Highlights
-
•
-Blastomycosis is a chronic granulomatous disease that usually starts as a pulmonary infection.
-
•
-A high percentage of patients will develop a disseminated disease if the initial lung infection is left untreated.
-
•
-Cutaneous blastomycosis is the most common extrapulmonary manifestation.
-
•
-Definitive diagnosis requires direct visualization of the yeast at staining or positive fungal culture.
Keywords: Blastomycosis, Cutaneous blastomycosis, Disseminated blastomycosis, Pneumonia, Scalp
Abstract
Cutaneous blastomycosis is the most common extrapulmonary manifestation of disseminated blastomycosis, a disease caused by Blastomyces dermatitidis, a dimorphic fungus endemic of North America. Initially, the organism enters the respiratory system by inhalation of the infectious conidia and produces an acute pulmonary infection that may eventually disseminate if it is left untreated. Blastomycosis may represent a diagnostic challenge and its definitive diagnosis requires direct visualization of the distinctive yeast or a positive fungal culture. The objective of this case report is to highlight the importance of the skin exam and tissue biopsy in the diagnosis of blastomycosis. We present a previously healthy patient with chronic pneumonia, evaluated at Pulmonary clinic with non-diagnostic thoracentesis and bronchoscopy, found to have disseminated blastomycosis after biopsy of a scalp lesion in Dermatology clinic.
Introduction
Blastomycosis is a systemic infection caused by the dimorphic fungus Blastomyces dermatitidis. It predominantly constitutes a disease of North America and is endemic in the Ohio and Mississippi River valleys, Midwestern states, and Canadian provinces that border the Great Lakes and the Saint Lawrence Riverway [1]. It is estimated to have an annual incidence of 1–40 cases per 100.000 population however, it is a reportable disease only in a few states hence, the true incidence is unknown.
Blastomycosis is most acquired by inhalation of the conidia, which represents the infectious component of Blastomyces dermatitidis [1,2]. For this reason, infection usually starts in the lungs where it can range from asymptomatic to severely symptomatic forms, including acute respiratory distress syndrome [3,4]. If this phase goes untreated, it disseminates hematogenous to other organs including the skin, bone, genitourinary tract, and central nervous system. The skin is the most affected organ and can manifest in up to 80 % of the cases [1,5]. Lesions are typically papulopustular and warty plaques that can have central ulceration. They usually appear in exposed areas, except for the scalp where involvement has hardly been reported [6,7].
We describe an atypical case of disseminated blastomycosis diagnosed in the Dermatology clinic, highlighting the importance of a comprehensive skin exam in every patient presenting with chronic pneumonia.
Case presentation
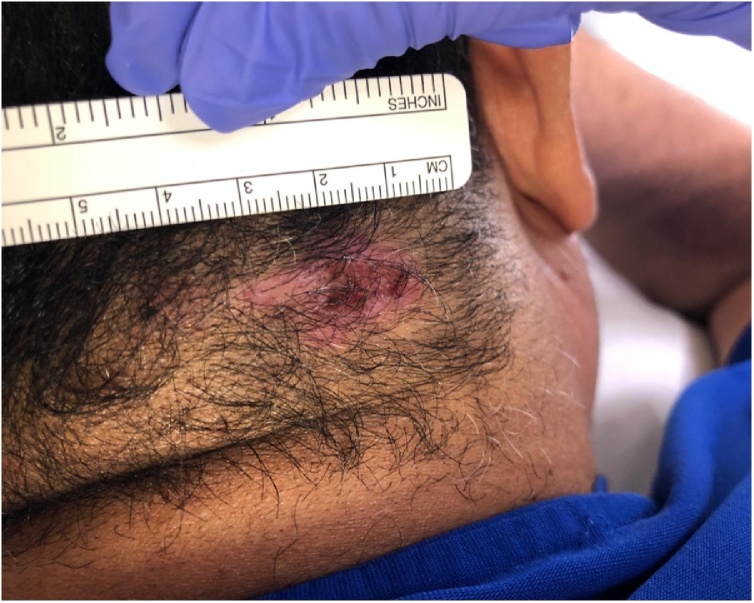
45 year old male with no chronic medical problems seen in the Dermatology clinic for evaluation of two skin lesions that have been gradually growing for 6 months. On examination, he had two erythematous plaques with hemorrhagic crusting located at the left shoulder (size 1.5 × 1 cm) and the right occipital scalp (2.5 × 1.5 cm). Both plaques were ulcerated in the middle and had sharp borders. The scalp lesion had intact hair follicles (Fig. 1, Fig. 2).
Fig. 1.
Erythematous plaque (1.5 × 1 cm) with hemorrhagic crusting and ulceration over the left shoulder.
Fig. 2.
Erythematous plaque (2.5 × 1.5 cm) with sharp borders with hemorrhagic crusting and ulceration over the right occipital scalp.
Of note, the patient was seen in Pulmonary clinic 8 months before this presentation for evaluation of persistent left pleural effusion and lingula opacity despite completing two courses of antibacterial therapy for community-acquired pneumonia. He had no pulmonary symptoms at this time and only complained of left pleuritic chest pain. The patient underwent thoracentesis with 700 mL of orange serous fluid obtained. The analysis revealed an exudative lymphocytic predominant pleural fluid with negative bacterial, fungal, and mycobacterial cultures. Cytology did not show any malignant cells. Further workup with bronchoscopy was done and transbronchial biopsy was included in the procedure. Bronchoalveolar lavage was lymphocytic predominant similar to the pleural fluid. Extensive culture and staining for bacteria, fungi, and acid-fast bacilli were negative. Pathology analysis of the lingula biopsy revealed granulomatous inflammation and previously done staining was repeated for this sample which was negative for bacterial, fungi, or mycobacterial elements. Given the inconclusive investigations with no definitive etiology and no major symptoms except for pleuritic chest pain, supportive management geared towards symptom control was pursued with the plan for close monitoring and imaging follow up.
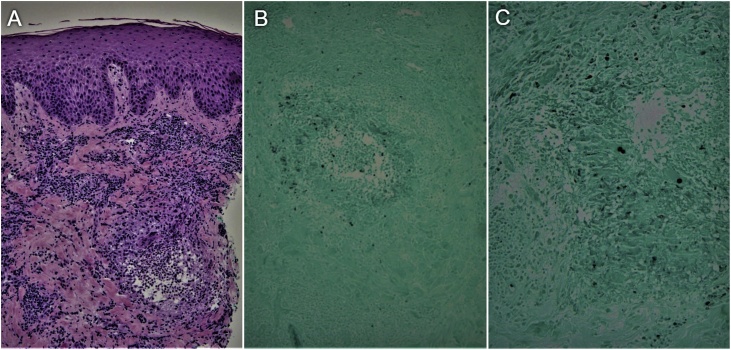
In the setting of his recent history of lung disease and new skin findings, a possible systemic infection connecting both presentations was suspected. For this reason, a shave biopsy of the left shoulder was performed which revealed acute and chronic inflammation with rare budding yeast. Due to inadequate sampling, culture was not done. A biopsy of the scalp lesion was subsequently done which showed dermal granulomatous inflammation and fungi morphologically consistent with blastomycosis (Fig. 3). This was confirmed with the fungal culture which grew Blastomyces dermatitidis. A diagnosis of disseminated blastomycosis with skin and pulmonary involvement was made and the patient was started on itraconazole for a total of 6 months. One month after starting antifungal therapy, the patient was seen in the dermatology clinic and his skin lesions had markedly improved without new respiratory symptoms.
Fig. 3.
A. Hematoxylin-and-eosin-stained section shows dermal granulomatous inflammation. B. Gomori methenamine silver (GMS) stained section shows one dermal granuloma where yeast can be seen in black. C. Closer view of the granuloma reveals the characteristic budding yeast of B. Dermatitidis.
Discussion
Cutaneous blastomycosis is the most common extrapulmonary manifestation of disseminated blastomycosis. Skin lesions have two classic presentations, ulcerative plaques and verruca with irregular and sharp borders. Lesions can occur anywhere and most of the described cases are typically found in exposed areas of the head and extremities [5,8]. The scalp lesion of our patient represents an uncommon location, as cutaneous blastomycosis affecting the head is usually described in the face. The plaque was not associated with alopecia or scarring. Skin lesions can also mimic several diseases including basal cell and squamous cell carcinoma and pyoderma gangrenosum. It should be noted that at the time of presentation, a significant number of patients may have resolved lung infection, constituting a diagnostic challenge for the dermatologist [8,9]. Secondly, blastomycosis can also appear as the result of direct traumatic inoculation of the fungus into the skin. This form is known as primary cutaneous blastomycosis. It usually presents as a pustule or papule with local lymphadenopathy that resolves spontaneously without intervention [10,11].
Given that the clinical picture of blastomycosis can be identical to other diseases, diagnosis requires direct visualization of the broad-based budding yeast or B. Dermatitidis growing in fungal culture. Staining can be performed using wet preparation, but yeast cells can be difficult to visualize, alternative methods include a potassium hydroxide (KOH) solution or Grocott-Gomori methenamine silver nitrate (GMS). The yeast forms are distinctive not only for the broad based budding but also for a doubly refractile cell wall. However, the sensitivity of these methods is lower than a culture, which should be included when analyzing each sample [1]. Learning the importance of culture for confirmatory diagnosis of blastomycosis could have contributed to earlier diagnosis in our patient, as fungal staining was not sensitive enough to identify the yeast, and culture of the transbronchial biopsy was not done. The definitive diagnosis was made after scalp biopsy where yeasts were identified at the direct examination and fungal culture. Besides, it is important to know the typical histopathologic features that include pseudoepitheliomatous hyperplasia and granulomatous inflammation [8].
As opposed to another endemic mycosis of North America such as histoplasmosis and coccidioidomycosis, pulmonary blastomycosis almost always requires therapy in the immunocompetent patient. This is because a high percentage of immunocompetent patients will develop disseminated blastomycosis if the initial pulmonary infection is left untreated. Often, histoplasmosis and coccidioidomycosis are usually self-limited unless patients have chronic pneumonia or immunocompromised state [12].
Treatment of blastomycosis will depend on the severity of the disease as well as organ involvement. In general, moderate disseminated blastomycosis can appropriately be treated with oral itraconazole for a total of 6 months. The same regimen was decided for our patient who showed improvement in his scalp lesion and did not develop new respiratory symptoms after one month of therapy. The present case highlights the importance of a thorough skin exam in patients with unresolved pneumonia, as extrapulmonary manifestations can help make a definitive diagnosis of blastomycosis. Although the scalp is an uncommon location for cutaneous blastomycosis, it should be considered as a differential in every patient with focal scalp disorders in the right epidemiologic setting. Furthermore, biopsy and culture from more than one specimen should be done to increase the diagnostic rate, as staining and culture from only one sample may be falsely negative delaying the diagnosis [13].
Patient consent
Written informed consent was obtained from the patient for publication of this case report and
accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Financial disclosure
This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author statement
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content.
CRediT authorship contribution statement
Elena G. Caldito: Writing - original draft, Data curation, Conceptualization, Resources. Oyintayo Ajiboye: Writing - review & editing, Validation. Estefania Flores: Visualization, Validation. Camila Antia: Resources, Validation. Patricia Demarais: Writing - review & editing, Conceptualization, Supervision.
Declaration of Competing Interest
None of the authors report any financial or personal conflicts of interest with this report.
Acknowledgements
None.
References
- 1.Saccente M., Woods G.L. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23(2):367–381. doi: 10.1128/CMR.00056-09. 2010/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo C.G., Kauffman C.A., Miceli M.H. Blastomycosis. Infect Clin North Am. 2016;30(1):247–264. doi: 10.1016/j.idc.2015.10.002. 2016/01/08. [DOI] [PubMed] [Google Scholar]
- 3.Sarosi G.A., Hammerman K.J., Tosh F.E., Kronenberg R.S. Clinical features of acute pulmonary blastomycosis. N Engl J Med. 1974;290(10):540–543. doi: 10.1056/NEJM197403072901004. 1974/03/07. [DOI] [PubMed] [Google Scholar]
- 4.Meyer K.C., McManus E.J., Maki D.G. Overwhelming pulmonary blastomycosis associated with the adult respiratory distress syndrome. N Engl J Med. 1993;329(17):1231–1236. doi: 10.1056/NEJM199310213291704. 1993/10/21. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Martinez R., Mendez-Tovar L.J. Blastomycosis. Clin Dermatol. 2012;30(6):565–572. doi: 10.1016/j.clindermatol.2012.01.002. 2012/10/17. [DOI] [PubMed] [Google Scholar]
- 6.Elewski B., Hunt L.H.K., Hay R. Fungal diseases. In: Bologna J., Cerroni J.S.L., editors. Dermatology. 4th ed. 2017. p. 1354. [Google Scholar]
- 7.Motswaledi H.M., Monyemangene F.M., Maloba B.R. Nemutavhanani DL. Blastomycosis: a case report and review of the literature. Int J Dermatol. 2012;51(9):1090–1093. doi: 10.1111/j.1365-4632.2011.05369.x. 2012/08/23. [DOI] [PubMed] [Google Scholar]
- 8.Smith J.A., Riddell J., Kauffman C.A. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15(5):440–449. doi: 10.1007/s11908-013-0352-2. [DOI] [PubMed] [Google Scholar]
- 9.Clinton TS, Timko AL Cutaneous blastomycosis without evidence of pulmonary involvement. Mil Med. 2003;168(8):651–653. 2003/08/29. [PubMed] [Google Scholar]
- 10.Gray N.A., Baddour L.M. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34(10):e44–9. doi: 10.1086/339957. [DOI] [PubMed] [Google Scholar]
- 11.Landay M.E., Schwarz J. Primary cutaneous blastomycosis. Arch Dermatol. 1971;104(4):408–411. [PubMed] [Google Scholar]
- 12.Chapman S.W., Dismukes W.E., Proia L.A. Clinical practice guidelines for the management of blastomycosis: 2008 update by the infectious diseases society of america. Clin Infect Dis. 2008;46(12):1801–1812. doi: 10.1086/588300. [DOI] [PubMed] [Google Scholar]