Abstract
Cognitive impairment is a prominent and difficult to treat symptom in schizophrenia (SZ), which is directly related to functional disability. A variant in the gene coding for the alpha 1C subunit of L-type voltage gated calcium channel (CACNA1C) has been shown to negatively affect several neurocognitive domains. We conducted a 4-week, open label, pilot study of isradipine, a calcium channel blocker, to determine its feasibility, safety, and efficacy in improving cognition in SZ patients.
Ten adults with stable SZ were started on a flexible dose of isradipine 5 mg/day (up to 10 mg/day) for 4 weeks. Weekly in-person visits tracked side effects and symptoms while neurocognition and functional capacity were assessed at baseline and week 4.
There were no serious adverse events reported. Newly emergent side effects were dizziness (1 new incidence at week 4); difficulty sleeping (2 new incidences at week 4); and decreased energy (3 new incidences at week 4). 1 patient discontinued medication and was withdrawn. Treatment did not exacerbate clinical symptoms. Although power is limited, results indicate no clear benefit on neurocognition but a positive effect (baseline mean = 6.8 ± 1.3 to week 4 mean = 7.9 ± 1.1; t = 2.91, p = 0.017) on functional capacity was noted.
This open label, pilot study provides preliminary evidence that isradipine is a relatively safe medication when used adjunctively in SZ patients. This study suggests that isradipine offers no clear cognitive and only minimal functional benefit; however, additional studies may be warranted in symptomatic patients, or those with specific CACNA1C genotypes.
Keywords: Calcium, Isradipine, Cognition, CACNAC1A
1. Introduction
Cognitive impairment is a prominent and difficult to treat feature of schizophrenia (SZ) and is directly related to functional disability. The lack of progress toward novel and effective interventions is due, at least in part, to the fact that the underlying etiology of cognitive dysfunction in SZ is not known; however, convergent data support a neurodevelopmental model in which genetic factors play a significant role (Davis et al., 2017).
Genome-wide association studies (GWAS) have reached beyond the candidate gene level in very large samples and have identified several novel risk variants with high statistical confidence. Among the most widely studied of these is a variant in the gene coding for the alpha 1C subunit of the L-type voltage-gated calcium channel (CACNA1C) (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011). A single nucleotide polymorphism (SNP; rs1006737) in CACNA1C; initially associated with bipolar disorder (BD) (Ferreira et al., 2008; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011; Sklar et al., 2008) has also been linked with SZ (Cross-Disorder Group of the Psychiatric Genomics Consortium et al., 2013; Green et al., 2010; Hamshere et al., 2013; Nyegaard et al., 2010) and recurrent major depression (Green et al., 2010). More recently, large-scale GWAS studies (Cross-Disorder Group of the Psychiatric Genomics Consortium et al., 2013; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011) have reported transdiagnostic associations with multiple SNPs within CACNA1C, highlighting its unique convergence across disorders and underscoring its probably importance for understanding the brain as affected by neuropsychiatric disturbance. Indeed, several subsequent studies have evaluated the effects of CACNA1C variation on neurocognitive capacity in healthy samples as well as in patients with SZ and bipolar disorder (BD); risk carriers evidence impairments in several cognitive domains. CACNA1C rs1006737 has been shown to affect neural networks underlying reward and emotional processing (Bigos et al., 2010; Wessa and Linke, 2009), verbal fluency (Krug et al., 2010), verbal memory (Cosgrove et al., 2017), alerting and orienting aspects of attention (Thimm et al., 2011), and executive functions (Bigos et al., 2010; Soeiro-de-Souza et al., 2013); however, not all studies have been positive (Hori et al., 2012; Rolstad et al., 2016). Table 1 summarizes the current literature (for recent review see Moon et al., 2018).
Table 1.
Literature on relationship between CACNA1C and cognition in BD and SZ.
| Publication | Subjects | Cognitive tests | Results: CACNA1C & cognition | SNP, rs# |
|---|---|---|---|---|
| (Cosgrove et al., 2017) | 157 healthy 557 broad psychosis 409 SZ/SZA |
SWM (CANTAB) In replication studies: MATRICS working memory (Cardiff) N-back, DS (WAIS), SS (WAIS) (Germany) |
|
2007044 |
| (Rolstad et al., 2016) | 104 healthy 114 BD |
TMT number sequencing (D-KEFS), Digit Symbol coding (WAIS-III), CDT, RCFT recall, DS (WAIS-III), LNS (WAIS-III), RCFT copy, BD (WAIS-III), Similarities (WAIS-III), Verbal Fluency Test (D-KEFS), Color-Word Interference (D-KEFS), Design Fluency (D-KEFS), Tower Test (D-KEFS), CPT |
|
1006737 |
| (Soeiro-de-Souza et al., 2013) | 96 healthy 109 BD |
LNS (WAIS-III), DS (WAIS-III), TMT, and WCST |
|
1006737 |
| (Hori et al., 2012) | 1132 healthy 552 SZ |
Japanese versions of WMS-R, WAIS-R, and WCST |
|
1006737 |
| (Zhang et al., 2012) | 401 healthy 318 SZ 74 BP |
Chinese versions of WAIS-R, N-back, DPX |
|
1006737 |
| (Thimm et al., 2011) | 82 healthy | ANT |
|
1006737 |
| (Krug et al., 2010) | 63 healthy men | Semantic verbal fluency |
|
1006737 |
| (Roussos et al., 2013) | 530 healthy men | CANTAB |
|
1006737 |
| (Erk et al., n.d.) | 110 healthy | 3 consecutive memory test (encoding, recall, and recognition),VIQ (MWT-B), -RAVLT |
|
1006737 |
| (Bigos et al., 2010) | 440 healthy 282 SZ |
|
Risk carriers had increased activity in hippocampus during emotion processing and in PFC during N-back | 1006737 |
| (Splawski et al., 2005) | 5 children | CELF, GFTA, NEPSY |
|
1006737 |
Key: HC = healthy controls, BD = bipolar disorder, SZ = schizophrenia, SZA = schizoaffective disorder, SWM = Spatial Working Memory, CANTAB = Cambridge Neuropsychological Test Automated Battery, MATRICS = Measurement and Treatment Research to Improve Cognition in Schizophrenia, DS = digit span, WAIS-III = Wechsler Adult Intelligence Scale version III, SS = spatial span, TMT = Trails Making Task, D-KEFS = Delis-Kaplan Executive Function System, CDT = Claeson-Dahl Test, RCFT = Rey Complex Figure Test, LNS = letter-number sequencing, BD = Block Design, CPT = Continuous Performance Test, WCST = Wisconsin Card Sorting Task, WMS-R = Wechsler Memory Scale-Revised, WAIS-R = Wechsler Adult Intelligence Scale-Revised, DPX = Dot Pattern expectancy, ANT = Attention Network Test, VIQ = Verbal Intelligence Quotient, MWT-B = Mehrfachwahl-Wortschatz-Intelligenztest, version B, RAVLT = Rey Auditory Learning Task, CELF = Clinical Evaluation of Language Fundamentals, GFTA = Goldman-Fristoe Test of Articulation, NEPSY = A Developmental Neuropsychological Assessment.
In addition to genetic evidence, peripheral and postmortem markers of calcium homeostasis have been found to be abnormal in patients with SZ, suggesting that calcium channel dysregulation may be a central feature of the disorder. Calcium channel blockers, and in particular dihydropyridines (DHPs) may serve to target this dysregulation. L-type channels are highly sensitive to DHPs, which, as a class, act to block the influx of Ca2+. DHPs have been widely used as anti-hypertensives, with more recent attention given to their potential to protect from neuronal damage associated with several pathological conditions (Hunter et al., 1997). Specifically, DHPs have been shown to block hippocampal (CA1) damage and to reduce memory impairment in animal models of hypoxia (Barhwal et al., 2009), diabetes (Tsukuda et al., 2008), cerebral ischemia (Iwasaki et al., 2007), and Parkinson's disease (Chan et al., 2007). Epidemiological data indicate that DHPs might prevent (Forette et al., 1998) or slow the progression of Alzheimer's disease (AD) in the general population (Fritze and Walden, 1995; Tollefson et al., 1998). Results from clinical trials however have been inconsistent (López-Arrieta and Birks, 2002); some agents do not cross the blood brain barrier (Anekonda et al., 2011) and some DHPs have shown benefits only for specific genotypes (Kennelly et al., 2011). In a high-throughput screening of candidate compounds for the treatment of dementia, which included several DHPs currently in clinical use (verapamil, diltiazem, nimodipine and isradipine), only isradipine provided a significant protective benefit and minimal toxicity at multiple levels of complexity (biochemical, cellular and whole organism), making it the most promising candidate in considering efforts to target brain-based phenotypes (Copenhaver et al., 2011).
Isradipine is a second-generation calcium channel blocker of the 1.4-dihydropyridine (DHP) class, which binds to both Cav1.2 and Cav1.3 channels. The Cav1.2 channel, which is specifically coded for by the CACNA1C gene, plays a critical role in hippocampal functioning and influences long term potentiation and spatial memory formation (Moosmang et al., 2005). Cav1.3 is encoded by CACNA1D and is primarily localized to the striatum (Tippens et al., 2008). Isradipine blocks Cav1.3 channel subtypes in dopamine (DA) cells in the substantia nigra (Chan et al., 2010) which is believed to be at least one of the its mechanisms of action resulting in neuroprotection in animal models of Parkinson's disease (PD) (Chan et al., 2010; Chang et al., 2010; Ritz et al., 2010). Isradipine has received some attention as a candidate for Alzheimer's Disease (AD) trials, due to data from animal models indicating its tolerability and superior blood-brain barrier penetrance, alongside its ability to attenuate beta amyloid toxicity, lower tau burden, and improve autophagy function in transgenic mouse models for AD (Anekonda et al., 2011). Isradipine has also been shown to protect against stroke (Lenhard et al., 2008) and ischemia (Campbell et al., 1997) in rat models of hypertension. Data from a pilot trial of isradipine in PD patients indicates safety and tolerability, with an optimal daily dose of 10 mg/day (Simuni et al., 2010).
We completed a four-week, open-label, pilot study of isradipine in a small sample (n = 10 completers) of symptomatically-stable patients with SZ. The primary goal of this pilot study was to assess the safety and feasibility of using isradipine in SZ. As a secondary aim, though underpowered to test in any definitive way, we investigated whether isradipine shows a positive signal for efficacy on the cognitive deficits in SZ.
2. Materials and methods
This study was reviewed and approved by the Institutional Review Board (IRB) at the Icahn School of Medicine at Mount Sinai. All patients signed an informed consent document before any study procedures were conducted.
This was an open-label study with a flexible dosing schedule, targeting an optimal dose of 10 mg/day (NCT01658150). Patients were screened and if deemed eligible, started on 5 mg/day at baseline and if tolerated, the dose was raised to 5 mg/BID with a total of 10 mg/day. The visit schedule included weekly in-person visits to track side effects and symptom ratings. Neurocognition and functional capacity were assessed by highly trained study staff at baseline and again at week 4 or end of study.
2.1. Patients
Patients were recruited through local advertisements in the New York City metropolitan area from January 2013 until August 2018. Inclusion criteria included age between 18 and 55; a diagnosis of schizophrenia or schizoaffective disorder as per the Structured Clinical Interview for the DSM-IV (SCID-IV); and symptom ratings indicating a residual phase of illness (Brief Psychiatric Rating Scale BPRS item scores of ≤4 on each of the following: hallucinatory behavior, unusual thought content, and conceptual disorganization, Hamilton Rating Scale for Depression HDRS total score < 12, and Clinician Administered Ratings Scale for Mania CARS-M total score < 5). Exclusion criteria were 1. History of CNS trauma, neurological disorder, attention deficit hyperactivity disorder (ADHD), mental retardation, learning disability, or other known non-schizophrenic cause of cognitive impairment; 2. DSM-IV diagnosis of substance abuse/dependence within 3 months or positive urine toxicology at screening that is not consistent with what participant reported; 3. pregnant women or women of child bearing potential who are not using a medically accepted means of contraception (including oral contraceptive or implant, condom, diaphragm, spermicide, intrauterine device, tubal ligation, or partner with vasectomy); 4. women who are breastfeeding; 5. active, unstable medical problem that may interfere with cognition; 6. current treatment for hypertension; 7. uncontrolled hypertension; 8. history of heart disease; 9. any drug known to interact with isradipine; 10. history of gastrointestinal strictures; 11. abnormal lab or electrocardiogram (ECG) at screen; and 12. significant suicidal ideation at baseline (HDRS item 3 > 2). Concomitant medications: Practical and ethical considerations prevent an exclusive focus on medication-free patients; however, we limited participation to individuals taking at least one and no >2 concomitant antipsychotic medications and we excluded subjects who have received ECT within 12 months.
2.2. Measures
Safety was assessed weekly through clinician-administered and self-report measures, in addition to laboratory measures (blood pressure; heart rate; weight). An ECG was conducted at screening and at week 4. Symptom severity was rated at each visit using the Scale for the Assessment of Negative Symptoms (SANS), BPRS, and the HDRS. Cognition was assessed at baseline and at week 4 using the MATRICS Consensus Cognitive Battery (MCCB), which taps into seven domains of cognitive functioning including processing speed, attention/vigilance, working memory, verbal memory, visual memory, reasoning/problem solving, and social cognition (Nuechterlein et al., 2008). Alternate forms were utilized at the week 4 visit to reduce practice effects. Everyday functioning was assessed using the UCSD Performance Skills Assessment (UPSA) and Quality of Life Scale at baseline and week 4. Blood samples were collected at baseline for DNA extraction.
2.3. Statistical analyses
The results reported here are largely descriptive for safety and feasibility outcomes. To assess for efficacy on cognitive and functional outcome measures, we conducted paired-sample t-tests and calculated effect size changes using Cohen's d.
3. Results
This proof-of-concept trial focused on feasibility and safety as primary outcomes. Efficacy on cognition was secondary and is considered very preliminary.
3.1. Demographics
The sample consisted of 10 adults with a mean age of 38 ± 13.4 years, 7/10 were male, 7/10 were diagnosed with schizophrenia, and 3/10 were diagnosed with schizoaffective disorder. Race, based upon self-report, was distributed as follows: 6 Black, 2 Asian, 1 White, and 1 Mixed-race.
3.2. Feasibility
Recruitment was difficult, but this was not necessarily unexpected as inclusion/exclusion criteria for clinical trials are often limiting. In the case of this trial, we specifically had difficulty identifying eligible patients due to the exclusion of patients who were taking any cardiac/hypertensive medications. The second most common reason for exclusion was the high percentage of patients not currently taking a stable dose of psychotropic medications. Most of the patients that we screened via their enrollment in a larger non-intervention-based study (of >100 patients who met diagnostic criteria) were ineligible due to these specific criteria. A total of 17 patients were consented to the study, with 6 screen fails. Screen fails were due to medication rule out, abnormal ECG, abnormal labs, positive urine toxicology, unwillingness to swallow pills, and scheduling complications (Supplemental Fig. 1).
3.3. Safety
There were no serious adverse events reported in the conduct of this trial. There was one patient who dropped out of the study after taking study drug for 3 days. This was a 33-year-old Hispanic female patient with schizophrenia who reported headache, flushing, and lightheadedness after 3 doses of 2.5 mg/day (she was taking half of the prescribed does of 5.0 mg/day). She discontinued medication and was withdrawn from the study. All of her labs/vitals/ECG were within normal limits and all reported AEs resolved by the time she was seen at a follow-up visit one week later.
Symptom severity measures were of interest in this trial primarily for safety assessment – to ensure that the study medication did not exacerbate psychosis, negative symptoms, or depression. Mean change for positive symptoms (BPRS) was −1.0 ± 2.5; negative symptoms (SANS) was −1.6 ± 3.3; and depressive symptoms (HDRS) was −0.6 ± 1.8. As all patients began the trial during a residual (non-acute) phase, no changes in symptom severity were of clinical concern for any patient. There were no cases of increased suicidality.
Blood pressure was closely monitored during the trial. At baseline, half of the patients (5/10) met strict criteria for hypertension (4 met Stage 1 criteria of >130–139 diastolic or >80–89 and 1 met Stage 2 criteria). At week 4, only 2 patients continued to be hypertensive (3 cases improved) and no new incidences were noted. The mean change in weight over the 4-week study was −0.16 (±4.1) pounds; however, there was a significant range in weight change (one patient lost 8 lbs., and another gained 5.6 lbs.).
Other common adverse events were assessed by direct query by the study physician at each visit. Details are shown in Table 2. The only newly emergent side effects (change since baseline report) that were reported were dizziness (1 new incidence at week 4); difficulty sleeping (2 new incidences at week 4); and decreased energy (3 new incidences at week 4, 2 of which reported difficulty sleeping as well). As this was an open-label study, we are unable to compare the frequency of these new reports with a placebo group.
Table 2.
Adverse events: Baseline vs. week 4 (end of study).
| Queried adverse events (AE) | Baseline (# reporting/10) | Week 4 (# reporting/10) | Note |
|---|---|---|---|
| Diarrhea | 0 | 0 | – |
| Constipation | 0 | 0 | – |
| Dry Mouth | 0 | 0 | – |
| Nausea | 1 | 0 | Resolved since baseline |
| Palpitations | 0 | 0 | – |
| Dizziness | 0 | 1 | New since baseline |
| Chest Pain | 0 | 0 | – |
| Rash | 0 | 0 | – |
| Perspiration | 0 | 0 | – |
| Itching | 0 | 0 | – |
| Dry skin | 1 | 0 | Resolved since baseline |
| Headache | 0 | 0 | – |
| Tremors | 0 | 0 | – |
| Poor coordination | 1 | 0 | Resolved since baseline |
| Blurred vision | 1 | 0 | Resolved since baseline |
| Tinnitus | 2 | 1 | No change from baseline |
| Difficulty urinating | 0 | 0 | – |
| Painful urination | 0 | 0 | – |
| Frequent urination | 2 | 1 | No change from baseline |
| Menstrual irregular | 3 | 3 | No change from baseline |
| Difficulty sleeping | 1 | 3 | Two new since baseline |
| Hypersomnia | 1 | 0 | Resolved since baseline |
| Loss of libido | 1 | 0 | Resolved since baseline |
| Anxiety | 2 | 2 | No change from baseline |
| Poor concentration | 2 | 1 | New since baseline |
| Malaise | 0 | 0 | – |
| Restlessness | 1 | 1 | No change from baseline |
| Fatigue | 2 | 1 | No change from baseline |
| Decreased energy | 0 | 3 | Three new since baseline |
3.4. Cognitive and functional outcomes
This was an open-label pilot study which is inherently underpowered to truly test for clinical efficacy; however, the goal was to assay systematic signals (both positive and negative effects) on cognition and functional capacity.
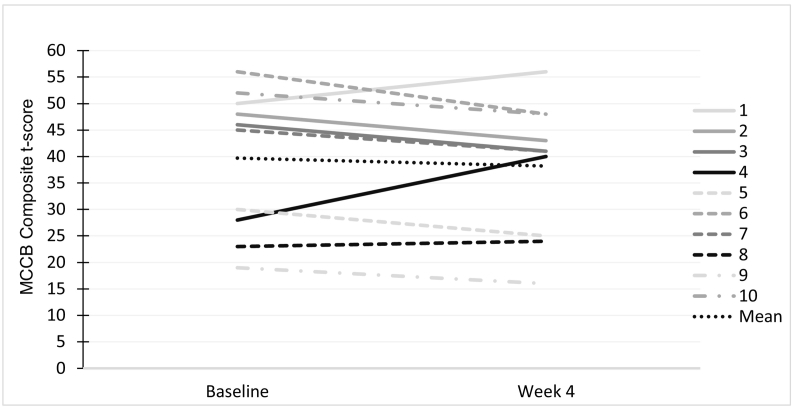
Preliminary evaluation of the data indicates no clear benefit on any measured aspect of cognition. When comparing baseline performance on the MCCB composite with performance at week 4, we see non-significant improvement as a group (t = 0.77, df = 9, p = 0.46). Similar non-significant changes were noted across all MCCB domains (Table 3). There was some variability/heterogeneity in cognitive response which is depicted at the individual patient level in Fig. 1; however, given the small sample, we are unable to determine any clear predictors of outcome.
Table 3.
Changes in neurocognitive domains: Baseline vs. week 4 (end of study).
| Domain | Baseline (n = 10) |
Week 4 (n = 10) |
t | p | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Speed of processing | 44.9 | 9.36 | 44.7 | 10.28 | 0.08 | 0.94 |
| Attention/vigilance | 39.2 | 10.80 | 39.5 | 11.29 | −0.08 | 0.94 |
| Working memory | 40.1 | 13.46 | 40.5 | 13.87 | −0.16 | 0.88 |
| Verbal learning | 44.4 | 9.61 | 39.3 | 4.11 | 1.69 | 0.13 |
| Visual learning | 48.9 | 14.06 | 50.5 | 10.97 | −0.51 | 0.62 |
| Reasoning and problem solving | 48.2 | 13.88 | 44.0 | 10.24 | 1.83 | 0.10 |
| Social cognition | 40.4 | 9.47 | 41.0 | 11.84 | −0.23 | 0.82 |
| Overall composite | 39.7 | 13.33 | 38.2 | 12.56 | 0.77 | 0.46 |
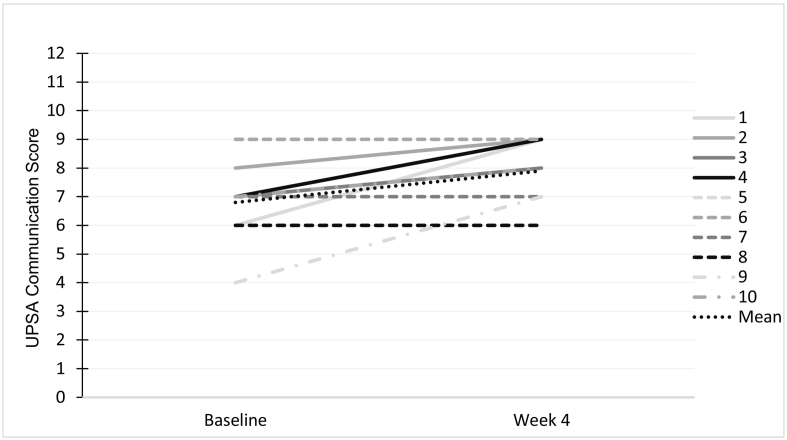
Fig. 1.
Variability in UPSA Communication Score, depicted at the individual patient level.
Of note, our functional capacity measure, the UPSA communication score did improve significantly overall (baseline mean = 6.8 ± 1.3 to week 4 mean = 7.9 ± 1.1; t = 2.91, p = 0.017). This is a large effect size (Cohen's d = 0.91) with a more consistent pattern of improvement across subjects (Fig. 2). A recent study estimated practice effects in SZ patients administered the UPSA to be considerably smaller (Cohen's d = 0.35) than the change that we are reporting here (Keefe et al., 2016). Nonetheless, given the lack of corroborating improvement on other measures of cognition and the preliminary nature of this study, it is unknown whether these gains represent clinically-meaningful change.
Fig. 2.
Variability in MCCB composite t-score (mean = 50 SD = 10, with higher scores indicating better performance), depicted at the individual patient level.
4. Discussion
This open-label pilot study provides preliminary evidence that isradipine is relatively safe when used adjunctively in patients with schizophrenia (SZ), as no serious adverse events were noted. Common side effects were minimal. By design, this study cannot determine whether the agent might be useful in treating positive or negative symptoms of the illness, as patients were very stable at the time of entry; however, there were no cases of clinical exacerbation after 4-weeks of treatment with isradipine. Mild hypertension that was present in 5 patients at baseline was normalized by week 4 in 3 of these cases.
The primary domain of interest for efficacy was cognition – and the study was quite convincingly negative on this outcome. These data, although very preliminary, may suggest that blocking the influx of calcium, at least with isradipine, is unlikely to produce a pronounced cognitive benefit in SZ. We did find a positive change on the UPSA, indicating some improvement in functional capacity; however, this result should be interpreted with caution given the preliminary and open-label nature of this trial.
The physiological effects of isradipine are fairly well understood, with broad effects on both long-term potentiation and dopaminergic tone in subcortical areas. These effects are among the reasons that we hypothesized that isradipine might influence cognitive functioning; however, given the lack of improvement on cognitive measures in our study, it is more difficult to speculate how isradipine's physiological effects would influence functional capacity. Thus, the most parsimonious explanation is that these effects may be a consequence of isradipine's effects on hypertension. There are several lines of evidence that would support this explanation, including a recent study (de Heus et al., 2019) which showed that elevated day-to-day variability in blood pressure was associated with greater cognitive and functional decline in patients with Alzheimer's disease. While SZ is not always considered to be a degenerative condition (aside from early definitions), it has a very high rate of stable cognitive impairment. Perhaps not uncoincidentally, schizophrenia also has high medical comorbidity and hypertension is common (Howell et al., 2019). In our study, our patients were symptomatically stable, but half of the patients met strict criteria for hypertension at baseline. Most of these patients showed significant stabilization of blood pressure by week 4, which could be a potential explanation for functional improvement in the absence of changes in symptom severity or cognitive impairment. Indeed, prior work has suggested that both psychiatric symptoms and physical functioning are major determinants of subjective disability in both patients with schizophrenia and bipolar disorder (Strassnig et al., 2018) and a recent meta-analysis demonstrates a consistent relationship between hypertension and cognitive and functional impairment in schizophrenia (Bora et al., 2017).
Prior studies of Ca2+ channel blockers in affective and substance disorders (Casamassima et al., 2010) are mixed and depend upon outcome measure (depression, mania, cycling, relapse) and the pharmacodynamics of the specific agent or class of agent utilized [e.g. verapamil (a phenylalkylamine), isradipine, nimodipine (DHPs)]. A pilot study of isradipine in depressed patients with bipolar disorder provides early evidence of a potential anti-depressant effect of this agent, but sample size was small and there were no measures of cognition or functioning reported (Ostacher et al., 2014). Of relevance to our work, Krupitsky et al. (2001) conducted a double-blind, placebo-controlled trial of nimodipine in 26 alcohol-dependent men as a pre-treatment to ketamine, an NMDA receptor antagonist which can produce SZ-like syndromes including psychosis, negative symptoms, and cognitive impairment in healthy humans. Nimodipine pre-treatment significantly attenuated the effects of ketamine with regard to psychosis, negative symptoms, dysphoria, verbal fluency impairment and learning deficits. In fact, nimodipine not only reduced the capacity of ketamine to induce memory deficits but actually improved memory performance in this cohort. These results alongside convergent evidence that calcium channel dysfunction represents a core pathogenic feature in SZ, support continued evaluation of DHPs, in general, in neuropsychiatric disorders.
Our study had several limitations including its open-label design and the small sample size. Although we only had a total of 10 completers, there was no compelling evidence of any positive cognitive effects in the first ten participants and the risk of exposing additional subjects to an experimental drug was deemed to outweigh the benefits of continuing to enroll for the sake of statistical power. In addition, the decision to focus on stable patients in the residual phase was done to reduce the chances of pseudospecificity and to isolate potential effects on neurocognition. This design did not allow us to test the effects of isradipine on clinical symptoms of SZ.
Despite the negative outcome, this was a proof of concept study that provides important early data on safety and feasibility of using isradipine in patients with SZ. There remains a possibility that the heterogeneity in cognitive response that was noted may be related to genetic variation in the CACNA1C gene. Given the small sample, we do not anticipate any measurable main effects of genotype, but a genotype-specific response remains a plausible, testable hypothesis – which could inform whether future studies stratified by genotype may be warranted.
The following are the supplementary data related to this article.
Most of the patients that were screened via their enrollment in a larger non-intervention-based study (of >100 patients who met diagnostic criteria) were ineligible. A total of 17 patients were consented to the study, with 6 screen fails. Screen fails were due to medication rule out, abnormal ECG, abnormal labs, positive urine toxicology, unwillingness to swallow pills, and scheduling complications.
Funding and disclosures
This work was funded by a NARSAD Independent Investigator Award (Brain and Behavior Research Foundation) to KEB and salaries were supported by R01MH100125 to KEB. None of the authors listed on this manuscript have any relevant conflicts to disclose. Senior author Pamela Sklar is deceased. The trial was registered at ClinicalTrials.gov with an identifier of: NCT01658150. The full protocol can be accessed by contacting Dr. Katherine Burdick at kburdick1@bwh.harvard.edu.
CRediT authorship contribution statement
Katherine E. Burdick: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing - original draft, Writing - review & editing. Mercedes Perez-Rodriguez: Data curation, Supervision. Rebecca Birnbaum: Data curation, Supervision, Writing - review & editing, Writing - original draft. Megan Shanahan: Data curation, Supervision. Emmett Larsen: Data curation. Cierra Harper: Writing - review & editing, Writing - original draft. Jessica Poskus: Writing - review & editing, Writing - original draft. Pamela Sklar: Conceptualization.
Declaration of competing interest
None.
References
- Anekonda T.S., Quinn J.F., Harris C., Frahler K., Wadsworth T.L., Woltjer R.L. L-type voltage-gated calcium channel blockade with isradipine as a therapeutic strategy for Alzheimer’s disease. Neurobiol. Dis. 2011;41:62–70. doi: 10.1016/j.nbd.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhwal K., Hota S.K., Baitharu I., Prasad D., Singh S.B., Ilavazhagan G. Isradipine antagonizes hypobaric hypoxia induced CA1 damage and memory impairment: complementary roles of L-type calcium channel and NMDA receptors. Neurobiol. Dis. 2009;34:230–244. doi: 10.1016/j.nbd.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Bigos K.L., Mattay V.S., Callicott J.H., Straub R.E., Vakkalanka R., Kolachana B., Hyde T.M., Lipska B.K., Kleinman J.E., Weinberger D.R. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch. Gen. Psychiatry. 2010;67:939–945. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Akdede B.B., Alptekin K. The relationship between cognitive impairment in schizophrenia and metabolic syndrome: a systematic review and meta-analysis. Psychol. Med. 2017;47:1030–1040. doi: 10.1017/S0033291716003366. [DOI] [PubMed] [Google Scholar]
- Campbell C.A., Mackay K.B., Patel S., King P.D., Stretton J.L., Hadingham S.J., Hamilton T.C. Effects of isradipine, an L-type calcium channel blocker on permanent and transient focal cerebral ischemia in spontaneously hypertensive rats. Exp. Neurol. 1997;148:45–50. doi: 10.1006/exnr.1997.6611. [DOI] [PubMed] [Google Scholar]
- Casamassima F., Hay A.C., Benedetti A., Lattanzi L., Cassano G.B., Perlis R.H. L-type calcium channels and psychiatric disorders: a brief review. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B:1373–1390. doi: 10.1002/ajmg.b.31122. [DOI] [PubMed] [Google Scholar]
- Chan H.-Y., Lin W.-W., Lin S.-K., Hwang T.-J., Su T.-P.T., Chiang S.-C., Hwu H.-G. Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: a randomized trial. J. Clin. Psychiatry. 2007;68:29–36. doi: 10.4088/jcp.v68n0104. [DOI] [PubMed] [Google Scholar]
- Chan C.S., Gertler T.S., Surmeier D.J. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov. Disord. 2010;25(Suppl. 1):S63–S70. doi: 10.1002/mds.22801. [DOI] [PubMed] [Google Scholar]
- Chang C.-C., Cao S., Kang S., Kai L., Tian X., Pandey P., Dunne S.F., Luan C.-H., Surmeier D.J., Silverman R.B. Antagonism of 4-substituted 1, 4-dihydropyridine-3,5-dicarboxylates toward voltage-dependent L-type Ca2+ channels Ca V 1.3 and Ca V 1.2. Bioorg. Med. Chem. 2010;18:3147–3158. doi: 10.1016/j.bmc.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Copenhaver P.F., Anekonda T.S., Musashe D., Robinson K.M., Ramaker J.M., Swanson T.L., Wadsworth T.L., Kretzschmar D., Woltjer R.L., Quinn J.F. A translational continuum of model systems for evaluating treatment strategies in Alzheimer’s disease: isradipine as a candidate drug. Dis. Model. Mech. 2011;4:634–648. doi: 10.1242/dmm.006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D., Mothersill O., Kendall K., Konte B., Harold D., Giegling I., Hartmann A., Richards A., Mantripragada K., Owen M.J., O’Donovan M.C., Gill M., Rujescu D., Walters J., Corvin A., Morris D.W., Donohoe G. Cognitive characterization of schizophrenia risk variants involved in synaptic transmission: evidence of CACNA1C’s role in working memory. Neuropsychopharmacology. 2017;42:2612–2622. doi: 10.1038/npp.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee S.H., Ripke S., Neale B.M., Faraone S.V., Purcell S.M., Perlis R.H., Mowry B.J., Thapar A., Goddard M.E., Witte J.S., Absher D., Agartz I., Akil H., Amin F., Andreassen O.A., Anjorin A., Anney R., Anttila V., Arking D.E., Asherson P., Azevedo M.H., Backlund L., Badner J.A., Bailey A.J., Banaschewski T., Barchas J.D., Barnes M.R., Barrett T.B., Bass N., Battaglia A., Bauer M., Bayés M., Bellivier F., Bergen S.E., Berrettini W., Betancur C., Bettecken T., Biederman J., Binder E.B., Black D.W., Blackwood D.H.R., Bloss C.S., Boehnke M., Boomsma D.I., Breen G., Breuer R., Bruggeman R., Cormican P., Buccola N.G., Buitelaar J.K., Bunney W.E., Buxbaum J.D., Byerley W.F., Byrne E.M., Caesar S., Cahn W., Cantor R.M., Casas M., Chakravarti A., Chambert K., Choudhury K., Cichon S., Cloninger C.R., Collier D.A., Cook E.H., Coon H., Cormand B., Corvin A., Coryell W.H., Craig D.W., Craig I.W., Crosbie J., Cuccaro M.L., Curtis D., Czamara D., Datta S., Dawson G., Day R., De Geus E.J., Degenhardt F., Djurovic S., Donohoe G.J., Doyle A.E., Duan J., Dudbridge F., Duketis E., Ebstein R.P., Edenberg H.J., Elia J., Ennis S., Etain B., Fanous A., Farmer A.E., Ferrier I.N., Flickinger M., Fombonne E., Foroud T., Frank J., Franke B., Fraser C., Freedman R., Freimer N.B., Freitag C.M., Friedl M., Frisén L., Gallagher L., Gejman P.V., Georgieva L., Gershon E.S., Geschwind D.H., Giegling I., Gill M., Gordon S.D., Gordon-Smith K., Green E.K., Greenwood T.A., Grice D.E., Gross M., Grozeva D., Guan W., Gurling H., De Haan L., Haines J.L., Hakonarson H., Hallmayer J., Hamilton S.P., Hamshere M.L., Hansen T.F., Hartmann A.M., Hautzinger M., Heath A.C., Henders A.K., Herms S., Hickie I.B., Hipolito M., Hoefels S., Holmans P.A., Holsboer F., Hoogendijk W.J., Hottenga J.-J., Hultman C.M., Hus V., Ingason A., Ising M., Jamain S., Jones E.G., Jones I., Jones L., Tzeng J.-Y., Kähler A.K., Kahn R.S., Kandaswamy R., Keller M.C., Kennedy J.L., Kenny E., Kent L., Kim Y., Kirov G.K., Klauck S.M., Klei L., Knowles J.A., Kohli M.A., Koller D.L., Konte B., Korszun A., Krabbendam L., Krasucki R., Kuntsi J., Kwan P., Landén M., Långström N., Lathrop M., Lawrence J., Lawson W.B., Leboyer M., Ledbetter D.H., Lee P.H., Lencz T., Lesch K.-P., Levinson D.F., Lewis C.M., Li J., Lichtenstein P., Lieberman J.A., Lin D.-Y., Linszen D.H., Liu C., Lohoff F.W., Loo S.K., Lord C., Lowe J.K., Lucae S., Mac Intyre D.J., Madden P.A.F., Maestrini E., Magnusson P.K.E., Mahon P.B., Maier W., Malhotra A.K., Mane S.M., Martin C.L., Martin N.G., Mattheisen M., Matthews K., Mattingsdal M., McCarroll S.A., McGhee K.A., McGough J.J., McGrath P.J., McGuffin P., McInnis M.G., McIntosh A., McKinney R., McLean A.W., McMahon F.J., McMahon W.M., McQuillin A., Medeiros H., Medland S.E., Meier S., Melle I., Meng F., Meyer J., Middeldorp C.M., Middleton L., Milanova V., Miranda A., Monaco A.P., Montgomery G.W., Moran J.L., Moreno-De-Luca D., Morken G., Morris D.W., Morrow E.M., Moskvina V., Muglia P., Mühleisen T.W., Muir W.J., Müller-Myhsok B., Murtha M., Myers R.M., Myin-Germeys I., Neale M.C., Nelson S.F., Nievergelt C.M., Nikolov I., Nimgaonkar V., Nolen W.A., Nöthen M.M., Nurnberger J.I., Nwulia E.A., Nyholt D.R., O’Dushlaine C., Oades R.D., Olincy A., Oliveira G., Olsen L., Ophoff R.A., Osby U., Owen M.J., Palotie A., Parr J.R., Paterson A.D., Pato C.N., Pato M.T., Penninx B.W., Pergadia M.L., Pericak-Vance M.A., Pickard B.S., Pimm J., Piven J., Posthuma D., Potash J.B., Poustka F., Propping P., Puri V., Quested D.J., Quinn E.M., Ramos-Quiroga J.A., Rasmussen H.B., Raychaudhuri S., Rehnström K., Reif A., Ribasés M., Rice J.P., Rietschel M., Roeder K., Roeyers H., Rossin L., Rothenberger A., Rouleau G., Ruderfer D., Rujescu D., Sanders A.R., Sanders S.J., Santangelo S.L., Sergeant J.A., Schachar R., Schalling M., Schatzberg A.F., Scheftner W.A., Schellenberg G.D., Scherer S.W., Schork N.J., Schulze T.G., Schumacher J., Schwarz M., Scolnick E., Scott L.J., Shi J., Shilling P.D., Shyn S.I., Silverman J.M., Slager S.L., Smalley S.L., Smit J.H., Smith E.N., Sonuga-Barke E.J.S., St Clair D., State M., Steffens M., Steinhausen H.-C., Strauss J.S., Strohmaier J., Stroup T.S., Sutcliffe J.S., Szatmari P., Szelinger S., Thirumalai S., Thompson R.C., Todorov A.A., Tozzi F., Treutlein J., Uhr M., van den Oord E.J.C.G., Van Grootheest G., Van Os J., Vicente A.M., Vieland V.J., Vincent J.B., Visscher P.M., Walsh C.A., Wassink T.H., Watson S.J., Weissman M.M., Werge T., Wienker T.F., Wijsman E.M., Willemsen G., Williams N., Willsey A.J., Witt S.H., Xu W., Young A.H., Yu T.W., Zammit S., Zandi P.P., Zhang P., Zitman F.G., Zöllner S., Devlin B., Kelsoe J.R., Sklar P., Daly M.J., O’Donovan M.C., Craddock N., Sullivan P.F., Smoller J.W., Kendler K.S., Wray N.R., International Inflammatory Bowel Disease Genetics Consortium (IIBDGC) Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.T., DellaGioia N., Matuskey D., Harel B., Maruff P., Pietrzak R.H., Esterlis I. Preliminary evidence concerning the pattern and magnitude of cognitive dysfunction in major depressive disorder using cogstate measures. J. Affect. Disord. 2017;218:82–85. doi: 10.1016/j.jad.2017.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heus R.A.A., Olde Rikkert M.G.M., Tully P.J., Lawlor B.A., Claassen J.A.H.R., NILVAD Study Group Blood pressure variability and progression of clinical Alzheimer disease. Hypertension. 2019;74:1172–1180. doi: 10.1161/HYPERTENSIONAHA.119.13664. [DOI] [PubMed] [Google Scholar]
- Erk, S., Meyer-Lindenberg, A., Schnell, K., Opitz von Boberfeld, C., Esslinger, C., Kirsch, P., Grimm, O., Arnold, C., Haddad, L., Witt, S.H., Cichon, S., Nothen, M., Rietschel, M., Walter, H., Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch. Gen. Psychiatry 67, 803–811. doi: 10.1001/archgenpsychiatry.2010.94. (n.d.). [DOI] [PubMed]
- Ferreira M.A.R., O’Donovan M.C., Meng Y.A., Jones I.R., Ruderfer D.M., Jones L., Fan J., Kirov G., Perlis R.H., Green E.K., Smoller J.W., Grozeva D., Stone J., Nikolov I., Chambert K., Hamshere M.L., Nimgaonkar V.L., Moskvina V., Thase M.E., Caesar S., Sachs G.S., Franklin J., Gordon-Smith K., Ardlie K.G., Gabriel S.B., Fraser C., Blumenstiel B., Defelice M., Breen G., Gill M., Morris D.W., Elkin A., Muir W.J., McGhee K.A., Williamson R., MacIntyre D.J., MacLean A.W., St C.D., Robinson M., Van Beck M., Pereira A.C.P., Kandaswamy R., McQuillin A., Collier D.A., Bass N.J., Young A.H., Lawrence J., Ferrier I.N., Anjorin A., Farmer A., Curtis D., Scolnick E.M., McGuffin P., Daly M.J., Corvin A.P., Holmans P.A., Blackwood D.H., Gurling H.M., Owen M.J., Purcell S.M., Sklar P., Craddock N., Wellcome Trust Case Control Consortium Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forette F., Seux M.L., Staessen J.A., Thijs L., Birkenhäger W.H., Babarskiene M.R., Babeanu S., Bossini A., Gil-Extremera B., Girerd X., Laks T., Lilov E., Moisseyev V., Tuomilehto J., Vanhanen H., Webster J., Yodfat Y., Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- Fritze J., Walden J. Clinical findings with nimodipine in dementia: test of the calcium hypothesis. J. Neural Transm. Suppl. 1995;46:439–453. [PubMed] [Google Scholar]
- Green E.K., Grozeva D., Jones I., Jones L., Kirov G., Caesar S., Gordon-Smith K., Fraser C., Forty L., Russell E., Hamshere M.L., Moskvina V., Nikolov I., Farmer A., McGuffin P., Wellcome Trust Case Control Consortium, Holmans P.A., Owen M.J., O’Donovan M.C., Craddock N. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol. Psychiatry. 2010;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamshere M.L., Walters J.T.R., Smith R., Richards A.L., Green E., Grozeva D., Jones I., Forty L., Jones L., Gordon-Smith K., Riley B., O’Neill F.A., O’Neill T., Kendler K.S., Sklar P., Purcell S., Kranz J., Schizophrenia Psychiatric Genome-wide Association Study Consortium, Wellcome Trust Case Control Consortium+, Wellcome Trust Case Control Consortium 2, Morris D., Gill M., Holmans P., Craddock N., Corvin A., Owen M.J., O’Donovan M.C. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol. Psychiatry. 2013;18:708–712. doi: 10.1038/mp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Yamamoto N., Fujii T., Teraishi T., Sasayama D., Matsuo J., Kawamoto Y., Kinoshita Y., Ota M., Hattori K., Tatsumi M., Arima K., Kunugi H. Effects of the CACNA1C risk allele on neurocognition in patients with schizophrenia and healthy individuals. Sci. Rep. 2012;2:634. doi: 10.1038/srep00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S., Yarovova E., Khwanda A., Rosen S.D. Cardiovascular effects of psychotic illnesses and antipsychotic therapy. Heart. 2019;105:1852–1859. doi: 10.1136/heartjnl-2017-312107. [DOI] [PubMed] [Google Scholar]
- Hunter R., McGill L., Bosanquet N., Johnson N. Alzheimer’s disease in the United Kingdom: developing patient and carer support strategies to encourage care in the community. Qual. Health Care. 1997;6:146–152. doi: 10.1136/qshc.6.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., Kato S., Monma Y., Niu K., Ohrui T., Okitsu R., Higuchi S., Ozaki S., Kaneko N., Seki T., Nakayama K., Furukawa K., Fujii M., Arai H. A pilot study of banxia houpu tang, a traditional Chinese medicine, for reducing pneumonia risk in older adults with dementia. J. Am. Geriatr. Soc. 2007;55:2035–2040. doi: 10.1111/j.1532-5415.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Davis V.G., Atkins A.S., Vaughan A., Patterson T., Narasimhan M., Harvey P.D. Validation of a computerized test of functional capacity. Schizophr. Res. 2016;175:90–96. doi: 10.1016/j.schres.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly S.P., Abdullah L., Paris D., Parish J., Mathura V., Mullan M., Crawford F., Lawlor B.A., Kenny R.A. Demonstration of safety in Alzheimer’s patients for intervention with an anti-hypertensive drug Nilvadipine: results from a 6-week open label study. Int. J. Geriatr. Psychiatry. 2011;26:1038–1045. doi: 10.1002/gps.2638. [DOI] [PubMed] [Google Scholar]
- Krug A., Nieratschker V., Markov V., Krach S., Jansen A., Zerres K., Eggermann T., Stöcker T., Shah N.J., Treutlein J., Mühleisen T.W., Kircher T. Effect of CACNA1C rs 1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage. 2010;49:1831–1836. doi: 10.1016/j.neuroimage.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Krupitsky E.M., Burakov A.M., Romanova T.N., Grinenko N.I., Grinenko A.Y., Fletcher J., Petrakis I.L., Krystal J.H. Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists. Neuropsychopharmacology. 2001;25:936–947. doi: 10.1016/S0893-133X(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Lenhard S.C., Strittmatter R., Price W.J., Chandra S., White R.F., Barone F.C. Brain MRI and neurological deficit measurements in focal stroke: rapid throughput validated with isradipine. Pharmacology. 2008;81:1–10. doi: 10.1159/000107661. [DOI] [PubMed] [Google Scholar]
- López-Arrieta J.M., Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst. Rev. 2002:CD000147. doi: 10.1002/14651858.CD000147. [DOI] [PubMed] [Google Scholar]
- Moon A.L., Haan N., Wilkinson L.S., Thomas K.L., Hall J. CACNA1C: association with psychiatric disorders, behavior, and neurogenesis. Schizophr. Bull. 2018;44:958–965. doi: 10.1093/schbul/sby096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., Stiess M., Marais E., Schulla V., Lacinova L., Goebbels S., Nave K.-A., Storm D.R., Hofmann F., Kleppisch T. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S., Baade L.E., Barch D.M., Cohen J.D., Essock S., Fenton W.S., Frese F.J., Gold J.M., Goldberg T., Heaton R.K., Keefe R.S.E., Kraemer H., Mesholam-Gately R., Seidman L.J., Stover E., Weinberger D.R., Young A.S., Zalcman S., Marder S.R. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Nyegaard M., Demontis D., Foldager L., Hedemand A., Flint T.J., Sørensen K.M., Andersen P.S., Nordentoft M., Werge T., Pedersen C.B., Hougaard D.M., Mortensen P.B., Mors O., Børglum A.D. CACNA1C (rs1006737) is associated with schizophrenia. Mol. Psychiatry. 2010;15:119–121. doi: 10.1038/mp.2009.69. [DOI] [PubMed] [Google Scholar]
- Ostacher M.J., Iosifescu D.V., Hay A., Blumenthal S.R., Sklar P., Perlis R.H. Pilot investigation of isradipine in the treatment of bipolar depression motivated by genome-wide association. Bipolar Disord. 2014;16:199–203. doi: 10.1111/bdi.12143. [DOI] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B., Rhodes S.L., Qian L., Schernhammer E., Olsen J.H., Friis S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 2010;67:600–606. doi: 10.1002/ana.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolstad S., Sellgren Majkowitz C., Joas E., Ekman C.J., Pålsson E., Landén M. Polymorphisms of BDNF and CACNA1C are not associated with cognitive functioning in bipolar disorder or healthy controls. Cogn. Neuropsychiatry. 2016;21:271–278. doi: 10.1080/13546805.2016.1185405. [DOI] [PubMed] [Google Scholar]
- Roussos P., McClure M.M., Hazlett E.A., New A.S., Siever L.J., Bitsios P., Giakoumaki S.G. CACNA1C as a risk factor for schizotypal personality disorder and schizotypy in healthy individuals. Psychiatry Res. 2013;206:122–123. doi: 10.1016/j.psychres.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simuni T., Borushko E., Avram M.J., Miskevics S., Martel A., Zadikoff C., Videnovic A., Weaver F.M., Williams K., Surmeier D.J. Tolerability of isradipine in early Parkinson’s disease: a pilot dose escalation study. Mov. Disord. 2010;25:2863–2866. doi: 10.1002/mds.23308. [DOI] [PubMed] [Google Scholar]
- Sklar P., Smoller J.W., Fan J., Ferreira M.a.R., Perlis R.H., Chambert K., Nimgaonkar V.L., McQueen M.B., Faraone S.V., Kirby A., de Bakker P.I.W., Ogdie M.N., Thase M.E., Sachs G.S., Todd-Brown K., Gabriel S.B., Sougnez C., Gates C., Blumenstiel B., Defelice M., Ardlie K.G., Franklin J., Muir W.J., McGhee K.A., MacIntyre D.J., McLean A., VanBeck M., McQuillin A., Bass N.J., Robinson M., Lawrence J., Anjorin A., Curtis D., Scolnick E.M., Daly M.J., Blackwood D.H., Gurling H.M., Purcell S.M. Whole-genome association study of bipolar disorder. Mol. Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro-de-Souza M.G., Bio D.S., Dias V.V., Vieta E., Machado-Vieira R., Moreno R.A. The CACNA1C risk allele selectively impacts on executive function in bipolar type I disorder. Acta Psychiatr. Scand. 2013;128:362–369. doi: 10.1111/acps.12073. [DOI] [PubMed] [Google Scholar]
- Splawski I., Timothy K.W., Decher N., Kumar P., Sachse F.B., Beggs A.H., Sanguinetti M.C., Keating M.T. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M., Kotov R., Fochtmann L., Kalin M., Bromet E.J., Harvey P.D. Associations of independent living and labor force participation with impairment indicators in schizophrenia and bipolar disorder at 20-year follow-up. Schizophr. Res. 2018;197:150–155. doi: 10.1016/j.schres.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm M., Kircher T., Kellermann T., Markov V., Krach S., Jansen A., Zerres K., Eggermann T., Stöcker T., Shah N.J., Nöthen M.M., Rietschel M., Witt S.H., Mathiak K., Krug A. Effects of a CACNA1C genotype on attention networks in healthy individuals. Psychol. Med. 2011;41:1551–1561. doi: 10.1017/S0033291710002217. [DOI] [PubMed] [Google Scholar]
- Tippens A.L., Pare J.-F., Langwieser N., Moosmang S., Milner T.A., Smith Y., Lee A. Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J. Comp. Neurol. 2008;506:569–583. doi: 10.1002/cne.21567. [DOI] [PubMed] [Google Scholar]
- Tollefson G.D., Sanger T.M., Lu Y., Thieme M.E. Depressive signs and symptoms in schizophrenia: a prospective blinded trial of olanzapine and haloperidol. Arch. Gen. Psychiatry. 1998;55:250–258. doi: 10.1001/archpsyc.55.3.250. [DOI] [PubMed] [Google Scholar]
- Tsukuda K., Mogi M., Li J.-M., Iwanami J., Min L.-J., Sakata A., Fujita T., Iwai M., Horiuchi M. Diabetes-associated cognitive impairment is improved by a calcium channel blocker, nifedipine. Hypertension. 2008;51:528–533. doi: 10.1161/HYPERTENSIONAHA.107.101634. [DOI] [PubMed] [Google Scholar]
- Wessa M., Linke J. Emotional processing in bipolar disorder: behavioural and neuroimaging findings. Int. Rev. Psychiatry. 2009;21:357–367. doi: 10.1080/09540260902962156. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Shen Q., Xu Z., Chen M., Cheng L., Zhai J., Gu H., Bao X., Chen X., Wang K., Deng X., Ji F., Liu C., Li J., Dong Q., Chen C. The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with schizophrenia or bipolar disorder. Neuropsychopharmacology. 2012;37:677–684. doi: 10.1038/npp.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Most of the patients that were screened via their enrollment in a larger non-intervention-based study (of >100 patients who met diagnostic criteria) were ineligible. A total of 17 patients were consented to the study, with 6 screen fails. Screen fails were due to medication rule out, abnormal ECG, abnormal labs, positive urine toxicology, unwillingness to swallow pills, and scheduling complications.