Abstract
Background/objectives
Articular cartilage erosion probably plays a substantial role in osteoarthritis (OA) initiation and development. Studies demonstrated that umbilical cord–derived mesenchymal stem cells (UCMSCs) could delay chondrocytes apoptosis and ameliorate OA progression in patients, but the detailed mechanisms are largely uncharacterised. In this study, we aimed to study the effects of UCMSCs on monosodium iodoacetate (MIA)–induced rat OA model, and explore the cellular mechanism of this effect.
Methods
Intra-articular injection of 0.3 mg MIA in 50 μL saline was performed on the left knee of the 200 g weight male Sprague-Dawley rat to induce rat knee OA. A single dose of 2.5 × 105 undifferentiated UCMSCs one day after MIA or three-time intra-articular injection of 2.5 × 105 UCMSCs on Days 1, 7 and 14 were given, respectively. Four weeks after MIA, joints were harvested and processed for paraffin sections. Safranine-O staining, haematoxylin and eosin staining and immunohistochemistry of MMP-13, ADAMTS-5, Col-2, CD68 and CD4 were performed to observe cartilage erosion and synovium. For in vitro studies, migration ability of cartilage superficial layer cells (SFCs) by UCMSCs were accessed by transwell assay. Furthermore, catabolism change of MIA-induced SFCs by UCMSCs was performed by real-rime polymerase chain reaction of Col-X and BCL-2 genes. CCK-8 assay was performed to check proliferation ability of SFCs by UCMSCs-conditioned media.
Result
In this study, we locally injected human UCMSCs, which is highly proliferative and noninvasively collectible, into MIA-induced rat knee OA. An important finding is on obviously ameliorated cartilage erosion and decreased OA Mankin score by repeated UCMSCs injection after MIA injection compared with single injection, both of which attenuated OA progression compared with vehicle. Interestingly, we observed significantly increased number of SFCs on the articular cartilage surface, probably related to elevated proliferation, mobilisation and inhibited catabolism marker: Col-X and BCL-2 gene expression of cultured SFCs by UCMSCs-conditioned media treatment in vitro. In addition to the change of unique SFCs, catabolism markers of ADAMTS-5 and MMP-13 were substantially upregulated in the whole cartilage layer chondrocytes as well. Strikingly, MIA-induced inflammatory cells infiltration, on both CD4+ Th cells and CD68+ macrophages, and hyperplasia of the synovium, which was alleviated by repeated UCMSCs injection.
Conclusion
Our study demonstrated a critical role of repeated UCMSCs dosing on preserving SFCs function, cartilage structure and inhibiting synovitis during OA progression, and thus provided mechanistic proof of evidence for the use of UCMSCs on OA patients in the future.
The translational potential of this article
UCMSCs are a relatively “young” stem cell, and noninvasively collectible. In our study, we clearly demonstrated that it could effectively delay OA progression, possibly through reserving SFCs function and inhibiting synovitis. Therefore, it could be a new promising therapeutic cell source for OA after further clinical trials.
Keywords: Cartilage superficial layer cells, Chondrocyte, Osteoarthritis, Synovitis, Umbilical cord–derived mesenchymal stem cells
Introduction
Osteoarthritis (OA) is the most common joint disease and places substantial physical and economic burden on affected individuals and the society as a whole [1]. All the treatments up to now are all symptom relieving, and no disease-modified treatment is available for OA [2].
OA is a whole joint disease, characterised by a complex and heterogeneous progress involving inflammatory, mechanical and metabolic factors [3], which could lead to articular cartilage deterioration, synovitis, subchondral bone sclerosis, osteophyte formation, muscle and ligament injury [4]. However, we incline that articular cartilage dysfunction is probably key to OA initiation and development, as we found that impaired mechanical property of articular cartilage surface occurred very early in DMM-induced mouse OA model [5], which led to abnormal mechanical loading of the joint along with abnormal subchondral bone remodelling [6]. In addition, breakdown of articular cartilage could trigger excessive release of inflammatory cytokines such as TNF-α and induced synovitis and hyperplasia [7,8].
It is well established that articular cartilage consists of three zones: the superficial zone, the middle zone and the deep zone, constituting uncalcified cartilage and deeper than calcified cartilage, characterised by distinct structure, composition and biomechanical properties [9,10]. The very superficial layer cells (SFCs), possibly two to four layers on the cartilage surface, are elongated and contain a subpopulation of cartilage progenitors [11] for secondary ossification centre formation [12]. Moreover, 70% of those cells are PRG4+, and substantially contribute to articular cartilage chondrocytes (CHOs) during development [13]. In addition, they could produce high levels of lubricin and hyaluronate acid to maintain joint function [14]. In pathologic conditions such as OA, degenerative changes initiate with decreased indentation modulus [5], cellular disorganisation and irregular surface of this layer [15]. Therefore, preserving SFCs could maintain the articular cartilage homeostasis and become a therapeutic target of OA.
Mesenchymal stem cells (MSCs), especially bone marrow and adipose tissue–derived MSCs, showed OA attenuation effects in both animal and human studies. Initially, it was thought that MSCs can differentiate the damaged tissue and replace them, but researchers gradually considered that secreted cytokines by MSCs can promote endogenous repair, which probably is a key mechanism. Umbilical cord–derived MSCs (UCMSCs) is an increasingly popular transplant cell source because of its high proliferation capacity, noninvasively harvesting method and relatively minor ethic issue [16]. Studies have showed ameliorated radiographic signs and improved joint function of UCMSCs on OA patients [17] and monosodium iodoacetate (MIA)–induced CHO apoptosis in rats OA knee [18], but the detailed effects and mechanism on OA development, especially at the histological and cellular level, were still largely uncharacterised. As UCMSCs exhibited improved chondrogenic factors [19], we therefore aimed to explore whether UCMSCs would attenuate OA process by rescuing SFCs function on a cellular and molecular basis. With respect to this, we induced rat knee OA by MIA local injection, and found a robust erosion of articular cartilage and synovial inflammation as previously described [20]. By UCMSCs treatment, we observed an obvious amelioration of cartilage deterioration and repeated UCMSCs dosing exhibited stronger effects than single injection. Moreover, we found increased SFC numbers on the articular cartilage surface, inhibited catabolism of the whole cartilage layer CHOs and alleviated synovitis by repeated UCMSCs injection. Further cell culture experiments revealed that UCMSCs-conditioned media (UCMSCs-CM) could promote SFCs proliferation, migration and downregulate Col-x and BCL-2 gene expression of SFCs induced by MIA treatment.
Materials and methods
Preparation of human UCMSCs
UCMSCs were purchased from Wuhan Hamilton Biotechnology Co., Ltd., Wuhan, Hubei, China, and cultured in growth media (Human Umbilical Cord Mesenchymal Stem Cells Serum-Free Basal Medium Cat. No. HUXUC-03061) containing 100 μg/mL streptomycin, and 100 U/mL penicillin at 37 °C in 5% CO2. Cells at passage 4–8 were used for experiments.
Isolation and expansion of rat SFCs and CHO
SFCs and CHO are harvested as previously described [21]. In brief, 3-day-old rats were euthanised, and their femur and tibia epiphyseal cartilage was dissected. The dissected cartilage was digested with 0.25% trypsin–EDTA (Invitrogen, Carlsbad, USA) for 1 h at 37 °C, and then further digested with 173 U/mL collagenase Type I (Worthington, Lakewood, USA, LS004196) in serum-free Dulbecco's Modified Eagle Medium (DMEM) for 2 h at 37 °C. After that, digestion solution was collected for SFCs and the remaining cartilage was used for CHO harvesting. Digested cells were filtrated through the cell strainer (80–100 μm) and centrifuged. Cell pellets after centrifugation were collected and resuspended in growth media until ready for use.
The remaining cartilage pieces were further digested with 86.5 U/mL collagenase Type I in serum-free DMEM overnight, and the digestion solution containing CHO was filtered and centrifuged. Supernatant was discarded and cell pellets were resuspended in growth media until ready for use.
MIA-induced OA in rats and UCMSCs transplantation
All animal experiments in this study followed the guidelines of Animal Welfare Act and were approved by the Animal Care and Use Committee of Union Hospital, Huazhong University of Science and Technology. Male Sprague-Dawley rats at 6 weeks of age were purchased from Huazhong University of Science and Technology (Wuhan, China), and were group-housed in ventilated cages with bedding and cotton pads, which were changed once every 3 days.
The rat OA model induced by local injection of MIA is a classic model for studying inflammation of OA [20]. On Day 0, intra-articular injection of 0.3 mg MIA in 50 μL saline was performed in the left knee of 200 g weight male, wild-type Sprague-Dawley rats. Only saline injection on contralateral right knees are used as normal joints. A single intra-articular injection of 2.5 × 105 UCMSCs in 50 μL saline was then given as single-dose injection (UCMSCs × 1) on Day 1, or 2.5 × 105 UCMSCs were repeatedly given on Days 1, 7 and 14 after MIA injection (UCMSCs × 3). In addition, 2.5 × 105 isolated rat CHOs in 50 μL saline were given on Days 1, 7 and 14 after MIA as CHO group. Fifty microlitre saline injection was used as vehicle.
Histology
Four weeks after MIA injection, rats were euthanised, and knee joints were harvested and processed for paraffine sections. After the joints were decalcified in 15% EDTA for 30 days, all samples were dehydrated in a graded series of ethanol (from 70% to 100%) and embedded in paraffin. Then, joints were cut into 6-μm-thick sections continuously by a microtome along the sagittal plane. Safranin O/Fast green staining, haematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) were performed on paraffine sections.
All sections were observed and scored by at least three independent researchers according to guidelines of the modified Mankin score [22].
To quantify the loss of articular cartilage, cartilage area (total) and Safranin-O stained area (uncalcified) were outlined and their thicknesses were determined by averaging five thickness values evenly distributed across the entire cartilage, and the uncalcified cartilage thickness percentage is uncal/total cartilage. To detect the inflammation of synovium, for each knee, the paraffin section near the one with maximal Mankin score was stained with H&E to examine the increase in the synovial lining cellular layers at the edge of synovium, the increase in cell density throughout the whole synovium membrane and the presence of inflammatory cells [23]. The score was calculated and averaged based on the results of each observer.
IHC
A portion of paraffin sections were left for immunohistochemical staining. For rat knee joint samples, slides were incubated with appropriate primary antibodies, such as rabbit anti-collagen II (Bioss, Beijing, China, bs-0709R), rabbit anti-ADAMTS-5 (Abcam, Cambridge, UK, ab41037) and rabbit anti-MMP-13 (Abcam, ab75606). After placed at 4 °C overnight, the slides were bonded with biotinylated secondary antibodies and reacted with DAB (DakoCytomation, Glostrup, Denmark, #K3465) for colour development later on. The stained sections were observed and recorded by light microscope (Olympus, Tokyo, Japan, IX73P1 F), processed and analysed by BIOQUANT, Nashville, USA software.
CCK-8 assay
SFCs were plated seeded in three 96-well plates at a density of 1 × 103 cells/well. There are three groups: blank, vehicle (DMEM) and UCMSCs-CM in the volume of 100 μL in each of the three plates, and the optical density (OD) value of each plate was read at 450 nm in a microplate reader after 10 μL CCK-8 solution was added to each hole for 1 h on ton days 1, 3, and 5, respectively. The OD difference value of every well between UCMSCs-CM and blank divided by the OD difference value between vehicle and blank is the growth rate. Growth rate curves were constructed after calculating the mean value of growth rate.
SFCs metabolism and real-rime polymerase chain reaction
To study the SFCs metabolic change by MIA (0.01 mg/mL) and UCMSCs, primary SFCs at P3–P5 were used. SFCs were seeded at 0.1 × 106 cells/well in two six-well plate. Cells were pretreated with MIA for 1 h. After that, cells were washed by PBS for three times and were either treated with UCMSCs-CM or DMEM for 12 h. Next, real-time polymerase chain reaction (RT-PCR) was performed to check Col-X and Bcl-2 gene expression.
After 12 h treatment, SFCs were washed by PBS for three times and treated by TRIzol (Invitrogen) to isolate total RNA according to manufacturer's instructions. The total RNA (200–500 ng) was performed reverse transcription synthesis using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, USA). The relative level of expression of each target gene was analysed using the 2−ΔΔCT method after RT-PCRs. The primer sequences used in this study are listed in the Supplementary Table.
Cell migration assay
CHOs in the lower chamber of transwell were serum starved overnight to induce catabolism, and then SFCs were seeded in the upper chamber in DMEM. Normal CHOs in the lower chamber without serum starvation were used as controls. After incubation for 16 h, SFCs on the upper chamber were removed and the migrated SFCs were fixed and stained with crystal violet. The migrated SFCs were observed and their number was counted using a microscope. To investigate whether UCMSCs could mobilise SFCs, we seeded SFCs in the upper chamber, and incubated for 16 h either in DMEM or in UCMSCs-CM. After that, migrated SFCs were observed under the microscope.
Statistical analysis
Summary statistics are expressed as means ± standard deviations and analysed by one-way repeated measures ANOVA for comparison among normal, vehicle, one-time UCMSCs injection, three-time UCMSCs injection and three-time CHO injection groups. The experiments were repeated independently at least three times and values of p < 0.05 denotes statistical significance.
Result
UCMSCs could ameliorate the cartilage degradation during MIA-induced OA progression
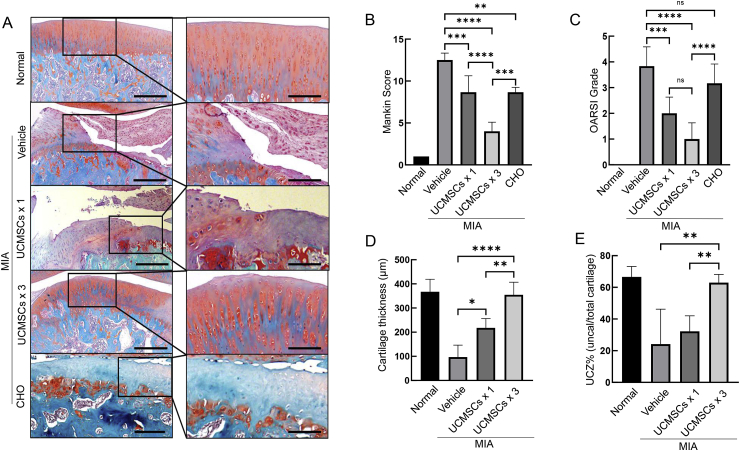
MIA induced the articular cartilage erosion down to the tidemark (Fig. 1A) and both the total cartilage layer and the uncalcified cartilage decreased significantly (Fig. 1D and E). By a single-dose injection, we saw the reserve of total and uncalcified cartilage (Fig. 1D and E) but diminished Safranin-O staining with a 31% decrease of Mankin score and Osteoarthritis Research Society International (OARSI) score compared with vehicle (Fig. 1B and C). Surprisingly, repeated injection of UCMSCs showed substantial cartilage preservation effects with mild destruction on the surface (Fig. 1A, D and E), and 68% and 54% decreases on Mankin score compared to vehicle and single dosing, respectively (Fig. 1A–C). Those data clearly showed that repeated UCMSCs could effectively preserve articular cartilage structure and delay MIA-induced rat knee OA progression.
Figure 1.
UCMSCs could preserve cartilage structure and attenuate OA progression induced by MIA injection. (A) Safranin O/Fast green staining of normal joints, vehicle, single injection of UCMSCs (UCMSCs × 1), three-time UCMSCs injection (UCMSCs × 3) and three-time CHO injection into MIA-induced rat knee OA. (Scale bars, 200 μm.) (B) and (C) Mankin and OARSI scores confirmed that both UCMSCs and CHO could significantly alleviate cartilage erosion triggered by MIA. Furthermore, repeated UCMSCs exhibited better effects compared with single dosing and three-time CHO injection. ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001. (D) Average thicknesses of articular cartilage in tibial plateau. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗∗p < 0.0001. (E) Percentages of uncalcified zone over the whole cartilage layer. ∗∗p < 0.01. Results are representative of at least three independent experiments and expressed as mean ± standard deviation. CHO = chondrocytes; MIA = monosodium iodoacetate; OA = osteoarthritis; UCMSCs = umbilical cord–derived mesenchymal stem cells; UCZ% = uncalcified cartilage zone thickness percentage, uncalcified cartilage thickness/total cartilage thickness × 100%.
UCMSCs switch articular cartilage from catabolism to anabolism after MIA treatment
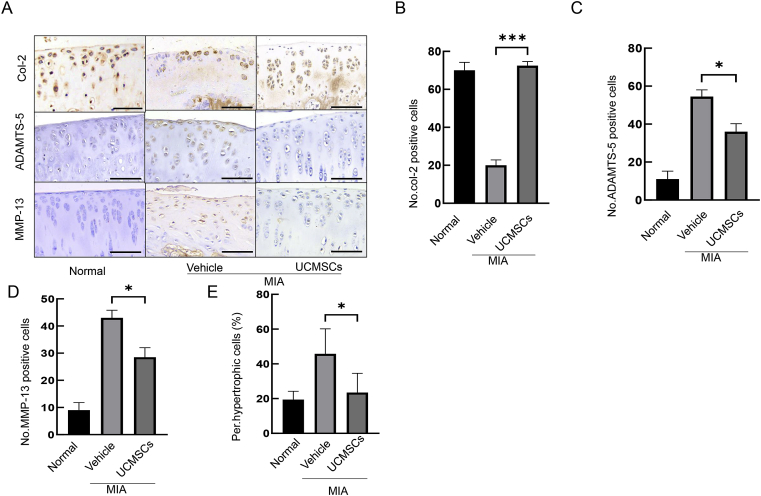
Loss of collagen-2 and cleavage of aggrecan is a key event in OA pathogenesis [5,24]. Therefore, we performed IHC staining on col-2, and revealed that repeated UCMSCs treatment significantly rescued the number of Col-2+ cells compared with vehicle (Fig. 2A and B). Next, we explored the examined aggrecan degradation by staining ADAMTS-5, a major aggrecanase of murine articular cartilage [25]. IHC revealed that ADAMTS-5 expression was markedly upregulated in the whole cartilage layer by MIA treatment, but was significantly brought down by UCMSCs treatment (Fig. 2A and C). MMP-13 is another critical proteinase responsible for cartilage degradation, and we found it was expressed widely in the articular cartilage after MIA treatment, but substantially abolished by UCMSCs (Fig. 2A and D).
Figure 2.
Immunostaining confirmed that UCMSCs could promote cartilage anabolism and alleviate cartilage catabolism. (A) Col-2 is evenly distributed on the whole cartilage, whereas MIA greatly decreased the expression, which is rescued by UCMSCs (upper panel). Aggrecanase ADAMTS-5 and collagen degradation enzyme MMP-13 is upregulated by MIA treatment, and UCMSCs effectively reduced its activity and attenuated catabolism (middle and lower panels). (Scale bars, 50 μm.) (B)–(D) Quantification of Col-2+, ADAMTS-5 and MMP-13+ cells in a fixed region. ∗p < 0.05 and ∗∗∗p < 0.001. (E) Quantification of hypertrophic chondrocytes in a fixed cartilage. ∗p < 0.05. Results are representative of at least three independent experiments and expressed as mean ± standard deviation. MIA = monosodium iodoacetate; UCMSCs = umbilical cord–derived mesenchymal stem cells.
CHO hypertrophy is an indicator of cartilage catabolism and routinely seen in the OA progression. We quantified the number of hypertrophic CHOs in each group and found a 136% increment of hypertrophic CHOs in MIA. This effect could be significantly diminished by UCMSCs treatment (23.47 vs. 45.75 in vehicle, p = 0.0234; Fig. 2E). These findings indicate that UCMSCs could effectively reverse the articular cartilage catabolism.
SFCs are substantially preserved by UCMSCs during OA pathogenesis
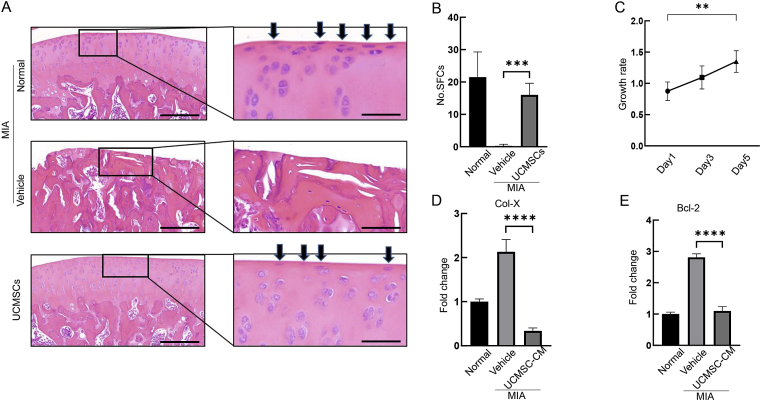
We have previously demonstrated that SFCs are critical to maintain articular cartilage homeostasis during OA development [26], so we first looked at the SFC number by H&E staining. Interestingly, MIA treatment almost abolished SFCs on the articular cartilage surface, whereas UCMSCs dramatically rescued SFCs number to almost normal level (16 vs. MIA 0.29, p = 0.0001; Fig. 3A and B). In line with this, we observed elevated proliferation ability in UCMSCs-CM (Fig. 3C).
Figure 3.
UCMSCs prohibited cartilage surface SFCs catabolism and preserved its number on the articular cartilage surface. (A) H&E staining showed typical elongated SFCs on the articular cartilage surface (arrows, upper panel). On the contrary, vehicle treated OA joint induced by MIA showed severe cartilage surface erosion and diminished SFCs (middle panel). UCMSCs administration moderately reserved the numbers of SFCs after MIA injection (lower panel). (Scale bars, 200 and 50 μm.) (B) Quantification of SFCs on the fixed cartilage area. ∗∗∗p< 0.001. (C) Effect of UCMSC-CM on SFCs' proliferation measured by CCK-8 assays. (D) and (E) Catabolism triggered by MIA treatment raise the Col-X and Bcl-2 gene expression, but conditioned media from UCMSCs could significantly downregulated those two catabolic genes. ∗∗∗∗p < 0.0001, one-way repeated measures ANOVA, results are representative of at least three independent experiments and expressed as mean ± standard deviation. H&E = haematoxylin and eosin; MIA = monosodium iodoacetate; SFCs = superficial layer cells; UCMSCs = umbilical cord–derived mesenchymal stem cells; UCMSCs-CM = UCMSCs-conditioned media.
Next, we explored the metabolism of SFCs by MIA and UCMSCs treatment. Col-X is a hypertrophic CHO marker to indicate CHO catabolism, so we treated SFCs with MIA to induce catabolism and found a 2.13-fold upregulation of Col-X by RT-PCR, whereas UCMSCs-CM treatment could bring it down to almost normal level (Fig. 3D).
Excessive CHO apoptotic rate is positively associated with OA progression. Therefore, we explored whether UCMSCs could rescue SFCs apoptosis induced by MIA treatment. RT-PCR revealed that UCMSCs substantially decreased the expression of Bcl-2, an apoptotic marker, by MIA treatment (Fig. 3E).
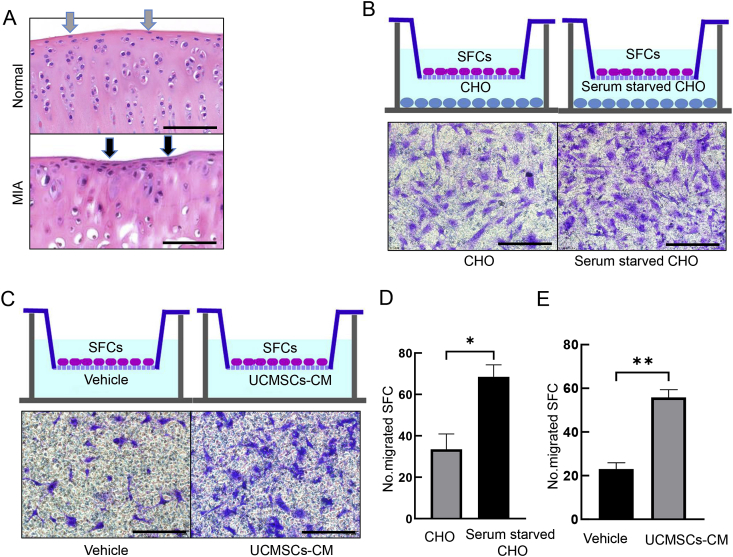
Mobilisation of cartilage stem/progenitor cells to the injured sites are crucial for endogenous repair initiation, so we injected MIA on Day 0, treated with vehicle or UCMSCs on Day 1 and harvested the joints on Day 7. Interestingly, we observed obvious enrichment of cells in the injured cartilage surface after MIA treatment, which are possibly SFCs (Fig. 4A). Further transwell study revealed that serum-starved CHOs significantly increased migration ability of SFCs (Fig. 4B and D), suggesting that injured CHOs could mobilise SFCs than healthy CHO. Next, we tested the effects of UCMSCs on SFCs mobilisation, and found UCMSCs-CM could significantly induce SFCs migration (Fig. 4C and E). Combining those in vivo and in vitro data, we demonstrated that UCMSCs could promote SFCs proliferation and effectively inhibit the catabolism of SFCs. In addition, CHOs with OA phenotype could induce those cells to the injured site, which is a key step for endogenous cartilage repair and UCMSCs could substantially promote this effect.
Figure 4.
Migration of UCMSCs to SFCs migration by CHO was enhanced after MIA induction and UCMSCs at early stage of OA. (A) One week after MIA treatment, we could see enriched elongated cells in the injured uneven cartilage surface (lower panel, arrow), whereas elongated SFCs evenly distributed on normal articular cartilage surface (upper panel). (Scale bars, 100 μm.) (B) Crystal violet staining of transwell assay revealed obviously more migrated SFCs by serum-starved CHOs in the lower chamber compared with vehicle-treated CHOs. (C) Transwell assay showed that UCMSCs-CM substantially promote SFCs migration compared with vehicle (DMEM). (Scale bars, 50 μm.) (D) Quantification of B. ∗p < 0.05. (E) Quantification of C and quantification data of E. ∗∗p < 0.01. Results are representative of at least three independent experiments and expressed as mean ± standard deviation. CHO = chondrocytes; MIA = monosodium iodoacetate; OA = osteoarthritis; SFCs = superficial layer cells; UCMSCs = umbilical cord–derived mesenchymal stem cells; UCMSCs-CM = UCMSCs-conditioned media.
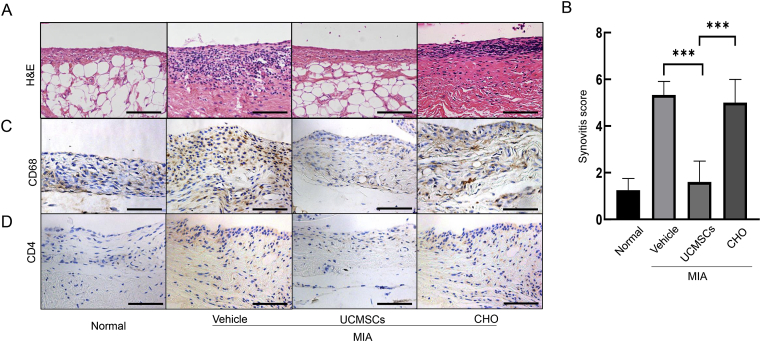
UCMSCs substantially inhibit synovitis induced by MIA treatment
Inflammatory cells infiltration during OA will release excessive proinflammatory cytokines and induce CHOs catabolism and cartilage erosion, and vice versa [27,28]. Therefore, we did H&E staining on the synovium, and revealed that MIA induced obvious inflammatory cell infiltration and obvious hyperplasia (Fig. 5A). On the contrary, UCMSCs treatment could greatly diminish these effects and significantly bring the synovitis score down (1.60 vs. vehicle 5.33, p = 0.0002; Fig. 5A and B). To further identify the identity of those infiltrated inflammatory cells in the synovium, we performed IHC staining of CD68 and CD4. Interestingly, both CD4+ Th cells and CD68+ macrophages were elevated by MIA, and obviously attenuated by multiple UCMSCs treatment (Fig. 5C and D).
Figure 5.
UCMSCs could alleviate synovitis during OA development. (A) H&E staining showed thin layer of membrane on the synovium and very few inflammatory cells in normal synovium. MIA injection significantly induced huge number of inflammatory cells and obvious hyperplasia. UCMSCs could substantially attenuate the inflammation and reverse it to almost normal level. CHO treatment exhibited mild attenuated but still obvious inflammatory cells infiltration and hyperplasia. (Scale bars, 100 μm.) (B) Quantification of synovitis score. ∗∗∗p < 0.001. Results are representative of at least three independent experiments and expressed as mean ± standard deviation. IHC clearly showed that multiple UCMSCs treatment could alleviate CD68+ macrophages (C) and CD4+ Th cells (D) infiltration induced by MIA, whereas CHO could not. (Scale bars, 100 μm.) CHO = chondrocytes; H&E = haematoxylin and eosin; IHC = immunohistochemistry; MIA = monosodium iodoacetate; OA = osteoarthritis; UCMSCs = umbilical cord–derived mesenchymal stem cells.
As CHOs transplantation for OA is recognised as an effective treatment for OA long before, we introduced those cells locally. Interestingly, we revealed much milder rescue effects on articular cartilage erosion compared with UCMSCs (Fig. 1A and B), but little effects on the synovial inflammation (Fig. 5). Combining those data, we conclude that UCMSCs could inhibit local inflammatory effects on synovitis, which also contribute to the articular cartilage preserving effects.
Discussion
Stem cells therapy has been introduced in OA since Murphy et al. [29] injected caprine bone marrow MSCs in a caprine OA model in 2003, which showed attenuated meniscus injury and OA phenotype. Because of limited resources and technical difficulty of harvesting cells from bone marrow, researchers gradually turned to other specific tissues such as adipose stem cell, which could protect cartilage and attenuate OA progression before culture [30] or after culture from different organs [31], mainly by protecting CHO viability, and by improving joint function in OA patients [32]. Recently, UCMSCs are becoming increasingly popular because of noninvasive harvesting method and shown preserved effects in reversing CHOs apoptosis and thus regenerating cartilage [18], and UCMSCs could directly differentiate into CHOs as well [33]. Our study demonstrated a new mechanism of UCMSCs on protecting specific CHOs: superficial layer cartilage cells and inhibiting synovitis in OA progression.
Initially, researchers thought that tissue-specific cell replacement therapy is the main mechanism of the regeneration effects as MSCs possess the multidifferentiation ability. Although the retention ability of MSCs was very low (possibly around 3%) and only appeared in meniscus, fat pad and synovium other than cartilage [34], researchers gradually accepted the concept that cytokines transplanted MSCs secreted to the host could mobilise the host stem cell and thus initiate the repair. Among different tissue-specific MSCs, UCMSCs are thought to be highly proliferative [35], noninvasively collectible [36], and probably can secrete high levels of chondro-protective cytokines such as GDF-5 [16]. Therefore, we explored UCMSCs' effects on OA progression, and indeed found well preserved cartilage structure and attenuated OA development. Further studies to compare the chondroprotective effects during OA progression with other MSCs are critical in the future.
Endogenous repair initiated by host stem cells locally are considered as a key mechanism of tissue regeneration. Articular cartilage surface contains cartilage chondroprogenitors, and those cells proliferate very slowly and could gradually populate other zones during development [13,21]. In pathologic conditions such as OA, those cells could be mobilised and migrated to injured region to initiate regeneration [37]. Dysfunction of those cells lead to accelerated cartilage erosion and OA progression [26]. Therefore, SFCs are crucial for OA endogenous repair. Our study, for the first time, revealed that UCMSCs could mobilise elongated SFCs to the injured sites (Fig. 4) to initiate the repair process at early time points and delay the hypertrophy and apoptosis of SFCs and promote its proliferation (Fig. 3C–E), and thus rescue the loss of SFC numbers by MIA at later stage of OA (Fig. 3A and B). Therefore, we hypothesised that UCMSCs could efficiently protect and modulate SFCs to promote endogenous cartilage repair. In addition to the unique SFCs, we also found inhibited aggrecanase activities but upregulated Col-2 expression of CHOs of the whole cartilage layer by UCMSCs treatment (Fig. 2A), which is in line with a previous study [18].
Synovial inflammation plays a substantial role in OA progression and symptom. On one hand, synovitis and articular cartilage could crosstalk with each other as a vicious cycle and accelerate OA progression. Abnormal CHOs catabolism could secrete a complexity of proinflammatory cytokines and extracellular vesicles to induce synovial inflammatory cells infiltration, such as CD4+ T cells and macrophages [28]. In turn, those abnormally infiltrated cells could produce excessive inflammatory cytokines and promote cartilage degradation enzyme expression such as MMPs, ADAMTS-4 and ADAMTS-5 [38]. Inhibiting synovitis could effectively protect cartilage and attenuate OA development. In line with this, we found MMP-13 and ADAMTS-5 expressions were greatly upregulated by MIA (Fig. 2A, C and D), together with obvious synovitis (Fig. 5). This phenotype is substantially abolished by UCMSCs treatment. This finding is consistent with previous studies that MSCs were reported to be capable of inhibiting inflammation in collagen-induced rheumatoid arthritis [39]. Interestingly, normal CHOs injection exhibited very mild attenuation effects on OA progression (Fig. 1A and B), and almost no effects in relieving synovitis (Fig. 5). This may be because normal CHOs could not effectively interfere with the crosstalk between articular cartilage deterioration and synovial inflammation, a vicious cycle triggered by MIA. On the other hand, synovitis is considered as a key factor contributing to OA pain: synovial inflammatory cells infiltration will trigger hyperalgesia due to elevated proinflammatory cytokines such as IL-6, TNF-α [2]and NGF, a major contributor to peripheral hypersensitivity [40]. Therefore, inhibiting synovitis could effectively relieve joint pain, the most disturbing symptom of OA patients. Further mechanistic studies regarding the role of UCMSCs on synovium and cartilage crosstalk, and pain assessment such as Von Frey assay are needed.
In conclusion, our study identified a novel role of UCMSCs on preserving articular cartilage CHOs anabolism, especially SFCs, and alleviating synovitis during OA progression. Based on this, it partially delineated the cellular and molecular mechanism of UCMSCs on OA pathology, which paved the road for the clinical application of UCMSCs on patients in the future.
Conflict of Interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
This study was supported by NSFC grant 81672235 (PI: Hongtao Tian), and NSFC grant 81702157 (PI: Wei Tong).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2020.03.007.
Contributor Information
Wei Tong, Email: tongwei312@126.com.
Xiaoguang Zhang, Email: zhangxiaoguang0095@163.com.
Quan Zhang, Email: 470558642@qq.com.
Jiarui Fang, Email: fangjiarui1992@163.com.
Yong Liu, Email: 961836059@qq.com.
Zengwu Shao, Email: 381712554@qq.com.
Shuhua Yang, Email: 1010948762@qq.com.
Dongcheng Wu, Email: bcdcwu@hotmail.com.
Xiaoming Sheng, Email: Xiaoming.Sheng@utah.edu.
Yingze Zhang, Email: 258974810@qq.com.
Hongtao Tian, Email: tianhongtao@vip.163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hunter D.J., Schofield D., Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 2.Malfait A.M., Schnitzer T.J. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9(11):654–664. doi: 10.1038/nrrheum.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu-Bryan R. Inflammation and intracellular metabolism: new targets in OA. Osteoarthritis Cartilage. 2015;23(11):1835–1842. doi: 10.1016/j.joca.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B. Osteoarthritis. Nat Rev Dis Prim. 2016;2:16072. doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 5.Doyran B., Tong W., Li Q., Jia H., Zhang X., Chen C. Nanoindentation modulus of murine cartilage: a sensitive indicator of the initiation and progression of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2017;25(1):108–117. doi: 10.1016/j.joca.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 7.Homandberg G.A., Hui F. Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch Biochem Biophys. 1996;334(2):325–331. doi: 10.1006/abbi.1996.0461. [DOI] [PubMed] [Google Scholar]
- 8.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacifici M., Koyama E., Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;75(3):237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- 10.Hunziker E.B., Michel M., Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37(4):271–284. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Dowthwaite G.P., Bishop J.C., Redman S.N., Khan I.M., Rooney P., Evans D.J. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 12.Tong W., Tower R.J., Chen C., Wang L., Zhong L., Wei Y. Periarticular mesenchymal progenitors initiate and contribute to secondary ossification center formation during mouse long bone development. Stem Cell. 2019;37(5):677–689. doi: 10.1002/stem.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozhemyakina E., Zhang M., Ionescu A., Ayturk U.M., Ono N., Kobayashi A. Identification of a Prg4-expressing articular cartilage progenitor cell population in mice. Arthritis Rheum. 2015;67(5):1261–1273. doi: 10.1002/art.39030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becerra J., Andrades J.A., Guerado E., Zamora-Navas P., López-Puertas J.M., Reddi A.H. Articular cartilage: structure and regeneration. Tissue Eng B Rev. 2010;16(6):617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 15.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Balasubramanian S., Venugopal P., Sundarraj S., Zakaria Z., Majumdar A.S., Ta M. Comparison of chemokine and receptor gene expression between Wharton's jelly and bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2012;14(1):26–33. doi: 10.3109/14653249.2011.605119. [DOI] [PubMed] [Google Scholar]
- 17.Matas J., Orrego M., Amenabar D., Infante C., Tapia-Limonchi R., Cadiz M. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–224. doi: 10.1002/sctm.18-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Y.H., Wu K.C., Liu H.W., Chu T.Y., Ding D.C. Human umbilical cord-derived mesenchymal stem cells reduce monosodium iodoacetate-induced apoptosis in cartilage. Ci Ji Yi Xue Za Zhi. 2018;30(2):71–80. doi: 10.4103/tcmj.tcmj_23_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González P.L., Carvajal C., Cuenca J., Alcayaga-Miranda F., Figueroa F.E., Bartolucci J. Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Transl Med. 2015;4(10):1109–1121. doi: 10.5966/sctm.2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guingamp C., Gegout-Pottie P., Philippe L., Terlain B., Netter P., Gillet P. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum. 1997;40(9):1670–1679. doi: 10.1002/art.1780400917. [DOI] [PubMed] [Google Scholar]
- 21.Yasuhara R., Ohta Y., Yuasa T., Kondo N., Hoang T., Addya S. Roles of beta-catenin signaling in phenotypic expression and proliferation of articular cartilage superficial zone cells. Lab Invest. 2011;91(12):1739–1752. doi: 10.1038/labinvest.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNulty M.A., Loeser R.F., Davey C., Callahan M.F., Ferguson C.M., Carlson C.S. Histopathology of naturally occurring and surgically induced osteoarthritis in mice. Osteoarthritis Cartilage. 2012;20(8):949–956. doi: 10.1016/j.joca.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haywood L., McWilliams D.F., Pearson C.I., Gill S.E., Ganesan A., Wilson D. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum. 2003;48(8):2173–2177. doi: 10.1002/art.11094. [DOI] [PubMed] [Google Scholar]
- 24.Billinghurst R.C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. 1997;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glasson S.S., Askew R., Sheppard B., Carito B., Blanchet T., Ma H.L. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 26.Jia H., Ma X., Tong W., Doyran B., Sun Z., Wang L. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc Natl Acad Sci U S A. 2016;113(50):14360–14365. doi: 10.1073/pnas.1608938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen P.C., Wu C.L., Jou I.M., Lee C.H., Juan H.Y., Lee P.J. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthritis Cartilage. 2011;19(6):728–736. doi: 10.1016/j.joca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Pearson M.J., Herndler-Brandstetter D., Tariq M.A., Nicholson T.A., Philp A.M., Smith H.L. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci Rep. 2017;7(1):3451. doi: 10.1038/s41598-017-03759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy J.M., Fink D.J., Hunziker E.B., Barry F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda Y., Matsumoto T., Hayashi S., Hashimoto S., Takayama K., Kirizuki S. Intra-articular autologous uncultured adipose-derived stromal cell transplantation inhibited the progression of cartilage degeneration. 2019;37(6):1376–1386. doi: 10.1002/jor.24174. [DOI] [PubMed] [Google Scholar]
- 31.Tang Y., Pan Z.Y., Zou Y., He Y., Yang P.Y., Tang Q.Q. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment, J Cell Mol Med. 2017;21(9):2153–2162. doi: 10.1111/jcmm.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pers Y.-M., Rackwitz L., Ferreira R., Pullig O., Delfour C., Barry F. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem cells Transl Med. 2016;5(7):847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Yan X., Jiang Y., Wang Z., Li Y., Shao Q. The human umbilical cord stem cells improve the viability of OA degenerated chondrocytes, Mol Med Rep. 2018;17(3):4474–4482. doi: 10.3892/mmr.2018.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry F. MSC therapy for osteoarthritis: an unfinished story. 2019;37(6):1229–1235. doi: 10.1002/jor.24343. [DOI] [PubMed] [Google Scholar]
- 35.Baksh D., Yao R., Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cell. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 36.Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Y., Tuan R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11(4):206–212. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathiessen A., Conaghan P.G. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. doi: 10.1186/s13075-017-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosenza S., Toupet K., Maumus M., Luz-Crawford P., Blanc-Brude O., Jorgensen C. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8(5):1399–1410. doi: 10.7150/thno.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svensson P., Cairns B.C., Wang K., Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104(1–2):241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.