Abstract
An electrophysiological technique that can record nerve impulses from a single nerve fiber is indispensable for studying modality-specific sensory receptors such as low threshold mechanoreceptors, thermal receptors, and nociceptors. The teased-fiber single-unit recording technique has long been used to resolve impulses that are likely to be from a single nerve fiber. The teased-fiber single-unit recording technique involves tedious nerve separation procedures, causes nerve fiber impairment, and is not a true single-fiber recording method. In the present study, we describe a new and true single-fiber recording technique, the pressure-clamped single-fiber recording method. We have applied this recording technique to mouse whisker hair follicle preparations with attached whisker afferents as well as to skin-nerve preparations made from mouse hindpaw skin and saphenous nerves. This new approach can record impulses from rapidly adapting mechanoreceptors (RA), slowly adapting type 1 mechanoreceptors (SA1), and slowly adapting type 2 mechanoreceptors (SA2) in these tissue preparations. We have also applied the pressure-clamped single-fiber recordings to record impulses on Aβ-fibers, Aδ-fibers, and C-fibers. The pressure-clamped single-fiber recording technique provides a new tool for sensory physiology and pain research.
Keywords: Mechanoreceptors, nociceptors, touch, pain, whisker hair follicles, impulses, skin nerve preparations
Introduction
Recordings of afferent nerve impulses are useful in studying sensory physiology and pain. Conventional extracellular recordings such as those using suction electrodes or with a pair of metal wires allow one to record compound action potentials propagated on an afferent nerve bundle. However, these recording techniques are not well suited for studying modality-specific sensory receptors such as mechanical receptors, thermal receptors, and nociceptors. This is because compound action potentials are impulses from many different types rather than a specific type of afferent nerve fibers. To solve this issue, researchers have developed several types of single-unit recording methods.1 For example, a sharp electrode can be inserted into a neuron of a sensory ganglion from which intracellular single-unit recordings are made.2 However, the probability is low to insert a recording electrode into the sensory neuron that innervates a receptive field of interest such as a particular area of the skin. The most widely used single-unit recording approach is the teased-fiber single-unit recording technique.1,3,4 In this recording method, a nerve trunk is carefully isolated, severed, and successively split or teased into fine filaments that contain a few nerve fibers. The teased-nerve fibers are then placed over a recording electrode made by a pair of platinum or silver wires. This recording approach has provided an important tool to study different sensory receptors and their physiological and pathophysiological functions including pain.3–6
Although the teased-fiber single-unit recording technique has continued to be a main method to study sensory receptors and their roles in pain,3,4,6,7 the technique suffers from a number of disadvantages. First, many nerve fibers in the nerve trunk were severely injured during mechanical separation procedures of preparing teased fibers.1 As such, only a few nerves can be recorded in each experiment, and these nerves may not fully represent those that innervate the receptive field of interest. Furthermore, in the teased-fiber single-unit recordings, the injured nerve fibers may have altered electrophysiological properties. Second, the procedures for preparing teased fibers are very delicate, tedious, and time consuming. In addition, spike discrimination and sorting need to be performed to analyze spikes in order to differentiate between single units and multiple units in a train of spikes recorded by this technique.1 Third, teased-fiber single-unit recording is not a true single-fiber recording method because there are still a number of fibers in each teased nerve. This compromises the precision in determining functional properties of a specific type of sensory receptors of interest. To avoid the technical weaknesses of the teased-fiber single-unit recording technique, we have developed the pressure-clamped single-fiber recording technique, a simple and reliable method to record impulses on individual nerve fibers in a nonteased nerve bundle. We have applied this new approach to successfully record impulses following the activation of mechanoreceptors in whisker hair follicles and in skin-nerve preparations of mice. The technique allowed us to record impulses conveyed by Aβ-, Aδ-, and C-afferent nerve fibers of mice.
Materials and methods
Whisker hair follicle and skin nerve preparations
Male C57BL/6 mice aged 8–10 weeks were used. Animal care and use conformed to National Institutes of Health guidelines for care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Whisker hair follicle preparations were made based on the method of our previous studies.8–10 In brief, mice were anesthetized with 5% isoflurane and then sacrificed by decapitation. Whisker pads were dissected out and placed in a 35-mm Petri dish that contained 5 ml ice-cold L-15 medium. Each whisker hair follicle together with its afferent bundle and hair shaft was then gently pulled out. The capsule of each whisker hair follicle was cut open to two small holes with one hole at the enlargement part and the other hole at the end of the capsule to facilitate solution exchange. The whisker hair follicle preparations were affixed in a recording chamber by a tissue anchor and submerged in a Krebs bath solution, and the recording chamber was then mounted on the stage of an Olympus BX50WI microscope and perfused with the Krebs bath solution at the flow rate of 2 ml/min. The Krebs solution contained (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose (pH 7.3 and osmolarity 325 mOsm) and was saturated with 95% O2 and 5% CO2. Unless otherwise indicated, the Krebs bath solution was maintained at a room temperature of 24°C.
Skin-nerve preparations were made based on previous studies with modifications.6,11 In brief, mice were euthanized by overdose of isoflurane followed by decapitation. Hairy skin of the hindlimb was shaved and then dissected out together with the saphenous nerve. The skin and its attached saphenous nerve were placed in a recording chamber that contained the aforementioned Krebs bath solution, and fat and connective tissues in the skin were carefully removed. The skin was then affixed by tissue pins and the saphenous nerve affixed by a tissue anchor in the recording chamber. The recording chamber was mounted on the stage of the Olympus BX50WI upright microscope. The skin-nerve preparation was continuously perfused by the Krebs bath solution at the room temperature of 24°C
The pressure-clamped single-fiber recording technique
For pressure-clamped single-fiber recordings made from afferent nerves innervating whisker hair follicles, whisker hair follicle preparations on the stage of the microscope were exposed to a mixture of 0.05% dispase II plus 0.05% collagenase for 3–5 min. The enzymes were then washed off with the continuous perfusion of Krebs bath solution. Recording electrodes were made by thin-walled borosilicate glass tubing without filament (inner diameter 1.12 mm, outer diameter 1.5 mm). They were fabricated using a P-97 Flaming/Brown Micropipette Puller and fire polished to make tip diameter at 3 to 6 µm. The recording electrode was filled with the Krebs bath solution, mounted onto an electrode holder which was connected to a high-speed pressure-clamp device (ALA Scientific Instruments, Farmingdale, NY). Under a 40× objective, individual fibers in the cutting end of the whisker afferent nerve bundle were separated by a positive pressure of approximately +10 mmHg delivered from the recording electrode. The end part of a single nerve fiber was then aspirated into the recording electrode by a negative pressure at approximately −10 mmHg. Once the nerve end reached approximately 10 µm in length within the recording electrode, the pressure in the recording electrode was readjusted to −5 to −1 mmHg and maintained throughout the experiment. Nerve impulses were recorded using a Multiclamp 700 A amplifier and signals sampled at 20 KHz with low pass filter set at 1 KHz. Unless otherwise indicated, all experiments were performed at 24°C.
For pressure-clamped single-fiber recordings made from saphenous nerves innervating the skin of the hindpaw, the aforementioned recording procedures were applied to the cutting end of the saphenous nerve. In some experiments, a small segment of the saphenous nerve was aspirated into a suction stimulation electrode. The suction stimulation electrode had a funnelform tip to help to aspirate nerve segment into the stimulation electrode. The stimulation site was approximately 15 mm away from the recording electrode. The stimulation electrode was used to deliver square pulse stimuli each at the duration of 50 µs to evoke nerve impulses. This allowed us to measure conduction velocity of each nerve fiber recorded so that a recorded nerve fiber could be categorized based on its conduction velocity.
In a different set of experiments, the pressure-clamped single-fiber recordings were applied to isolated sciatic nerves without attached skin. In these experiments, animals were euthanized, the skin was cut open at the gluteal area, and the sciatic nerve trunk was dissected out. The sciatic nerve trunk was from proximal site at sacral foramen level to distal site at the ankle level, which was in the length of approximately 30 mm. The distal end of the nerve was then divided into several fascicles manually and enzymatically treated as described above, and the pressure-clamped single-fiber recording was then applied to individual fibers. Impulses were evoked by electrical stimulation at the proximal site of the sciatic nerve trunk using a suction electrode. The individual nerve fibers recorded were classified into Aβ-, Aδ-, or C-fiber based on the conduction velocity of the antidromic impulses.
Stimulation of mechanoreceptors in whisker hair follicles
Mechanical stimulation was applied to the body of each whisker hair follicle using a blunted 20-gauge needle as a probe. The needle was mounted on a holder and attached to a piezo device. The tip of the needle was positioned at an angle of 45° to the surface of the whisker hair follicle. The piezo device with the mechanical probe was mounted on a Sutter MPC-200 micromanipulator. The piezo device was computer-programmable with the pCLAMP10 software to deliver forward stepwise mechanical stimulation. In each experiment, a receptive field was first probed manually with the mechanical probe controlled by the micromanipulator. Once identified, the vertical position of the probe tip was adjusted such that no nerve impulses were evoked at this position but a 1-µm forward movement of the probe would evoke nerve impulses. Unless otherwise indicated, the stepwise forward movement of the probe consisted of a 100-ms ramp to 38-µm step (dynamic phase) followed by a 2500-ms holding position at the 38-µm step (static phase) and then a 100-ms ramp back to baseline.
Stimulation of mechanoreceptors in the skin
Receptive fields of mechanoreceptors were identified by skin indentation using either von Frey filaments or a mechanical probe fabricated by a glass electrode. The mechanical probe was fire-polished to 500 µm in diameter. It was attached to an electrode holder and positioned vertically to the surface of the skin. The movement of mechanical probe was controlled by a Sutter MPC-200 micromanipulator. In some experiments, the mechanical probe was attached to a piezo device whose movement was computer programmed to produce displacement steps in a ramp-and-hold manner. Unless otherwise indicated, the stepwise forward movement of the probe consisted of a 100-ms ramp to 38-µm step followed by a 2500-ms holding position at the 38-µm step (static phase) and then a 100-ms ramp back to baseline.
Results
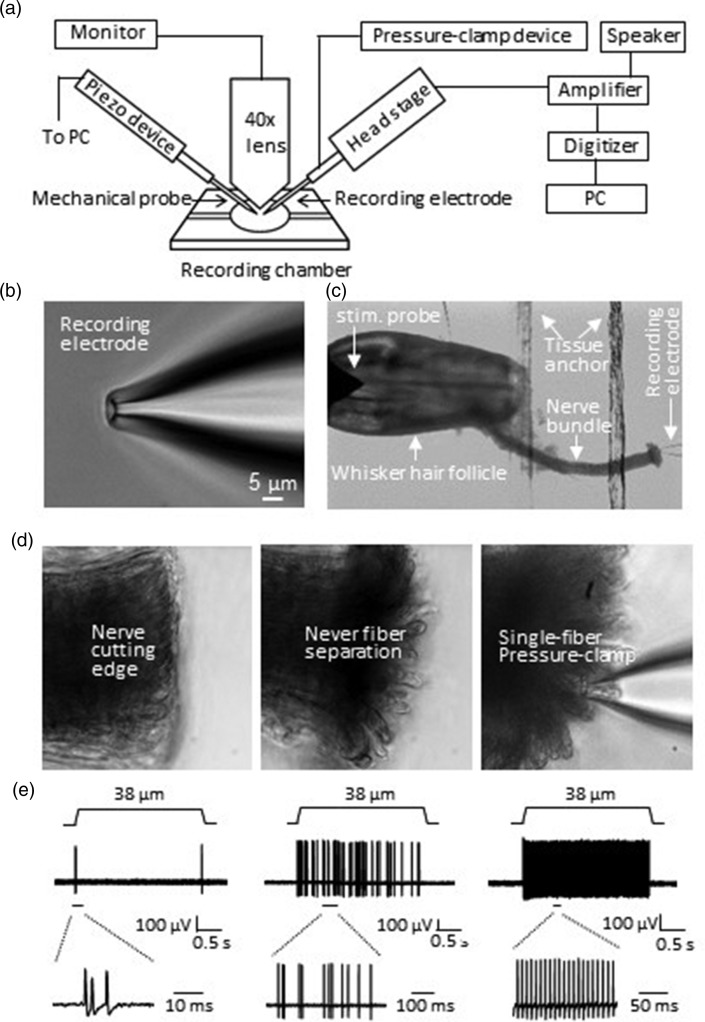
Figure 1(a) shows the arrangement of the pressure-clamped single-fiber recording setup. The setup consists of an Olympus BX50WI upright microscope, a custom-made recording chamber, an electrophysiology recording system whose recording electrode holder was connected with a pressure-clamp device, and a computer-programmable piezo device (Figure 1(a)). The microscope was equipped with a 4× objective for observing tissue samples and a 40× water immersion objective for observing individual nerve fibers. The recording chamber with tissue preparations was mounted on the stage of the microscope and perfused with Krebs bath solution. The pressure-clamp device was used for aspirating a single nerve fiber into recording electrode and for clamping the nerve fiber within the recording electrode during recordings of nerve impulses. In each recording, a fire-polished recording electrode was used and the tip size of the electrode was 3–6 µm in diameters (Figure 1(b)). The recording electrode could be reused multiple times for the pressure-clamped single-fiber recordings. Figure 1(c) to (e) shows an example of our pressure-clamped single-fiber recordings from whisker afferent nerves to study mechanoreceptors in mouse whisker hair follicles. In each experiment, the whisker afferent nerve bundle was first transected with a sharp surgical knife and then briefly enzyme-treated to facilitate the aspiration of individual nerve fibers at the cutting end (Figure 1(d)). To access a single fiber by the recording electrode, we applied a positive pressure of approximately +10 mmHg into the recording electrode to separate individual nerve fibers at the cutting end of the whisker afferent nerve bundle. Then, a negative pressure of approximately −10 mmHg was applied into the recording electrode to gently aspirate the end of a single afferent fiber into the recording electrode. The negative pressure was then adjusted to −5 mmHg to −1 mmHg in the recording electrode, which could maintain stable recordings for more than 2 h (Figure 1(c) to (d)). Sample traces in Figure 1(e) show three types of mechanical responses recorded from three different individual whisker afferent nerve fibers following mechanical displacements of the capsules of whisker hair follicles (Figure 1(e)). These three types of mechanical responses were rapidly adapting (RA) impulses (Figure 1(e), left), slowly adapting type 1 (SA1) impulses (Figure 1(e), middle), and slowly adapting type 2 (SA2) impulses (Figure 1(e), right). In a recent study, we have characterized these three types of mechanoreceptors by using this single-fiber recording technique.9 In the present study, we focused on the technical aspects of this new recording method.
Figure 1.
Setup of the pressure-clamped single-fiber recordings and its application to study mechanical receptors in whisker hair follicles of mice. (a) Schematic diagram illustrating the setup of the pressure-clamped single-fiber recordings. (b) Image showing the tip of a recording electrode used for the pressure-clamped single-fiber recordings. The tip was fire-polished to final tip size of ∼5 µm in diameter. (c) Image taken under a 4× objective shows experimental arrangement for applying the pressure-clamped single-fiber recordings to study low threshold mechanical receptors in an ex vivo whisker hair follicle preparation. (d) Images taken under a 40× objective show the preparation of the cutting end of a whisker afferent nerve bundle and the aspiration of a single fiber into the recording electrode. Individual nerve fibers of the cutting edge (left) was loosened enzymatically and separated by a positive pressure applied through the recording electrode (middle). A single afferent fiber was then aspirated into the recording electrode by a negative pressure of approximately −10 mmHg, and then a negative pressure of −1 to −5 mmHg was maintained during the recordings. (e) Three sets of sample traces show rapidly adapting (RA, left), slowly adapting type 1 (SA1, middle), and slowly adapting type 2 (SA2, right) impulses recorded using the pressure-clamped single-fiber recordings from three different whisker afferent fibers. The lower panels were impulses at expanded time scales.
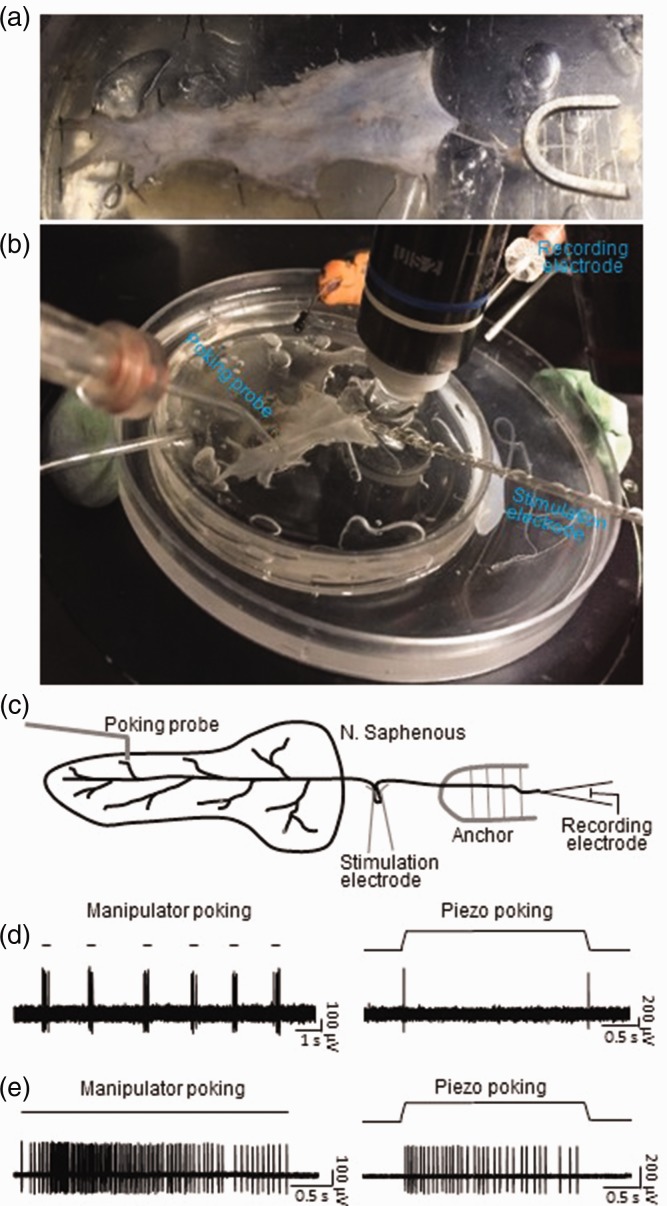
We explored feasibility of applying the pressure-clamped single-fiber recordings to study mechanoreceptors in the skin-nerve preparations of mice. Figure 2(a) shows the skin-nerve preparation made with the shaved hairy skin of a hindpaw and the saphenous nerve that innervated the skin area of the hindpaw. The skin was affixed by tissue pins to the bottom of a recording chamber with the inside of the skin facing up. The saphenous nerve bundle was affixed in the recording chamber with a tissue anchor. Figure 2(b) shows recording setup in the recording chamber, and Figure 2(c) is the schematic diagram of the recording setup. In this set of experiments, the pressure-clamped single-fiber recordings were applied to individual afferent fibers while mechanical stimuli were applied onto the skin. In addition, a suction stimulation electrode was used to deliver electrical stimulation, which allowed us to classify afferent nerve types based on their conduction velocities (Figure 2(c)). Figure 2(d) shows an example of impulses elicited by mechanical stimuli. Impulses were evoked by multiple brief indentations of the skin at 100 µm displacement with the mechanical probe fabricated with a glass tubing (Figure 2(d), left panel). When the indentation was applied stepwise in a ramp-and-hold manner, the mechanical response was shown to be rapidly adapting (Figure 2(d), right panel). The site was also probed with von Frey filaments, which shows a mechanical threshold of 0.07 g in this receptive field. In addition, the conduction velocity of the nerve fiber was measured to be 2.86 m/s based on impulses elicited by electrical stimulation. In a different site, probing with von Frey filament elicited mechanical responses with a threshold of 0.16 g. We applied skin indentation at this site either manually via a manipulator (Figure 2(e), left) or using computerized piezo movement (Figure 2(e), right) for a prolonged period of time, both stimulation methods elicited slowly adapting responses. The conduction velocity of this fiber was 4.56 m/s based on impulse elicited by electrical stimulation.
Figure 2.
Application of the pressure-clamped single-fiber recordings to study mechanical receptors in skin-nerve preparations of mice. (a) Image showing a skin-nerve preparation made from a piece of shaved hairy skin from a mouse hindpaw and the attached saphenous nerve. The skin was affixed to the bottom of the recording chamber by tissue pins and the saphenous nerve was affixed with a tissue anchor. An actual image (b) and schematic diagram (c) of experimental settings on the stage of an upright microscope during the pressure-clamped single-fiber recordings from the skin-nerve preparation. (d) Sample traces showing impulses recorded from a single saphenous nerve fiber following mechanical probing manually operated by a manipulator (left, manipulator probing) or by a computer-programmable piezo device (right, piezo probing). Manipulator probing was applied briefly for multiple times with the indentation step of 100 µm (left). Piezo probing was applied in a ramp-and-hold indentation step of 38 µm for a duration of 2.5 s (right). The mechanoreceptor of this recording shows to be a typical RA type. Similar results were obtained in seven other single-fiber recordings. (e) Sample traces showing impulses recorded from another single saphenous nerve fiber following manipulator probing (left) or piezo probing (right). Manipulator probing was applied with indentation step of 100 µm for a prolonged period of 3.2 s (left). Piezo probing was applied in a ramp-and-hold step of 38 µm and for a duration of 2.5 s (right). The mechanoreceptor of this recording shows to be a typical SA1 type. Similar results were obtained in two other single-fiber recordings.
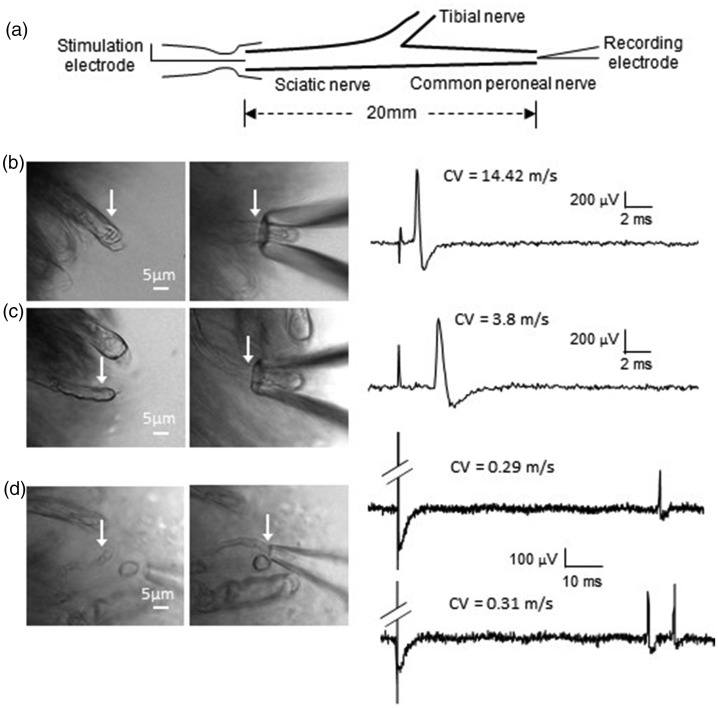
We determined whether the pressure-clamped single-fiber recording may be applied to different types of afferent fibers classified by their conduction velocities. In this set of experiments, sciatic nerve fibers with different diameters were tested (Figure 3, Table 1). The recordings were made from distal end of the common peroneal branch of the sciatic nerve while electrical stimulation was delivered to the proximal site of the sciatic nerve (Figure 3(a)). When recordings were made from fibers of large diameters, we found that the latency of the impulses elicited by electrical stimulation was very short (Figure 3(b)). The conduction velocities calculated based on the latencies and the lengths of the nerve fibers were 17.41 ± 1.1 m/s (n = 15), fell into the category of Aβ-fibers of mice (Figure 3(b), Table 1). Overall, these Aβ-fibers had the diameters of 5.76 ± 0.57 µm (n = 15, Table 1). When recordings were made from fibers of medium diameter, the latencies of the impulses were longer (Figure 3(c)) and conduction velocities of these fibers were 7.4 ± 0.49 ms (n = 23, Figure 3(c), Table 1), fell into the category of Aδ-fibers. These Aδ-fibers had the diameters of 3.96 ± 0.27 µm (n = 23, Table 1). We made recordings from the finest fibers in the sciatic nerve bundle whose diameters were 1.41 ± 0.06 µm (n = 11). We found that the latencies of the impulses were very long (Figure 3(d)) and the conduction velocities were 0.33 ± 0.02 µm (n = 11, Figure 3(d), Table 1), fell into the category of C-fibers. Interestingly, some of the C-fibers fired two to three impulses in response to a single electrical stimulation (Figure 3(d)). Overall, of the 11 C-fibers recorded in response to a single electrical stimulation, five of them each fired a single impulse, and 6 of them each fired two to three impulses (Figure 3(d), Table 1).
Figure 3.
Application of the pressure-clamped single-fiber recordings to record impulses conveyed by Aβ-, Aδ-, and C-fibers of sciatic nerves of mice. (a) Schematic diagram illustrating experimental settings. Impulses are generated at the proximal end of a sciatic nerve by a suction electrode and recorded by the pressure-clamped single-fiber recordings at the distal end of the sciatic nerve (common peroneal nerve). (b) Images showing a large-sized nerve fiber (6.52 µm in diameter, left) whose end was pressure-clamped in a recording electrode (right). Sample trace on right shows an impulse elicited by a suction stimulation electrode as illustrated in (a). The latency between the stimulation artifact and the impulse was short. Impulse conduction velocity was calculated to be 14.42 m/s, fell into Aβ-fiber conduction range. (c) Images showing a medium-sized nerve fiber (4.1 µm in diameter, left) whose end was pressure-clamped in a recording electrode (right). Sample trace on right shows an impulse elicited by a suction stimulation electrode. The latency between the stimulation artifact and the impulse was longer. Impulse conduction velocity was calculated to be 3.8 m/s, fell into Aδ-fiber conduction range. (d) Images showing a small-sized nerve fiber (1.74 µm in diameter, left) whose end was pressure-clamped in a recording electrode (right). Sample trace on top right shows an impulse elicited by a suction electrode. The latency between the stimulation artifact and the impulse was very longer. Impulse conduction velocity was calculated to be 0.29 m/s, fell into C-fiber conduction range. Sample trace on bottom right shows the recording from a different small-sized nerve fiber. In this recording, two impulses were evoked by a single stimulus. The conduction velocity of the first impulse was 0.31 m/s, fell into C-fiber conduction range. CV: conduction velocities.
Table 1.
Types of afferent nerve fibers recorded from mouse sciatic nerves by the pressure-clamped single-fiber recording technique.
| Nerve fiber diameters (µm) | Conduction velocities (m/s) | Impulse numbers | Fiber type classification |
|---|---|---|---|
| 5.76 ± 0.57 (n = 15) | 17.41 ± 1.1 (n = 15) | 1 (n = 15) | Aβ-afferent fibers |
| 3.96 ± 0.27 (n = 23) | 7.4 ± 0.49 (n = 23) | 1 (n = 23) | Aδ-afferent fibers |
| 1.41 ± 0.06 (n = 11) | 0.33 ± 0.02 (n =11) | 1 (n = 5)2 (n = 4) 3 (n = 2) | C-afferent fibers |
Note: Impulses were evoked by electrical stimulation to sciatic nerve bundles with 50-µs square wave pulses. Conduction velocities were calculated based on the lengths of the nerve fibers and the impulse latencies. Afferent fiber types were classified based on conduction velocities (CV) with CV > 10 m/s being Aβ-afferent fibers, 10 m/s ≥ CV ≥ 1 m/s being Aδ-afferent fibers, and CV < 1 m/s being C-afferent fibers.
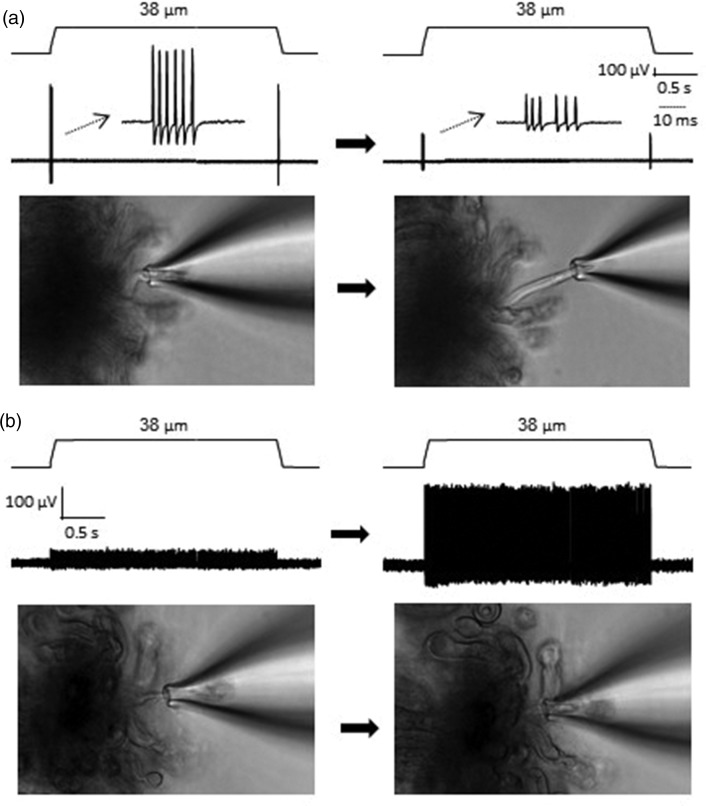
Impulses recorded by the pressure-clamped single-fiber recording usually showed high signal-to-noise ratio. However, the signal, i.e., amplitude of the impulses, could gradually become smaller over the time of recordings in some experiments (Figure 4(a)). We found that the reduction of impulse amplitudes was usually due to recording electrode drift such that the nerve segment inside the recording electrode became too short. In this case, repositioning the recording electrode and aspirating an appropriate length of nerve segment back into the recording electrode usually could restore the amplitude of the impulses. We also found that the amplitudes of impulses could be small if a nerve segment was aspirated too long into the recording electrode. In this case, readjusting the pressure within the electrode is needed to optimize the length of the nerve segment within the electrode, which could enhance the amplitude of the impulses (Figure 4(b)). Overall, the optimal length of the nerve segment within the recording electrode should be kept in approximately 10 to 15 µm.
Figure 4.
Technical considerations of the pressure-clamped single-fiber recordings. (a) Two sample traces on the top show RA impulses recorded from a single whisker afferent nerve fiber at the beginning (left) and 1 h later (right); the RA impulses were evoked by a 38-µm displacement step applied onto the whisker hair follicle in both recordings. The two images on the bottom show the nerve end that was pressure-clamped within the recording electrode at the beginning (left) and 1 h later (right). (b) Sample trace on the top left showing SA2 impulses recorded from a single whisker afferent nerve fiber which was aspirated too deep into the recording electrode (image on the bottom left). The amplitudes of the impulses were increased (top right) after reducing the internal pressure in the recording electrode to readjust the fiber fitting in the recording electrode (bottom right). The SA2 impulses were evoked by a 38-µm displacement step applied onto the whisker hair follicle in both recordings.
Discussion
In the present study, we have described methodological aspects of our newly developed pressure-clamped single-fiber recording technique. This recording method is a true single-fiber recording approach, a key feature different from the commonly used teased-fiber single-unit recording method.12 We have validated our new recording method on tissue preparations including whisker hair follicles, skin-nerve preparations, and sciatic nerves. We show that this new recording method can be conveniently used to reliably record responses of mechanoreceptors in both whisker hair follicle and skin nerve preparations. Furthermore, we have demonstrated that the pressure-clamped single-fiber recording method is applicable for recording impulses carried by different types of afferents including Aβ-, Aδ-, and C-afferent fibers. Thus, the recording method described here provides a new tool that can be used to explore functions of specific sensory receptors including mechanoreceptors, thermal receptors, and nociceptors.
Using the pressure-clamped single-fiber recording technique, we have recently characterized properties of low threshold mechanoreceptors in whisker hair follicles.9 In the present study, we have further illustrated technical details of this recording method so that other researchers in the field can adopt this new recording technique for their studies in sensory physiology and pain. We have demonstrated that our new recording technique is not only suitable for studying mechanical receptors in whisker hair follicles but also can be used for investigating sensory receptors in the skin with the use of nerve-skin preparations. Previously, the teased-fiber single-unit recording technique is the main approach to study mechanoreceptors as well as nociceptors in the skin using nerve-skin preparations.5,6 Although we have not applied our new recording method to study nociceptors in the present study, we have shown with sciatic nerve preparations that the new recording technique can be used to record impulses conveyed by different types of afferents including Aβ-, Aδ-, and C-fibers. Therefore, the pressure-clamped single-fiber recording technique is ready to be used to study nociceptors whose signals are conveyed by Aδ- and C-fibers.
There are a number of technical advantages of our pressure-clamped single-fiber recordings over the teased-fiber single-unit recording method. First, unlike the teased-fiber single-unit recording which is tedious and very time consuming for nerve preparations, our new recording method is simple and takes short time to make nerve preparations. While mechanically splitting nerve bundle into fine filaments is required for teased-fiber single-unit recordings,1 this delicate procedure is not required in our pressure-clamped single-fiber recording technique. This avoids mechanical damage of nerve fibers that would inevitably occur in teased-fiber single-unit recording technique. In teased-fiber single-unit recordings, spike analysis including spike discrimination and sorting are needed to differentiate between single-unit spikes and multiple unit spikes. In contrast, such spike analysis is not needed for the pressure-clamped single-fiber recordings because all impulses recorded come from a single nerve fiber in each recording. The pressure-clamped single-fiber recording technique allows prolonged and stable recordings of impulses for hours in a manner of high signal-to-noise ratio.9 However, in some cases, the amplitudes of impulses became reduced over long time of recordings, which appears to be mainly due to the changes in the fitting of the nerve segment within the recording electrode. This weakness can be solved by monitoring and readjusting the fitting of the nerve segment within the recording electrode, which can be easily achieved by adjusting the pressures in the recording electrode using the pressure clamp device.
In conclusion, the pressure-clamped single-fiber recording technique provides a novel, reliable, and convenient approach to study different types of sensory receptors in different structures including whisker hair follicles, skin tissues, and other sensory organs.
Author Contributions
MS designed and performed the experiments and then analyzed the data. HY participated data interpretation and discussion. JGG conceived the study and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research grants DE018661 and DE023090 to JGG.
ORCID iD
Jianguo G Gu https://orcid.org/0000-0002-8404-9850
References
- 1.Schafers M, Cain D. Single-fiber recording: in vivo and in vitro preparations. Methods Mol Med 2004; 99: 155–166. [DOI] [PubMed] [Google Scholar]
- 2.Tonomura S, Ebara S, Bagdasarian K, Uta D, Ahissar E, Meir I, Lampl I, Kuroda D, Furuta T, Furue H, Kumamoto K. Structure-function correlations of rat trigeminal primary neurons: emphasis on club-like endings, a vibrissal mechanoreceptor. Proc Jpn Acad Ser B Phys Biol Sci 2015; 91: 560–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osteen JD, Herzig V, Gilchrist J, Emrick JJ, Zhang C, Wang X, Castro J, Garcia-Caraballo S, Grundy L, Rychkov GY, Weyer AD, Dekan Z, Undheim EA, Alewood P, Stucky CL, Brierley SM, Basbaum AI, Bosmans F, King GF, Julius D. Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain. Nature 2016; 534: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AK, O’Hara CL, Stucky CL. Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model. Mol Pain 2013; 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014; 509: 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy SE, Loud MC, Daou I, Marshall KL, Schwaller F, Kuhnemund J, Francisco AG, Keenan WT, Dubin AE, Lewin GR, Patapoutian A. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med 2018; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehto SG, Weyer AD, Youngblood BD, Zhang M, Yin R, Wang W, Teffera Y, Cooke M, Stucky CL, Schenkel L, Geuns-Meyer S, Moyer BD, Wild KD, Gavva NR. Selective antagonism of TRPA1 produces limited efficacy in models of inflammatory- and neuropathic-induced mechanical hypersensitivity in rats. Mol Pain 2016; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci USA 2016; 113: E5491–E5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonekatsu M, Gu JG. Functional properties of mechanoreceptors in mouse whisker hair follicles determined by the pressure-clamped single-fiber recording technique. Neurosci Lett 2019; 707: 134321. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell 2014; 157: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, Clapham DE, Reeh PW. Phenotyping sensory nerve endings in vitro in the mouse. Nat Protoc 2009; 4: 174–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng YB, Ringkamp M, Campbell JN, Meyer RA. Electrophysiological assessment of the cutaneous arborization of Adelta-fiber nociceptors. J Neurophysiol 1999; 82: 1164–1177. [DOI] [PubMed] [Google Scholar]