Summary
Successful integration of proteins in solid-state electronics requires contacting them in a non-invasive fashion, with a solid conducting surface for immobilization as one such contact. The contacts can affect and even dominate the measured electronic transport. Often substrates, substrate treatments, protein immobilization, and device geometries differ between laboratories. Thus the question arises how far results from different laboratories and platforms are comparable and how to distinguish genuine protein electronic transport properties from platform-induced ones. We report a systematic comparison of electronic transport measurements between different laboratories, using all commonly used large-area schemes to contact a set of three proteins of largely different types. Altogether we study eight different combinations of molecular junction configurations, designed so that Ageoof junctions varies from 105 to 10−3 μm2. Although for the same protein, measured with similar device geometry, results compare reasonably well, there are significant differences in current densities (an intensive variable) between different device geometries. Likely, these originate in the critical contact-protein coupling (∼contact resistance), in addition to the actual number of proteins involved, because the effective junction contact area depends on the nanometric roughness of the electrodes and at times, even the proteins may increase this roughness. On the positive side, our results show that understanding what controls the coupling can make the coupling a design knob. In terms of extensive variables, such as temperature, our comparison unanimously shows the transport to be independent of temperature for all studied configurations and proteins. Our study places coupling and lack of temperature activation as key aspects to be considered in both modeling and practice of protein electronic transport experiments.
Subject Areas: Electrochemistry, Bioelectronics, Bioelectrical Engineering
Graphical Abstract

Highlights
-
•
Junction geometry determines effective contact area
-
•
Mechanism of charge transport is independent of junction platform
-
•
Electrode-molecule coupling determines transport efficiency across interfaces
-
•
Tunneling dominates solid-state electron transport across protein-based junctions
Electrochemistry; Bioelectronics; Bioelectrical Engineering
Introduction
A long-standing goal in (bio)molecular electronics is the development of a reliable approach to integrate proteins and peptides into electrical circuits (McCreery et al., 2013, Ratner, 2013). Understanding the mechanism of electron transport (ETp) through biomolecules in a solid-state configuration, with different device geometries, is an important step toward controlling ETp for designing bioelectronic circuits that incorporate proteins as active components.
Compared with synthetic molecules often studied in molecular electronics, proteins are much larger, which decreases the currents that pass, mostly well below what can be measured by “single-molecule” methods based on, e.g., scanning probe microscopes or break junctions. Also, as their tertiary structure may affect transport efficiency across them, the top electrode should induce no or a minimal stress to the immobilized proteins to ensure that protein structure (and orientation) on the surface can be investigated. Thus, ETp through proteins is predominantly studied in large-area configurations such as liquid metal (EGaIn and Hg) or ready-made contacts (lift-off float-on, LOFO or nanorods) rather than by scanning probe microscopies (especially scanning tunneling microscopy [STM] and conducting probe atomic force microscopy [AFM]) or mechanical break junctions. However, the reproducibility of experimental results of a given molecule or protein across different platforms is not really known, although former meta-data analyses could find some rough agreements for biomolecular (Amdursky et al., 2014a) and molecular junctions (Salomon et al., 2003). However, both those compilations showed in several cases significant differences between methods in the measured current densities, which is an intensive variable, and, in principle, depends on the effective electrical contact area (Aelec), which may be orders of magnitude smaller than the geometrical contact area (Ageo) of the junction, and contact resistance. Consequently, comparison of the current density derived from Ageo across platforms is challenging as Aelec depends on the details of the roughness of the molecule-electrode interfaces and the types of contacts used in the devices (Holm, nd, Timsit, 1982).
The mechanism of electron transport depends on how strongly the molecules are coupled to the electrodes, which affects the activation energy for transport (Ea) and/or the tunneling barrier height (), although normally one of these two energies will dominate ETp. The way molecules contact and interact with the electrodes is important, and at times critical (Moulton et al., 2003, Sayed et al., 2012). This observation that merely expresses what is well known in solid-state electronics, viz. the importance of the contact resistance. Because the contact made will likely vary between methods and possibly between the applications of a given method in different laboratories, we set out to compare ETp results (essentially, currents at given, preferably low, bias voltages) among laboratories, as obtained with different device geometries. We also compare the shape of current density, J-V, curves of a given protein, as measured with different junction configurations to understand how the shape of the J-V curve differs as a function of the protein-electrode configurations of the two contacts, required for all methods (except for junctions based on STM, which will not be considered). To establish the mechanism of charge transport across a given protein, we measured the magnitude of the current not only as a function of applied bias voltage but also as a function of temperature (T) to determine the activation energy (an extensive variable that does not depend on the effective contact area). We used proteins that yielded molecular films with similar thickness, i.e., imposed a similar separation between the electrodes (d). To get corrected current densities we further normalized J ( = Imeasured/Ageo)to the effective electrical contact area (Aelec) in different junction configurations.
Toward that end, we performed a cross-laboratory study—a first of its kind to our knowledge—aimed to compare the instruments and measurement methods and contact configurations used for electrical transport characterization at different laboratories, and to establish how the intensive and extensive (if at all) charge transport parameters change across different junction platforms, involving the University of Alberta (UoA), Canada; University of Groningen (UoG), The Netherlands; National University of Singapore (NUS), Singapore; and Weizmann Institute of Science (WIS), Israel, with standard molecular junctions as-fabricated at UoA. We conceptualized experimental studies with three different proteins, namely bacteriorhodopsin (bR), photosystem-I (PSI), and ferritin, in different device configurations, using fabrication expertise available in the different laboratories. Analyzing statistically significant numbers of J-V data obtained from nine different platforms, three different proteins, and J(V,T) data (in total we used 100–400 J-V curves for each type of molecular junction in our analyses; see Table S1 in Transparent ), we extracted tunneling parameters, mainly energy offset/barrier height (), conductance (Geq), and electronic coupling () and used these results to conclude about the universal nature of the electrical transport across (bio)molecular junctions (Vilan et al., 2013, Bâldea, 2018, Xie et al., 2015, Vilan, 2017). The major conclusion is that although the extrinsic variable of current density varies greatly across platforms (due to changes in contact resistances and effective contact areas studied over a dynamic range of 8 orders of magnitude), intrinsic variables do not change; from this we conclude that the different methods probe the same mechanisms of charge transport for a given protein. The report reflects the ongoing interest and efforts in developing efficient, reliable protein-based molecular junction fabrication methods by combining top-down micro/nanofabrication with bottom-up molecular assembly.
Results
Standard Molecular Junction Fabrication and Measurements
Different research groups prepare and measure molecular junctions using distinct protocols and equipment, which complicates the comparison of results of electrical characterization studies over different laboratories. As a reference standard, we choose two types of samples: (1) a sample made of carbon-NAB//e-C//Au junctions (NAB is 4-[2-(4-nitrophenyl) diazenyl]-phenyl groups; e-C is thermally evaporated carbon) and (2) a sample made of carbon-NAB (i.e., without top contact) (Yan et al., 2011). In this molecular junction, pyrolyzed photoresist film (PPF) is used as the bottom electrode, on which a ∼5-nm-thick multilayer of NAB was formed via diazonium chemistry followed by deposition of a layer of carbon and then Au, both via thermal evaporation, which served as the top contact (Figure 1A). The conjugated NAB layers are highly stable, and the junctions have been proved to be highly reproducible (Sayed et al., 2012, Yan et al., 2011). To establish that all the electrical measurement equipment used in the different laboratories are the same, three samples of the type (1), each sample containing 25 junctions, were circulated and measured among the different laboratories. We also measured the current density of the sample type (2) with the tip-EGaIn method to determine the ratio of Aelec/Ageo. The root-mean-square (rms) surface roughness of the reference sample was characterized with tapping mode AFM (see Figure S1 in Transparent Method).
Figure 1.
Illustration of the Junction Configurations Used in This Study
(A–H) (A) Carbon-NAB//e-C//Au, (B) TSAu-linker-protein//GaOx/μch-EGaIn, (C) TSAu-linker-protein//GaOx/tip-EGaIn, (D) Si/SiOx/protein//evap-Pb//Au, (E) Si-SiOx-linker-protein//LOFO-Au, (F) TSAu-linker-protein//GaOx/th-EGaIn, and (G) Si/SiOx-linker-protein//Hg and (H) Au-linker-protein//Au nanowire junction. Note, “-” indicates a covalent contact, “/” indicated the interface between GaOx and the bulk EGaIn alloy or Si and SiOx, and “//” indicates a van der Waals contact.
The Proteins
Biomolecules exhibiting different functional properties were preferred for this study as they already represent extreme examples of efficient, long-range temperature-independent charge transport in solid-state device configurations (Castañeda Ocampo et al., 2015, Kumar et al., 2016, Ron et al., 2010). Here we compare monolayers of the following three proteins inside the junctions.
-
1.
Ferritin is a highly symmetrical, primary intracellular, iron storage protein, which forms via self-assembly of 24 subunits. The protein shell is 12 nm in diameter with an 8-nm hollow interior and possesses channels that traverse the 2-nm-thick protein shell to allow metal ions to enter and exit. The ferritin was isolated from a hyperthermophilic archaeon Archaeoglobus fulgidus, which has high thermal stability (up to 80°C), making it favorable for room-temperature operation, once incorporated into solid-state devices. This type of ferritin can store up to ∼7,000 Fe ions (Sana et al., 2010), but in this study we used ferritin loaded with 4,800 Fe ions (see Transparent Methods Section 1a for details procedure for iron oxide loading inside the ferritin).
-
2
bR is a protein-chromophore (= retinal) complex that serves as a light-driven proton pump in the purple membrane of the archaeon Halobacterium salinarum, a remarkably stable primordial converter of solar energy (into a proton gradient) (Jin et al., 2006, Jin et al., 2008)
-
3
PSI is a multi-subunit protein complex located in the thylakoid membranes of green plants and algae, which contains both metal atoms and photoactive molecules. It absorbs solar energy via its antenna chlorophyll molecules and transfers energy to a special chlorophyll pair, where charge separation occurs; from there, driven by a free energy slope, the electron is transported in steps to the next stage in the photosynthetic processes (Castañeda Ocampo et al., 2015, Singhal et al., 1999).
Protein Monolayer Formation
The proteins were adsorbed on the template-stripped gold surface (TSAu, with glass support) or doped silicon surface (p++-Si/SiOx) either by physisorption or chemisorption via short linker molecules following previously reported methods (see Table 2 for details). We used self-assembled monolayers (SAM) of 6-mercaptohexanoic acid or 3-mercaptopropionic acid (MPA) on TSAu to immobilize ferritin. Ferritin was covalently bound to the linker layer utilizing carbodiimide cross-linker chemistry (EDC (carbodiimide)/N-hydroxysuccinimide (NHS) crosslinking reaction). For bR, we used cysteamine linker SAMs on TSAu. PSI monolayer was prepared on TSAu utilizing 2-mercaptoethanol or MPA linker followed by EDC/NHS. On p++-Si/SiOx, all the proteins were anchored via (3-aminopropyl) trimethoxysilane linker (Bostick et al., 2018). Before electrical measurements in different laboratories, protein monolayers were characterized using a variety of methods, such as AFM, ellipsometry, and infrared spectroscopy, provided in the respective references (Ron et al., 2010; Kumar et al., 2016; Castañeda Ocampo et al., 2015, Jin et al., 2008, Jin et al., 2006). We confirmed the monolayer quality of three different proteins on TSAu and p++-Si/SiOx substrates with tapping mode AFM measurements, which were summarized in the Supplemental Information Section 2b (see Transparent Methods Section 1a and Figures S2–S8 for more details).
Table 2.
AFM Analysis of the Protein Films Prepared by the Different Laboratories
| Protein | Substrate | Linkera | Lab | Protein Thickness (nm; ±1) | Roughnessb (nm; ±0.1) | Coverage (%; ±5) |
|---|---|---|---|---|---|---|
| bR | TSAu | 6-Amino-1-hexanethiol | NUS | 7 | 1.3 | 65 |
| Cysteamine | NUS | 7 | 1.6 | 60 | ||
| Cysteamine | WIS | 7 | 1.6 | 95 | ||
| Cysteamine | UoG | 8 | 3.2 | 65 | ||
| Si/SiOx | (3-Aminopropyl)trimethoxysilane | WIS | 7 | 2.3 | 85 | |
| Ferritin | TSAu | 6-Mercaptohexanoic acid | NUS | 7 | 2.0 | 50 |
| 3-Mercaptopropionic acid | WIS | 5 | 2.6 | 60 | ||
| 6-Mercaptohexanoic acid | UoG | 7 | 2.8 | 65 | ||
| Si/SiOx | (3-Aminopropyl)trimethoxysilane | WIS | 7 | 1.4 | 95 | |
| PSI | TSAu | 3-Mercaptopropionic acid | NUS | 7 | 1.4 | 80 |
| 2- Mercaptoethanol | WIS | 7 | 2.4 | 95 | ||
| Si/SiOx | 2- Mercaptoethanol | UoG | 7 | 2.1 | 80 | |
| (3-Aminopropyl)trimethoxysilane | WIS | 8 | 2.8 | 60 |
See Supplemental Information, Section SA2b and Figures S2–S8 for further detail.
Roughness value is the rms roughness over the scanned area of 1 μm2.
The Junctions
We focus our comparison on device fabrication methods that allow for making soft contacts to surface-adsorbed protein films in a non-destructive manner, have good yields in working junctions (up to ∼90%), have high reproducibility, yield statistically large numbers of J-V measurements, and have withstood the test of time (Castañeda Ocampo et al., 2015, Jin et al., 2008; Karuppannan et al., 2016; Ron et al., 2010). Figure 1 illustrates the various different junction platforms and methods to form the top contacts employed in this work, and Table 1 summarizes their Ageo, and exact composition, while throughout the text we will use shorter designations (first column of Table 1). All architectures have been used earlier and are well accepted for large-area molecular junctions (Ron et al., 2010, Kumar et al., 2016, Castañeda Ocampo et al., 2015, Jin et al., 2008, Jin et al., 2006). In the following discussion we summarize their preparation and unique features, focusing on their values of geometrical contact area (Ageo) ranging eight orders of magnitude and electrical contact area (Aelec) in the junctions, and surface roughness of protein-modified bottom conducting substrates and corresponding top electrodes.
Table 1.
Device Configurations Used in the Different Laboratories
| Name | Junctions Descriptiona | Contact Areac [μm2] |
||
|---|---|---|---|---|
| Geometric | Uncertainty | Methodb | ||
| LOFO-Au | Si/SiOx-linker-protein//LOFO-Au | 2 × 105 | ±10% | Img |
| Hg | Si/SiOx-linker-protein//Hg | 5000 | ±5% | Img |
| evap-Pb | Si/SiOx-linker-protein//evap-Pb/Au | 5000 | ±10% | Ptr |
| th-EGaIn | TSAu-linker-protein//GaOx/th-EGaIn | 1000 | ±10% | Ptr |
| μch-EGaIn | TSAu-linker-protein//GaOx/μch-EGaIn | 500 | ±10% | Ptr |
| tip-EGaIn | TSAu-linker-protein//GaOx/tip-EGaIn | 300 | ±10% | Img |
| Au nanorod | Au-linker-protein//Au nanorod | ~5 × 10−3 | ±50% | AFM Img |
| NAB | Carbon-NAB//evap-C//Au | 1.25× 105 | ±10% | Ptr |
Protein: ferritin, PSI; bR.
Img or Ptr mark whether the geometric area was obtained from a microscopy image (Img) or AFM tapping mode imaging (AFM Img) or dictated by feature patterning (Ptr).
Refer to Figure 1.
mg or Ptr mark whether the geometric area was obtained from a microscopy image (Img) or AFM tapping mode imaging (AFM Img) or dictated by feature patterning (Ptr).
Refer to Figure 2.
EGaIn Techniques
EGaIn is a liquid-metal alloy of a Ga-In eutectic (75% Ga and 25% In by weight), which is widely used as an electronic top contact to large-area molecular junctions due to its ease in junction fabrication, non-toxicity of EGaIn, ambient stability, non-damaging nature to the monolayers, and high reproducibility of measured junction currents. EGaIn has a passivating Ga-oxide layer on the surface. The oxide layer is predominantly amorphous Ga2O3 with a thickness of about ∼0.7 nm (at least on smooth surfaces) and is highly conducting (Kumar et al., 2016, Regan et al., 1997, Rothemund et al., 2018) and therefore adds only negligibly to the net resistance. However, the oxide skin floats on the EGaIn and behaves like a solid (resulting in non-Newtonian properties), which yields a much rougher surface than, e.g., Hg, because it wrinkles. The EGaIn surface roughness is sensitive to the preparation method, but recently the effective contact areas for the different variations of the EGaIn technique have been quantified as indicated later (see for details Chen et al., 2019). Here, we used the EGaIn top electrode in the following three configurations to contact the protein monolayers on TSAu.
Tip-EGaIn
A tip-shaped EGaIn (Figure 1C) is fabricated by pulling out a microneedle of a drop of EGaIn (Cademartiri et al., 2012, Chiechi et al., 2008). The Ageo is determined by recording the diameter of the footprint of the EGaIn tip with the monolayer and by assuming that the footprint is circular. In this case, the tip apex is very rough due to the rupture and wrinkling of the GaOx during the tip formation process (Chen et al., 2019). For Ageo larger than 1,000 μm2, the Aelec/Ageo ratio for tip-EGaIn contacts is ∼10−4 (Rothemund et al., 2018, Chen et al., 2019, Simeone et al., 2013, Kumar et al., 2019). Recently, it was shown that for Ageo larger than 1,000 μm2 leakage current across defect can become important (Chen et al., 2019).
μch-EGaIn
The EGaIn was injected into a Polydimethylsiloxane microchannel perpendicularly aligned over an array of TSAu electrodes supporting the SAMs (Nijhuis et al., 2010a). The Ageo, i.e., the crossing area, was ∼500 μm2 (Chen et al., 2019, Kumar et al., 2019). The GaOx forms in situ during the injection of the EGaIn in the microchannels, and therefore the GaOx is smooth and gives about a factor of 102 higher Aelec/Ageo ratio for μch-EGaIn (Figure 1B) than for tip-EGaIn contacts as deduced from modeling of I-V curves, in Kumar et al. (2019), i.e., for μch-EGaIn contacts Aelec/Ageo = ∼10−2. The rate at which the EGaIn is injected into the channel is slow, relative to the formation rate of the GaOx, and, given that PDMS is permeable to O2, the GaOx layer is continuous (Dickey, 2017, Nijhuis et al., 2010a). This device geometry may suffer from leakage currents flowing across the defects for very large values of Ageo >1,000 μm2 (Jiang et al., 2015, Wan et al., 2014).
th-EGaIn
In this configuration (Figure 1F), EGaIn is stabilized in a through-hole in PDMS. The EGaIn is injected into a network of microchannels in PDMS, connected to a through-hole where the GaOx/EGaIn is exposed and can contact the protein monolayer (Sangeeth et al., 2014, Wan et al., 2014). The bottom TSAu is non-patterned, and therefore Ageo is defined by the diameter of the through-hole, which was ∼1,000 μm2 for the experiments reported here. The top-contact can be placed at any place on the TSAu surface supporting the protein layer. Although this method does not suffer from electrode edges where the molecules do not pack well, the GaOx layer is formed ex situ and therefore is rough due to wrinkling and handling of the EGaIn (Kumar et al., 2019). In the th-EGaIn junction configuration, Aelec is very similar to that of the tip-EGaIn method (Aelec/Ageo = 10−4) (Kumar et al., 2019, Sangeeth et al., 2016, Wan et al., 2014). The second and third setups can both be used for temperature-dependent studies.
Junctions with Si/SiOx Bottom Electrodes
LOFO-Au
In this “lift-off, float-on” (LOFO) method (Haick and Cahen, 2008, Vilan and Cahen, 2002), the top contact was prepared by deposition of ready-made Au pads onto the protein film from a liquid (Figure 1E). The pads float on water, into which the Si substrate, covered with regrown <1.0-nm SiOx, supporting the protein monolayer was immersed. The value of Ageo was 2 × 105 μm2, and its inner, glass-stripped surface has an average rms roughness of <1 nm (1.0 μm × 1.0 μm AFM scan area) (Mukhopadhyay et al., 2015). LOFO is a low-pressure, low-temperature method that works well for water-compatible molecules such as proteins and is vacuum compatible, as required for low-temperature measurements (Sepunaru et al., 2012). Its major disadvantage is the non-negligible skill required to prepare these contacts; in addition, adventitious materials from the ambient environment are present on the electrodes, which can reduce the work function and/or limit electrode-protein coupling (Reus et al., 2012). This method was used for temperature-dependent studies.
Hg Drop
The protein monolayers were contacted with a hanging drop of Hg (Figure 1G) (Ron et al., 2010, Haick and Cahen, 2008). This method was used for room-temperature measurements only. The Ageo is ∼5,000 μm2 as determined optically from the diameter of the circle made by the Hg drop onto the surface. The high surface tension of Hg (the contact angle between Hg and an alkyl monolayer is 150°) (Seitz et al., 2006) implies that Hg follows the large surface terrain, and Aelec is determined by the roughness of the protein layers on the Si/SiOx substrate.
evap-Pb/Au
The top contact was made by direct evaporation of lead (Pb) on the protein layers via a shadow mask (Figure 1D). Pb can be vacuum evaporated under very mild (low-temperature) conditions. This method, demonstrated earlier for organic molecules with an exposed labile group (Yu et al., 2014) was used to contact bR protein films on Si/SiOx substrate. The contact area, defined by a shadow mask, is ∼5,000 μm2. Here, we can only assume that Pb layer forms continuous and conformal contact with the protein monolayer. In principle, such contacts might be transferable to future practical devices.
Au-Nanorod Junction
Au-nanorod junction (Figure 1H) of Au-linker-protein//Au was made by dielectrophoresis trapping of Au nanorods between two micropatterned Au leads on a silicon substrate; the protein SAM is adsorbed on the micropatterned Au leads connected to external electronics. Although both ends of the nanorod contact a protein layer, one end is always shorted, yielding a single active molecular junction (Guo et al., 2016, Yu et al., 2015). The geometrical contact area for nanorod junction is a narrow rectangle with length dictated by the overlap between the nanorod and the protein-covered Au lead (500–2,000 nm long); its width is defined by the rod's diameter (200 nm) reduced to only 10–20 nm, due to curvature. In contrast to spherical liquid contacts (EGaIn and Hg), the solid nanorod does not flatten by adhesive forces. Therefore the geometrical contact area for nanorod junction has both significant junction-to-junction variation (varying rod-pad overlap) and large uncertainty (curved contact); as a rough estimate it is set to 5,000 nm2(Guo et al., 2016). Electrical measurements with this method were restricted to 0 ± 0.5 V range to avoid damage due to junction heating.
Effective Contact Area of the Biomolecular Junctions
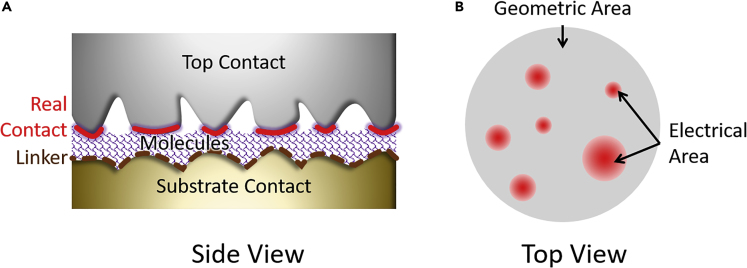
The “bottom” contact is always the conductive substrate on which the proteins were adsorbed. Fabrication of the “top” contact is challenging, as it must not damage the soft protein material. As mentioned in the Introduction, determining the value of Aelec, or how many molecules contribute in parallel to the measured current, is a major challenge in molecular electronics on ensembles of molecules. Figure 2 shows schematically the difference between Ageo and Aelec. The Ageo refers to the macroscopic dimension of the overlap between the bottom and top electrodes, as determined by imaging or patterning. In practice, however, surface roughness limits the value of the Aelec to a rather small fraction of the Ageo. The value of Aelec can be up to 2 to 6 orders of magnitude smaller than the Ageo for contacts between two solid-state electrodes or a solid and liquid electrode material (Holm, nd, Timsit, 1982). Although this issue is recognized also in molecular junctions (Cademartiri et al., 2012, Nijhuis et al., 2010b, Rothemund et al., 2018, Salomon et al., 2003, Simeone et al., 2013), it has been only rarely experimentally determined. In this report, we verified the value of Ageo for all techniques, and, therefore, all current density values reported here are based on the relevant Ageo values. Nonetheless, we will argue that Ageo/Aelec variations for junction to junction originates in our inability to know, let alone control, the Aelec, with the exception of EGaIn top electrodes for which the ratio (Ageo/Aelec) is statistically known (and has been used before to rationalize the differences in current density measured across molecular junctions using different platforms) (Chen et al., 2019, Sangeeth et al., 2016).
Figure 2.
Geometric vs. Electrical Contact Area
(A) Schematic illustration of the side view of the junction shows how the roughness in the bottom and top contacts results in Aelec << Ageo leaving room for air (in experiments performed in ambient environments) or vacuum (for experiments performed under reduced pressure); red lines indicate Aelec.
(B) Top view illustrates that the junction is a collection of few contact points in parallel as indicated by fuzzy red regions.
Electrical Characterization
Room-temperature charge transport measurements were carried out in ambient conditions. For statistical analysis, 100–400 J-V traces were obtained from 20–30 molecular junctions, which were then used to determine the log-average J-V curves and log-standard deviation following previously reported methods (see Section A3 and Table S1 in Transparent Method). Temperature-dependent transport measurements were carried out with th-EGaIn and LOFO-Au junctions (see Table 1 and Figure 1) in temperature-controlled cryogenic probe stations (pressures varied between ∼10−6 and 3 × 10−5 mbar). The currents across the junctions did not change upon changing the pressure from ambient to vacuum, which indicates that the contacts were stable at low pressure also (see section A4 in Transparent Method). The devices were slowly cooled, and their J-V characteristics measured at intervals of 5 K (GaOx/th-EGaIn junctions) and 10 K (LOFO-Au junctions), allowing the devices to stabilize before performing each scan.
Discussion
Calibration by “Standard” Molecular Junction
Different research groups prepare and measure MJs using distinct protocols and equipment, which complicates the comparison of the results of electrical characterization studies over different laboratories. As a reference junction, we choose carbon-NAB//e-C//Au as this junction is robust and stable and can be readily shipped. This reference molecular junction had a value of Ageo of 1.25 × 105 μm2 (Figure 1A; for details see Supplemental Information Sections A2a and A3a) and was measured in all the participating laboratories, starting with its “home,” in Edmonton (UoA, Canada); followed by WIS, Israel; NUS, Singapore; and UoG, The Netherlands. Figure 3 shows a semi-log plot of the J-V traces measured in the different laboratories, where 1 to 4 mark the order of measurement. Overall, the reproducibility between the different laboratories is good, with some time degradation at the high-voltage range, likely due to changes in the probe/PPF contact resistance with time (fully encapsulated NAB junctions last for years without change). This result establishes that the electronic measurement systems between the laboratories are comparable. We note that the J-V of Figure 3 was measured using only two probes because the biomolecular junctions could only be measured in 2-probe configurations (molecules were sandwiched between electrodes). Still, we note that if moderately conducting leads, such as the carbon-based electrodes in this reference device, are used, they are better characterized by four probes to eliminate the series resistance contribution.
Figure 3.
Validation of Measurement Equipment
Current density (A/cm2) (on log scale) versus voltage (V) curves for carbon-NAB (5 nm)//e-C(10nm)/Au(15 nm) junctions, measured in different laboratories. The error bars represent the standard deviations in current densities over ~140 traces. We collected a similar number of J-V traces from the same junctions at different laboratories.
As an additional control, the current density across a reference sample of carbon-NAB (i.e., lacking the carbon-Au top contact) was also measured with tip-EGaIn in direct contact with NAB (i.e., carbon/NAB//GaOx/tip-EGaIn junctions) with an Ageo of 3.8 × 103 μm2. For this junction, the current density was ∼3.5 orders of magnitude lower (see section A3a and Figure S9) than for the junctions shown in Figure 3, an effect that overwhelms the above-mentioned increase in contact resistance with time. This difference is very similar to the 4 orders of magnitude difference between the Aeff and Ageo due to the roughness of the electrodes (i.e., the ratio Aelec/Ageo is 10−4) as illustrated in Figure 2; A factor of 10−4 was also reported by the group of Whitesides (Rothemund et al., 2018, Simeone et al., 2013) and confirmed by the group of Nijhuis (Chen et al., 2019, Sangeeth et al., 2014, Yuan et al., 2018), which is attributed to the surface roughness of the cone-shaped tips (tip-EGaIn) (Chen et al., 2019).
Protein Monolayer Characterization
Before considering the transport characteristics across the different platforms and laboratories, we determined the quality of the protein films, prepared by each laboratory, as shown in Table 2. From AFM or ellipsometry, the thicknesses of the monolayers of ferritin, bR, and PSI layers were comparable with the size of the corresponding single proteins, indicating the formation of well-packed protein monolayers. However, the monolayer coverages vary among different laboratories as prepared by different methods or linker molecules. Each protein-SAM was analyzed by tapping-mode AFM imaging from which we calculated the protein surface coverage as well as rms surface roughness from the AFM topography over an area of 1 μm2 (see Section A2b and Figures S2–S8). The rms surface roughness of the self-assembled protein surfaces on the bottom electrode changes from 0.5 nm (for TSAu or Si/SiOx substrates) to 1.3–2.7 nm (Table 2), which could also lead to variation in the ratio Aelec/Ageo over the different platforms. The surface coverage of ferritin monolayers ranges from ∼40% to ∼95%, that of bR films is from ∼60% to ∼95%, and that of PSI monolayers is from ∼50% to ∼80%. Such differences can originate from the grade of chemicals used, environments, and person-to-person variation in fabrication methods. Based on earlier work (Castañeda Ocampo et al., 2015), the differences in monolayer coverages affect the measured current magnitudes by at most a factor of 2–3, which can explain the results shown later (Figure 4). Given the spread between J-V on the same protein junctions (which can reach up to an order of magnitude), we can ignore variations in the monolayer coverages across the different laboratories (Table 2).
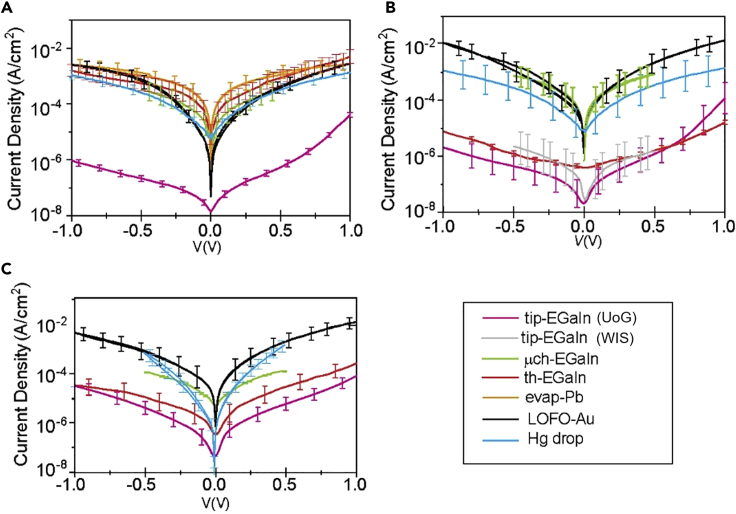
Figure 4.
Protein Junction Transport Results at Room Temperature
(A–C) Current density (A/cm2) versus voltage (J-V) data for different junction configurations with (A) bR (B) ferritin, and (C) PSI. The tip-EGaIn junction measurements were reproduced at WIS (gray) and UoG (magenta), showing the reproducibility between the different laboratories (B). Error bars represents statistical variations in current densities over measured I-V traces for different devices (as in Table - S1)
Room-Temperature Current Density-Voltage (J-V) Characteristics
Figure 4 summarizes the room-temperature J-V characteristics of the junctions made with the different proteins, bR (Figure 4A), ferritin (Figure 4B), and PSI (Figure 4C), in different laboratories. The legend lists the different configurations and laboratories that prepared them (as summarized in Figure 1, Table 2, and Figures S10 and S11 and section A3b for measurement details to specific device configurations) (Castañeda Ocampo et al., 2015, Garg et al., 2018, Kumar et al., 2016, Rothemund et al., 2018, Vilan et al., 2017, Wan et al., 2014). The I-V data for Au nanorod junctions are shown on Figure 5, and will be discussed separately.
Figure 5.
Transport Results for Gold Nanorod Junctions Experimentally Measured Currents versus Applied Bias for Junctions Fabricated with Gold Nanorod on Protein SAM on Patterned Gold Electrodes (At Room Temperature)
Average currents were obtained from at least 10 different junctions where error bar represent variation of measured currents over junctions. 10−10A corresponds to ~2 A/cm2 (cf. Table 1).
The bR junctions yield rather reproducible current densities, as shown in Figure 4A, where all J-V curves are within error from one another; the only exception is the tip-EGaIn junction, which yields a few orders of magnitude lower nominal current density (Cademartiri et al., 2012, Chen et al., 2019, Nijhuis et al., 2012, Rothemund et al., 2018). Once corrected for the ratio Aelec/Ageo of 10−4, all data fall within one order of magnitude (Figure S12 in section A3b).
In few tip-EGaIn junctions we observed rectification, with rectification ratios of up to 50, whereas those junctions with μch-EGaIn and th-EGaIn top contacts did not rectify significantly (Table S2). We ascribe this to the fine details of the SAM//EGaIn contact, which, in the experiments with tip-EGaIn, result in a large potential drop at the SAM//EGaIn interface relative to the Au-SAM interface, leading to rectification (Kumar et al., 2019).
The current densities across ferritin (Figure 4B) fall in two distinct ranges, with roughly the same 104 factor between them (see also Figure S12B). Generally, each mode of EGaIn was prepared by a different laboratory. To verify that the much lower current density of tip-EGaIn is not due to human operation, two laboratories prepared tip-EGaIn contacts to ferritin: UoG and WIS; the resulting I-V curves (Figure 4) are fairly reproducible between them, and the different bias windows did not affect the measurements. In comparison, the WIS-prepared μch-EGaIn has ∼104 higher current than the tip-EGaIn prepared by the same laboratory. The th-EGaIn contact (red curves in Figure 4) represents an interesting case: when it contacts bR, it yields a high current density, similar to the majority of top contacts; however, the same contact to ferritin yielded a low current density, similar to that of tip-EGaIn and in agreement with previous findings for n-alkanethiolate SAMs (Wan et al., 2014). This can be understood if globular ferritin produces significant roughness by its own (see Table 2). For the tip-EGaIn and th-EGaIn the top electrode, along with the GaOx layer, is formed ex situ, and thus the GaOx layer wrinkles and buckles during handling, which lowers Aelec. In contrast, the μch-EGaIn top contact, along with the GaOx layer, is formed in situ for which the Aelec increases considerably by about 102 times ( Jiang et al., 2015). We postulate that because the ex situ-formed oxide of th-EGaIn is confined in a through-hole, it is too rigid to adapt to the protein roughness, and therefore the current density via th-EGaIn is more sensitive to the roughness and mechanical properties of the bottom substrate than other types of contacts. Unlike tip-EGaIn, which can easily deform and release pressure (Rothemund et al., 2018), th-EGaIn cannot yield due to this confinement, and therefore this top electrode may result in exerting a significant pressure on the monolayer during the formation of the top contact.
Finally, the current density across PSI (Figure 4C), based on Ageo, showed the smallest net spread in current densities. However, after correcting for the differences in Aelec/Ageo for the different junction configurations (see Figure S12C), the spread is similar to that obtained for ferritin and bR, especially if single outlier curves are excluded.
Role of the Linker
Several linker molecules were used to achieve reproducible SAM of the examined proteins. On Si the linker was identical for the three tested proteins, whereas linkers to Au were adjusted to the protein's chemical structure, electrostatic charge distribution of protein surfaces, and methods of preparation. As reported in Table 2, apart from a few exceptions, the linker was identical for each protein. Importantly, the SAM quality was similar between the different laboratories, supporting the choice of linker for each protein. Although Table 2 shows variability in binding density and roughness, there is no correlation between these structural characterizations and the net current of Figure 4, which, for the protein density on the substrate surface, confirms earlier results (Castañeda Ocampo et al., 2015). The role of the linker was directly tested in two occasions (see Figure S13 in secti). Such comparative experiments are reported here for completeness' sake; their results do not alter the general picture.
Role of Protein Orientation
The arrangement or orientation of the proteins on a gold/Si substrate and their structural and dynamic properties have been simulated using molecular dynamics studies as reported (Boussaad and Tao, 1999, Tao, 2006, Waleed Shinwari et al., 2010). Modification of electrodes with linker molecules eliminates unpredictable orientations of proteins on the electrode surface, because then the proteins can bind to the linkers via specific (bioengineered) positions. An appropriate choice of linker molecules can alter protein orientation in a controlled fashion (Gaigalas and Niaura, 1997, Schnyder et al., 2002)—as we have done here for PSI (Castañeda Ocampo et al., 2015) and bR (Jin et al., 2006), based on our previous reports—and thus reduce unknowns in the junction structure related to the orientation of the protein. An extensive study on cytochrome c, with wild-type and seven mutants of the included cysteine for directed binding to the substrate, showed at most four times difference in currents at 0.05 V (Amdursky et al., 2014b). When proteins are attached to substrate surfaces through an organized monomolecular layer with site-specific immobilization, it provides better reproducibility and better control over electron transfer and transport measurements than approaches based on physisorption of proteins on surfaces. Relevant to our study reported here, we have explored a detailed comparison with PSI, where protein orientation was altered by varying the organic linker molecules (see Figure S13 in section A3b) on TSAu substrates, and a 3-fold change in current was observed between the two orientations (Castañeda Ocampo et al., 2015). Although certainly significant, this effect is much smaller than what we measure here between different junction contact configurations, i.e., in comparing junctions between different metal/protein/metal configurations, we will neglect the effect of different linker molecules. The effects related to the orientation are not applicable for ferritin given its globular tertiary structure. For bR we have shown that the orientation is always directional with the linkers used in the present study. For these reasons, the orientation of the proteins in the junction plays only a minor role in the measured currents in the present study Figure S13 in Section SA3b and detailed discussion in Section SB1.
Top Electrode Effects
When comparing normalized current densities, the above-mentioned issue of Ageo versus Aelec is always a problem, and the issue appears most pronounced in the range between nanoscopic and macroscopic areas (Cademartiri et al., 2012, Simeone et al., 2013). In the following we point to some of the differences between the contact methods, relevant to this issue.
Comparison of EGaIn Techniques
Because in “μch-EGaIn” the liquid metal is actively pushed against the protein and confined in small channel, its ability to yield and deform is limited, which might well result in some pressure on the protein film (similar to the gravity push in the Hg drop configuration). In tip-EGaIn junctions the alloy exerts negligible pressure on the proteins (as suggested by AFM measurements on EGaIn tips Chen et al., 2019). The th-EGaIn junctions differ from the μch-EGaIn ones in that in the former the GaOx layer is formed ex situ, and in the latter in situ, which is therefore smooth (and, as mentioned earlier, gives ∼2,000× larger Aelec) (Kumar et al., 2019). In terms of pressure, EGaIn that is injected in hole-modified microchannels (th-EGaIn) will likely also exert negligible pressure on the proteins (although shear pressure may become an issue at extremely large flow rates).
The LOFO-Au Method
This method (Figure S11) uses the peeled (and thus smoother) side of the metallic film, which was measured to have rms ∼1.0 nm over a 10 × 10 μm2 area (Mukhopadhyay et al., 2015) as its active surface. The interface is then formed by repulsion of the floating solvent, which may lead to wrinkling of the metallic leaf. Such wrinkling introduces long-range corrugation of the top electrode interface, an annoying feature that, though, is unlikely to change Aelec by even one order of magnitude. Another effect can be due to the physisorption of the leaf from the solution on the protein surface, viz. trapped pockets of solvents that prevent direct Au-protein contact after drying can lower Aelec (Vilan and Cahen, 2002).
Evaporated Contact
The electrode/protein contact should be less of an issue by using low-temperature metal evaporation, possible with Pb (or Bi), which was shown to work well on an organic molecular monolayer (Lovrinčić et al., 2013). The roughness of the Pb top electrode will be a convolution of the roughness of the protein monolayer, which is ∼2.5 nm (Table 2), and the granularity of the metal. Judging from transmission electron microscopic cross-sectional images (unpublished data) the latter can decrease the contact area by a single-digit factor.
With the Hg drop method (Figure S10 in section A3b), the seminoble metal might be expected to follow the roughness of the protein monolayer, but Hg's high surface tension does not make that possible on a scale of nm-s.
For all these larger top contact area methods, LOFO-Au, Hg, EGaIn, and evap-Pb, the Aelec/Ageo ratio is within 2 orders of magnitude, a range that also reflects that th- and tip-EGaIn have larger macroscopic roughness than the other methods.
Nanoscopic, Pure Metal Junctions
Figure 5 shows averaged I-V curves for the three proteins contacted by Au nanorod technique. It differs in the following two main aspects from all the above-mentioned techniques: (1) it includes no oxide layer compared with GaOx in EGaIn contacts and SiOx for Si substrates and (2) its geometrical contact area is at least ∼ 105 smaller than the above-mentioned configurations (see Table 1). Because of the uncertainty in the exact contact area of nanorod junctions, Figure 5 shows current (in A) rather than current-density (A/cm2). This comparison shows similar currents for PSI and bR proteins and almost 10-fold higher current for ferritin. We could attribute the higher conductance for the iron-loaded ferritin protein cage to the strong electronic coupling between Au-ferritin (iron)-Au configuration.
Temperature-Dependent Transport Measurements
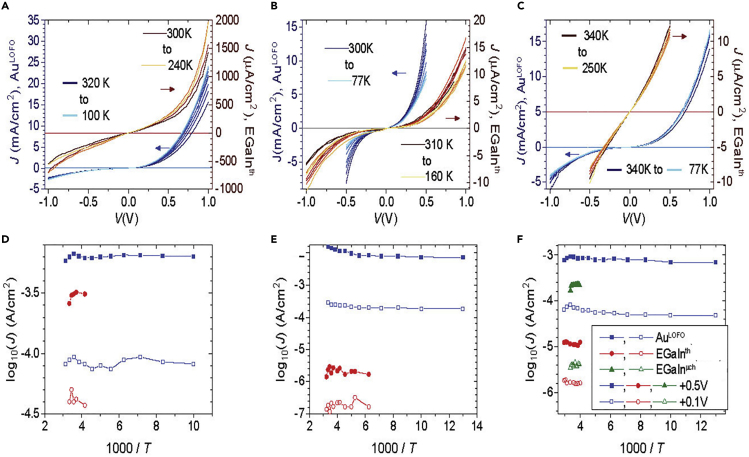
To elucidate the transport mechanism across the proteins for each device configuration, we measured junction current density as a function of temperature (Figure 6). We compare the temperature-dependent current density of protein films with th-EGaIn (reddish traces in panels A–C/red symbols in panels D–F), μch-EGaIn (green symbols in panel F), and LOFO-Au (bluish traces in panels A–C/blue symbols in panels D–F), for the three different proteins (see section A4). The three top panels show that the directly measured current varies only mildly with the applied temperature, much less than the magnitude change induced by the contacts. The latter explains the use of separate y axis in panels (A–C), and it originates in different Aelec to Ageo ratios, as explained in the former section. Here we have the opportunity to investigate whether the activation energy—an extensive parameter that is independent of Aelec and Ageo—depends on the device configuration, as done in the three lower panels of Figure 5. Here, we plot current density values at bias voltages of 0.1 V (Figures 5D–5F, hollow symbols) and 0.5 V (Figures 5D–5F, filled symbols) versus (1,000/T), i.e., an Arrhenius plot.
Figure 6.
Temperature Dependent Transport across Solid-State Protein Junctions
Temperature-dependent J-V characteristics of junctions with (A, D) bR, (B, E) ferritin, and (C, F) PSI, showing the direct J-V response (A–C) and their Arrhenius plots (D–F) for values of J measured at +0.5 V (filled symbols) and +0.1 V (hollow symbols). The plots show two electrode configurations: Si/SiOx-protein//LOFO-Au (bluish traces, left y axis in top panels, blue symbols in bottom panels), TSAu-protein//μch-EGaIn (green symbols in bottom panels), and TSAu-protein//th-EGaIn (reddish traces, right –y axis in top panels, red symbols in bottom panels).
The results show that charge transport across the junctions formed with th-EGaIn top electrodes is temperature independent for all the proteins used in this study (Garg et al., 2018). For the Si/SiOx-linker-ferritin//LOFO-Au junctions, log(J) at +0.5 V decreases from −1.80 to −2.13, which may be due to occluded and surface charges of the Si oxide on the clean Si surface (Garg et al., 2018). Therefore, the most straightforward interpretation of these results is that transport across all the junctions is dominated at all temperatures by a temperature-independent charge transport process; the one mechanism that could fit this behavior is quantum mechanical tunneling. Note that this temperature-independent charge transport behavior does not necessarily imply that all the transport is tunneling, but that quantum mechanical tunneling is the dominant process, the rate-determining (current is a rate) step (Sepunaru et al., 2012).
Numerical Analysis
All the laboratories that tested the different proteins reported, separately, temperature-independent transport for each of the three proteins studied here, and the present comparative studies confirm these findings. Such behavior is consistent with tunneling of the electronic carriers as the most efficient transport mechanism over the temperature range that is studied. Naturally, we are well aware of the fact that the separation between the electrodes, imposed by the widths of the protein films, deposited onto the substrates, as measured by AFM (scratching) and deduced from ellipsometry, is well beyond the maximal electrode separation of ∼4.5 nm to yield measurable tunneling currents across fully conjugated organics (Xie et al., 2015). Reconciling these findings or forwarding different models to explain the temperature independence of solid-state protein junctions is intensively studied these days; we will not enter into this, but refer the reader to recent literature (Bostick et al., 2018, Yuan et al., 2018).
The uncertainty regarding the exact ETp mechanism calls for empirical modeling of the experimental current density (A/cm2)-voltage (V), J-V, curves of the junctions. Such an approach translates the raw J-V curves into a few characteristic parameters that can be compared between different junction configurations. Taylor expansion of junction current density as a function of applied bias is one such empirical approach that was very popular in the early days of tunneling research (Brinkman et al., 1970, Simmons, 1963). In practice, expanding up to the third power is sufficient to describe various molecular junctions (Vilan, 2007, Vilan et al., 2013). In addition, the exponential nature of tunneling J-V relations allows to factorize the Taylor coefficients in the following manner (Vilan, 2007, Vilan et al., 2013):
| (Equation 1) |
where , , and (dimensionless) are empirical fitting parameters, called equilibrium conductance, scaling voltage, and asymmetry factor, respectively. The scaled nature of Equation (1) implies that its fitting parameters are orthogonal to each other and their values are independent. This procedure is demonstrated in Figure 7 for one type of junction (μch-EGaIn) for the three different proteins. The semi-log J-V presentation for μch-EGaIn (Figure 7A) follows a similar trend to that of Au nanorod (Figure 5) and is dominated by the large variation in transmission probability between the proteins. Within our empirical terminology, it implies variation in , where is simply the slope of J versus V close to 0 V (Wold and Frisbie, 2001). Dividing each set of J values by their corresponding value eliminates the orders of magnitude differences in J (without the need to know the value of Aelec) and allows comparing the J-V traces on a linear scale (Figure 7B). This reveals an almost linear response (Ohmic), where the individual protein identity is expressed at a positive voltage (>0.3 V). Equation (1) was fitted to all measured J-V traces (after averaging the traces, for each junction type), and the results are summarized in Table 3 (extended information is given in Table S2 in section B3).
Figure 7.
Analysis of Current-Voltage Response of Different Proteins
Comparison of transport characteristics through TSAu-linker-protein//GaOx/μch-EGaIn junctions with ferritin (red), bR (purple), and PSI (green) as the protein, showing (A) current density versus voltage on a semi-log scale (from Figure 4, where the errors are also shown); (B) normalized current-voltage, where the equilibrium conductance, Geq, is used as the normalization factor (i.e., the slope at V = 0 equals ~1).
Table 3.
Summary of Extracted Parameters for Different Junction Configurations (Rows) with Three Different Proteins (Columns)
| Junction | a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Bottom | Top | bR | PSI | Ferritin | bR | PSI | Ferritin | bR | PSI | Ferritin |
| n-rod | Au | Au | 5,500 | 7,500 | 5.2 × 105 | 130 | 75 | 850 | 0.53 | 0.20 | 0.42 |
| LOFO | p++−Si | Au | 0.1 | 0.65 | 0.83 | 0.076 | 0.49 | 0.34 | 0.07 | 0.14 | 0.14 |
| Hg | p++−Si | Hg | 0.03 | 0.03 | 0.27 | 0.036 | 0.073 | 0.31 | 0.07 | 0.10 | 0.21 |
| μch | TSAu | EGaIn | 0.45 | 0.15 | 0.97 | 0.75 | 0.45 | 0.96 | 0.35 | 0.27 | 0.35 |
| Th | TSAu | EGaIn | 1.3 | 0.02 | 0.001 | 1.3 | 0.14 | 0.023 | 0.34 | 0.24 | 0.24 |
| tipb | TSAu | EGaIn | 0.0005 | 0.002 | 0.001 | 0.024 | 0.04 | 0.018 | 0.34 | 0.20 | 0.19 |
| 0.003 | 0.049 | 0.34 | |||||||||
was computed using Eq. 3b from and and number of molecules, with protein's footprint of 80, 140, and 60 nm2 for bR, PS-I, and ferritin, respectively. These values are based on protein's diameter and include a factor of 2 for circular surface filling; the bR is further multiplied by 2 to account for surrounding OTG matrix.
tip-EGaIn shows two values for Ferritin: the upper one was measured by UoG (as were the other two proteins) and the lower one by WIS.
The second parameter V0 was extracted by fitting the J-V curve to Equation (1); to comply with the near-zero expansion nature of Equation (1) the fitting procedure gave larger weight to the low-signal range (normally the fitting range was limited to: |V| < 0.7 V) as further explained in Section B3a of the Supplemental Information. The asymmetry parameter, S, was also extracted, but approached zero (ideal, symmetric) in most cases (see Table S2), and therefore will not be discussed further.
In a purist approach, the empirical parameters (Geq, V0) can be compared across the different junctions directly, as shown on Table 3. As this approach is a bit abstract, we have translated these empirical observables into specific ETp process parameters. For this purpose, we have chosen the well-accepted single-level Landauer model, where a realistic transmission function is approximated by a single, Lorentzian-shaped peak representing only the nearest molecular level (Bâldea, 2012):
| (Equation 2) |
Equation 2 is a simplified version of Landauer model under the assumption of Γ≪|ε0 | and |V|≤|ε0 |.
Here, is the energy of the transmission peak and represents the effective energy difference between the electrode Fermi level and the closest molecular level at zero applied bias; , where are the level broadenings by the molecule-electrode couplings at the two (i. j) electrode/biomolecule interfaces (including organic linker molecules, where applicable); and is a dimensionless parameter for the deviation from the symmetric partition of the applied voltage between the two contacts, ranging between for symmetric voltage distribution. Considering that Equation (1) is a modified Taylor expansion, its coefficients are derived from ; this allows a direct translation (or mapping) of the empirical coefficients (Equation (1)) into Landauer-tunneling ones (Equation (2)):
| (Equation 3a) |
and
| (Equation 3b) |
, , and are the parameters extracted from the cubic fit (Equation 1); is the quantum of conductance; and N (molecules/cm2) is the number of molecules per unit area (as Equation 2 refers to current density; if the fit is to direct current, N would be the absolute number of molecules in the junction). Thus, N essentially reflects the effective electrical contact area (Aelec = N × molecular footprint). As argued earlier, the uncertainty in N (Aelec) can be orders of magnitude, which explains why Equation 3b yields only effective values of ; this restriction is stressed by changing its subscript to effective (). Table 3 gives the values of the equilibrium conductance, ; its translation into effective coupling energy, (using Equation 3b); and the effective energy barrier (Equation 3a).
The most surprising aspect of Table 3 is its low values, both in terms of coupling () and energy barrier . The energy barrier was in general less than 0.5 eV (which is confirmed by an alternative extraction method of transition voltage spectroscopy (Vilan et al., 2013) (see Section B3b and Table S2). Technically, these low values contradict the model of Equation (2), because the model predicts sharp current onsets (conductance resonances) at , and none are observed, although the voltage range is sufficiently large. This suggests that the potential drop across the junction and its variation with the applied external voltage are different than what is assumed in the simplified model (Equation 2).
The physical intuition suggests that low-lying energy levels (close to the contact's Fermi level) will have strong coupling to the metal's density of states, namely large values. Instead, most values are extremely low (0.02–1 μeV), way below what is commonly assumed in the field (). Note, though, that the oxide-free junction (those with Au nanorods) is much closer to this range () and also has a much larger Geq than the other junctions. The larger value of Geq is likely also because a smaller Ageo increases the Aelec/Ageo ratio (Akkerman and de Boer, 2007).
The very small values for all junctions except the nanorod ones are likely an artifact because their electric contact area is far smaller than their geometric one. In such a case the number of molecules, N, that participates in the transport is much smaller than the nominal one. Therefore, the apparent values of , in Table 3 (and in Table S1), should be considered as the lower limit to . Still, considering that (Equation 3b) and assuming a realistic , of few meV, implies a ratio of Aelec over Ageo in the order of 10−3 to 10−6, supporting our understanding that a very small fraction of the molecules participates in the transport.
Thus, the far higher values for nanorod Au-linker-protein//Au junctions can be attributed to the combined effect of a much higher Aelec/Ageo ratio and better coupling between the protein and electrode energy state (oxide free). A higher Aelec/Ageo ratio can be understood as follows: the estimation of the nanorod contact area assumes a strip only 10 nm wide; this value is based on geometrical consideration of the distance where a cylinder of 200 nm diameter retracts 1 Å in distance. This width is already at the dimension of a single protein, and therefore in the case of rigid nanorods the effective electrical contacts approaches the nominal one.
In addition to geometrical considerations, chemical and physical details may also influence the coupling. First, nanogold is characterized by strong gold plasmon interactions with the molecular levels (Du et al., 2016, Wu et al., 2016). In addition, this is the only junction in which both contacts are purely metallic without an oxide buffer (GaOx for EGaIn junctions and SiOx for Si junctions). Although it can (and has been) argued that these oxides are sufficiently thin so that electrons can tunnel through them efficiently, the oxides do decrease the electronic coupling between the metal that they cover and the proteins and therefore even a thin, poor-quality oxide will reduce considerably.
Table 3 also shows very clearly a factor of ∼102–103 between Geq of μch-EGaIn and tip-EGaIn, an effect that was discussed earlier and is attributed to the much rougher surface, and its effect on Ageo and Aelec/Ageo of tip-EGaIn compared with μch-EGaIn. Obviously, the values, derived for tip-EGaIn junctions, are severely underestimated.
Table 3 confirms the conductance trend of ferritin ≫ PSI > bR (as is qualitatively observed in Figures 5 and 7) for few types of contacts (nanorod, μch, Hg), but there are many exceptions. We note that reduced contact area cannot explain such trend crossing. We generally ascribe these variations to a combination of factors, including rigidity of the protein layer and differences in the linker chemistry (electrostatic versus covalent binding) for different contact types.
The scaling voltage V0 and its translation into an effective energy, are higher for bR-based junctions than for those with the other two proteins (namely bR's J-V response is more linear) with the exception of the ferritin junctions with Si/SiOx electrodes. Uncertainties (error bar) in V0 are large, and V0 values are rather sensitive to the voltage range used to extract them. Interestingly, all three proteins show reasonable to good reproducibility in despite a wide distribution of values of . We note that also reflect interface effects and as such do not reflect protein-only parameters, explaining why we refer to their values as strictly effective ones. Given the spread of values between different contacts configurations for likely both are dominated by the electrical properties of the contact-protein interface rather than the body of the protein, whereas seems robust, indicating that the energy barriers are less affected (the small values for junctions with Si-based contacts can be contributed to electrostatic barriers; cf. Garg et al., 2018). This agrees with the evolving notion of highly efficient charge propagation along the protein and points to the degree of interfacial electronic coupling as a key player in dictating both the conductance magnitude and its voltage sensitivity.
Conclusion
Comparing charge transport characteristics of (bio)molecular junctions formed with different junction configurations and/or in different laboratories, we find differences of up to three orders of magnitude in geometric area-based (Ageo varies in the range of 105–10−3 μm2) nominal current densities between different junction geometries for the same protein. The variation in current densities is likely due to differences in the actual contacts, i.e., real electrical contact area, compared with geometric one (which often is difficult to define), and in electronic contact-protein coupling. Still, current densities across all different protein-based molecular junctions are temperature independent, which suggests tunneling as the dominant transport process. The efficiency with which the protein is electronically coupled to the electrodes likely varies between contact materials, including their roughness, ways the contact with nominally the same material is made (e.g., nature of GaOx, cleanliness of Au), the linker used to immobilize the protein, and the orientation of the protein (a polyelectrolyte) on the contact material; all these factors can affect what is termed in electrical engineering, the contact resistance (Fereiro et al., 2018). Overall, our observations lead to the conclusion that for devices with , the ratios between the “electrical” to measured “geometrical” contact area were relatively uniform, as shown by the small (and in terms of the order of magnitude insignificant) differences between measured current densities for junctions prepared by different fabrication methods in different laboratories. Our conclusions are based on the first set of data from different molecular bioelectronic contacting configurations, which also is an unprecedented set of molecular electronic data of biomolecular tunnel junctions. In terms of temperature dependence, the results match quite well, and as such, studies that use this tool to learn about transport mechanisms, as well as studies that do not require absolute values for current densities, are transferable from one laboratory to the other. Likely, also length-dependent measurements, wherever possible without subjecting the proteins to tensile or compressive stress, can yield robust results (e.g., of the so-called length decay, β parameter). On the downside, it is hard to compare results using absolute current values as obtained with different junction types and geometries, which calls into question the concept of “conductivity” of a given protein, which often pervades the field. Instead, there is likely a junction conductivity, derived with specific assumptions for the junction conductance, which requires specifying the way the proteins are contacted.
Limitations of the Study
Ill-defined micro-structure of the interface between the proteins and the top-contact is a major limitation of solid-state molecular junctions in general. In addition, the shape of the current-voltage response remains close to linear even at relatively high applied voltage, which hinders our ability to identify clear differences in the electronic response of different proteins. Reconciling the efficient long-distance charge transport with lack of temperature activation, is yet challenging.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sabyasachi Mukhopadhyay (sabyasachi.m@srmap.edu.in).
Materials Availability
This study did not generate new unique reagents, however proteins used in this study are available from the Lead Contact without restriction.
Data and Code Availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplemental Information. Additional data related to this paper may be requested from S.M. (sabyasachi.m@srmap.edu.in). Code for NDC analysis and data fitting may be requested from A.V. (ayelet.vilan@weizmann.ac.il).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
S.M. thanks SERB-DST, Govt. of India (award No. ECR/2017/001937) research grants, and the Council for Higher Education (Israel) for a postdoctoral research fellowship at the initial stage of this work. J.F. thanks the Azrieli Foundation for a PD fellowship; C.G. acknowledges a Dean's PD fellowship. At WIS, we thank Dr. Noga Friedman for bR samples, the Minerva Foundation (Munich) and the Israel Science Foundation for partial support. NUS research groups acknowledge the Ministry of Education (MOE) for supporting this research under award No. MOE2015-T2-2-134. Prime Minister’s Office, Singapore, under its medium-sized centre program, is also acknowledged for supporting this research. At Groningen the Zernike Institute of Advanced Materials is gratefully acknowledged for financial support. R.M. thanks the Zernike Institute of Advanced Materials; R.C. and V.M. thank the University of Alberta & Alberta Innovates, and R.C. and A.B. thank the National Research Council Canada for financial support.
Author Contributions
Conceptualization, A.V., C.A.N., and D.C.; Methodology, S.M., S.K.K., C.G., and A.V.; Investigation, S.M., S.K.K., C.G., J.A.F., A.B., V.M., X.Q., O.E.C.O., X.C., R.R.P., and S.L.; Writing – Original Draft, S.M., S.K.K., and A.V.; Writing –Review & Editing, S.M., S.K.K., A.V., R.C.C., C.G., R.M., C.A.N., and D.C.; Funding Acquisition, S.M., R.C.C., R.M., M.S., C.A.N., and D.C.; Resources, R.C.C., R.M., I.P., M.S., R.R.P., C.A.N., A.V., and D.C.; Supervision, A.B., R.C.C., R.M., I.P., M.S., C.A.N., A.V., and D.C.
Declaration of Interests
The authors declare no competing interests.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101099.
Contributor Information
Sabyasachi Mukhopadhyay, Email: sabyasachi.m@srmap.edu.in.
Christian A. Nijhuis, Email: chmnca@nus.edu.sg.
Ayelet Vilan, Email: ayelet.vilan@weizmann.ac.il.
David Cahen, Email: david.cahen@weizmann.ac.il.
Supplemental Information
References
- Akkerman H.B., de Boer B. Electrical conduction through single molecules and self-assembled monolayers. J. Phys. Condens. Matter. 2007;20:013001. [Google Scholar]
- Amdursky N., Marchak D., Sepunaru L., Pecht I., Sheves M., Cahen D. Electronic transport via proteins. Adv. Mater. 2014;26:7142–7161. doi: 10.1002/adma.201402304. [DOI] [PubMed] [Google Scholar]
- Amdursky N., Ferber D., Bortolotti C.A., Dolgikh D.A., Chertkova R.V., Pecht I., Sheves M., Cahen D. Solid-state electron transport via cytochrome c depends on electronic coupling to electrodes and across the protein. Proc. Natl. Acad. Sci. U S A. 2014;111:5556–5561. doi: 10.1073/pnas.1319351111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bâldea I. Ambipolar transition voltage spectroscopy: Analytical results and experimental agreement. Phys. Rev. B. 2012;85:035442. [Google Scholar]
- Bâldea I. Pan Stanford Publishing Pte Ltd.; 2018. Molecular Electronics - an Experimental and Theoretical Approach. [Google Scholar]
- Bostick C.D., Mukhopadhyay S., Pecht I., Sheves M., Cahen D., Lederman D. Protein bioelectronics: a review of what we do and do not know. Rep. Prog. Phys. 2018;81:026601. doi: 10.1088/1361-6633/aa85f2. [DOI] [PubMed] [Google Scholar]
- Boussaad S., Tao N.J. Electron transfer and adsorption of myoglobin on self-assembled surfactant films: an electrochemical tapping-mode AFM study. J. Am. Chem. Soc. 1999;121:4510–4515. [Google Scholar]
- Brinkman W.F., Dynes R.C., Rowell J.M. Tunneling conductance of asymmetrical barriers. J. Appl. Phys. 1970;41:1915–1921. [Google Scholar]
- Cademartiri L., Thuo M.M., Nijhuis C.A., Reus W.F., Tricard S., Barber J.R., Sodhi R.N.S., Brodersen P., Kim C., Chiechi R.C. Electrical resistance of AgTS–S(CH2)n−1CH3//Ga2O3/EGaIn tunneling junctions. J. Phys. Chem. C. 2012;116:10848–10860. [Google Scholar]
- Castañeda Ocampo O.E., Gordiichuk P., Catarci S., Gautier D.A., Herrmann A., Chiechi R.C. Mechanism of orientation-dependent asymmetric charge transport in tunneling junctions comprising photosystem I. J. Am. Chem. Soc. 2015;137:8419–8427. doi: 10.1021/jacs.5b01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Hu H., Trasobares J., Nijhuis C.A. Rectification ratio and tunneling decay coefficient depend on the contact geometry revealed by in situ imaging of the formation of EGaIn junctions. ACS Appl. Mater. Interfaces. 2019;11:21018–21029. doi: 10.1021/acsami.9b02033. [DOI] [PubMed] [Google Scholar]
- Chiechi R.C., Weiss E.A., Dickey M.D., Whitesides G.M. Eutectic gallium–indium (EGaIn): a moldable liquid metal for electrical characterization of self-assembled monolayers. Angew. Chem. Int. Ed. 2008;47:142–144. doi: 10.1002/anie.200703642. [DOI] [PubMed] [Google Scholar]
- Dickey M.D. Stretchable and soft electronics using liquid metals. Adv. Mater. 2017;29:1606425. doi: 10.1002/adma.201606425. [DOI] [PubMed] [Google Scholar]
- Du W., Wang T., Chu H.-S., Wu L., Liu R., Sun S., Phua W.K., Wang L., Tomczak N., Nijhuis C.A. On-chip molecular electronic plasmon sources based on self-assembled monolayer tunnel junctions. Nat. Photon. 2016;10:274. [Google Scholar]
- Fereiro J.A., Porat G., Bendikov T., Pecht I., Sheves M., Cahen D. Protein electronics: chemical modulation of contacts control energy level alignment in gold-azurin-gold junctions. J. Am. Chem. Soc. 2018;140:13317–13326. doi: 10.1021/jacs.8b07742. [DOI] [PubMed] [Google Scholar]
- Gaigalas A.K., Niaura G. Measurement of electron transfer rates between adsorbed azurin and a gold electrode modified with a hexanethiol layer. J. Colloid Interface Sci. 1997;193:60–70. doi: 10.1006/jcis.1997.5034. [DOI] [PubMed] [Google Scholar]
- Garg K., Raichlin S., Bendikov T., Pecht I., Sheves M., Cahen D. Interface electrostatics dictates the electron transport via bioelectronic junctions. ACS Appl. Mater. Interfaces. 2018;10:41599–41607. doi: 10.1021/acsami.8b16312. [DOI] [PubMed] [Google Scholar]
- Guo C., Yu X., Refaely-Abramson S., Sepunaru L., Bendikov T., Pecht I., Kronik L., Vilan A., Sheves M., Cahen D. Tuning electronic transport via hepta-alanine peptides junction by tryptophan doping. Proc. Natl. Acad. Sci. U S A. 2016;113:10785–10790. doi: 10.1073/pnas.1606779113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haick H., Cahen D. Contacting organic molecules by soft methods: towards molecule-based electronic devices. Acc. Chem. Res. 2008;41:359–366. doi: 10.1021/ar700099n. [DOI] [PubMed] [Google Scholar]
- Holm, R. n.d., Electric Contacts: Theory and Application (Springer-Verlag Berlin Heidelberg).
- Jiang L., Sangeeth C.S.S., Wan A., Vilan A., Nijhuis C.A. Defect scaling with contact area in EGaIn-based junctions: impact on quality, joule heating, and apparent injection current. J. Phys. Chem. C. 2015;119:960–969. [Google Scholar]
- Jin Y., Friedman N., Sheves M., He T., Cahen D. Bacteriorhodopsin (bR) as an electronic conduction medium: current transport through bR-containing monolayers. Proc. Natl. Acad. Sci. U S A. 2006;103:8601–8606. doi: 10.1073/pnas.0511234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Honig T., Ron I., Friedman N., Sheves M., Cahen D. Bacteriorhodopsin as an electronic conduction medium for biomolecular electronics. Chem. Soc. Rev. 2008;37:2422–2432. doi: 10.1039/b806298f. [DOI] [PubMed] [Google Scholar]
- Kumar K.S., Pasula R.R., Lim S., Nijhuis C.A. Long-range tunneling processes across ferritin-based junctions. Adv. Mater. 2016;28:1824–1830. doi: 10.1002/adma.201504402. [DOI] [PubMed] [Google Scholar]
- Kumar K.S., Troadec C., Vilan A., Nijhuis C.A. Ultra-smooth photoresist-free micropore-based EGaIn molecular junctions: fabrication and how roughness determines voltage response. Adv. Funct. Mater. 2019;29:1904452. [Google Scholar]
- Lovrinčić R., Kraynis O., Har-Lavan R., Haj-Yahya A., Li W., Vilan A., Cahen D. A new route to nondestructive top-contacts for molecular electronics on Si: Pb evaporated on organic monolayers. J. Phys. Chem. Lett. 2013;4:426–430. doi: 10.1021/jz302153z. [DOI] [PubMed] [Google Scholar]
- McCreery R.L., Yan H., Bergren A.J. A critical perspective on molecular electronic junctions: there is plenty of room in the middle. Phys. Chem. Chem. Phys. 2013;15:1065–1081. doi: 10.1039/c2cp43516k. [DOI] [PubMed] [Google Scholar]
- Moulton S.E., Barisci J.N., Bath A., Stella R., Wallace G.G. Investigation of protein adsorption and electrochemical behavior at a gold electrode. J. Colloid Interface Sci. 2003;261:312–319. doi: 10.1016/S0021-9797(03)00073-0. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S., Dutta S., Pecht I., Sheves M., Cahen D. Conjugated cofactor enables efficient temperature-independent electronic transport across ∼6 nm long halorhodopsin. J. Am. Chem. Soc. 2015;137:11226–11229. doi: 10.1021/jacs.5b06501. [DOI] [PubMed] [Google Scholar]
- Nijhuis C.A., Reus W.F., Barber J.R., Dickey M.D., Whitesides G.M. Charge transport and rectification in arrays of SAM-based tunneling junctions. Nano Lett. 2010;10:3611–3619. doi: 10.1021/nl101918m. [DOI] [PubMed] [Google Scholar]
- Nijhuis C.A., Reus W.F., Whitesides G.M. Mechanism of rectification in tunneling junctions based on molecules with asymmetric potential drops. J. Am. Chem. Soc. 2010;132:18386–18401. doi: 10.1021/ja108311j. [DOI] [PubMed] [Google Scholar]
- Nijhuis C.A., Reus W.F., Barber J.R., Whitesides G.M. Comparison of SAM-based junctions with Ga2O3/EGaIn top electrodes to other large-area tunneling junctions. J. Phys. Chem. C. 2012;116:14139–14150. [Google Scholar]
- Ratner M. A brief history of molecular electronics. Nat. Nano. 2013;8:378–381. doi: 10.1038/nnano.2013.110. [DOI] [PubMed] [Google Scholar]
- Regan M.J., Tostmann H., Pershan P.S., Magnussen O.M., DiMasi E., Ocko B.M., Deutsch M. X-ray study of the oxidation of liquid-gallium surfaces. Phys. Rev. B. 1997;55:10786–10790. [Google Scholar]
- Reus W.F., Nijhuis C.A., Barber J.R., Thuo M.M., Tricard S., Whitesides G.M. Statistical tools for analyzing measurements of charge transport. J. Phys. Chem. C. 2012;116:6714–6733. [Google Scholar]
- Ron I., Pecht I., Sheves M., Cahen D. Proteins as solid-state electronic conductors. Acc. Chem. Res. 2010;43:945–953. doi: 10.1021/ar900161u. [DOI] [PubMed] [Google Scholar]
- Rothemund P., Morris Bowers C., Suo Z., Whitesides G.M. Influence of the contact area on the current density across molecular tunneling junctions measured with EGaIn top-electrodes. Chem. Mater. 2018;30:129–137. [Google Scholar]
- Salomon A., Cahen D., Lindsay S., Tomfohr J., Engelkes V.B., Frisbie C.D. Comparison of electronic transport measurements on organic molecules. Adv. Mater. 2003;15:1881–1890. [Google Scholar]
- Sana B., Johnson E., Sheah K., Pho C.L., Lim S. Biointerphases. 2010;5:48. doi: 10.1116/1.3483216. [DOI] [PubMed] [Google Scholar]
- Sangeeth C.S.S., Wan A., Nijhuis C.A. Equivalent circuits of a self-assembled monolayer-based tunnel junction determined by impedance spectroscopy. J. Am. Chem. Soc. 2014;136:11134–11144. doi: 10.1021/ja505420c. [DOI] [PubMed] [Google Scholar]
- Sangeeth C.S.S., Demissie A.T., Yuan L., Wang T., Frisbie C.D., Nijhuis C.A. Comparison of DC and AC transport in 1.5–7.5 nm oligophenylene imine molecular wires across two junction platforms: eutectic Ga–In versus conducting probe atomic force microscope junctions. J. Am. Chem. Soc. 2016;138:7305–7314. doi: 10.1021/jacs.6b02039. [DOI] [PubMed] [Google Scholar]
- Sayed S.Y., Fereiro J.A., Yan H., McCreery R.L., Bergren A.J. Charge transport in molecular electronic junctions: Compression of the molecular tunnel barrier in the strong coupling regime. Proc. Natl. Acad. Sci. U S A. 2012;109:11498–11503. doi: 10.1073/pnas.1201557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder B., Kotz R., Alliata D., Facci P. Comparison of the self-chemisorption of azurin on gold and on functionalized oxide surfaces. Surf. Interf. Anal. 2002;34:40–44. [Google Scholar]
- Seitz O., Böcking T., Salomon A., Gooding J.J., Cahen D. Importance of monolayer quality for interpreting current transport through organic molecules: alkyls on oxide-free Si. Langmuir. 2006;22:6915–6922. doi: 10.1021/la060718d. [DOI] [PubMed] [Google Scholar]
- Sepunaru L., Friedman N., Pecht I., Sheves M., Cahen D. Temperature-dependent solid-state electron transport through bacteriorhodopsin: experimental evidence for multiple transport paths through proteins. J. Am. Chem. Soc. 2012;134:4169–4176. doi: 10.1021/ja2097139. [DOI] [PubMed] [Google Scholar]
- Simeone F.C., Yoon H.J., Thuo M.M., Barber J.R., Smith B., Whitesides G.M. Defining the value of injection current and effective electrical contact area for EGaIn-based molecular tunneling junctions. J. Am. Chem. Soc. 2013;135:18131–18144. doi: 10.1021/ja408652h. [DOI] [PubMed] [Google Scholar]
- Simmons J.G. Low-voltage current-voltage relationship of tunnel junctions. J. Appl. Phys. 1963;34:238–239. [Google Scholar]
- Singhal G.S., Renger G., Sopory S.K., Irrgang K.D., Govindjee R. Springer Netherlands; 1999. Concepts in Photobiology: Photosynthesis and Photomorphogenesis. [Google Scholar]
- Tao N.J. Electron transport in molecular junctions. Nat. Nano. 2006;1:173–181. doi: 10.1038/nnano.2006.130. [DOI] [PubMed] [Google Scholar]
- Timsit R.S. The true area of contact at a liquid metal-solid interface. Appl. Phys. Lett. 1982;40:379–381. [Google Scholar]
- Vilan A. Analyzing molecular current-voltage characteristics with the simmons tunneling model: scaling and linearization. J. Phys. Chem. C. 2007;111:4431–4444. [Google Scholar]
- Vilan A. Revealing tunnelling details by normalized differential conductance analysis of transport across molecular junctions. Phys. Chem. Chem. Phys. 2017;19:27166–27172. doi: 10.1039/c7cp05536f. [DOI] [PubMed] [Google Scholar]
- Vilan A., Cahen D. Soft contact deposition onto molecularly modified GaAs. thin metal film flotation: principles and electrical effects. Adv. Funct. Mater. 2002;12:795–807. [Google Scholar]
- Vilan A., Cahen D., Kraisler E. Rethinking transition voltage spectroscopy within a generic taylor expansion view. ACS Nano. 2013;7:695–706. doi: 10.1021/nn3049686. [DOI] [PubMed] [Google Scholar]
- Vilan A., Aswal D., Cahen D. Large-area, ensemble molecular electronics: motivation and challenges. Chem. Rev. 2017;117:4248–4286. doi: 10.1021/acs.chemrev.6b00595. [DOI] [PubMed] [Google Scholar]
- Waleed Shinwari M., Jamal Deen M., Starikov E.B., Cuniberti G. Electrical conductance in biological molecules. Adv. Funct. Mater. 2010;20:1865–1883. [Google Scholar]
- Wan A., Jiang L., Sangeeth C.S.S., Nijhuis C.A. Reversible soft top-contacts to yield molecular junctions with precise and reproducible electrical characteristics. Adv. Funct. Mater. 2014;24:4442–4456. [Google Scholar]
- Wold D.J., Frisbie C.D. Fabrication and characterization of metal−molecule−metal junctions by conducting probe atomic force microscopy. J. Am. Chem. Soc. 2001;123:5549–5556. doi: 10.1021/ja0101532. [DOI] [PubMed] [Google Scholar]
- Wu L., Tan S.F., Bosman M., Yang J.K.W., Nijhuis C.A., Bai P. Charge transfer plasmon resonances across silver–molecule–silver junctions: estimating the terahertz conductance of molecules at near-infrared frequencies. RSC Adv. 2016;6:70884–70894. [Google Scholar]
- Xie Z., Bâldea I., Smith C.E., Wu Y., Frisbie C.D. Experimental and theoretical analysis of nanotransport in oligophenylene dithiol junctions as a function of molecular length and contact work function. ACS Nano. 2015;9:8022–8036. doi: 10.1021/acsnano.5b01629. [DOI] [PubMed] [Google Scholar]
- Yan H., Bergren A.J., McCreery R.L. All-carbon molecular tunnel junctions. J. Am. Chem. Soc. 2011;133:19168–19177. doi: 10.1021/ja206619a. [DOI] [PubMed] [Google Scholar]
- Yu X., Lovrinčić R., Kraynis O., Man G., Ely T., Zohar A., Toledano T., Cahen D., Vilan A. Fabrication of reproducible, integration-compatible hybrid molecular/si electronics. Small. 2014;10:5151–5160. doi: 10.1002/smll.201400484. [DOI] [PubMed] [Google Scholar]
- Yu X., Lovrincic R., Sepunaru L., Li W., Vilan A., Pecht I., Sheves M., Cahen D. Insights into solid-state electron transport through proteins from inelastic tunneling spectroscopy: the case of azurin. ACS Nano. 2015;9:9955–9963. doi: 10.1021/acsnano.5b03950. [DOI] [PubMed] [Google Scholar]
- Yuan L., Wang L., Garrigues A.R., Jiang L., Annadata H.V., Anguera Antonana M., Barco E., Nijhuis C.A. Transition from direct to inverted charge transport Marcus regions in molecular junctions via molecular orbital gating. Nat. Nanotechnol. 2018;13:322–329. doi: 10.1038/s41565-018-0068-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplemental Information. Additional data related to this paper may be requested from S.M. (sabyasachi.m@srmap.edu.in). Code for NDC analysis and data fitting may be requested from A.V. (ayelet.vilan@weizmann.ac.il).