Highlights
-
•
Pathological basis of primary progressive aphasia is heterogeneous.
-
•
Logopenic primary progressive aphasia can precede dementia with Lewy bodies (DLB).
-
•
Cholinesterase inhibitor can improve logopenic aphasia with DLB.
Keywords: Logopenic aphasia, Lewy body disease, Alzheimer's disease, Primary progressive aphasia, Cholinesterase inhibitor
Dear Editor,
Logopenic progressive aphasia (LPA) is a major subtype of primary progressive aphasia (PPA), characterized by frequent word-finding pauses and impaired sentence repetition [1]. Alzheimer's disease (AD) was initially thought to be the primary cause of LPA [2]. However, accumulating evidence suggests that non-AD disorders often cause LPA, and prediction of underlying pathology in LPA remains difficult in clinical practice.
Recently, several case studies reported that LPA can precede the development of dementia with Lewy bodies (DLB) [[3], [4], [5]]. DLB is the second most common cause of dementia after AD, and which shows a better treatment response to cholinesterase inhibitors than AD. It is therefore important to elucidate the clinical features and management strategies of LPA with DLB.
In this report, we describe a patient with LPA who had imaging evidence of both AD and DLB and showed a dramatic treatment response to donepezil, a major cholinesterase inhibitor. To the best of our knowledge, there is currently no effective treatment for PPA. This case report provides insight into the neural basis of aphasia and offers potential therapeutic options, especially for LPA.
1. Case report
A seventy-five-year-old trained-right-handed man presented to our hospital with a 1-year history of mild memory problems and progressive speech impairment. He complained of misplacing objects, but his memory deficits remained mild and his functional independence was well-preserved. On the other hand, his word-finding difficulties became progressively worse, and he had difficulty communicating thoughts with his wife because of severe anomia in conversation. In addition to these problems, his wife described a 7-year history of episodic sleep talking with periodic limb movements. His only past medical history was chronic obstructive pulmonary disease treated with tiotropium.
On neurological examination, his speech was frequently interrupted with pauses, prominent anomia for nouns and occasional phonemic paraphasias, but his articulation was not distorted. He also showed poor confrontation naming ability. He was unable to correctly repeat sentences of more than 10 syllables and to follow complex verbal commands. On the other hand, he showed no semantic deficit or agrammatism in conversation or testing. According to these results, we diagnosed the patient as having logopenic type of primary progressive aphasia. Additionally, he showed severe olfactory impairment and mild postural tremor in his hands. He had no signs of parkinsonism, and the remainder of his neurological examination was within normal limits.
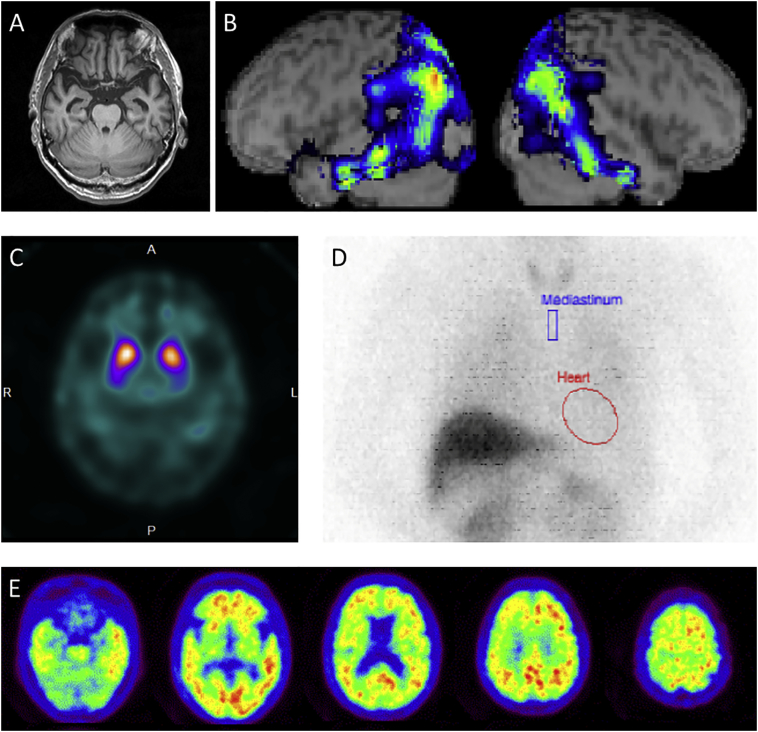
A T1-weighted magnetic resonance imaging (MRI) scan revealed mild hippocampal atrophy (Fig. 1A). 123I-IMP single photon emission computed tomography (SPECT) brain imaging showed hypoperfusion in the left-predominant bilateral temporoparietal region and in the bilateral precuneus/posterior cingulate cortex (Fig. 1B). 123I-ioflupane SPECT (dopamine transporter scan) showed reduced striatal uptake (Fig. 1C). His electrocardiogram showed decreased heart rate variability suggesting parasympathetic dysfunction, and 123I-metaiodobenzylguanidine (123I-MIBG) myocardial scintigraphy showed significantly reduced cardiac uptake (Fig. 1D).
Fig. 1.
A: Mild hippocampal atrophy. B: Left-predominant temporoparietal hypoperfusion. C: Reduced striatal uptake on dopamine transporter imaging. D: Significantly reduced cardiac uptake on 123I-metaiodobenzylguanidine myocardial scintigraphy. E: Left temporoparietal-predominant extensive cortical PiB binding.
Cerebrospinal fluid β-amyloid 1-42 (Aβ42) was reduced (392 pg/mL, Fig. 1); however, tau proteins and phosphorylated tau proteins (P-tau) were within normal limits (243 pg/mL and 37.6 pg/mL, respectively). 11C-PIB PET imaging showed a left temporoparietal-predominant extensive cortical PiB binding (neocortical SUVR = 1.72; Fig. 1E).
After the initial evaluation, we started treating the patient with donepezil. Seven weeks later, his language impairment was dramatically improved, and his Western Aphasia Battery-Aphasia Quotient (WAB AQ) score increased from 66.8 to 85. On the other hand, scores on the general cognitive tests showed only very minor improvement (Table 1).
Table 1.
| A. Longitudinal change in the Western Aphasia Battery score | |||
|---|---|---|---|
| Baseline | 7 weeks after treatment | ||
| Western Aphasia Battery | Aphasia Quotient | 66.8 | 85 |
| Information Content | 8 | 8 | |
| Fluency | 6 | 9 | |
| Auditory comprehension | 6 | 8.3 | |
| Repetition | 6.8 | 8.8 | |
| Naming | 6.6 | 8.4 | |
| Reading | 3.5 | 7.1 | |
| Writing | 0.9 | 7.6 | |
| B. Longitudinal changes in general and domain-specific cognitive functions | |||
|---|---|---|---|
| Baseline | 6 months after treatment | ||
| General cognition | MoCA-J | 7 | 8 |
| MMSE-J | 13 | 15 | |
| Raven's Colored Progressive Materices | 10 | 8 | |
| Atttention | Digit span (forward/backward) | 5/2 | 5/2 |
| Executive functions | Phonemic fluency (letter ‘ka’) | 4 | 6 |
| Semantic fluency (animal category) | 7.5 | 7 | |
| Language | Modified Boston Naming Test (60items) | 42 | 56 |
MoCA-J, Japanese version of the Montreal Cognitive Assessment; MMSE-J, Japanese version of the Mini Mental State Examination.
2. Discussion
This is the first case of LPA who exhibited a significant treatment response to donepezil. The patient showed a constellation of language symptoms typical of LPA, such as impaired single-word retrieval and sentence repetition, without frank agrammatism, motor speech impairment or semantic deficit. The SPECT finding of left temporoparietal predominant hypoperfusion supported the clinical diagnosis of LPA [1]. To date, there is no effective pharmacological treatment for progressive aphasia, including LPA [1]. However, our patient showed dramatic improvement of language dysfunction 7 weeks after administration of donepezil.
Imaging and CSF biomarker results may provide insights into the possible underlying pathology in this case. LPA has been mainly linked to AD or frontotemporal lobar degeneration [2]. Increased PiB uptake and reduced CSF Aβ42 in this patient indicated underlying AD pathology [6]. However, the CSF P-tau level was within the normal range, and cortical PiB uptake was modest for AD-related dementia [7]. These findings suggest that the burden of AD pathology is relatively low in this case. On the other hand, the LBD pathology may have played a more predominant role in the development of donepezil-responsive LPA symptoms. Our patient had REM sleep behavior disorder and showed abnormal cardiac 123I-metaiodobenzylguanidine uptake and decreased striatal dopamine transporter binding. These findings are known as diagnostic features of DLB [8] and indicate the presence of LBD pathology in this patient. Aphasia is not common even in the advanced stages of DLB. However, a recent study analyzing narrative discourse demonstrated that patients with Parkinson's disease dementia and DLB had significant anomia and phonemic paraphasia [9], known as core features of LPA. Furthermore, recent case reports and pathological studies demonstrated that LPA-like symptoms precede DLB [[3], [4], [5]]. Moreover, cognitive dysfunction in LBD is more responsive to cholinesterase inhibitors than cognitive dysfunction in AD in general [10]. Taken together, these results suggest that our patient had both AD and LBD pathologies and that the LBD pathology may be a primary cause for treatable LPA.
There are some limitations in this study. We did not perform sleep recordings, and there remains the possibility that sleep apnea could elicit abnormal night behavior and adversely affect cognitive function to some extent [11].
In summary, we describe a patient with LPA whose language dysfunction resolved immediately after administration of donepezil. He showed imaging and CSF evidence of both AD and LBD pathology. Our case may shed light on the treatment of progressive aphasia, especially for LPA. We propose a careful imaging work-up for screening LBD in LPA patients not to miss a potential therapeutic opportunity.
Ethics approval and informed consent
Ethics committee of Tohoku University Hospital.
Written informed consent was obtained from the patient.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (C) (17K09789) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and Grant-in-Aid for Scientific Research on Innovative Areas (No. 17H05936, 18H05058) from MEXT to KS, Japan.
References
- 1.Gorno-Tempini M.L., Hillis A.E., Weintraub S. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spinelli E.G., Mandelli M.L., Miller Z.A. Typical and atypical pathology in primary progressive aphasia variants. Ann. Neurol. 2017;81:430–443. doi: 10.1002/ana.24885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picková T., Matej R., Bezdicek O. Genetic Alzheimer disease and sporadic dementia with Lewy bodies: a comorbidity presenting as primary progressive aphasia. Cogn. Behav. Neurol. 2017;30:23–29. doi: 10.1097/WNN.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 4.Teichmann M., Migliaccio R., Kas A., Dubois B. Logopenic progressive aphasia beyond Alzheimer’s--an evolution towards dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry. 2013;84:113–114. doi: 10.1136/jnnp-2012-302638. [DOI] [PubMed] [Google Scholar]
- 5.Caselli R.J., Beach T.G., Sue L.I., Connor D.J., Sabbagh M.N. Progressive aphasia with Lewy bodies. Dement. Geriatr. Cogn. Disord. 2002;14:55–58. doi: 10.1159/000064925. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B., Feldman H.H., Jacova C. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 7.Leyton C.E., Villemagne V.L., Savage S. Subtypes of progressive aphasia: application of the international consensus criteria and validation using beta-amyloid imaging. Brain. 2011;134:3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 8.McKeith I.G., Boeve B.F., Dickson D.W. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ash S., McMillan C., Gross R.G. The organization of narrative discourse in Lewy body spectrum disorder. Brain Lang. 2011;119:30–41. doi: 10.1016/j.bandl.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emre M., Cummings J.L., Lane R.M. Rivastigmine in dementia associated with Parkinson’s disease and Alzheimer’s disease: similarities and differences. J. Alzheimers Dis. 2007;11:509–519. doi: 10.3233/jad-2007-11412. [DOI] [PubMed] [Google Scholar]
- 11.Kaminska M., Lafontaine A.L., Kimoff R.J. The interaction between obstructive sleep apnea and Parkinson’s disease: possible mechanisms and implications for cognitive function. Parkinsons Dis. 2015;2015:849472. doi: 10.1155/2015/849472. [DOI] [PMC free article] [PubMed] [Google Scholar]