 s. A break of ∼5 min was then taken, before this was repeated. The purpose of this break was to avoid long blocks of stimuli during which the participant’s attention or level of arousal might decline. Five sets of stimuli were performed in total. At each target, the experimenter viewed real-time information on the position of the coil and the error from the target position and did not stimulate until there was <2 mm error in coil position. The position of the coil was recorded for each stimulation. The same experimenter (JD) performed TMS for all participants.
Fig. 1.
Target stimulation sites. Target stimulation sites were arranged in a 9 × 7 cm grid with 1-cm spacings. The front-rightmost corner of the grid was positioned 1 cm to the right and 5 cm anterior to the vertex, and the back-leftmost corner was 5 cm to the left and 3 cm posterior to the vertex. Grey shading indicates the four stimulation sites (from midline to 3-cm lateral to midline) at the vertex, 3 cm anterior to the vertex, and 3 cm posterior to the vertex over which motor evoked potential latency was averaged for the exploratory analysis.
2.5. Data analysis
All processing and analyses were performing using custom-written code in Matlab (versions 2015a and 2019a, MathWorks, Natick, MA, USA). All code used to process and analyse the data is available on the Open Science Framework (https://osf.io/qrsp5/).
2.5.1. Planned analyses
Background EMG was quantified as mean absolute EMG in the 250 ms prior to stimulus onset. Trials were automatically discarded if there was background muscle activity, defined as background EMG greater than three median absolute deviations above the median background EMG from all trials for that muscle. Trials were also excluded if the stimulus was delivered >2 mm from the target. EMG data from the remaining trials were visualised and trials were manually excluded if there were visible artefacts. EMG traces for all muscles of all participants (https://osf.io/3k74p/), and the raw data on which all processing and analyses were performed (https://osf.io/y49uj/) are available on the Open Science Framework (doi 10.17605/osf.io/e7nmk).
EMG data from the remaining trials were averaged across all stimuli at each scalp site. Planned analysis was that the amplitude of the MEP be quantified as the peak-to-peak amplitude of the EMG signal between 10 and 70 ms after stimulus onset. In three participants, this window included the beginning of a second MEP and was a posteriori shortened to finish at 60 ms after stimulus onset. Visual inspection of EMG data confirmed that the windows captured the MEP for all participants and muscles.
Within each participant and each muscle, MEP amplitude was scaled to peak MEP amplitude (Tsao et al., 2011b, Tsao et al., 2008). Map volume was calculated as the sum of scaled MEP amplitudes across all sites (Te et al., 2017). Discrete peaks were identified if the scaled MEP amplitude at a grid site was greater than 50%, was at least 5% greater than the scaled MEP amplitude at all but one of the surrounding grid sites, and was not adjacent to another peak (Schabrun et al., 2015, Te et al., 2017).
The location of each grid site was expressed relative to the vertex. The scaled MEP amplitude, and the medial–lateral (ML) and anterior-posterior (AP) coordinates of the grid site were spline interpolated in two dimensions to obtain a resolution of one millimetre for each axis (Borghetti et al., 2008, Ward et al., 2016a, Ward et al., 2016b). The interpolated data were used to create a topographical map and calculate CoG. CoG is the amplitude-weighted indication of map position, and was calculated using the following formulae:
where is the medial–lateral location of the grid site, is the anterior-posterior location of the grid site, and is the scaled amplitude of the MEP at that grid site.
For each outcome and each muscle, the distribution of the data was evaluated using visual inspection of histograms in conjunction with the Anderson-Darling test at the 5% significance level. CoGML, CoGAP and map volume were not different to the normal distribution. Within each muscle group, CoGML, CoGAP and map volume were compared across muscles using a repeated-measures one-way analysis of variance (quadricep muscle group [vastus lateralis, rectus femoris, vastus medialis]) or paired t-test (hamstring [medial, lateral] and plantar flexor [medial gastrocnemius, lateral gastrocnemius] muscle groups). Effect size was calculated as η2 for one-way analysis of variance (Tomczak and Tomczak, 2014) and dz for paired t-test (G*Power 3.1 manual, http://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower.html; last accessed 15/10/2019). The number of discrete peaks was compared across muscles using a Friedman test (quadriceps) or a Wilcoxon signed-rank test (hamstrings, plantar flexors). The signed-rank test was performed using the approximate method (specified in Matlab software). Effect size was calculated as Kendall’s coefficient of concordance (W) for Friedman test and r for signed-rank test (Tomczak and Tomczak, 2014).
2.5.2. Exploratory analyses
The following analyses were conceived and performed after the data had been viewed.
CoG can be influenced by the presence of multiple discrete peaks in the topography. The stimulation location that elicited the largest MEP (hotspot) was quantified as an additional measure of the cortical representation.
For each participant, the onset latency of the MEP was determined for each stimulation site. Latency was defined as the first point after stimulation at which the full-wave rectified EMG signal was more than five median absolute deviations above the median full-wave rectified EMG signal in the 250 ms prior to stimulation and stayed above this threshold for at least 1 ms. The full-wave rectified EMG from each simulation site was then visually inspected to ensure that latency was accurately identified. Latency was averaged across four stimulation sites (from midline to 3-cm lateral to midline) at the vertex (central), 3 cm anterior to the vertex (anterior), and 3 cm posterior to the vertex (posterior; see Fig. 1).
In some muscles of some participants, a second MEP was present with a latency of ∼60 ms. For each participant and muscle, the presence or absence of a late MEP was determined by visual inspection of the EMG data. The onset latency of this late MEP was determined manually for each muscle by clicking a cursor on a graph where the first deviation from ongoing EMG was visible.
For several muscles, hotspotML and hotspotAP were different from the normal distribution. Within each muscle group, hotspotML and hotspotAP were compared across muscles using a Friedman test (quadriceps) or a Wilcoxon signed rank test (hamstrings, plantar flexors). For several muscles, MEP latency at central, posterior, and/or anterior stimulation locations were different from the normal distribution. For each muscle, onset latency was compared across stimulation locations using a Friedman test. Effect sizes were calculated as described in Section 2.5.1.
3. Results
Data were collected from 16 participants (12 women, four men; mean [SD] age 23.0 [2.6] years). All participants completed the testing session and did not report any adverse effects. In six participants, stimulation at the most anterior row of grid sites was uncomfortable due to large twitches of the facial muscles. In one of these participants and one additional participant, stimulation at the most lateral column of grid sites was also uncomfortable due to large twitches in hand muscles. These grid sites were not stimulated for these participants. In the remaining nine participants all 63 grid sites were stimulated.
For one participant (#9), EMG data from the medial gastrocnemius were of poor quality. For another participant (#15), EMG data from the medial gastrocnemius and the lateral hamstring were of poor quality. For a third participant (#7) EMG data from the medial gastrocnemius and medial hamstring were of poor quality. These five muscles (from three participants) were excluded from further analysis.
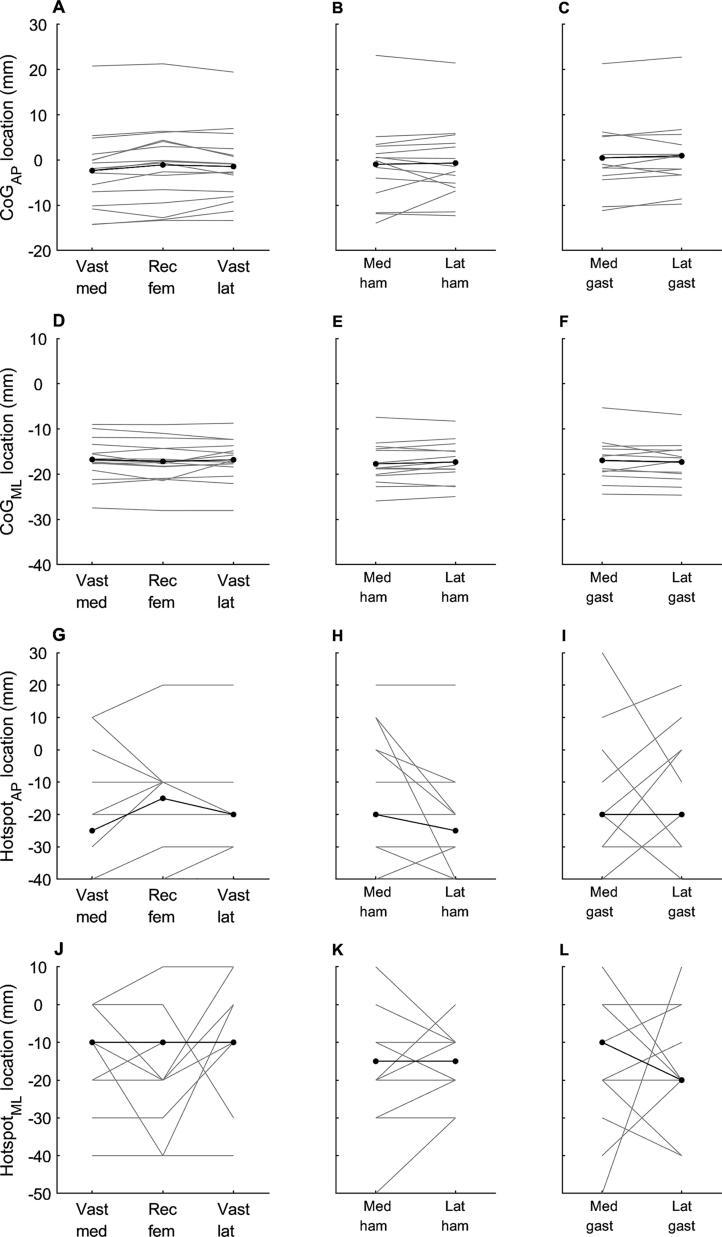
MEPs were present in all muscles from all participants. CoG and hotspot are shown in Fig. 2. MEP latency, CoG, hotspot, map volume and the number of discrete peaks for each muscle are shown in Table 1. Within the quadriceps muscle group there was a significant main effect of muscle on CoGAP (analysis of variance p = 0.010), but the effect size was very small (η2 = 0.003). Post-hoc tests showed that CoGAP was more negative (posterior) for vastus medialis than for vastus lateralis (Bonferroni corrected p = 0.035) and rectus femoris (Bonferroni corrected p = 0.018), but the magnitude of this difference was very small (Table 1 and Fig. 2A). CoGAP was similar for rectus femoris and vastus lateralis (Bonferroni corrected p > 1).
Fig. 2.
Location of the cortical representation for each muscle. Anterior-posterior (AP) and medial–lateral (ML) centre of gravity (CoG; A–F) and hotspot (G–L) for each muscle in the quadriceps (left column; A, D, G, J), hamstring (centre column; B, E, H, K) and gastrocnemius (right column; C, F, I, L) muscle groups. Grey lines indicate data for individual participants. Black lines indicate mean (for CoG) or median (for hotspot) across all participants. Vast med, vastus medialis; Rec fem, rectus femoris; Vast lat, vastus lateralis; Med ham, medial hamstring; Lat ham, lateral hamstring; Med gast, medial gastrocnemius; Lat gast, lateral gastrocnemius.
Table 1.
Characteristics of the cortical representation of each muscle.
| Quadriceps |
Hamstrings |
Plantar flexors |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vastus medialis | Rectus femoris | Vastus lateralis | p | Effect size | Medial hamstring | Lateral hamstring | p | Effect size | Medial gastrocnemius | Lateral gastrocnemius | p | Effect size | |
| Latency (ms) | 23.9 (1.4) | 21.7 (1.3) | 24 (3.2) | – | – | 25.4 (1.7) | 26.1 (2.2) | – | – | 33.5 (3) | 32.1 (3) | – | – |
| AP CoG (mm) | −2 (9) | −1 (9) | −1 (8) | 0.01 | 0.003 | −1 (9) | −1 (9) | 0.75 | 0.086 | 0 (8) | 1 (8) | 0.30 | 0.086 |
| ML CoG (mm) | −17 (5) | −17 (5) | −17 (4) | 0.33 | 0.000 | −18 (5) | −17 (5) | 0.16 | 0.395 | −17 (5) | −17 (5) | 0.35 | 0.395 |
| AP hotspot (mm)* | −15 (30) | −5 (30) | −10 (25) | 0.18 | 0.106 | −10 (40) | −15 (20) | 0.07 | 0.482 | −10 (32.5) | −10 (40) | 1 | 0.000 |
| ML hotspot (mm)* | −10 (10) | −10 (10) | −10 (5) | 0.03 | 0.225 | −15 (20) | −15 (10) | 0.62 | 0.131 | −10 (22.5) | −20 (12.5) | 0.37 | 0.241 |
| Map volume (%) | 1645 (577) | 1866 (770) | 1789 (656) | 0.05 | 0.014 | 1596 (694) | 1560 (796) | 0.76 | 0.084 | 2085 (960) | 2131 (739) | 0.8 | 0.084 |
| Number of discrete peaks* | 3.5 (2) | 4 (2.5) | 3 (3) | 0.32 | 0.071 | 4 (5) | 3 (3) | 0.96 | 0.012 | 4 (3.3) | 5 (3.3) | 0.07 | 0.481 |
Data are mean (standard deviation) for variables evaluated with parametric statistics and median (interquartile range) for variables evaluated with non-parametric statistics. Effect size is η2 for one-way analysis of variance, dz for paired t-test, Kendall’s coefficient of concordance (W) for Friedman test and r for signed-rank test.
AP, anterior-posterior; CoG, centre of gravity; ML, medial–lateral.
N = 16 for quadriceps, 14 for hamstrings, and 13 for plantar flexors.
Evaluated using non-parametric statistics.
Within the quadriceps muscle group there was also a significant main effect of muscle on hotspotML (Friedman test p = 0.027; W = 0.225). No post-hoc tests were significant, and there was no clear trend in the data (Fig. 2J). Within the quadriceps muscle group there was a significant main effect of muscle on map volume (analysis of variance p = 0.047, η2 = 0.014). No post-hoc tests were significant, but there was a trend for greater map volume in rectus femoris than in vastus medialis (Bonferroni corrected p = 0.07; Table 1). There was no significant effect of muscle for any other outcome measure in any other muscle group (Table 1).
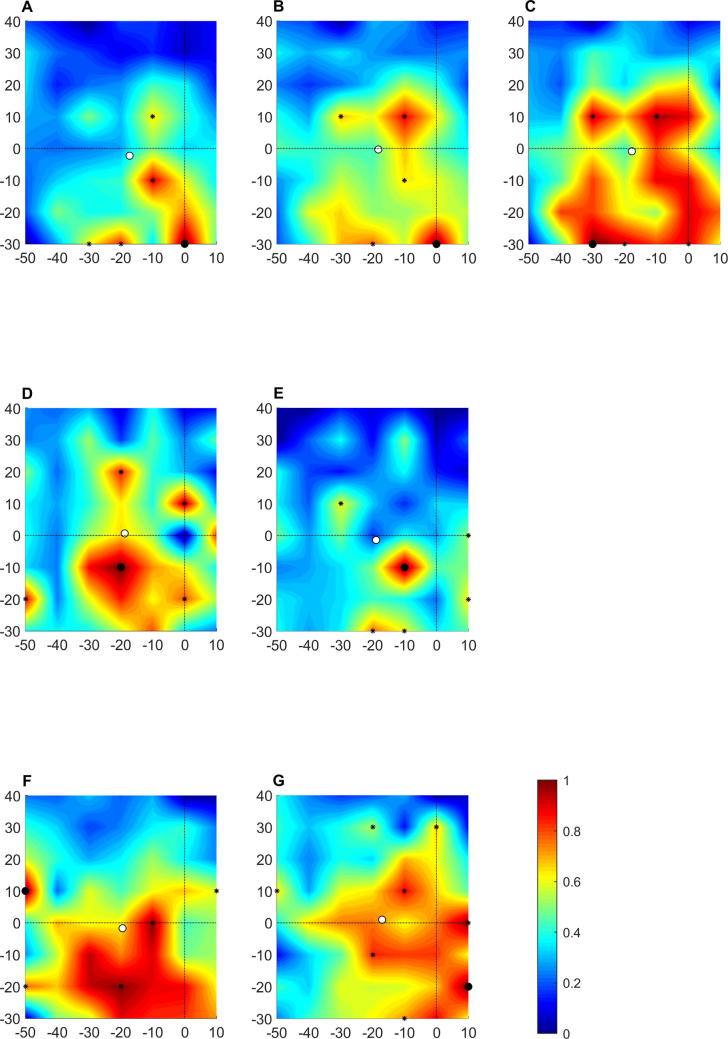
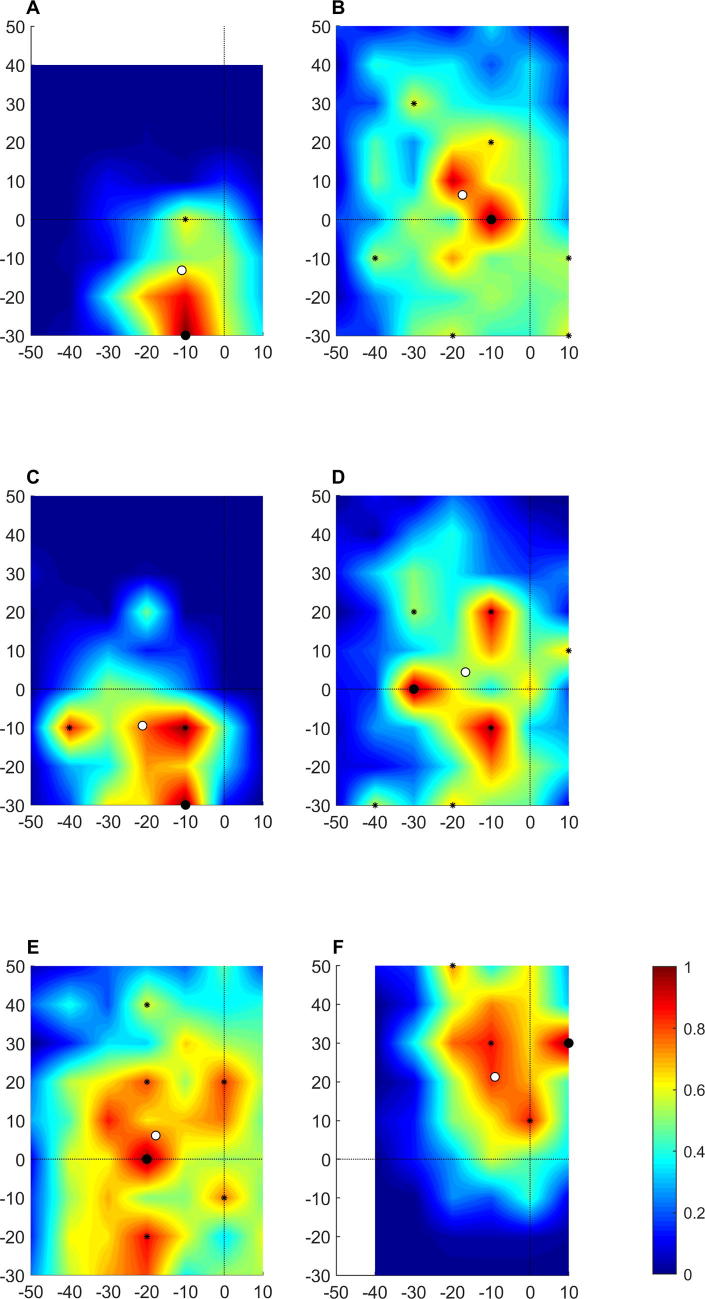
Topographical maps for all muscles from one participant are shown in Fig. 3. Topographical maps for the rectus femoris muscle from several participants are shown in Fig. 4. These show a complex and variable topography with multiple peaks present across the stimulation grid, including at the boundaries of the grid. Topographical maps for all muscles from all participants are available on the Open Science Framework (https://osf.io/4m2x9/).
Fig. 3.
Topographical maps for each muscle from one participant. A: vastus medialis; B: rectus femoris; C: vastus lateralis; D: medial hamstring; E: lateral hamstring; F medial gastrocnemius; G: lateral gastrocnemius. Colour represents scaled amplitude of the motor evoked potential, as indicated in the colour bar. Large black dot indicates the centre of gravity of the cortical representation. Small black dots indicate discrete peaks in the cortical representation. White dot indicates hotspot. Solid black lines indicate the interaural line and the line connecting the nasion and inion.
Fig. 4.
Topographical maps for the rectus femoris muscle from several participants. Large black dot indicates the centre of gravity of the cortical representation. Colour represents scaled amplitude of the motor evoked potential, as indicated in the colour bar. Small black dots indicate discrete peaks in the cortical representation. White dot indicates hotspot. Solid black lines indicate the interaural line and the line connecting the nasion and inion.
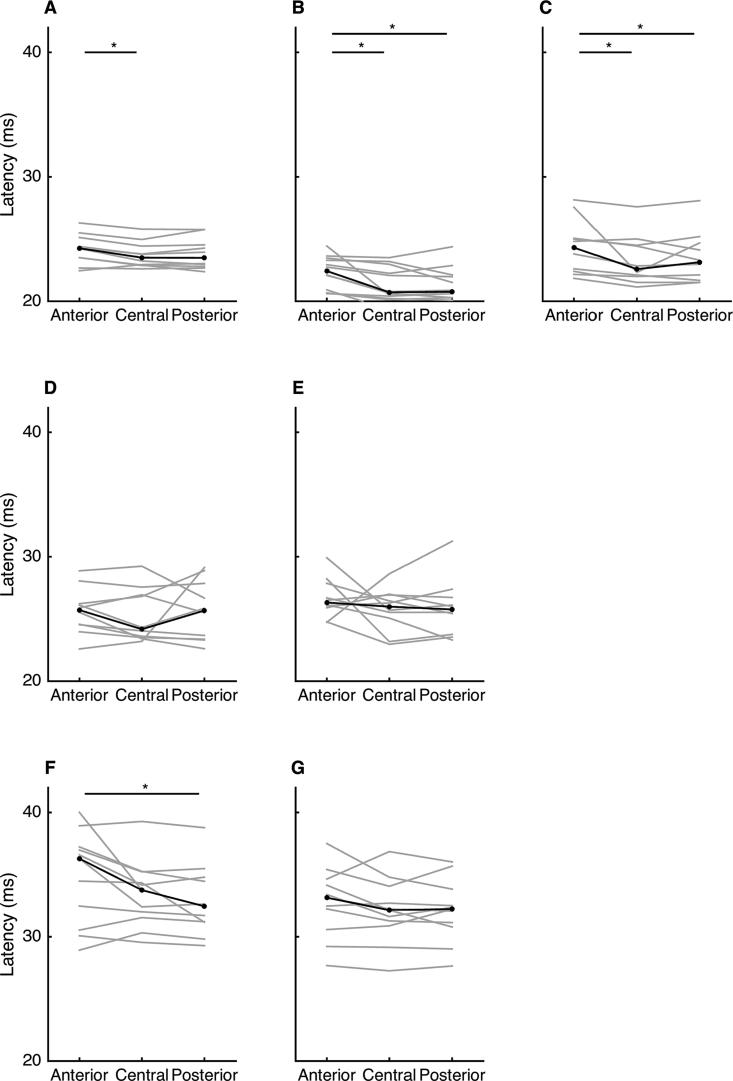
There was a significant main effect of stimulation location on MEP latency for the vastus medialis (p = 0.03), rectus femoris (p = 0.002), vastus lateralis (p = 0.006) and medial gastrocnemius (p = 0.04; Table 2 and Fig. 5). There was no significant main effect for medial hamstring (p = 0.20), lateral hamstring (p = 0.67) or lateral gastrocnemius (p = 0.31; Table 2 and Fig. 5). In all muscles, the tendency was for latency to be longer for stimuli delivered at the anterior location than for stimuli delivered at the central and posterior locations. The detailed results of post-hoc tests are provided in Table 2.
Table 2.
MEP latency at anterior, central and posterior stimulation sites for each muscle.
| Latency (ms) |
Post-hoc pairwise comparison (corrected p) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Anterior | Central | Posterior | p | Effect size (W) | Anterior-central | Anterior-posterior | Central-posterior | |
| Vastus Medialis | 10 | 24.3 (1.6)* | 23.5 (1.5) | 23.5 (1.7) | 0.03 | 0.356 | 0.03 | 0.22 | >1 |
| Rectus Femoris | 12 | 22.4 (2.6)* | 20.7 (2.3)* | 20.8 (1.8) | 0.002 | 0.528 | 0.001 | 0.02 | >1 |
| Vastus Lateralis | 10 | 24.3 (2.7)* | 22.6 (2.5)* | 23.1 (3) | 0.006 | 0.515 | 0.02 | 0.03 | >1 |
| Medial Hamstring | 10 | 25.7 (1.7) | 24.2 (3.4) | 25.7 (4.5) | 0.20 | 0.160 | – | – | – |
| Lateral Hamstring | 10 | 26.3 (2) | 26.0 (1.9) | 25.8 (3) | 0.67 | 0.040 | – | – | – |
| Medial Gastrocnemius | 11 | 36.3 (6.1) | 33.8 (3.3)* | 32.5 (3.5) | 0.04 | 0.298 | 0.25 | 0.06 | 0.16 |
| Lateral Gastrocnemius | 11 | 33.1 (3.5) | 32.1 (2.7) | 32.2 (2.6) | 0.31 | 0.107 | – | – | – |
Latency data are median (interquartile range). Main effect p was obtained from Friedman’s test. Post-hoc p were obtained from Wilcoxon signed-rank tests. Effect size is Kendall’s coefficient of concordance.
MEP, motor evoked potential.
Different from latency of MEP evoked from stimulation at posterior stimulation sites.
Fig. 5.
Latency of motor evoked potential evoked from stimulation at anterior, central and posterior stimulation sites. Data are shown for the quadriceps (top row), hamstring (middle row) and gastrocnemius (bottom row) muscles. A: vastus medialis; B: rectus femoris; C: vastus lateralis; D: medial hamstring; E: lateral hamstring; F medial gastrocnemius; G: lateral gastrocnemius. Grey lines indicate data for individual participants. Black lines indicate median across all participants. Asterisks indicate significant difference (p < 0.05).
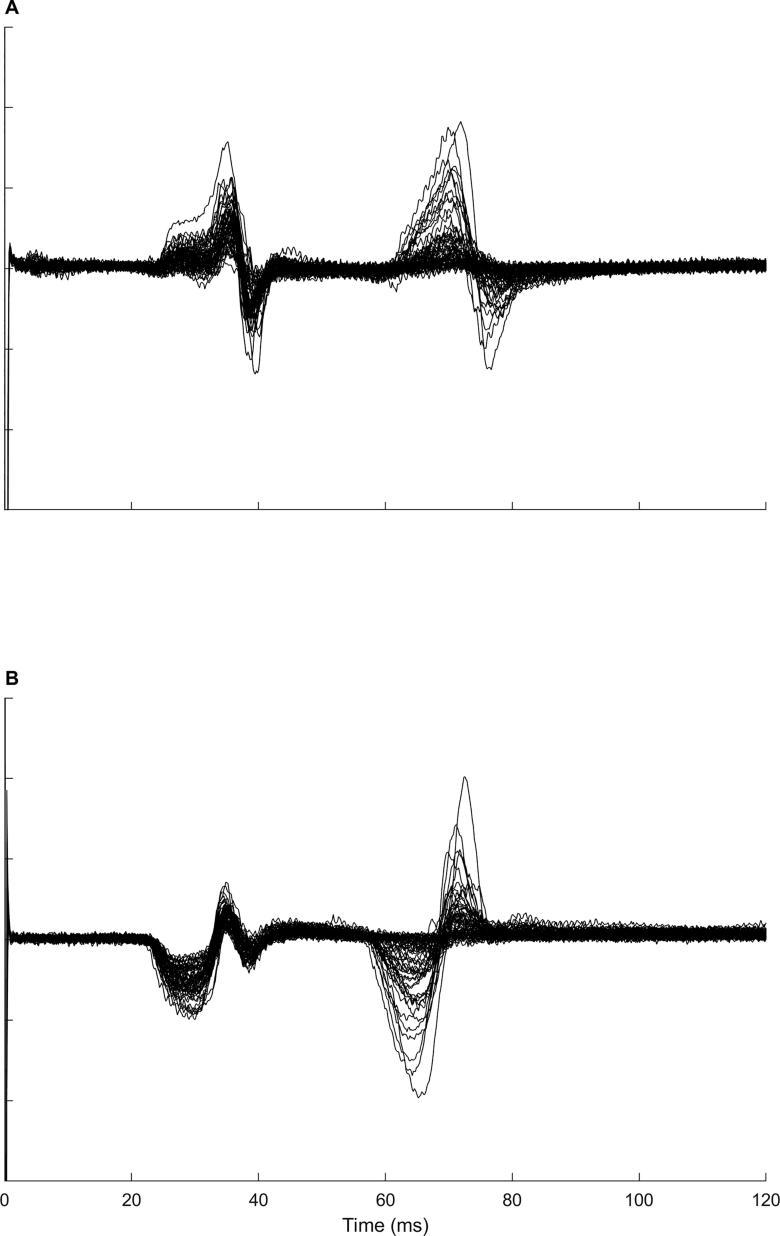
EMG data from the medial and lateral hamstring muscles from one participant are shown in Fig. 6. In these muscles, a second MEP was present after the primary MEP. This late MEP was present in the lateral hamstring of five participants, the medial hamstring of four participants, the vastus medialis and rectus femoris of two participants and the vastus lateralis of one participant. The late MEPs observed in the quadriceps muscles were all very small, whereas those observed in the hamstring muscles could be sizeable (see Fig. 6). The mean (SD) estimated onset latency for the late MEP was 61 (3) ms for the lateral hamstring (n = 5), 62 (7) ms for the medial hamstring (n = 4), 68 (2) ms for the vastus medialis (n = 2), 67 (0) ms for the rectus femoris (n = 2) and 65 ms for the vastus lateralis (n = 1).
Fig. 6.
Late motor evoked potential. Surface electromyography data from the medial (A) and lateral (B) hamstring of one participant. A late motor evoked potential is clearly evident with a latency of approximately 60 ms.
4. Discussion
In this study I used TMS to map the cortical representation of seven resting lower-limb muscles in healthy individuals. The results provide a comprehensive description of the cortical representation of these lower-limb muscles as revealed by TMS, advancing our knowledge in this area. The size, CoG, hotspot and number of discrete peaks were largely similar across muscles within each group (quadriceps, hamstrings, plantar flexors). There was a statistically significant difference in CoGAP and hotspotML across the quadriceps muscles but the effect size and magnitude of differences was very small. The magnitude of the difference means that it would not be practically possible to differentially target one of the three quadriceps muscles with navigated TMS. Overall, the results demonstrate considerable overlap in the cortical representations of lower limb-muscles identified by TMS delivered with a double-cone coil and MEPs measured with bipolar surface EMG, and provide normative data to inform future clinical comparisons.
Since the early studies demonstrating broad somatotopic organisation of the primary motor cortex in humans there has been much work on the organisation of the motor cortex, and the extent to which there is somatotopic (discrete) vs. distributed organisation of individual muscles. There is considerable overlap in the cortical representation of different muscles (Donoghue et al., 1992, Schieber, 2001), but within-limb somatotopic organisation may still exist (Schellekens et al., 2018). Although the majority of evidence for distributed representation has been obtained from the upper limbs (Schieber, 2001), the principles are thought to extend to other body areas. However, TMS has provided evidence of discrete organisation of the deep and superficial fascicles of the paraspinal muscles (Tsao et al., 2011a), consistent with their distinct functional roles, and this is reduced in individuals with low back pain (Tsao et al., 2011b). TMS has also provided evidence of cortical reorganisation in elbow pain (Schabrun et al., 2015), rotator cuff tendinopathy (Ngomo et al., 2015), patellofemoral pain (Te et al., 2017), traumatic single-leg amputation (Schwenkreis et al., 2003), upper-limb amputation (Schwenkreis et al., 2001), and ankle immobilisation (Liepert et al., 1995). The use of TMS to investigate the cortical representation of muscles is therefore clinically relevant, and the current study provides a detailed description of the cortical representation of multiple lower limb muscles that advances the position of this field.
The topography of the seven lower-limb muscle studied was often complex, displaying multiple peaks that were present across the stimulation grid, and variable across individuals. This may reflect a large and complex anatomical representation of these muscles within the cortex, with considerable inter-individual variability. However, the impact of the techniques used to quantify the cortical representation must also be considered, particularly the volume of cortical tissue excited by the TMS and the potential for peripheral volume conduction (crosstalk) in the surface EMG recordings.
4.1. Complexity of cortical representation
For all muscles, the average CoG was located at, or slightly posterior to, the vertex. Despite the large area covered by the target grid, large MEPs were often observed in response to stimuli delivered at the edge of the grid. This is in contrast to previous mapping studies of the quadriceps muscles, which have reported an anterior CoG (Al Sawah et al., 2014, Schwenkreis et al., 2003, Te et al., 2017, Ward et al., 2016a, Ward et al., 2016b) and relatively constrained map boundaries (see Fig. 1 in Te et al., 2017) and Fig. 2 in Ward et al. (2016a). These previous studies have all used a figure-of-eight stimulation coil, in contrast to the double-cone stimulation coil used here. The figure-of-eight coil provides a focal stimulation in superficial cortical regions, but minimal stimulation at increasing depths. By contrast, the double-cone coil can stimulate deeper regions of the brain, but at the expense of focality (Lu and Ueno, 2017). If a portion of the cortical representation of lower-limb muscles is inaccessible to the figure-of-eight coil, the resulting topography will appear less complex than that obtained with a double-cone coil. It is possible that the double-cone coil accessed corticospinal neurones that could not be accessed with the figure-of-eight coil, and thus the complexity of the cortical representation reflects true anatomical complexity within the motor cortex that cannot be uncovered with a figure-of-eight coil. Alternatively, it is possible that the double-cone coil excited cortical tissue beyond the motor cortex, and this resulted in MEPs and contributed to the apparent expansion of the cortical representation beyond the borders of the target grid and larger area of cortical representation than previously reported (Al Sawah et al., 2014, Schwenkreis et al., 2003, Te et al., 2017, Ward et al., 2016a, Ward et al., 2016b).
The latency of MEPs observed in response to stimulation at the anterior of the target grid was often slightly longer than that of MEPs observed in response to stimulation at the centre or posterior of the target grid. This may suggest that a different pathway was involved in the generation of these MEPs, supporting the latter hypothesis that the double-cone coil excited cortical tissue beyond the motor cortex. Corticospinal neurones innervating the lower limb spinal motoneurones are present in the premotor cortex (He et al., 1993) and supplementary motor area, caudal cingulate motor area on the dorsal bank and the rostral cingulate motor area (He et al., 1995), as well as the primary motor cortex. However, it is also possible that the longer latency at anterior stimulation sites is an artefact of a smaller MEP size at the grid boundary, and this requires further investigation.
The presence of multiple discrete peaks in the cortical representation of each muscle extends previous reports of this phenomenon in the wrist extensor (Schabrun et al., 2015) and knee extensor (Te et al., 2017) muscles. One possible reason for this observation is that the peaks represent different functional representations of the muscle (Ejaz et al., 2015, Leo et al., 2016). It would be interesting to identify if there are different areas of peak activation in functional magnetic resonance imaging data when performing different type of motor task with the lower-limb muscles, and whether these correspond to discrete peaks in the cortical representation identified by TMS.
This study focussed on muscles involved in the control of the knee joint due to their relevance for the study of various knee pain conditions and whole-body movements such as walking. The complex topography of the cortical representation was observed for all seven muscles. The tibialis anterior muscle receives a large number of monosynaptic corticomotoneuronal projections (Petersen et al., 2003) and is considered a good target muscle for TMS (Groppa et al., 2012). It would be interesting to compare the cortical representation of distal leg muscles, which may receive more corticomotoneuronal projections than proximal muscles, to that of proximal leg muscles evaluated using the same methods.
4.2. Surface EMG
High-density surface EMG recordings suggest that MEPs recorded in forearm muscles using conventional surface EMG may contain crosstalk from neighbouring muscles (Gallina et al., 2017, Neva et al., 2017, van Elswijk et al., 2008). No analogous studies have been performed in lower-limb muscles. The identification of crosstalk in voluntary contractions without using high-density surface EMG is difficult (Talib et al., 2019), and studies with high-density surface EMG are required to evaluate the influence of crosstalk on MEPs evoked from lower-limb muscles.
Although careful electrode placement can minimise crosstalk, it does not provide a guarantee to eliminate it completely. Nonetheless, bipolar surface EMG has been used to map the representation of lower-limb muscles in healthy (Al Sawah et al., 2014, Liepert et al., 1995, Lotze et al., 2003, Ward et al., 2016a, Weiss et al., 2013) and clinical (Schwenkreis et al., 2003, Te et al., 2017, Ward et al., 2016b) populations, and continues to be used to evaluate motor responses to TMS in the overwhelming majority of studies. Understanding what can and cannot be understood from bipolar surface EMG and providing normative data on TMS responses recorded using bipolar surface EMG in healthy individuals is of great practical relevance, despite the inherent limitations of this technique. The current results indicate bipolar surface EMG used with TMS delivered through a double-cone coil cannot reliably identify discrete cortical representation of lower-limb muscles in young, healthy individuals.
4.3. Resting vs. active muscle
Some previous lower-limb mapping studies have studied active muscles (Te et al., 2017, Ward et al., 2016a, Ward et al., 2016b), in contrast to the resting muscles studied here. This is in line with guidelines to increase the accessibility of lower-limb muscles to TMS (Groppa et al., 2012). However, if as has been suggested, the cortical representation of muscles is functional, rather than anatomical (Ejaz et al., 2015, Leo et al., 2016, Schieber, 2001), then requiring the participant to perform a motor task will engage the specific subset of cortical neurones relevant for that task. The cortical representation revealed by TMS may then be biased towards the representation for that specific task, at the expense of the representations for other functions of the target muscle. For example, requiring the participant to perform an isometric contraction of the quadriceps would increase excitability of the cortical areas involved in generating this type of contraction. The cortical representation of the quadriceps revealed by TMS will reflect this, and may fail to include the cortical representation relevant for a dynamic movement such as gait. For this reason, I chose to study resting muscles in the present study. Ward et al., 2016a, Ward et al., 2016b, Te et al., 2017 studied muscles performing a low-intensity isometric contraction, and this may have contributed to the more focussed topographical maps in these previous studies.
By contrast, Schwenkreis et al., 2003, Al Sawah et al., 2014 studied resting muscles. The topographical maps are not described in detail, but Schwenkreis et al. report that, across six healthy subjects, they observed an MEP in the resting quadriceps muscle response to stimulation at, on average, ∼15 sites (cf map area results) (Schwenkreis et al., 2003). Similarly, Al Sawah observed an MEP in the resting vastus lateralis muscle in response to stimulation at, on average, ∼8 sites (n = 10 healthy participants, cf map area results) (Al Sawah et al., 2014). The size of these MEPs, and whether the topography incorporated multiple discrete peaks, is not clear. However, the available data suggest that the cortical representations uncovered were smaller and less complex than those revealed in the present study. This suggests that the difference between the current and previous studies cannot be explained by the state of the muscle, and is more likely a function of the stimulating coil.
4.4. Late MEPs
In some muscles in some participants, there was a second MEP that occurred with a latency of 60–70 ms. This late MEP has previously been reported in the resting hamstrings, quadriceps, tibialis anterior and triceps surae muscles, with a latency of 59 ms, 64 ms, 79 ms and 72 ms, respectively (Dimitrijević et al., 1992), and in resting and active tibialis anterior and triceps surae muscles with a latency of ∼100 ms (Holmgren et al., 1990). Dimitrijevic et al. reported that this late MEP was most prevalent in the hamstrings, where it was of higher amplitude than the primary MEP. This is in line with the current findings, where the late MEP was observed most frequently and with the largest amplitude in the hamstring muscles. The late MEP is not exclusive to the lower limbs, and has been observed in resting and active forearm muscles (Holmgren et al., 1990) and a resting, but not active, intrinsic hand muscle (Wilson et al., 1995).
The source of the late MEP is not known, and could be central or peripheral. Indirect cortico-spinal or cortico-brainstem-spinal pathways, which originate either from the targeted areas of the motor cortex or from wider cortical areas excited by the stimulation, could play a role. Proprioceptive information arising from the primary MEP could also play a role. Based on the latency of responses from several lower-limb muscles, Dimitrijevic et al. argued against a segmental or transcortical stretch reflex origin of the late MEP, and against the involvement of gamma motor neurones (Dimitrijević et al., 1992). The difference in the latency of the late MEP between upper- and lower-limb muscles is ∼ 5 ms greater than the difference in latency of the early, primary MEP (Holmgren et al., 1990), lending the possibility that a slow central pathway is involved. Recent evidence indicates the primary motor cortex includes slow pyramidal tract neurones (Innocenti et al., 2019), which comprise the majority of the pyramidal tract but are not well studied (Firmin et al., 2014, Kraskov et al., 2019). This may provide one such candidate pathway, but this remains to be studied.
4.5. Limitations
Responses to TMS were evaluated using bipolar surface EMG, and the results may have been influenced by peripheral volume conduction (crosstalk) from other muscles. Studies using high-density surface EMG recordings, similar to those conducted in the forearm muscles (Gallina et al., 2017, Neva et al., 2017, van Elswijk et al., 2008), are required to elucidate the contribution of cross-talk to MEPs recorded from lower-limb muscles. However, the finding that TMS could not identify discrete cortical representations of lower-limb muscles measured with bipolar surface EMG is highly relevant as the overwhelming majority of TMS studies of the lower-limb are performed using bipolar surface EMG.
TMS was delivered through a double-cone coil. The ability of other coil designs, such as the figure-of-eight coil, to identify discrete cortical representations of lower-limb muscles remains to be determined. When using any coil, the volume of cortical tissue excited by the stimulus, and whether this is likely to encompass the full cortical representation of the target muscle, should be considered. This is particularly relevant for flat coils, where the depth of electric field penetration is lower than for the double-cone coil (Lu and Ueno, 2017). The current results are particularly relevant in light of a recent study recommending the standard use of the double-cone coil for lower-limb studies, in preference to a figure-of-eight or circular coil (Dharmadasa et al., 2019). The gold standard for cortical motor mapping in humans is direct electrical stimulation (Borchers et al., 2012, Desmurget et al., 2013); however, such studies are rare and only possible in a small subset of individuals. Ideally, information obtained from TMS studies should be combined with that obtained from other modalities such as direct electrical stimulation, positron emission topography and functional magnetic resonance imaging to build our understanding of cortical organisation.
The double-cone coil was orientated such that the current in the coil at the intersection of the two windings flowed from anterior to posterior. This is in line with previous studies (Roy et al., 2010, Sivaramakrishnan et al., 2016, Stokić et al., 1997). The shape of the coil used prevents it from being placed over the scalp at an angle allowing current flow in the medial–lateral direction, yet it is still more efficient at stimulating the corticospinal pathway to the lower limbs than a figure-of-eight coil delivering medial–lateral current (Dharmadasa et al., 2019, Groppa et al., 2012). However, a recent report used a double-cone coil from a different manufacturer that was oriented such that the current flow was medial–lateral, directed towards the hemisphere to be stimulated (Schecklmann et al., 2020). Further studies are needed to evaluate the effect of double-cone coil type and orientation on the cortical representation of lower-limb muscles.
4.6. Conclusions
The results of this study indicate that TMS delivered with a 110-mm double-cone coil cannot reliably identify discrete cortical representations of resting lower-limb muscles when MEPs are measured using bipolar surface EMG. The characteristics of the cortical representation of lower-limb muscles reported here advance our knowledge in this area and provide a basis against which to evaluate cortical reorganisation in clinical populations.
Funding source
J Davies is funded by the Biomechanics and Bioengineering Research Centre Versus Arthritis at Cardiff University. Funding for this project was provided by a Wellcome Trust Institutional Strategic Support award from Cardiff University to J Davies. Study sponsors had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al Sawah M., Rimawi M., Concerto C., Amer B., Cao Y., D’Antoni A.V., Chusid E., Battaglia F. Symmetric corticospinal excitability and representation of vastus lateralis muscle in right-handed healthy subjects. Clin. Anat. 2014;27:1053–1057. doi: 10.1002/ca.22438. [DOI] [PubMed] [Google Scholar]
- Borchers S., Himmelbach M., Logothetis N., Karnath H.-O. Direct electrical stimulation of human cortex — the gold standard for mapping brain functions? Nat. Rev. Neurosci. 2012;13:63–70. doi: 10.1038/nrn3140. [DOI] [PubMed] [Google Scholar]
- Borghetti D., Sartucci F., Petacchi E., Guzzetta A., Piras M.F., Murri L., Cioni G. Transcranial magnetic stimulation mapping: A model based on spline interpolation. Brain Res. Bull. 2008;77:143–148. doi: 10.1016/j.brainresbull.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Desmurget M., Song Z., Mottolese C., Sirigu A. Re-establishing the merits of electrical brain stimulation. Trends Cogn. Sci. 2013;17:442–449. doi: 10.1016/j.tics.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Dharmadasa T., Matamala J.M., Howells J., Simon N.G., Vucic S., Kiernan M.C. The effect of coil type and limb dominance in the assessment of lower-limb motor cortex excitability using TMS. Neurosci. Lett. 2019;699:84–90. doi: 10.1016/j.neulet.2019.01.050. [DOI] [PubMed] [Google Scholar]
- Dimitrijević M.R., Kofler M., McKay W.B., Sherwood A.M., Van der Linden C., Lissens M.A. Early and late lower limb motor evoked potentials elicited by transcranial magnetic motor cortex stimulation. Electroencephalogr. Clin. Neurophysiol. 1992;85:365–373. doi: 10.1016/0168-5597(92)90049-H. [DOI] [PubMed] [Google Scholar]
- Donoghue J.P., Leibovic S., Sanes J.N. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp. Brain Res. 1992;89:1–19. doi: 10.1007/bf00228996. [DOI] [PubMed] [Google Scholar]
- Ejaz N., Hamada M., Diedrichsen J. Hand use predicts the structure of representations in sensorimotor cortex. Nat. Neurosci. 2015;18:1034–1040. doi: 10.1038/nn.4038. [DOI] [PubMed] [Google Scholar]
- Firmin L., Field P., Maier M.A., Kraskov A., Kirkwood P.A., Nakajima K., Lemon R.N., Glickstein M. Axon diameters and conduction velocities in the macaque pyramidal tract. J. Neurophysiol. 2014;112:1229–1240. doi: 10.1152/jn.00720.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina A., Peters S., Neva J.L., Boyd L.A., Garland S.J. Selectivity of conventional electrodes for recording motor evoked potentials: An investigation with high-density surface electromyography. Muscle Nerve. 2017;55:828–834. doi: 10.1002/mus.25412. [DOI] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V., Kaelin-Lang A., Mima T., Rossi S., Thickbroom G.W., Rossini P.M., Ziemann U., Valls-Solé J., Siebner H.R. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Dum R., Strick P. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J. Neurosci. 1995;15:3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.Q., Dum R.P., Strick P.L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J. Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren H., Larsson L.-E., Pedersen S. Late muscular responses to transcranial cortical stimulation in man. Electroencephalogr. Clin. Neurophysiol. 1990;75:161–172. doi: 10.1016/0013-4694(90)90170-O. [DOI] [PubMed] [Google Scholar]
- Innocenti G.M., Caminiti R., Rouiller E.M., Knott G., Dyrby T.B., Descoteaux M., Thiran J.-P. Diversity of Cortico-descending Projections: Histological and Diffusion MRI Characterization in the Monkey. Cereb. Cortex. 2019;1991(29):788–801. doi: 10.1093/cercor/bhx363. [DOI] [PubMed] [Google Scholar]
- Kraskov A., Baker S., Soteropoulos D., Kirkwood P., Lemon R. The Corticospinal Discrepancy: Where are all the Slow Pyramidal Tract Neurons? Cereb. Cortex. 2019;29:3977–3981. doi: 10.1093/cercor/bhy278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo A., Handjaras G., Bianchi M., Marino H., Gabiccini M., Guidi A., Scilingo E.P., Pietrini P., Bicchi A., Santello M., Ricciardi E. A synergy-based hand control is encoded in human motor cortical areas. eLife. 2016;5 doi: 10.7554/eLife.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J., Tegenthoff M., Malin J.P. Changes of cortical motor area size during immobilization. Electroencephalogr. Clin. Neurophysiol. 1995;97:382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- Lotze M., Kaethner R.J., Erb M., Cohen L.G., Grodd W., Topka H. Comparison of representational maps using functional magnetic resonance imaging and transcranial magnetic stimulation. Clin. Neurophysiol. 2003;114:306–312. doi: 10.1016/s1388-2457(02)00380-2. [DOI] [PubMed] [Google Scholar]
- Lu M., Ueno S. Comparison of the induced fields using different coil configurations during deep transcranial magnetic stimulation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva J.L., Gallina A., Peters S., Garland S.J., Boyd L.A. Differentiation of motor evoked potentials elicited from multiple forearm muscles: An investigation with high-density surface electromyography. Brain Res. 2017;1676:91–99. doi: 10.1016/j.brainres.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Ngomo S., Mercier C., Bouyer L.J., Savoie A., Roy J.-S. Alterations in central motor representation increase over time in individuals with rotator cuff tendinopathy. Clin. Neurophysiol. 2015;126:365–371. doi: 10.1016/j.clinph.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Petersen N.T., Pyndt H.S., Nielsen J.B. Investigating human motor control by transcranial magnetic stimulation. Exp. Brain Res. 2003;152:1–16. doi: 10.1007/s00221-003-1537-y. [DOI] [PubMed] [Google Scholar]
- Roy F.D., Yang J.F., Gorassini M.A. Afferent Regulation of Leg Motor Cortex Excitability After Incomplete Spinal Cord Injury. J. Neurophysiol. 2010;103:2222–2233. doi: 10.1152/jn.00903.2009. [DOI] [PubMed] [Google Scholar]
- Schabrun S.M., Hodges P.W., Vicenzino B., Jones E., Chipchase L.S. Novel Adaptations in Motor Cortical Maps: The Relation to Persistent Elbow Pain. Med. Sci. Sports Exerc. 2015;47:681–690. doi: 10.1249/MSS.0000000000000469. [DOI] [PubMed] [Google Scholar]
- Schabrun S.M., Stinear C.M., Byblow W.D., Ridding M.C. Normalizing motor cortex representations in focal hand dystonia. Cereb. Cortex. 2009;1991(19):1968–1977. doi: 10.1093/cercor/bhn224. [DOI] [PubMed] [Google Scholar]
- Schecklmann M., Schmaußer M., Klinger F., Kreuzer P.M., Krenkel L., Langguth B. Resting motor threshold and magnetic field output of the figure-of-8 and the double-cone coil. Sci. Rep. 2020;10:1644. doi: 10.1038/s41598-020-58034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellekens W., Petridou N., Ramsey N.F. Detailed somatotopy in primary motor and somatosensory cortex revealed by Gaussian population receptive fields. NeuroImage. 2018;179:337–347. doi: 10.1016/j.neuroimage.2018.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M.H. Constraints on Somatotopic Organization in the Primary Motor Cortex. J. Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P., Pleger B., Cornelius B., Weyen U., Dertwinkel R., Zenz M., Malin J.P., Tegenthoff M. Reorganization in the ipsilateral motor cortex of patients with lower limb amputation. Neurosci. Lett. 2003;349:187–190. doi: 10.1016/s0304-3940(03)00838-3. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P., Witscher K., Janssen F., Pleger B., Dertwinkel R., Zenz M., Malin J.P., Tegenthoff M. Assessment of reorganization in the sensorimotor cortex after upper limb amputation. Clin. Neurophysiol. 2001;112:627–635. doi: 10.1016/s1388-2457(01)00486-2. [DOI] [PubMed] [Google Scholar]
- Shanahan C.J., Hodges P.W., Wrigley T.V., Bennell K.L., Farrell M.J. Organisation of the motor cortex differs between people with and without knee osteoarthritis. Arthritis Res. Ther. 2015;17:164. doi: 10.1186/s13075-015-0676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan A., Tahara-Eckl L., Madhavan S. Spatial localization and distribution of the TMS-related “hotspot” of the tibialis anterior muscle representation in the healthy and post-stroke motor cortex. Neurosci. Lett. 2016;627:30–35. doi: 10.1016/j.neulet.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokić D.S., McKay W.B., Scott L., Sherwood A.M., Dimitrijević M.R. Intracortical inhibition of lower limb motor-evoked potentials after paired transcranial magnetic stimulation. Exp. Brain Res. 1997;117:437–443. doi: 10.1007/s002210050238. [DOI] [PubMed] [Google Scholar]
- Talib I., Sundaraj K., Lam C.K., Hussain J., Ali Md.A. A review on crosstalk in myographic signals. Eur. J. Appl. Physiol. 2019;119:9–28. doi: 10.1007/s00421-018-3994-9. [DOI] [PubMed] [Google Scholar]
- Te M., Baptista A.F., Chipchase L.S., Schabrun S.M. Primary Motor Cortex Organization Is Altered in Persistent Patellofemoral Pain. Pain Med. 2017;18:2224–2234. doi: 10.1093/pm/pnx036. [DOI] [PubMed] [Google Scholar]
- Tomczak M., Tomczak E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014;1:19–25. [Google Scholar]
- Tsao H., Danneels L., Hodges P.W. Individual fascicles of the paraspinal muscles are activated by discrete cortical networks in humans. Clin. Neurophysiol. 2011;122:1580–1587. doi: 10.1016/j.clinph.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Tsao H., Danneels L.A., Hodges P.W. ISSLS prize winner: Smudging the motor brain in young adults with recurrent low back pain. Spine. 2011;36:1721–1727. doi: 10.1097/BRS.0b013e31821c4267. [DOI] [PubMed] [Google Scholar]
- Tsao H., Galea M.P., Hodges P.W. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain J. Neurol. 2008;131:2161–2171. doi: 10.1093/brain/awn154. [DOI] [PubMed] [Google Scholar]
- van Elswijk G., Kleine B.U., Overeem S., Eshuis B., Hekkert K.D., Stegeman D.F. Muscle imaging: Mapping responses to transcranial magnetic stimulation with high-density surface electromyography. Cortex. 2008;44:609–616. doi: 10.1016/j.cortex.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ward S., Bryant A.L., Pietrosimone B., Bennell K.L., Clark R., Pearce A.J. Cortical motor representation of the rectus femoris does not differ between the left and right hemisphere. J. Electromyogr. Kinesiol. 2016;28:46–52. doi: 10.1016/j.jelekin.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Ward S.H., Pearce A., Bennell K.L., Pietrosimone B., Bryant A.L. Quadriceps cortical adaptations in individuals with an anterior cruciate ligament injury. Knee. 2016;23:582–587. doi: 10.1016/j.knee.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Weiss C., Nettekoven C., Rehme A.K., Neuschmelting V., Eisenbeis A., Goldbrunner R., Grefkes C. Mapping the hand, foot and face representations in the primary motor cortex — Retest reliability of neuronavigated TMS versus functional MRI. NeuroImage. 2013;66:531–542. doi: 10.1016/j.neuroimage.2012.10.046. [DOI] [PubMed] [Google Scholar]
- Wilson S.A., Thickbroom G.W., Mastaglia F.L. An investigation of the late excitatory potential in the hand following magnetic stimulation of the motor cortex. Electroencephalogr. Clin. Neurophysiol. 1995;97:55–62. doi: 10.1016/0924-980X(94)00274-B. [DOI] [PubMed] [Google Scholar]